Innovative Methodology for Antimicrobial Susceptibility Determination in Mycoplasma Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Media
2.2. Bacterial Isolates
2.3. Culture Methods
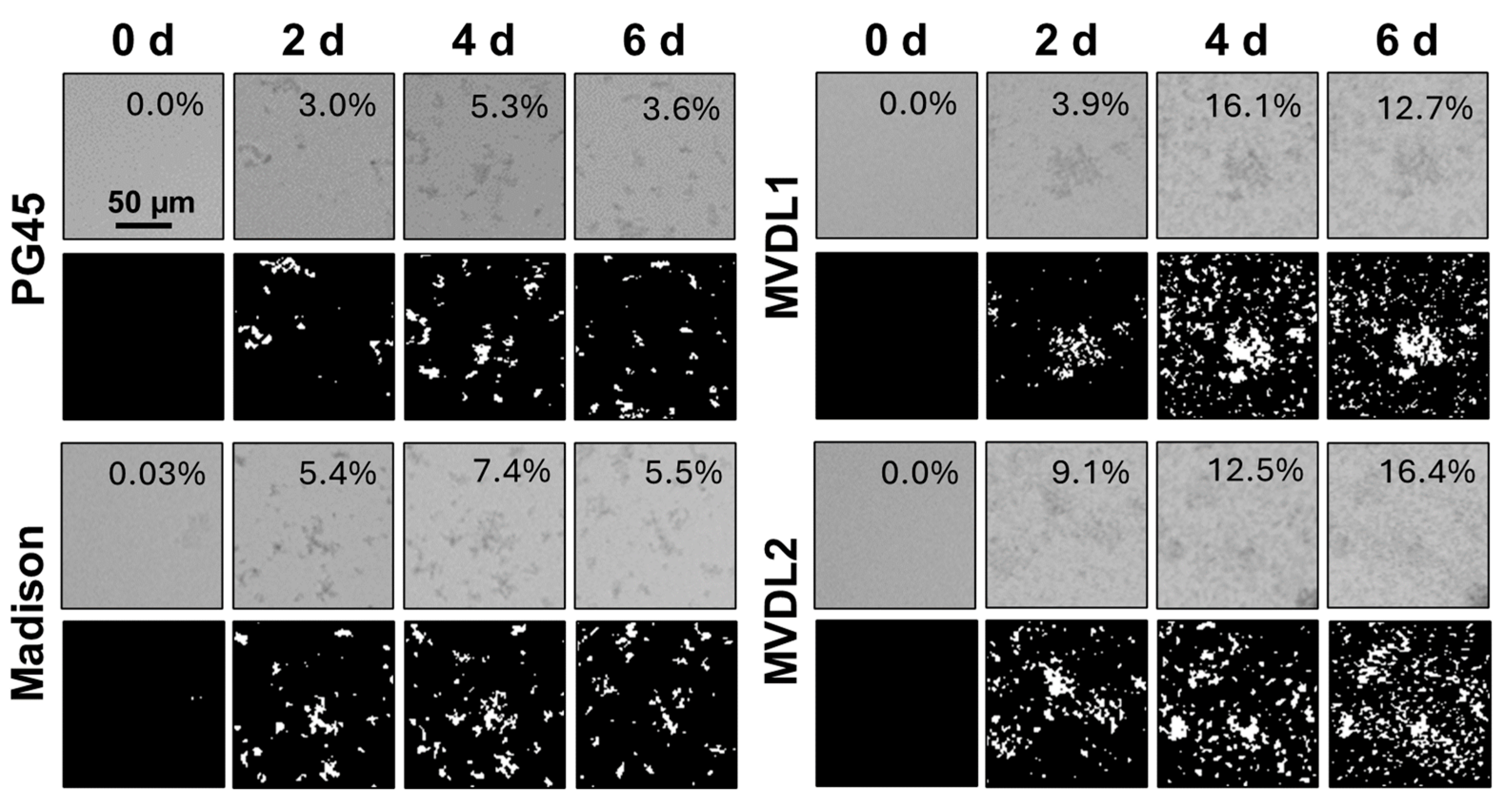
2.4. Biofilm Image Analysis
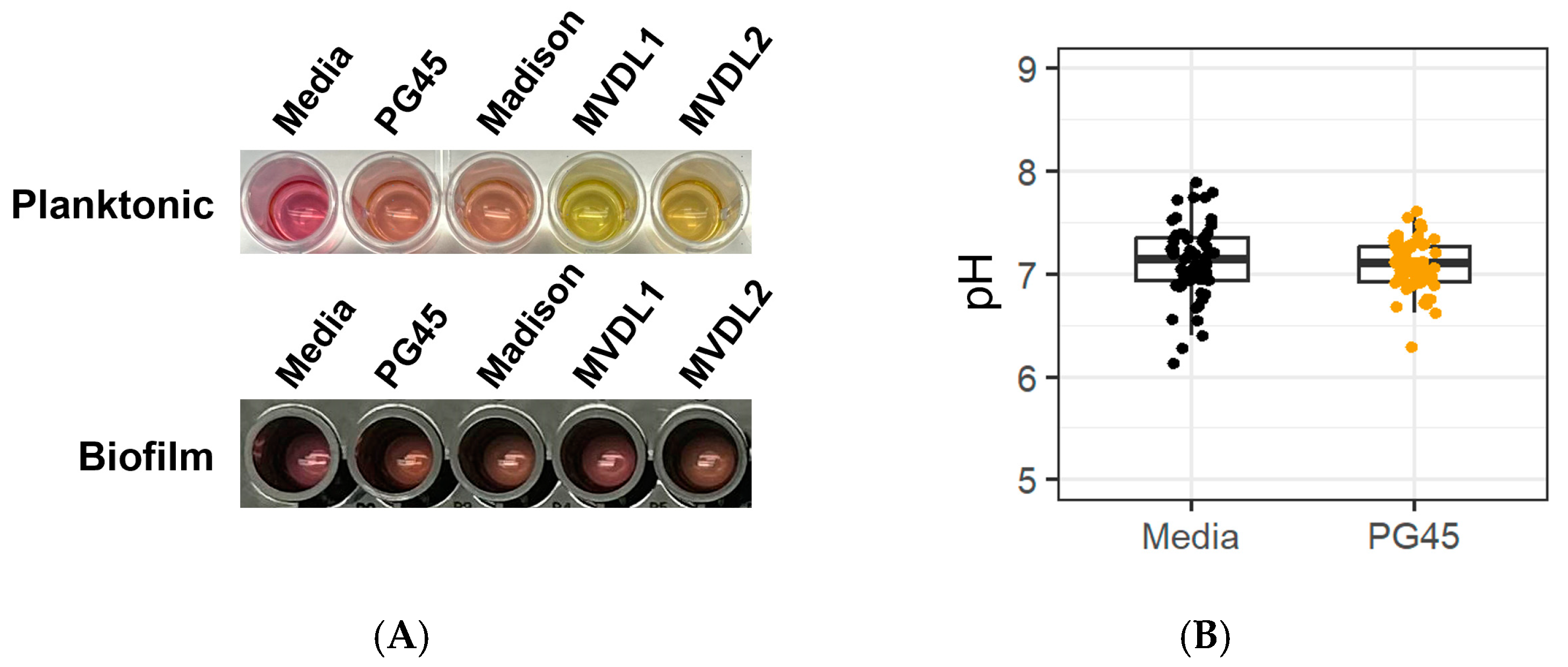
2.5. Acid Production Assay
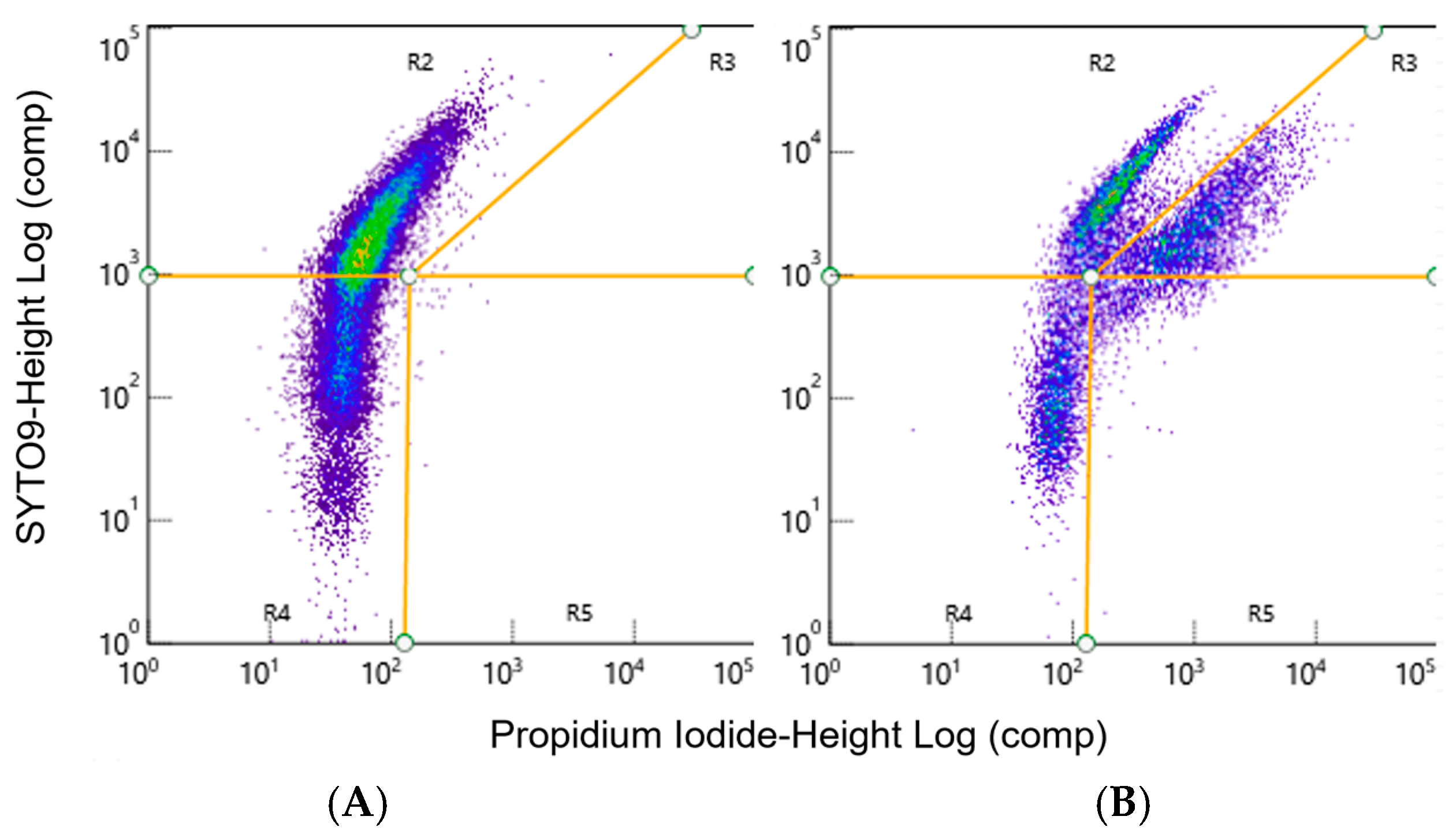
2.6. Quantification of Cells with Flow Cytometry
2.7. Biofilm Disruption
2.8. Imaging Cytometry Analysis
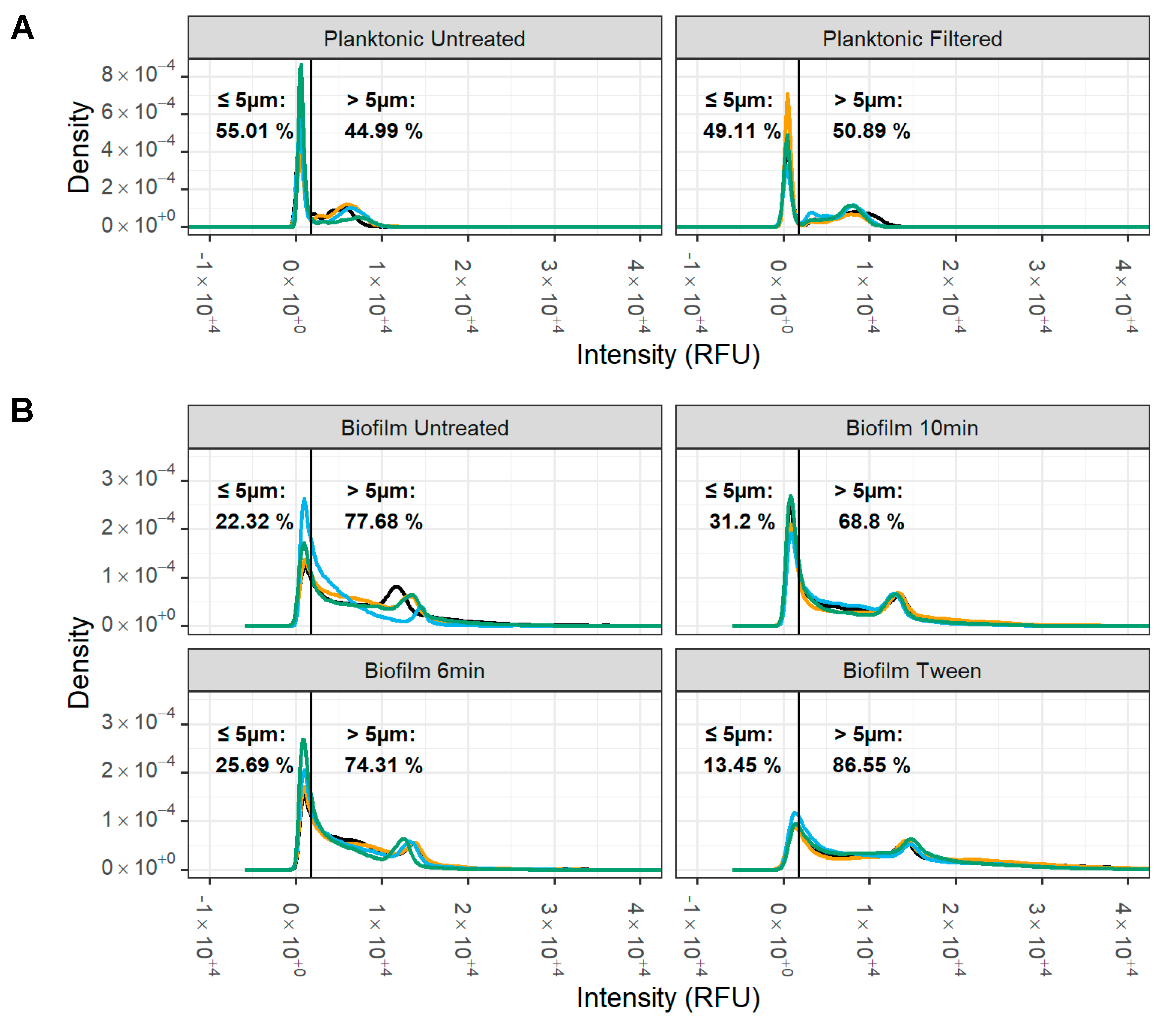
2.9. Small Particle Analysis with Flow Cytometry
2.10. Live/Dead Percentage Quantification
2.11. Disruption Survival Determination
2.12. Cell Adhesion Rate for Newly Adhered Biofilms
2.13. Broth Microdilutions
2.14. LD-AST Assays for Planktonic Mycoplasma spp., Newly Adhered and Mature Biofilm
2.15. Statistical Analysis
3. Results
3.1. Biofilm Maturity Determination
3.2. Acid Production
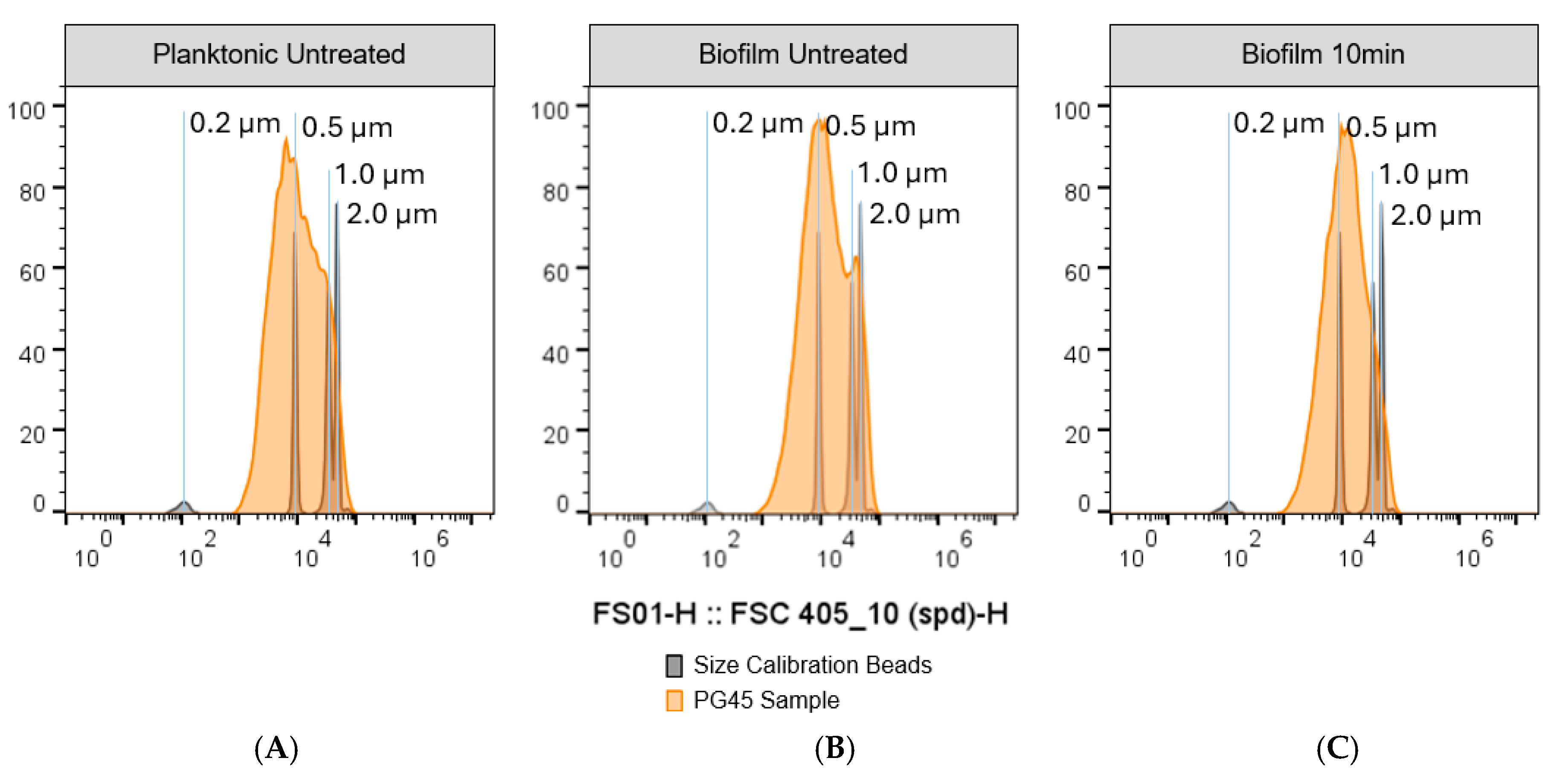
3.3. Particle Size Standardization
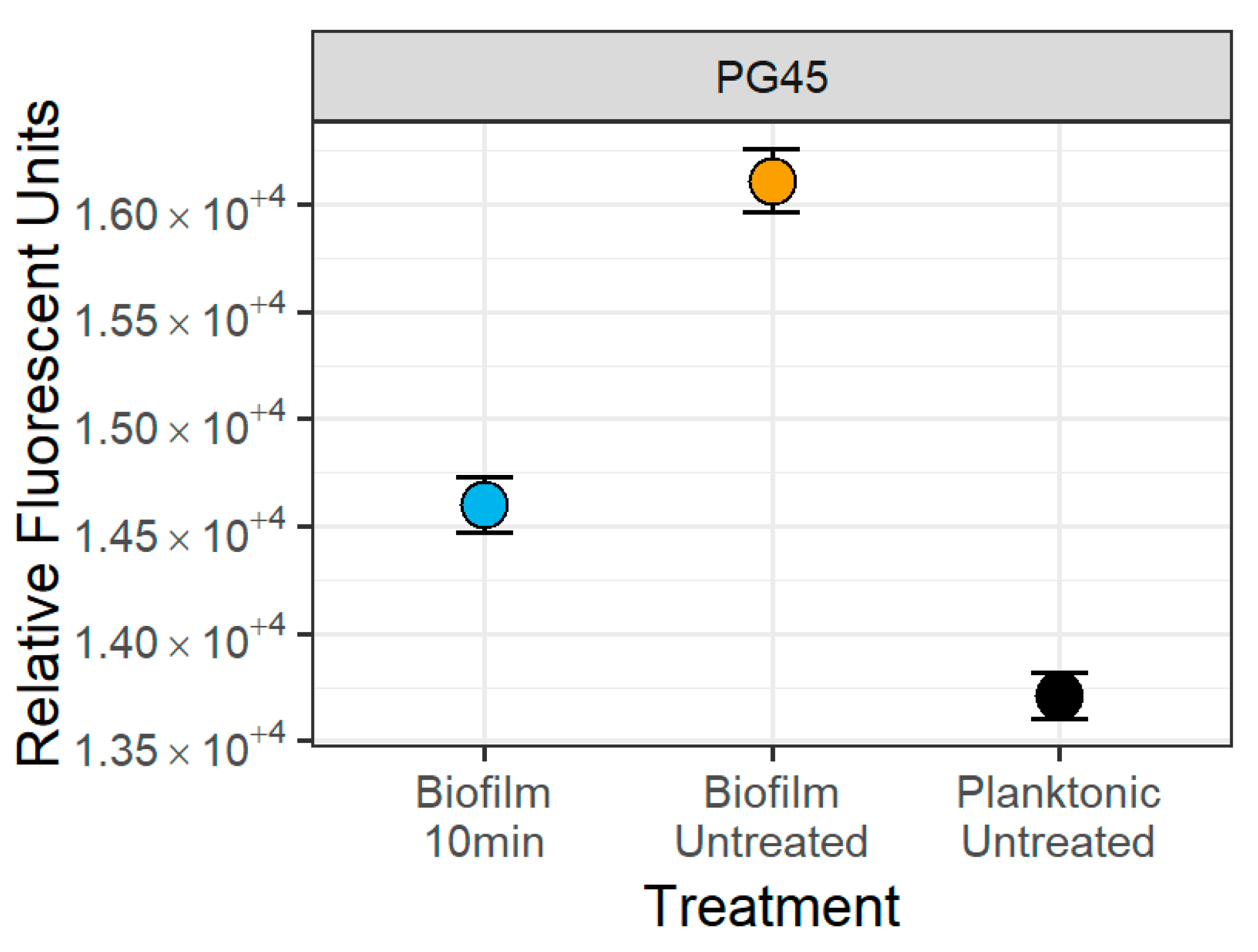
3.4. Small Particle Analysis
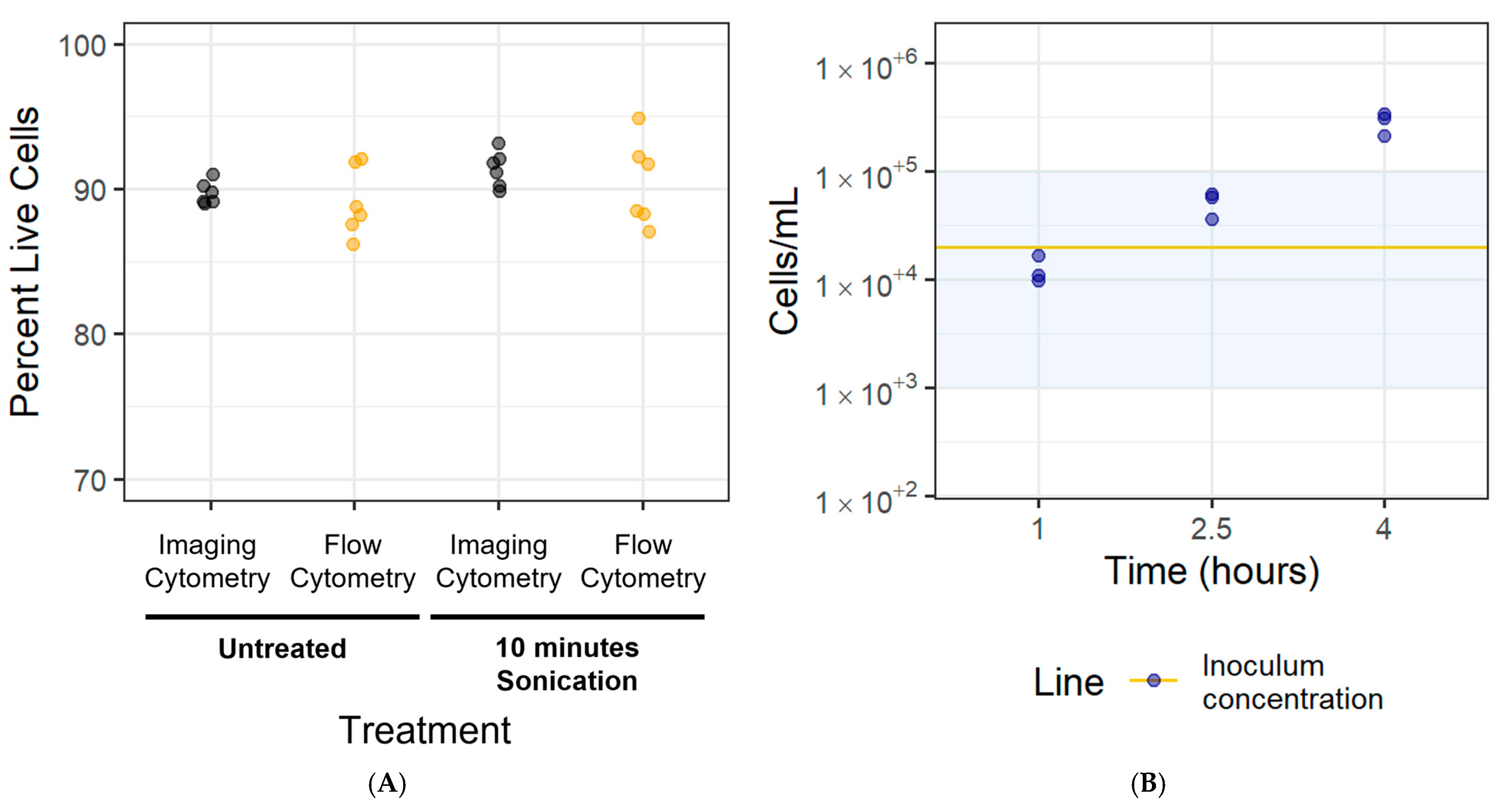
3.5. Disaggregation Method Survival
3.6. Cell Adhesion Rate for Newly Adhered Biofilms
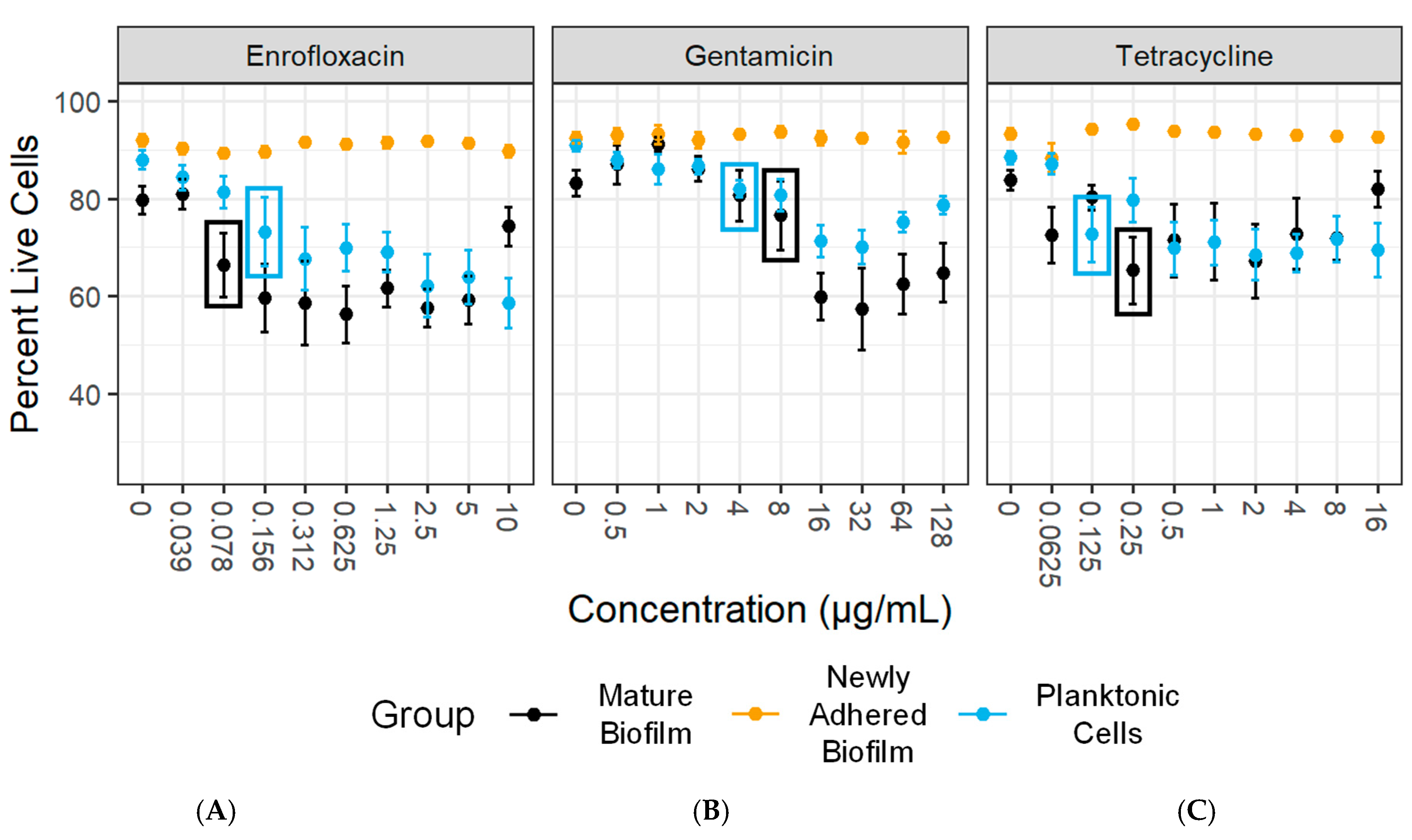
3.7. Antimicrobial Susceptibility Testing
3.8. Statistical Diagnostics Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, B.J.; Larson, B.L. Impact of bovine respiratory disease in U.S. beef cattle. Anim. Health Res. Rev. 2020, 21, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Cusack, P. Evaluation of practices used to reduce the incidence of bovine respiratory disease in Australian feedlots (to November 2021). Aust. Vet. J. 2023, 101, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Punyapornwithaya, V.; Fox, L.K.; Hancock, D.D.; Gay, J.M.; Alldredge, J.R. Association between an outbreak strain causing mycoplasma bovis mastitis and its asymptomatic carriage in the herd: A case study from Idaho, USA. Prev. Vet. Med. 2010, 93, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.J.; Skirpstunas, R.T.; Trujillo, J.D.; Cavender, K.B.; Bagley, C.V.; Harding, R.L. Unusual history and initial clinical signs of Mycoplasma bovis mastitis and arthritis in first-lactation cows in a closed commercial dairy herd. J. Am. Vet. Med. Assoc. 2007, 230, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Bednarek, D.; Lisiecka, U.; Kycko, A.; Reichert, M.; Kostro, K.; Winiarczyk, S. Analysis of the Leukocyte Response in Calves Suffered from Mycoplasma bovis Pneumonia. Pathogens 2020, 9, 407. [Google Scholar] [CrossRef]
