The Daunomycin: Biosynthesis, Actions, and the Search for New Solutions to Enhance Production
Abstract
1. Introduction
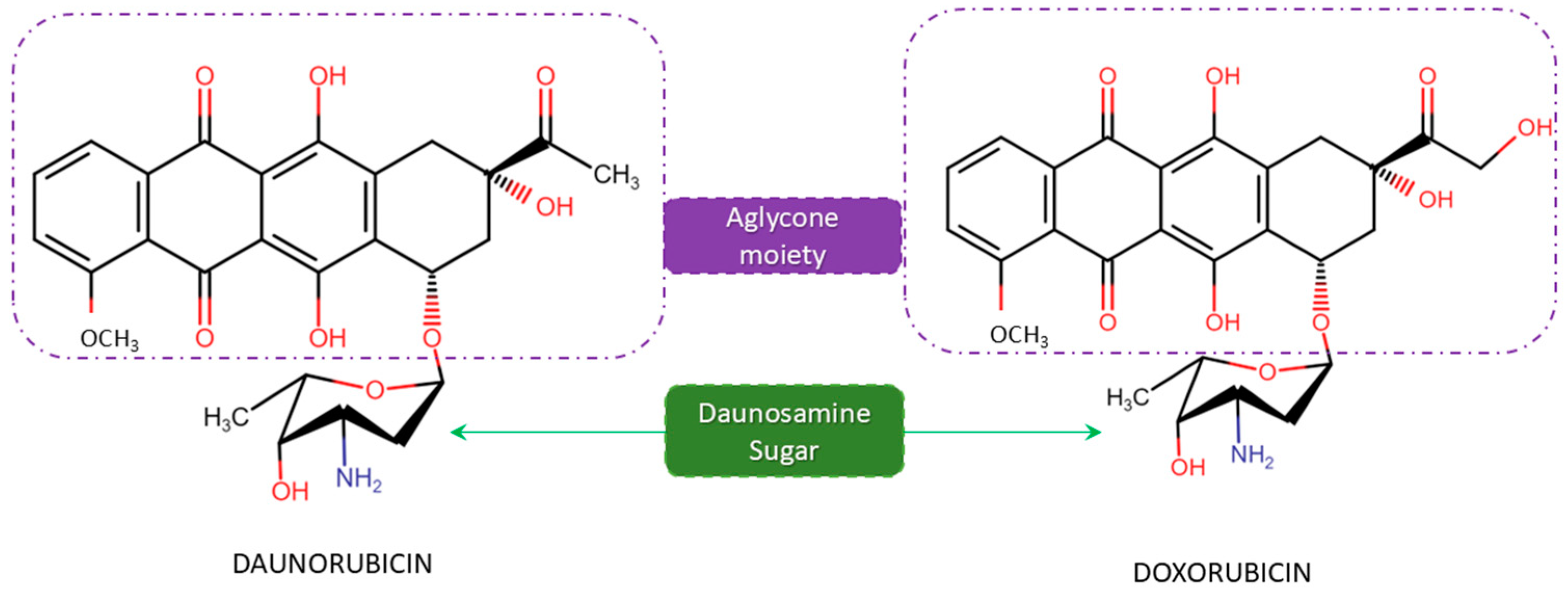
2. The Anthracycline Producers and Daunomycin Synthesis
2.1. Biosynthetic Gene Clusters (BGCs)
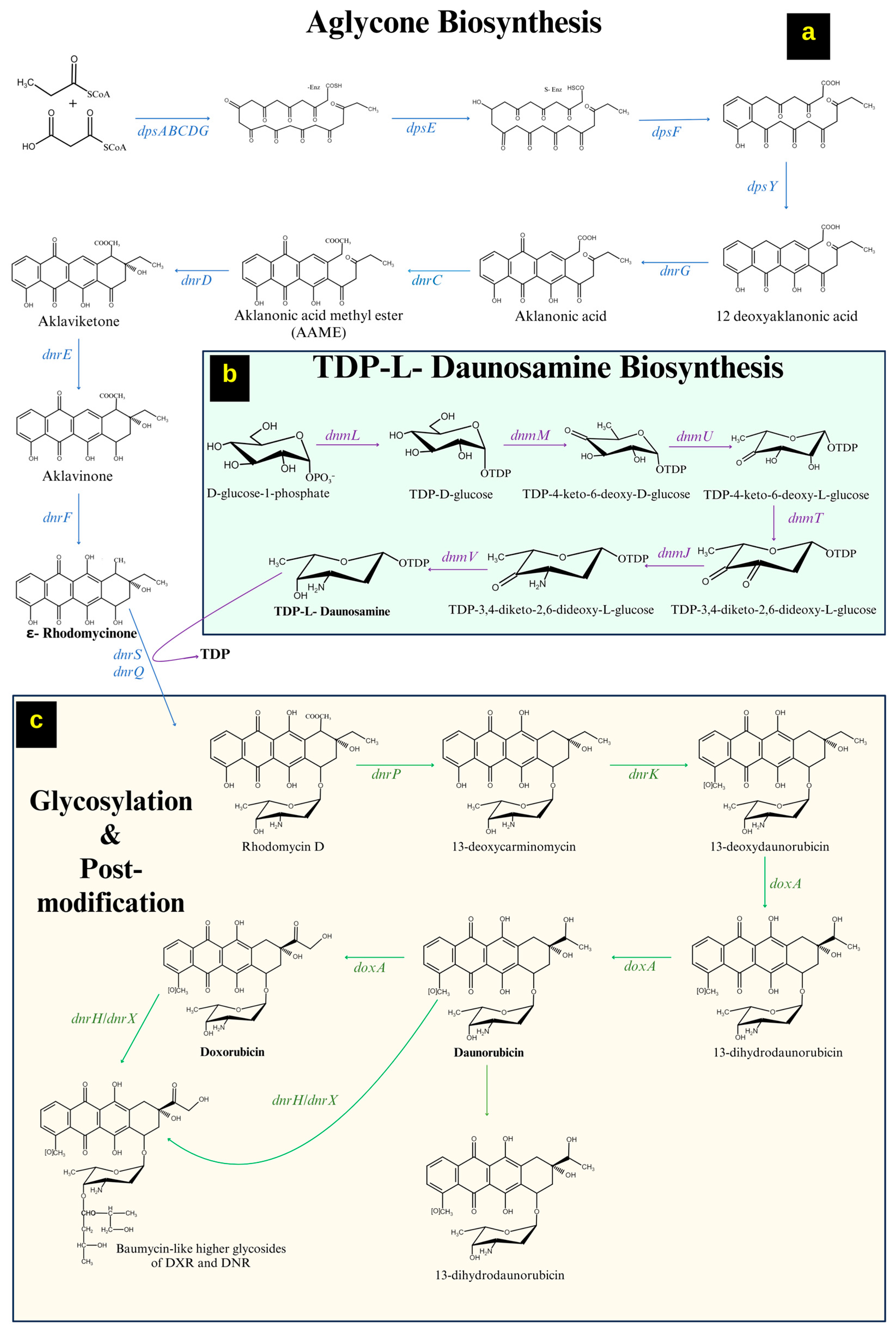
2.1.1. Formation of ε-Rhodomycinone
2.1.2. Formation of a Sugar Moiety (Thymidine Diphosphate-L-Daunosamine)
2.1.3. Tailoring Reactions/Modifications in DNR/DOX Biosynthesis
2.2. Gene Regulation in DNR/DOX Biosynthesis
3. Daunomycin Mode of Action
3.1. DNA Intercalation
3.2. Topoisomerase II (Topo II) Poisoning
3.3. Formation of DNA Adducts
4. Side Effects of DNR/DOX
4.1. Cardiotoxicity
4.2. Redox Mechanisms and Oxidative Stress
4.3. ROS Alleviation and Mitochondrial Dysfunction
4.4. Lipid Dysfunction and Cell Membrane Alterations
5. Self-Resistance in Microbial Factories/Non-Target Species
5.1. Resistance Genes
5.2. Efflux Pumps
5.3. Inactivation of Drug by Enzymatic Reaction
5.4. Alteration of Drug Targets
6. Interaction of DNR/DOX with Iron
7. Interaction of DNR/DOX with Oil
8. Culture Media for Metabolites Production in Streptomyces spp.
8.1. Carbon Source
8.2. Nitrogen Source
9. Engineering Culture Media—In Prospect of Improved DNR Production
9.1. Perturbation of Metabolite Biosynthesis in Streptomycetes
9.2. Media Construction for Three-Way Interaction (DNR–Iron–Oligolipid)
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinis, P.; Tirkkonen, H.; Wandi, B.N.; Siitonen, V.; Niemi, J.; Grocholski, T.; Metsä-Ketelä, M. Evolution-Inspired Engineering of Anthracycline Methyltransferases. PNAS Nexus 2023, 2, pgad009. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Recht, A. Side Effects of Adjuvant Treatment of Breast Cancer. N. Engl. J. Med. 2001, 344, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.B. The Anthracyclines: Will We Ever Find a Better Doxorubicin? Semin. Oncol. 1992, 19, 670–686. [Google Scholar]
- Murabito, A.; Russo, M.; Ghigo, A. Mitochondrial Intoxication by Anthracyclines. In Mitochondrial Intoxication; Elsevier: Amsterdam, The Netherlands, 2023; pp. 299–321. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologie Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- McGowan, J.v.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, H.; Brockmann, H., Jr. Rhodomycine, VIII; Antibiotica Aus Actinomyceten, L. δ-Rhodomycinon. Chem. Ber. 1963, 96, 1771–1778. [Google Scholar] [CrossRef]
- Metsä-Ketelä, M.; Niemi, J.; Mäntsälä, P.; Schneider, G. Anthracycline Biosynthesis: Genes, Enzymes and Mechanisms. In Anthracycline Chemistry and Biology I: Biological Occurence and Biosynthesis, Synthesis and Chemistry; Krohn, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 101–140. ISBN 978-3-540-75815-0. [Google Scholar]
- Fujiwara, A.; Hoshino, T.; Westley, J.W. Anthracycline Antibiotics. Crit. Rev. Biotechnol. 1985, 3, 133–157. [Google Scholar] [CrossRef]
- Hortobágyi, G.N. Anthracyclines in the Treatment of Cancer. Drugs 1997, 54, 1–7. [Google Scholar] [CrossRef]
- Arcamone, F.; Franceschi, G.; Orezzi, P.; Cassinelli, G.; Barbieri, W.; Mondelli, R. Daunomycin. I. The Structure of Daunomycinone. J. Am. Chem. Soc. 1964, 86, 5334–5335. [Google Scholar] [CrossRef]
- Bayles, C.E.; Hale, D.E.; Konieczny, A.; Anderson, V.D.; Richardson, C.R.; Brown, K.V.; Nguyen, J.T.; Hecht, J.; Schwartz, N.; Kharel, M.K.; et al. Upcycling the Anthracyclines: New Mechanisms of Action, Toxicology, and Pharmacology. Toxicol. Appl. Pharmacol. 2023, 459, 116362. [Google Scholar] [CrossRef]
- Drevin, G.; Briet, M.; Bazzoli, C.; Gyan, E.; Schmidt, A.; Dombret, H.; Orvain, C.; Giltat, A.; Recher, C.; Ifrah, N.; et al. Daunorubicin and Its Active Metabolite Pharmacokinetic Profiles in Acute Myeloid Leukaemia Patients: A Pharmacokinetic Ancillary Study of the BIG-1 Trial. Pharmaceutics 2022, 14, 792. [Google Scholar] [CrossRef] [PubMed]
- Aubel-Sadron, G.; Londos-Gagliardi, D. Daunorubicin and Doxorubicin, Anthracycline Antibiotics, a Physicochemical and Biological Review. Biochimie 1984, 66, 333–352. [Google Scholar] [CrossRef] [PubMed]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New Insights into the Activities and Toxicities of the Old Anticancer Drug Doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Arcamone, F. Antitumor Anthracyclines: Recent Developments. Med. Res. Rev. 1984, 4, 153–188. [Google Scholar] [CrossRef]
- Ainger, L.E.; Bushore, J.; Johnson, W.W.; Ito, J. Daunomycin: A Cardiotoxic Agent. J. Natl. Med. Assoc. 1971, 63, 261–267. [Google Scholar]
- Bossa, R.; Galatulas, I.; Mantovani, E. Cardio-Toxicity of Daunomycin and Adriamycin. Neoplasma 1977, 24, 405–409. [Google Scholar]
- Von Hoff, D.D.; Rozencweig, M.; Layard, M.; Slavik, M.; Muggia, F.M. Daunomycin-Induced Cardiotoxicity in Children and Adults: A Review of 110 Cases. Am. J. Med. 1977, 62, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.; Ghaleb, S.; Das, B.B. Diagnosis and Management of Cancer Treatment-Related Cardiac Dysfunction and Heart Failure in Children. Children 2023, 10, 149. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive Heart Failure in Patients Treated with Doxorubicin. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A Double-Edged Sword in Cancer Treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Chen, R.; Wang, Z.; Huang, Z.; Kong, N.; Zhang, M.; Han, W.; Lou, F.; Yang, J.; Zhang, Q.; et al. Autophagy and Chemotherapy Resistance: A Promising Therapeutic Target for Cancer Treatment. Cell Death Dis. 2013, 4, e838. [Google Scholar] [CrossRef]
- Xiao, M.; Cai, J.; Cai, L.; Jia, J.; Xie, L.; Zhu, Y.; Huang, B.; Jin, D.; Wang, Z. Let-7e Sensitizes Epithelial Ovarian Cancer to Cisplatin through Repressing DNA Double Strand Break Repair. J. Ovarian Res. 2017, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef]
- Bagnyukova, T.; Serebriiskii, I.G.; Zhou, Y.; Hopper-Borge, E.A.; Golemis, E.A.; Astsaturov, I. Chemotherapy and Signaling: How Can Targeted Therapies Supercharge Cytotoxic Agents? Cancer Biol. Ther. 2010, 10, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Niraula, N.P.; Kim, S.H.; Sohng, J.K.; Kim, E.S. Biotechnological Doxorubicin Production: Pathway and Regulation Engineering of Strains for Enhanced Production. Appl. Microbiol. Biotechnol. 2010, 87, 1187–1194. [Google Scholar] [CrossRef]
- Sarkar, G.; Suthindhiran, K. Diversity and Biotechnological Potential of Marine Actinomycetes from India. Indian. J. Microbiol. 2022, 62, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Jensen, P.R. Developing a New Resource for Drug Discovery: Marine Actinomycete Bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sandiford, S.K.; van Wezel, G.P. Triggers and Cues That Activate Antibiotic Production by Actinomycetes. J. Ind. Microbiol. Biotechnol. 2014, 41, 371–386. [Google Scholar] [CrossRef]
- Hulst, M.B.; Grocholski, T.; Neefjes, J.J.C.; van Wezel, G.P.; Metsä-Ketelä, M. Anthracyclines: Biosynthesis, Engineering and Clinical Applications. Nat. Prod. Rep. 2022, 39, 814–841. [Google Scholar] [CrossRef]
- Elshahawi, S.I.; Shaaban, K.A.; Kharel, M.K.; Thorson, J.S. A Comprehensive Review of Glycosylated Bacterial Natural Products. Chem. Soc. Rev. 2015, 44, 7591–7697. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z. The SnogI Gene Is Necessary for the Proper Functioning of the Nogalamycin Biosynthesis Pathway. Indian J. Microbiol. 2021, 61, 467–474. [Google Scholar] [CrossRef]
- Kandula, S.K.; Terli, R. Production, Purification and Characterization of an Antimicrobial Compound from Marine Streptomyces coeruleorubidus BTSS-301. J. Pharm. Res. 2013, 7, 397–403. [Google Scholar] [CrossRef]
- Bundale, S.; Begde, D.; Nashikkar, N.; Kadam, T.; Upadhyay, A. Optimization of Culture Conditions for Production of Bioactive Metabolites by Streptomyces spp. Isolated from Soil. Adv. Microbiol. 2015, 5, 441–451. [Google Scholar] [CrossRef]