- Bringhenti, L.; Pallu, M.; Silva, J.C.; Tomazi, T.; Tomazi, A.C.C.H.; Rodrigues, M.X.; Cruzado-Bravo, M.; Bilby, T.R.; Bicalho, R.C. Effect of treatment of pneumonia and otitis media with tildipirosin or florfenicol + flunixin meglumine on health and upper respiratory tract microbiota of preweaned Holstein dairy heifers. J. Dairy Sci. 2021, 104, 10291–10309. [Google Scholar] [CrossRef]
- Ayling, R.; Bashiruddin, S.; Nicholas, R. Mycoplasma species and related organisms isolated from ruminants in Britain between 1990 and 2000. Vet. Rec. 2004, 155, 413–416. [Google Scholar] [CrossRef]
- Dudek, K.; Nicholas, R.A.J.; Szacawa, E.; Bednarek, D. Mycoplasma bovis Infections—Occurrence, Diagnosis and Control. Pathogens 2020, 9, 640. [Google Scholar] [CrossRef]
- Bokma, J.; Gille, L.; De Bleecker, K.; Callens, J.; Haesebrouck, F.; Pardon, B.; Boyen, F. Antimicrobial Susceptibility of Mycoplasma bovis Isolates from Veal, Dairy and Beef Herds. Antibiotics 2020, 9, 882. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, S.; Li, M.; Zhou, M.; Huo, W.; Gao, J.; Liu, G.; Kastelic, J.P.; Han, B. Molecular characteristics and antibiotic susceptibility profiles of Mycoplasma bovis associated with mastitis on dairy farms in China. Prev. Vet. Med. 2020, 182, 105106. [Google Scholar] [CrossRef]
- García-Galán, A.; Seva, J.; Gómez-Martín, Á.; Ortega, J.; Rodríguez, F.; García-Muñoz, Á.; De la Fe, C. Importance and Antimicrobial Resistance of Mycoplasma bovis in Clinical Respiratory Disease in Feedlot Calves. Animals 2021, 11, 1470. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, A.; McAllister, T.A.; Zaheer, R.; Waldner, M.; Ruzzini, A.C.; Andrés-Lasheras, S.; Parker, S.; Hill, J.E.; Jelinski, M.D. Investigation of Macrolide Resistance Genotypes in Mycoplasma bovis Isolates from Canadian Feedlot Cattle. Pathogens 2020, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Ayling, R.D.; Baker, S.E.; Nicholas, R.A.J.; Peek, M.L.; Simon, A.J. Comparison of in vitro activity of danofloxacin, florfenicol, oxytetracycline, spectinomycin and tilmicosin against recent field isolates of Mycoplasma bovis. Vet. Rec. 2000, 146, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Francoz, D.; Fortin, M.; Fecteau, G.; Messier, S. Determination of Mycoplasma bovis susceptibilities against six antimicrobial agents using the E test method. Vet. Microbiol. 2005, 105, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Klein, U.; de Jong, A.; Youala, M.; El Garch, F.; Stevenin, C.; Moyaert, H.; Rose, M.; Catania, S.; Gyuranecz, M.; Pridmore, A.; et al. New antimicrobial susceptibility data from monitoring of Mycoplasma bovis isolated in Europe. Vet. Microbiol. 2019, 238, 108432. [Google Scholar] [CrossRef]