- Ohnuki, T.; Imanaka, T.; Aiba, S. Self-Cloning in Streptomyces Griseus of an Str Gene Cluster for Streptomycin Biosynthesis and Streptomycin Resistance. J. Bacteriol. 1985, 164, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Madduri, K.; Ali, A.; Hutchinson, C.R. Characterization of the Streptomyces peucetius ATCC 29050 Genes Encoding Doxorubicin Polyketide Synthase. Gene 1994, 151, 1–10. [Google Scholar] [CrossRef]
- Dickens, M.L.; Ye, J.; Strohl, W.R. Analysis of Clustered Genes Encoding Both Early and Late Steps in Daunomycin Biosynthesis by Streptomyces Sp. Strain C5. J. Bacteriol. 1995, 177, 536–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parajuli, N.; Basnet, D.B.; Chan Lee, H.; Sohng, J.K.; Liou, K. Genome Analyses of Streptomyces peucetius ATCC 27952 for the Identification and Comparison of Cytochrome P450 Complement with Other Streptomyces. Arch. Biochem. Biophys. 2004, 425, 233–241. [Google Scholar] [CrossRef]
- Hutchinson, C.R. Biosynthetic Studies of Daunorubicin and Tetracenomycin C. Chem. Rev. 1997, 97, 2525–2536. [Google Scholar] [CrossRef]
- Shrestha, B.; Pokhrel, A.R.; Darsandhari, S.; Parajuli, P.; Sohng, J.K.; Pandey, R.P. Engineering Streptomyces peucetius for Doxorubicin and Daunorubicin Biosynthesis. In Pharmaceuticals from Microbes: The Bioengineering Perspective; Arora, D., Sharma, C., Jaglan, S., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 191–209. ISBN 978-3-030-01881-8. [Google Scholar] [CrossRef]
- Wohlert, S.-E.; Lomovskaya, N.; Kulowski, K.; Fonstein, L.; Occi, J.L.; Gewain, K.M.; MacNeil, D.J.; Hutchinson, C.R. Insights about the Biosynthesis of the Avermectin Deoxysugar Oleandrose through Heterologous Expression of Streptomyces Avermitilis Deoxysugar Genes in Streptomyces Lividans. Chem. Biol. 2001, 8, 681–700. [Google Scholar] [CrossRef]
- Madduri, K.; Hutchinson, C.R. Functional Characterization and Transcriptional Analysis of the DnrR1 Locus, Which Controls Daunorubicin Biosynthesis in Streptomyces Peucetius. J. Bacteriol. 1995, 177, 1208–1215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gallo, M.A.; Wardlt, J.; Hutchinson, C.R. The DnrM Gene in Streptomyces peucetius Contains a Naturally Occurring Frameshift Mutation That Is Suppressed by Another Locus Outside of the Daunorubicin-Production Gene Cluster. Microbiology 1996, 142, 269–275. [Google Scholar] [CrossRef]
- Otten, S.L.; Gallo, M.A.; Madduri, K.; Liu, X.; Hutchinson, C.R. Cloning and Characterization of the Streptomyces peucetius DnmZUV Genes Encoding Three Enzymes Required for Biosynthesis of the Daunorubicin Precursor Thymidine Diphospho-L-Daunosamine. J. Bacteriol. 1997, 179, 4446–4450. [Google Scholar] [CrossRef][Green Version]
- Furuya, K.; Richard Hutchinson, C. The DrrC Protein of Streptomyces peucetius, a UvrA-like Protein, Is a DNA-Binding Protein Whose Gene Is Induced by Daunorubicin. FEMS Microbiol. Lett. 1998, 168, 243–249. [Google Scholar] [CrossRef]
- Dickens, M.L.; Priestley, N.D.; Strohl, W.R. In Vivo and in Vitro Bioconversion of Epsilon-Rhodomycinone Glycoside to Doxorubicin: Functions of DauP, DauK, and DoxA. J. Bacteriol. 1997, 179, 2641–2650. [Google Scholar] [CrossRef]
- Walczak, R.J.; Dickens, M.L.; Priestley, N.D.; Strohl, W.R. Purification, Properties, and Characterization of Recombinant Streptomyces sp. Strain C5 DoxA, a Cytochrome P-450 Catalyzing Multiple Steps in Doxorubicin Biosynthesis. J. Bacteriol. 1999, 181, 298–304. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Kattusamy, K.; Prasad, R. Regulation of Daunorubicin Biosynthesis in Streptomyces peucetius–Feed Forward and Feedback Transcriptional Control. J. Basic. Microbiol. 2013, 53, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yin, C.; Zhu, C.; Zhu, B.; Hu, Y. Improvement of Antibiotic Productivity by Knock-out of DauW in Streptomyces Coeruleobidus. Microbiol. Res. 2011, 166, 539–547. [Google Scholar] [CrossRef]
- Guilfoile, P.G.; Hutchinson, C.R. A Bacterial Analog of the Mdr Gene of Mammalian Tumor Cells Is Present in Streptomyces peucetius, the Producer of Daunorubicin and Doxorubicin. Proc. Natl. Acad. Sci. USA 1991, 88, 8553–8557. [Google Scholar] [CrossRef]
- Kaur, P. Expression and Characterization of DrrA and DrrB Proteins of Streptomyces peucetius in Escherichia coli: DrrA Is an ATP Binding Protein. J. Bacteriol. 1997, 179, 569–575. [Google Scholar] [CrossRef]
- Kaur, P.; Rao, D.K.; Gandlur, S.M. Biochemical Characterization of Domains in the Membrane Subunit DrrB That Interact with the ABC Subunit DrrA: Identification of a Conserved Motif. Biochemistry 2005, 44, 2661–2670. [Google Scholar] [CrossRef]
- Li, W.; Sharma, M.; Kaur, P. The DrrAB Efflux System of Streptomyces peucetius Is a Multidrug Transporter of Broad Substrate Specificity*. J. Biol. Chem. 2014, 289, 12633–12646. [Google Scholar] [CrossRef]
- Srinivasan, P.; Palani, S.N.; Prasad, R. Daunorubicin Efflux in Streptomyces peucetius Modulates Biosynthesis by Feedback Regulation. FEMS Microbiol. Lett. 2010, 305, 18–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Doxorubicin and Other Anthracyclines in Cancers: Activity, Chemoresistance and Its Overcoming. Mol. Asp. Med. 2023, 93, 101205. [Google Scholar] [CrossRef]
- Comings, D.E.; Drets, M.E. Mechanisms of Chromosome Banding. Chromosoma 1976, 56, 199–211. [Google Scholar] [CrossRef]
- Chaires, J.B.; Herrera, J.E.; Waring, M.J. Preferential Binding of Daunomycin to 5’TACG and 5’TAGC Sequences Revealed by Footprinting Titration Experiments. Biochemistry 1990, 29, 6145–6153. [Google Scholar] [CrossRef]
- Nunn, C.M.; Van Meervelt, L.; Zhang, S.; Moore, M.H.; Kennard, O. DNA-Drug Interactions: The Crystal Structures of d(TGTACA) and d(TGATCA) Complexed with Daunomycin. J. Mol. Biol. 1991, 222, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Ashley, N.; Poulton, J. Mitochondrial DNA Is a Direct Target of Anti-Cancer Anthracycline Drugs. Biochem. Biophys. Res. Commun. 2009, 378, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Zlatanova, J.; Tomschik, M. Nucleosome Assembly Depends on the Torsion in the DNA Molecule: A Magnetic Tweezers Study. Biophys. J. 2009, 97, 3150–3157. [Google Scholar] [CrossRef]
- Martins-Teixeira, M.B.; Carvalho, I. Antitumour Anthracyclines: Progress and Perspectives. ChemMedChem 2020, 15, 933–948. [Google Scholar] [CrossRef]
- Pang, B.; de Jong, J.; Qiao, X.; Wessels, L.F.A.; Neefjes, J. Chemical Profiling of the Genome with Anti-Cancer Drugs Defines Target Specificities. Nat. Chem. Biol. 2015, 11, 472–480. [Google Scholar] [CrossRef]
- Nitiss, J.L. DNA Topoisomerase II and Its Growing Repertoire of Biological Functions. Nat. Rev. Cancer 2009, 9, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the Molecular Basis of Doxorubicin-Induced Cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Lyu, Y.L.; Kerrigan, J.E.; Lin, C.-P.; Azarova, A.M.; Tsai, Y.-C.; Ban, Y.; Liu, L.F. Topoisomerase IIβ–Mediated DNA Double-Strand Breaks: Implications in Doxorubicin Cardiotoxicity and Prevention by Dexrazoxane. Cancer Res. 2007, 67, 8839–8846. [Google Scholar] [CrossRef]
- Kato, S.; Burke, P.J.; Koch, T.H.; Bierbaum, V.M. Formaldehyde in Human Cancer Cells: Detection by Preconcentration-Chemical Ionization Mass Spectrometry. Anal. Chem. 2001, 73, 2992–2997. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, C.; Phillips, D.R. In Vitro Transcription Analysis of DNA Adducts Induced by Cyanomorpholinoadriamycin. Biochemistry 1992, 31, 9513–9519. [Google Scholar] [CrossRef]
- Forrest, R.A.; Swift, L.P.; Rephaeli, A.; Nudelman, A.; Kimura, K.-I.; Phillips, D.R.; Cutts, S.M. Activation of DNA Damage Response Pathways as a Consequence of Anthracycline-DNA Adduct Formation. Biochem. Pharmacol. 2012, 83, 1602–1612. [Google Scholar] [CrossRef]
- Bilardi, R.A.; Kimura, K.-I.; Phillips, D.R.; Cutts, S.M. Processing of Anthracycline-DNA Adducts via DNA Replication and Interstrand Crosslink Repair Pathways. Biochem. Pharmacol. 2012, 83, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.M.S.; Bilardi, R.A.; Koch, T.H.; Post, G.C.; Nafie, J.W.; Kimura, K.-I.; Cutts, S.M.; Phillips, D.R. DNA Repair in Response to Anthracycline–DNA Adducts: A Role for Both Homologous Recombination and Nucleotide Excision Repair. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2008, 638, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Barthel, B.L.; Mooz, E.L.; Wiener, L.E.; Koch, G.G.; Koch, T.H. Correlation of in Situ Oxazolidine Formation with Highly Synergistic Cytotoxicity and DNA Cross-Linking in Cancer Cells from Combinations of Doxorubicin and Formaldehyde. J. Med. Chem. 2016, 59, 2205–2221. [Google Scholar] [CrossRef]