- Ayling, R.D.; Rosales, R.S.; Barden, G.; Gosney, F.L. Changes in antimicrobial susceptibility of Mycoplasma bovis isolates from Great Britain. Vet. Rec. 2014, 175, 486. [Google Scholar] [CrossRef]
- Barber, D.M.; Jones, G.E.; Wood, A. Microbial flora of the eyes of cattle. Vet. Rec. 1986, 118, 204–206. [Google Scholar] [CrossRef]
- Kahane, I.; Adoni, A. Rapid Diagnosis of Mycoplasmas; Springer: New York, NY, USA, 2012. [Google Scholar]
- Levisohn, S.; Garazi, S.; Gerchman, I.; Brenner, J. Diagnosis of a mixed mycoplasma infection associated with a severe outbreak of bovine pinkeye in young calves. J. Vet. Diagn. Invest. 2004, 16, 579–581. [Google Scholar] [CrossRef]
- Loy, J.D.; Clothier, K.A.; Maier, G. Component Causes of Infectious Bovine Keratoconjunctivitis—Non-Moraxella Organisms in the Epidemiology of Infectious Bovine Keratoconjunctivitis. Vet. Clin. Food Anim. Pract. 2021, 37, 295–308. [Google Scholar] [CrossRef]
- Midla, L. Infectious bovine keratoconjunctivitis: A practical yet evidence-based approach to treatment and prevention on dairy farms. In Proceedings of the 54th Annual Conference Proceedings of the American Library Association, New Orleans, LA, USA, 25–30 April 2021; pp. 80–83. [Google Scholar]
- Rosenbusch, R.; Knudtson, W. Bovine mycoplasmal conjunctivitis: Experimental reproduction and characterization of the disease. Cornell Vet. 1980, 70, 307–320. [Google Scholar]
- Friis, N.F.; Pedersen, K.B. Isolation of Mycoplasma bovoculi from cases of infectious bovine keratoconjunctivitis. Acta Vet. Scand. 1979, 20, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Schnee, C.; Heller, M.; Schubert, E.; Sachse, K. Point prevalence of infection with Mycoplasma bovoculi and Moraxella spp. in cattle at different stages of infectious bovine keratoconjunctivitis. Vet. J. 2015, 203, 92–96. [Google Scholar] [CrossRef]
- Thacker, E.L. Mycoplasmal diseases. Dis. Swine 2006, 9, 701–717. [Google Scholar]
- Besser, T.E.; Levy, J.; Ackerman, M.; Nelson, D.; Manlove, K.; Potter, K.A.; Busboom, J.; Benson, M. A pilot study of the effects of Mycoplasma ovipneumoniae exposure on domestic lamb growth and performance. PLoS ONE 2019, 14, e0207420. [Google Scholar] [CrossRef] [PubMed]
- Goltz, J.P.; Rosendal, S.; McCraw, B.M.; Ruhnke, H.L. Experimental studies on the pathogenicity of Mycoplasma ovipneumoniae and Mycoplasma arginini for the respiratory tract of goats. Can. J. Vet. Res. 1986, 50, 59–67. [Google Scholar]
- Resende, T.P.; Pieters, M.; Vannucci, F.A. Swine conjunctivitis outbreaks associated with Mycoplasma hyorhinis. J. Vet. Diagn. Investig. 2019, 31, 766–769. [Google Scholar] [CrossRef]
- Dhondt, A.A.; States, S.L.; Dhondt, K.V.; Schat, K.A. Understanding the origin of seasonal epidemics of mycoplasmal conjunctivitis. J. Anim. Ecol. 2012, 81, 996–1003. [Google Scholar] [CrossRef]
- Baumworcel, N.; Soares, A.M.B.; Silva, S.B.; Almeida, N.K.O.; de Castro, T.X. Correlation between clinical signs of feline conjunctivitis and molecular detection of felid herpesvirus-1, feline calicivirus, Chlamydophila felis and Mycoplasma felis in cats from shelters in Rio de Janeiro. Braz. J. Vet. Res. Anim. Sci. 2017, 54, 18–26. [Google Scholar] [CrossRef]
- CLSI—Clinical and Laboratory Standards Institute. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Hannan, P.C. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. International Research Programme on Comparative Mycoplasmology. Vet. Res. 2000, 31, 373–395. [Google Scholar] [CrossRef]
- Gütgemann, F.; Müller, A.; Churin, Y.; Kumm, F.; Braun, A.S.; Yue, M.; Eisenberg, T.; Entorf, M.; Peters, T.; Kehrenberg, C. Toward a Method for Harmonized Susceptibility Testing of Mycoplasma bovis by Broth Microdilution. J. Clin. Microbiol. 2023, 61, e01905-22. [Google Scholar] [CrossRef]
- Balish, M.F.; Chopra-Dewasthaly, R.; Pereyre, S.; Ramírez, A.S.; Viver, T.; Spergser, J. Mycoplasma. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2024; pp. 1–101. [Google Scholar]
- Waites, K.B.; Crabb, D.M.; Duffy, L.B.; Jensen, J.S.; Liu, Y.; Paukner, S. In Vitro Activities of Lefamulin and Other Antimicrobial Agents against Macrolide-Susceptible and Macrolide-Resistant Mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob. Agents Chemother. 2017, 61, e02008-16. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Crabb, D.M.; Duffy, L.B. Inhibitory and bactericidal activities of gemifloxacin and other antimicrobials against Mycoplasma pneumoniae. Int. J. Antimicrob. Agents 2003, 21, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Tavío, M.M.; Poveda, C.; Assunção, P.; Ramírez, A.S.; Poveda, J.B. In vitro activity of tylvalosin against Spanish field strains of Mycoplasma hyopneumoniae. Vet. Rec. 2014, 175, 539. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cai, Y.; Slimmen, L.J.M.; de Bruijn, A.; van Rossum, A.M.C.; Folkerts, G.; Braber, S.; Unger, W.W.J. Galacto-Oligosaccharides as an Anti-Infective and Anti-Microbial Agent for Macrolide-Resistant and -Sensitive Mycoplasma pneumoniae. Pathogens 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.J., Jr.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 2013, 13, 47. [Google Scholar] [CrossRef]
- Feng, C.; Huang, Y.; Yu, Y.; Duan, G.; Dai, Y.; Dongs, K.; Li, Q. Effects on quinolone resistance due to the biofilm formation activity in Ureaplasma urealyticum. Turk. J. Med. Sci. 2015, 45, 55–59. [Google Scholar] [CrossRef]
- Marchi, A.P.; Farrel Côrtes, M.; Vásconez Noguera, S.; Rossi, F.; Levin, A.S.; Figueiredo Costa, S.; Perdigão Neto, L.V. Chlorhexidine susceptibility and Eagle effect in planktonic cells and biofilm of nosocomial isolates. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 787–792. [Google Scholar] [CrossRef]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial Persister Cell Formation and Dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef]
- McAuliffe, L.; Ellis, R.J.; Miles, K.; Ayling, R.D.; Nicholas, R.A.J. Biofilm formation by mycoplasma species and its role in environmental persistence and survival. Microbiology 2006, 152, 913–922. [Google Scholar] [CrossRef]
- Burki, S.; Frey, J.; Pilo, P. Virulence, persistence and dissemination of Mycoplasma bovis. Vet. Microbiol. 2015, 179, 15–22. [Google Scholar] [CrossRef]
- Jacques, M.; Aragon, V.; Tremblay, Y.D.N. Biofilm formation in bacterial pathogens of veterinary importance. Anim. Health Res. Rev. 2010, 11, 97–121. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Saavedra, M.J.; Henriques, M. Bovine mastitis disease/pathogenicity: Evidence of the potential role of microbial biofilms. Pathog. Dis. 2016, 74, ftw006. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hao, H.; Zhao, P.; Ji, W.; Li, M.; Liu, Y.; Chu, Y. Differential Immunoreactivity to Bovine Convalescent Serum Between Mycoplasma bovis Biofilms and Planktonic Cells Revealed by Comparative Immunoproteomic Analysis. Front. Microbiol. 2018, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Schaff, A.C.; Balish, M.F. Mycoplasma pneumoniae biofilms grown in vitro: Traits associated with persistence and cytotoxicity. Microbiology 2020, 166, 629–640. [Google Scholar] [CrossRef]
- Kang, T.; Zhou, M.; Yan, X.; Song, S.; Yuan, S.; Yang, H.; Ding, H.; Jiang, H.; Zhang, D.; Bai, Y.; et al. Biofilm formation and correlations with drug resistance in Mycoplasma synoviae. Vet. Microbiol. 2023, 283, 109777. [Google Scholar] [CrossRef]