- Swift, L.P.; Rephaeli, A.; Nudelman, A.; Phillips, D.R.; Cutts, S.M. Doxorubicin-DNA Adducts Induce a Non-Topoisomerase II–Mediated Form of Cell Death. Cancer Res. 2006, 66, 4863–4871. [Google Scholar] [CrossRef]
- Grenier, M.A.; Lipshultz, S.E. Epidemiology of Anthracycline Cardiotoxicity in Children and Adults. Semin. Oncol. 1998, 25, 72–85. [Google Scholar] [PubMed]
- Weingart, S.N.; Zhang, L.; Sweeney, M.; Hassett, M. Chemotherapy Medication Errors. Lancet Oncol. 2018, 19, e191–e199. [Google Scholar] [CrossRef]
- Henriksen, P.A. Anthracycline Cardiotoxicity: An Update on Mechanisms, Monitoring and Prevention. Heart 2018, 104, 971. [Google Scholar] [CrossRef]
- Su, É.; Villard, C.; Manneville, J.-B. Mitochondria: At the Crossroads between Mechanobiology and Cell Metabolism. Biol. Cell 2023, 115, e2300010. [Google Scholar] [CrossRef]
- Xie, S.; Sun, Y.; Zhao, X.; Xiao, Y.; Zhou, F.; Lin, L.; Wang, W.; Lin, B.; Wang, Z.; Fang, Z.; et al. An Update of the Molecular Mechanisms Underlying Anthracycline Induced Cardiotoxicity. Front. Pharmacol. 2024, 15, 1406247. [Google Scholar] [CrossRef]
- Sala, V.; Della Sala, A.; Hirsch, E.; Ghigo, A. Signaling Pathways Underlying Anthracycline Cardiotoxicity. Antioxid. Redox Signal. 2020, 32, 1098–1114. [Google Scholar] [CrossRef]
- Henninger, C.; Fritz, G. Statins in Anthracycline-Induced Cardiotoxicity: Rac and Rho, and the Heartbreakers. Cell Death Dis. 2018, 8, e2564. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Lin, H.; Zhang, J.; Lin, N.; Sun, Z.; Gao, F.; Luo, H.; Ni, T.; Luo, W.; Chi, J.; et al. Doxorubicin Induces Cardiomyocyte Pyroptosis via the TINCR-Mediated Posttranscriptional Stabilization of NLR Family Pyrin Domain Containing 3. J. Mol. Cell Cardiol. 2019, 136, 15–26. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Cui, M.; Jin, L.; Wang, Y.; Lv, F.; Liu, Y.; Zheng, W.; Shang, H.; Zhang, J.; et al. CaMKII Is a RIP3 Substrate Mediating Ischemia- and Oxidative Stress–Induced Myocardial Necroptosis. Nat. Med. 2016, 22, 175–182. [Google Scholar] [CrossRef]
- Bansal, N.; Adams, M.J.; Ganatra, S.; Colan, S.D.; Aggarwal, S.; Steiner, R.; Amdani, S.; Lipshultz, E.R.; Lipshultz, S.E. Strategies to Prevent Anthracycline-Induced Cardiotoxicity in Cancer Survivors. Cardio-Oncololgy 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.E.; Gianni, L.; Simone, C.B.; Klecker, R.; Greene, R. Oxidative Destruction of Erythrocyte Ghost Membranes Catalyzed by the Doxorubicin-Iron Complex. Biochemistry 1982, 21, 1707–1713. [Google Scholar] [CrossRef]
- Xu, X.; Persson, H.L.; Richardson, D.R. Molecular Pharmacology of the Interaction of Anthracyclines with Iron. Mol. Pharmacol. 2005, 68, 261. [Google Scholar] [CrossRef]
- Bertorello, N.; Luksch, R.; Bisogno, G.; Haupt, R.; Spallarossa, P.; Cenna, R.; Fagioli, F. Cardiotoxicity in Children with Cancer Treated with Anthracyclines: A Position Statement on Dexrazoxane. Pediatr. Blood Cancer 2023, 70, e30515. [Google Scholar] [CrossRef]
- Szponar, J.; Niziński, P.; Dudka, J.; Kasprzak-Drozd, K.; Oniszczuk, A. Natural Products for Preventing and Managing Anthracycline-Induced Cardiotoxicity: A Comprehensive Review. Cells 2024, 13, 1151. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a Target for Protection against Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.V.; Kim, J. Iron Promotes Cardiac Doxorubicin Retention and Toxicity Through Downregulation of the Mitochondrial Exporter ABCB8. Front. Pharmacol. 2022, 13, 817951. [Google Scholar] [CrossRef]
- Berthiaume, J.M.; Wallace, K.B. Adriamycin-Induced Oxidative Mitochondrial Cardiotoxicity. Cell Biol. Toxicol. 2007, 23, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, J.H. Mechanisms of Anthracycline-Enhanced Reactive Oxygen Metabolism in Tumor Cells. Oxid. Med. Cell Longev. 2019, 2019, 9474823. [Google Scholar] [CrossRef]