- Andrés-Lasheras, S.; Zaheer, R.; Jelinski, M.; McAllister, T.A. Role of biofilms in antimicrobial resistance of the bacterial bovine respiratory disease complex. Front. Vet. Sci. 2024, 11, 1353551. [Google Scholar] [CrossRef]
- Awadh, A.A.; Le Gresley, A.; Forster-Wilkins, G.; Kelly, A.F.; Fielder, M.D. Determination of metabolic activity in planktonic and biofilm cells of Mycoplasma fermentans and Mycoplasma pneumoniae by nuclear magnetic resonance. Sci. Rep. 2021, 11, 5650. [Google Scholar] [CrossRef]
- Carvalhais, V.; França, A.; Cerca, F.; Vitorino, R.; Pier, G.B.; Vilanova, M.; Cerca, N. Dormancy within Staphylococcus epidermidis biofilms: A transcriptomic analysis by RNA-seq. Appl. Microbiol. Biotechnol. 2014, 98, 2585–2596. [Google Scholar] [CrossRef]
- Kim, J.; Hahn, J.-S.; Franklin, M.J.; Stewart, P.S.; Yoon, J. Tolerance of dormant and active cells in Pseudomonas aeruginosa PA01 biofilm to antimicrobial agents. J. Antimicrob. Chemother. 2008, 63, 129–135. [Google Scholar] [CrossRef]
- Chernova, O.A.; Medvedeva, E.S.; Mouzykantov, A.A.; Baranova, N.B.; Chernov, V.M. Mycoplasmas and Their Antibiotic Resistance: The Problems and Prospects in Controlling Infections. Acta Naturae 2016, 8, 24–34. [Google Scholar] [CrossRef]
- Parray, O.R.; Haq, I.U.; Muhee, A.; Shaheen, M.; Yatoo, M.I. A mini-review on antimycoplasma antibiotics. Appl. Vet. Res. 2022, 1, e2022002. [Google Scholar] [CrossRef]
- Van Duijkeren, E.; Schwarz, C.; Bouchard, D.; Catry, B.; Pomba, C.; Baptiste, K.E.; Moreno, M.A.; Rantala, M.; Ružauskas, M.; Sanders, P.; et al. The use of aminoglycosides in animals within the EU: Development of resistance in animals and possible impact on human and animal health: A review. J. Antimicrob. Chemother. 2019, 74, 2480–2496. [Google Scholar] [CrossRef] [PubMed]
- Giguère, S.; Dowling, P.M. Fluoroquinolones. Antimicrob. Ther. Vet. Med. 2013, 295–314. [Google Scholar]
- Del Castillo, J.R. Tetracyclines. Antimicrob. Ther. Vet. Med. 2013, 257–268. [Google Scholar]
- Apley, M.D.; Coetzee, J.F. Antimicrobial drug use in cattle. Antimicrob. Ther. Vet. Med. 2013, 495–518. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Busschots, S.; O’Toole, S.; O’Leary, J.J.; Stordal, B. Non-invasive and non-destructive measurements of confluence in cultured adherent cell lines. MethodsX 2015, 2, 8–13. [Google Scholar] [CrossRef]
- Bio-Rad. Everest Software, 3.2.12.0; Bio-Rad: Hercules, CA, USA, 2024.