- Pourahmad, J.; Salimi, A.; Seydi, E. Role of Oxygen Free Radicals in Cancer Development and Treatment. In Free Radicals and Diseases; Ahmad, R., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 17; ISBN 978-953-51-2747-5. [Google Scholar]
- Goormaghtigh, E.; Huart, P.; Praet, M.; Brasseur, R.; Ruysschaert, J.-M. Structure of the Adriamycin-Cardiolipin Complex: Role in Mitochondrial Toxicity. Biophys. Chem. 1990, 35, 247–257. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An Update on Anticancer Molecular Action, Toxicity and Novel Drug Delivery Systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yu, X.; Wang, Y.; Wang, F.; Li, H.; Wang, Y.; Lu, D.; Qi, R.; Wang, H. Berberine Inhibits Doxorubicin-Triggered Cardiomyocyte Apoptosis via Attenuating Mitochondrial Dysfunction and Increasing Bcl-2 Expression. PLoS ONE 2012, 7, e47351. [Google Scholar] [CrossRef]
- Bellance, N.; Furt, F.; Melser, S.; Lalou, C.; Thoraval, D.; Maneta-Peyret, L.; Lacombe, D.; Moreau, P.; Rossignol, R. Doxorubicin Inhibits Phosphatidylserine Decarboxylase and Modifies Mitochondrial Membrane Composition in Hela Cells. Int. J. Mol. Sci. 2020, 21, 1317. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, D.A. How Do Antibiotic-Producing Bacteria Ensure Their Self-Resistance before Antibiotic Biosynthesis Incapacitates Them? Mol. Microbiol. 2007, 63, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Julian, D.; Dorothy, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Mak, S.; Xu, Y.; Nodwell, J.R. The Expression of Antibiotic Resistance Genes in Antibiotic-Producing Bacteria. Mol. Microbiol. 2014, 93, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Lomovskaya, N.; Hong, S.K.; Kim, S.U.; Fonstein, L.; Furuya, K.; Hutchinson, R.C. The Streptomyces peucetius DrrC Gene Encodes a UvrA-like Protein Involved in Daunorubicin Resistance and Production. J. Bacteriol. 1996, 178, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.A.; Piddock, L.J. V The Importance of Efflux Pumps in Bacterial Antibiotic Resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef]
- Bazzi, W.; Abou Fayad, A.G.; Nasser, A.; Haraoui, L.-P.; Dewachi, O.; Abou-Sitta, G.; Nguyen, V.-K.; Abara, A.; Karah, N.; Landecker, H.; et al. Heavy Metal Toxicity in Armed Conflicts Potentiates AMR in A. Baumannii by Selecting for Antibiotic and Heavy Metal Co-Resistance Mechanisms. Front. Microbiol. 2020, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.V. Clinically Relevant Chromosomally Encoded Multidrug Resistance Efflux Pumps in Bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug Efflux Pumps: Structure, Function and Regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of Bacterial Efflux Pumps in Antibiotic Resistance, Virulence, and Strategies to Discover Novel Efflux Pump Inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef] [PubMed]
- Abdi, S.N.; Ghotaslou, R.; Ganbarov, K.; Mobed, A.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Kafil, H.S. Acinetobacter baumannii Efflux Pumps and Antibiotic Resistance. Infect. Drug Resist. 2020, 13, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Zack, K.M.; Sorenson, T.; Joshi, S.G. Types and Mechanisms of Efflux Pump Systems and the Potential of Efflux Pump Inhibitors in the Restoration of Antimicrobial Susceptibility, with a Special Reference to Acinetobacter baumannii. Pathogens 2024, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Tampé, R. Annual Review of Biochemistry Structural and Mechanistic Principles of ABC Transporters. Annu. Rev. Biochem. 2024, 89, 605–636. [Google Scholar] [CrossRef] [PubMed]
- Jacek, L.; Konings, W.N.; Driessen, A.J.M. Distribution and Physiology of ABC-Type Transporters Contributing to Multidrug Resistance in Bacteria. Microbiol. Mol. Biol. Rev. 2007, 71, 463–476. [Google Scholar] [CrossRef]
- Kaur, P.; Russell, J. Biochemical Coupling between the DrrA and DrrB Proteins of the Doxorubicin Efflux Pump of Streptomyces peucetius. J. Biol. Chem. 1998, 273, 17933–17939. [Google Scholar] [CrossRef]
- Méndez, C.; Salas, J.A. The Role of ABC Transporters in Antibiotic-Producing Organisms: Drug Secretion and Resistance Mechanisms. Res. Microbiol. 2001, 152, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Rao, D.K.; Kaur, P. Dual Role of the Metalloprotease FtsH in Biogenesis of the DrrAB Drug Transporter. J. Biol. Chem. 2013, 288, 11854–11864. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ning, J.; Tian, Y.; Li, H.; Chen, H.; Guan, W. The Involvement of Multiple ABC Transporters in Daunorubicin Efflux in Streptomyces coeruleorubidus. Microb. Biotechnol. 2024, 17, e70023. [Google Scholar] [CrossRef] [PubMed]
- Scotti, C.; Hutchinson, C.R. Enhanced Antibiotic Production by Manipulation of the Streptomyces peucetius DnrH and DnmT Genes Involved in Doxorubicin (Adriamycin) Biosynthesis. J. Bacteriol. 1996, 178, 7316–7321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Westman, E.L.; Canova, M.J.; Radhi, I.J.; Koteva, K.; Kireeva, I.; Waglechner, N.; Wright, G.D. Bacterial Inactivation of the Anticancer Drug Doxorubicin. Chem. Biol. 2012, 19, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; North, B.J.; Shu, J. Regulation of Topoisomerase II Stability and Activity by Ubiquitination and SUMOylation: Clinical Implications for Cancer Chemotherapy. Mol. Biol. Rep. 2021, 48, 6589–6601. [Google Scholar] [CrossRef]
- Zweier, J.L.; Gianni, L.; Muindi, J.; Myers, C.E. Differences in O2 Reduction by the Iron Complexes of Adriamycin and Daunomycin: The Importance of the Sidechain Hydroxyl Group. Biochim. Biophys. Acta Gen. General. Subj. 1986, 884, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Fiallo, M.; Laigle, A.; Garnier-Suillerot, A.; Amirand, C.; Ballini, J.-P.; Chinsky, L.; Duquesne, M.; Jolles, B.; Sureau, F.; Turpin, P.-Y.; et al. Interactions of Iron-Anthracycline Complexes with Living Cells: A Microspectrofluorometric Study. Biochim. Biophys. Acta Mol. Cell Res. 1993, 1177, 236–244. [Google Scholar] [CrossRef]
- Fiallo, M.M.L.; Garnier-Suillerot, A. Metal Anthracycline Complexes as a New Class of Anthracycline Derivatives. Palladium(II)-Adriamycin and Palladium(II)-Daunorubicin Complexes: Physicochemical Characteristics and Antitumor Activity. Biochemistry 1986, 25, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Gosálvez, M.; Blanco, M.F.; Vivero, C.; Vallés, F. Quelamycin, a New Derivative of Adriamycin with Several Possible Therapeutic Advantages. Eur. J. Cancer 1978, 14, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Beraldo, H.; Garnier-Suillerot, A.; Tosi, L.; Lavelle, F. Iron(III)-Adriamycin and Iron(III)-Daunorubicin Complexes: Physicochemical Characteristics, Interaction with DNA, and Antitumor Activity. Biochemistry 1985, 24, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Funes, H.; Brugarolas, A.; Gosálvez, M. Quelamycin: A Summary of Phase I Clinical Trials. In Cancer Chemo- and Immunopharmacology: 1. Chemopharmacology; Mathé, G., Muggia, F.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1980; pp. 200–206. ISBN 978-3-642-81488-4. [Google Scholar]
- Alves, A.C.; Nunes, C.; Lima, J.; Reis, S. Daunorubicin and Doxorubicin Molecular Interplay with 2D Membrane Models. Colloids Surf. B Biointerfaces 2017, 160, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Matyszewska, D. The Influence of Charge and Lipophilicity of Daunorubicin and Idarubicin on Their Penetration of Model Biological Membranes–Langmuir Monolayer and Electrochemical Studies. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183104. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Silva, F.; Pereira, C.M. Electrochemical Study of the Anticancer Drug Daunorubicin at a Water/Oil Interface: Drug Lipophilicity and Quantification. Anal. Chem. 2013, 85, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Peretz, T.; Sulkes, A.; Amselem, S.; Ben-Yosef, R.; Ben-Baruch, N.; Catane, R.; Biran, S.; Barenholz, Y. Systemic Administration of Doxorubicin-Containing Liposomes in Cancer Patients: A Phase I Study. Eur. J. Cancer Clin. Oncol. 1989, 25, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Amselem, S.; Cohen, R.; Druckmann, S.; Gabizon, A.; Goren, D.; Abra, R.M.; Huang, A.; New, R.; Barenholz, Y. Preparation and Characterization of Liposomal Doxorubicin for Human Use. J. Liposome Res. 1992, 2, 93–123. [Google Scholar] [CrossRef]