- Totten, A.H.; Xiao, L.; Crabb, D.M.; Ratliff, A.E.; Dybvig, K.; Waites, K.B.; Atkinson, T.P. Shaken or stirred?: Comparison of methods for dispersion of Mycoplasma pneumoniae aggregates for persistence in vivo. J. Microbiol. Methods 2017, 132, 56–62. [Google Scholar] [CrossRef]
- Alsharif, R.; Godfrey, W.; Bacterial Detection and Live/Dead Discrimination by Flow Cytometry. Microbial Cytometry Application Note. BD Biosciences, Immunocytometry Systems. 2002. Available online: https://www.technologynetworks.com/diagnostics/application-notes/bacterial-detection-and-livedead-discrimination-by-flow-cytometry-228589 (accessed on 23 July 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Assunção, P.; Diaz, R.; Comas, J.; de Galarreta, C.M.R.; González-Llamazares, O.R.; Poveda, J.B. Evaluation of Mycoplasma hyopneumoniae growth by flow cytometry. J. Appl. Microbiol. 2005, 98, 1047–1054. [Google Scholar] [CrossRef]
- Uenoyama, A.; Miyata, M. Gliding ghosts of Mycoplasma mobile. Proc. Natl. Acad. Sci. USA 2005, 102, 12754–12758. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Goode, K.; Rey, K. ggResidpanel: Panels and Interactive Versions of Diagnostic Plots Using ‘ggplot2’, R package version 0.3.0; Comprehensive R Archive Network: Online, 2019.
- Lüdecke, D. sjPlot: Data Visualization for Statistics in Social Science, R package version 2.8.15; Comprehensive R Archive Network: Online, 2023.
- Wickham, H.; Henry, L. purrr: Functional Programming Tools, R package version 1.0.2; Comprehensive R Archive Network: Online, 2023.
- Wickham, H. stringr: Simple, Consistent Wrappers for Common String Operations, R package version 1.5.1; Comprehensive R Archive Network: Online, 2023.
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests, R package version 0.7.2; Comprehensive R Archive Network: Online, 2023.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Comprehensive R Archive Network: Online, 2016. [Google Scholar]
- Arnold, J.B. ggthemes: Extra Themes, Scales and Geoms for ‘ggplot2’; R Package Version 5.1.0; Comprehensive R Archive Network: Online, 2024. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots; R Package Version 0.6.0; Comprehensive R Archive Network: Online, 2023. [Google Scholar]
- Kleinschmidt, S.; Spergser, J.; Rosengarten, R.; Hewicker-Trautwein, M. Long-term survival of Mycoplasma bovis in necrotic lesions and in phagocytic cells as demonstrated by transmission and immunogold electron microscopy in lung tissue from experimentally infected calves. Vet. Microbiol. 2013, 162, 949–953. [Google Scholar] [CrossRef]
- Maniloff, J.; Morowitz, H.J. Cell biology of the mycoplasmas. Bacteriol. Rev. 1972, 36, 263–290. [Google Scholar] [CrossRef]
- Schielzeth, H.; Dingemanse, N.J.; Nakagawa, S.; Westneat, D.F.; Allegue, H.; Teplitsky, C.; Réale, D.; Dochtermann, N.A.; Garamszegi, L.Z.; Araya-Ajoy, Y.G. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol. Evol. 2020, 11, 1141–1152. [Google Scholar] [CrossRef]
- Dapore, Z. Hydrogen peroxide and the Mycoplasma pneumoniae biofilm. Master’s Thesis, Miami University, Oxford, OH, USA, 2022. [Google Scholar]
- Wang, Y.; Yi, L.; Zhang, F.; Qiu, X.; Tan, L.; Yu, S.; Cheng, X.; Ding, C. Identification of genes involved in Mycoplasma gallisepticum biofilm formation using mini-Tn4001-SGM transposon mutagenesis. Vet. Microbiol. 2017, 198, 17–22. [Google Scholar] [CrossRef]
- Stewart, P.S. Diffusion in biofilms. J. Bacteriol. 2003, 185, 1485–1491. [Google Scholar] [CrossRef]
- Soehnlen, M.K.; Kunze, M.E.; Karunathilake, K.E.; Henwood, B.M.; Kariyawasam, S.; Wolfgang, D.R.; Jayarao, B.M. In vitro antimicrobial inhibition of Mycoplasma bovis isolates submitted to the Pennsylvania Animal Diagnostic Laboratory using flow cytometry and a broth microdilution method. J. Vet. Diagn. Investig. 2011, 23, 547–551. [Google Scholar] [CrossRef]
- Assunção, P.; Antunes, N.T.; Rosales, R.S.; Poveda, C.; De La Fe, C.; Poveda, J.B.; Davey, H.M. Application of flow cytometry for the determination of minimal inhibitory concentration of several antibacterial agents on Mycoplasma hyopneumoniae. J. Appl. Microbiol. 2007, 102, 1132–1137. [Google Scholar] [CrossRef]
- Sulyok, K.M.; Kreizinger, Z.; Fekete, L.; Hrivnák, V.; Magyar, T.; Jánosi, S.; Schweitzer, N.; Turcsányi, I.; Makrai, L.; Erdélyi, K.; et al. Antibiotic susceptibility profiles of Mycoplasma bovis strains isolated from cattle in Hungary, Central Europe. BMC Vet. Res. 2014, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Trunk, T.; Khalil, H.S.; Leo, J.C. Bacterial autoaggregation. AIMS Microbiol. 2018, 4, 140–164. [Google Scholar] [CrossRef] [PubMed]
- Caceres Silvia, M.; Malcolm Kenneth, C.; Taylor-Cousar Jennifer, L.; Nichols David, P.; Saavedra Milene, T.; Bratton Donna, L.; Moskowitz Samuel, M.; Burns Jane, L.; Nick Jerry, A. Enhanced In Vitro Formation and Antibiotic Resistance of Nonattached Pseudomonas aeruginosa Aggregates through Incorporation of Neutrophil Products. Antimicrob. Agents Chemother. 2014, 58, 6851–6860. [Google Scholar] [CrossRef] [PubMed]
- Wirth, S.M.; Lowry, G.V.; Tilton, R.D. Natural Organic Matter Alters Biofilm Tolerance to Silver Nanoparticles and Dissolved Silver. Environ. Sci. Technol. 2012, 46, 12687–12696. [Google Scholar] [CrossRef]
- Folkesson, A.; Haagensen, J.A.J.; Zampaloni, C.; Sternberg, C.; Molin, S. Biofilm Induced Tolerance towards Antimicrobial Peptides. PLoS ONE 2008, 3, e1891. [Google Scholar] [CrossRef]
- Mah, T.-F. Biofilm-Specific Antibiotic Resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Lynch, S.S. Tolerance and Resistance; Merck Manual Professional Version: Rahway, NJ, USA, 2022. [Google Scholar]
- Stewart, P.S.; William Costerton, J. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [PubMed]
- Prasetyoputri, A.; Jarrad, A.M.; Cooper, M.A.; Blaskovich, M.A.T. The Eagle Effect and Antibiotic-Induced Persistence: Two Sides of the Same Coin? Trends Microbiol. 2019, 27, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Walraven, C.J.; Bernardo, S.M.; Wiederhold, N.P.; Lee, S.A. Paradoxical antifungal activity and structural observations in biofilms formed by echinocandin-resistant Candida albicans clinical isolates. Med. Mycol. 2013, 52, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Nahum, Y.; Gross, N.; Cerrone, A.; Matouš, K.; Nerenberg, R. Effect of biofilm physical characteristics on their susceptibility to antibiotics: Impacts of low-frequency ultrasound. npj Biofilms Microbiomes 2024, 10, 70. [Google Scholar] [CrossRef]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Maslowska, K.H.; Makiela-Dzbenska, K.; Fijalkowska, I.J. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 2019, 60, 368–384. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Yang, F.; Li, J.; Guo, L.; Guo, Y.; He, S. Drug sensitivity and genome-wide analysis of two strains of Mycoplasma gallisepticum with different biofilm intensity. Front. Microbiol. 2023, 14, 1196747. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, H.; Liu, Y.; Jiang, Y.; Xin, J.; Chen, W.; Song, Z. The Complete Genome Sequence of Mycoplasma bovis Strain Hubei-1. PLoS ONE 2011, 6, e20999. [Google Scholar] [CrossRef]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA Chelates Cations and Induces Antibiotic Resistance in Pseudomonas aeruginosa Biofilms. PLOS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef]
- Raymond, B.B.A.; Jenkins, C.; Turnbull, L.; Whitchurch, C.B.; Djordjevic, S.P. Extracellular DNA release from the genome-reduced pathogen Mycoplasma hyopneumoniae is essential for biofilm formation on abiotic surfaces. Sci. Rep. 2018, 8, 10373. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [PubMed]
- Ledger, L.; Eidt, J.; Cai, H.Y. Identification of Antimicrobial Resistance-Associated Genes through Whole Genome Sequencing of Mycoplasma bovis Isolates with Different Antimicrobial Resistances. Pathogens 2020, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Simmons, W.L.; Bolland, J.R.; Daubenspeck, J.M.; Dybvig, K. A stochastic mechanism for biofilm formation by Mycoplasma pulmonis. J. Bacteriol. 2007, 189, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Barkhatova, O.I.; Andreevskaya, S.G.; Alekseeva, N.V.; Zhukhovitsky, V.G.; Rakovskaya, I.V. In vitro Biofilm Formation by the Respiratory Mycoplasmosis Pathogen Mycoplasma pneumonia. Mol. Genet. Microbiol. Virol. 2019, 34, 170–175. [Google Scholar] [CrossRef]
- Chen, H.; Yu, S.; Hu, M.; Han, X.; Chen, D.; Qiu, X.; Ding, C. Identification of biofilm formation by Mycoplasma gallisepticum. Vet. Microbiol. 2012, 161, 96–103. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Hendrickson, G.R.; Lyon, L.A. Microgel Translocation through Pores under Confinement. Angew. Chem. Int. Ed. 2010, 49, 2193–2197. [Google Scholar] [CrossRef]

| Mycoplasma Isolate | Source | Isolation Source | Year Isolated |
|---|---|---|---|
| M. bovis strain Donetta PG45 | American Type Culture Collection (ATCC) 25523 | Bovine mastitis | 1962 |
| M. bovis strain Madison | ATCC 27368 | Dairy products | 1971 |
| M. bovoculi Clinical Isolate MVDL1 | Montana Veterinary Diagnostic Lab (MVDL) | Bovine conjunctivitis | 2023 |
| M. bovoculi Clinical Isolate MVDL2 | MVDL | Bovine conjunctivitis | 2023 |
| Mycoplasma Isolate | Mature Biofilm Confluence | Largest Structure Size |
|---|---|---|
| PG45 | 10.42 ± 1.89% (4 days) | 29.50 ± 8.10 µm (4 days) |
| Madison | 8.99 ± 0.81% (5 days) | 30.40 ± 9.38 µm (2 days) |
| MVDL1 | 26.38 ± 4.12% (5 days) | 60.48 ± 25.18 µm (3 days) |
| MVDL2 | 22.84 ± 2.94% (5 days) | 50.09 ± 13.82 µm (2 days) |
| Antimicrobial Agent | Mycoplasma bovis State | |||
|---|---|---|---|---|
| Mycoplasma Isolate | Planktonic | Newly Adhered Biofilm | Mature Biofilm | |
| PG45 | Enrofloxacin | 0.156 | >10 1 | 0.078 |
| Gentamicin | 4 | >128 1 | 8 | |
| Tetracycline | 0.125 | >16 1 | 0.25 | |
| Madison | Enrofloxacin | 0.078 | 0.078 | 0.078 |
| Gentamicin | 8 | 16 | 2 | |
| Tetracycline | 0.125 | <0.0625 2 | <0.0625 2 | |
| MVDL1 | Enrofloxacin | <0.039 2 | >10 1 | 0.312 |
| Gentamicin | 2 | >128 1 | 16 | |
| Tetracycline | <0.0625 2 | >16 1 | 0.5 | |
| MVDL2 | Enrofloxacin | 0.078 | >10 1 | 0.078 |
| Gentamicin | 16 | >128 1 | 4 | |
| Tetracycline | 0.125 | >16 1 | <0.0625 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobson, B.T.; DeWit-Dibbert, J.; Selong, E.T.; Quirk, M.; Throolin, M.; Corona, C.; Sonar, S.; Zanca, L.; Schwarz, E.R.; Bimczok, D. Innovative Methodology for Antimicrobial Susceptibility Determination in Mycoplasma Biofilms. Microorganisms 2024, 12, 2650. https://doi.org/10.3390/microorganisms12122650
Jacobson BT, DeWit-Dibbert J, Selong ET, Quirk M, Throolin M, Corona C, Sonar S, Zanca L, Schwarz ER, Bimczok D. Innovative Methodology for Antimicrobial Susceptibility Determination in Mycoplasma Biofilms. Microorganisms. 2024; 12(12):2650. https://doi.org/10.3390/microorganisms12122650
Chicago/Turabian StyleJacobson, B. Tegner, Jessica DeWit-Dibbert, Eli T. Selong, McKenna Quirk, Michael Throolin, Chris Corona, Sobha Sonar, LaShae Zanca, Erika R. Schwarz, and Diane Bimczok. 2024. "Innovative Methodology for Antimicrobial Susceptibility Determination in Mycoplasma Biofilms" Microorganisms 12, no. 12: 2650. https://doi.org/10.3390/microorganisms12122650
APA StyleJacobson, B. T., DeWit-Dibbert, J., Selong, E. T., Quirk, M., Throolin, M., Corona, C., Sonar, S., Zanca, L., Schwarz, E. R., & Bimczok, D. (2024). Innovative Methodology for Antimicrobial Susceptibility Determination in Mycoplasma Biofilms. Microorganisms, 12(12), 2650. https://doi.org/10.3390/microorganisms12122650