- Gabizon, A.; Meshorer, A.; Barenholz, Y. Comparative Long-Term Study of the Toxicities of Free and Liposome-Associated Doxorubicin in Mice After Intravenous Administration. JNCI J. Natl. Cancer Inst. 1986, 77, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Stamp, D. Pharmacokinetics of Liposome-Encapsulated Anti-Tumor Drugs: Studies with Vinblastine, Actinomycin D, Cytosine Arabinoside, and Daunomycin. Biochem. Pharmacol. 1978, 27, 21–27. [Google Scholar] [CrossRef]
- Mussi, S.V.; Sawant, R.; Perche, F.; Oliveira, M.C.; Azevedo, R.B.; Ferreira, L.A.M.; Torchilin, V.P. Novel Nanostructured Lipid Carrier Co-Loaded with Doxorubicin and Docosahexaenoic Acid Demonstrates Enhanced in Vitro Activity and Overcomes Drug Resistance in MCF-7/Adr Cells. Pharm. Res. 2014, 31, 1882–1892. [Google Scholar] [CrossRef]
- Rodrigues da Silva, G.H.; de Moura, L.D.; de Carvalho, F.V.; Geronimo, G.; Mendonça, T.C.; de Lima, F.F.; de Paula, E. Antineoplastics Encapsulated in Nanostructured Lipid Carriers. Molecules 2021, 26, 6929. [Google Scholar] [CrossRef] [PubMed]
- Rokem, J.S.; Lantz, A.E.; Nielsen, J. Systems Biology of Antibiotic Production by Microorganisms. Nat. Prod. Rep. 2007, 24, 1262–1287. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, S.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Ávalos, M.; Guzmán-Trampe, S. Carbon Source Regulation of Antibiotic Production. J. Antibiot. 2010, 63, 442–459. [Google Scholar] [CrossRef]
- Bilyk, O.; Luzhetskyy, A. Metabolic Engineering of Natural Product Biosynthesis in Actinobacteria. Curr. Opin. Biotechnol. 2016, 42, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Bentley, S.D.; Chater, K.F.; Cerdeño-Tárraga, A.-M.; Challis, G.L.; Thomson, N.R.; James, K.D.; Harris, D.E.; Quail, M.A.; Kieser, H.; Harper, D. Complete Genome Sequence of the Model Actinomycete Streptomyces coelicolor A3(2). Nature 2002, 417, 141–147. [Google Scholar] [CrossRef]
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S. Production of Microbial Secondary Metabolites: Regulation by the Carbon Source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.A. Primary Metabolism and Its Control in Streptomycetes: A Most Unusual Group of Bacteria. In Advances in Microbial Physiology; Academic Press: Cambridge, MA, USA, 2000; Volume 42, pp. 47–238. ISBN 0065-2911. [Google Scholar]
- Romero-Rodríguez, A.; Rocha, D.; Ruiz-Villafán, B.; Guzmán-Trampe, S.; Maldonado-Carmona, N.; Vázquez-Hernández, M.; Zelarayán, A.; Rodríguez-Sanoja, R.; Sánchez, S. Carbon Catabolite Regulation in Streptomyces: New Insights and Lessons Learned. World J. Microbiol. Biotechnol. 2017, 33, 162. [Google Scholar] [CrossRef] [PubMed]
- Escalante, L.; Ramos, I.; Imriskova, I.; Langley, E.; Sanchez, S. Glucose Repression of Anthracycline Formation in Streptomyces peucetius Var. Caesius. Appl. Microbiol. Biotechnol. 1999, 52, 572–578. [Google Scholar] [CrossRef]
- Wang, X.; Tian, X.; Wu, Y.; Shen, X.; Yang, S.; Chen, S. Enhanced Doxorubicin Production by Streptomyces peucetius Using a Combination of Classical Strain Mutation and Medium Optimization. Prep. Biochem. Biotechnol. 2018, 48, 514–521. [Google Scholar] [CrossRef]
- Tiffert, Y.; Franz-Wachtel, M.; Fladerer, C.; Nordheim, A.; Reuther, J.; Wohlleben, W.; Mast, Y. Proteomic Analysis of the GlnR-Mediated Response to Nitrogen Limitation in Streptomyces coelicolor M145. Appl. Microbiol. Biotechnol. 2011, 89, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Krysenko, S. Impact of Nitrogen-Containing Compounds on Secondary Metabolism in Streptomyces spp.—A Source of Metabolic Engineering Strategies. SynBio 2023, 1, 204–225. [Google Scholar] [CrossRef]
- Kiviharju, K.; Leisola, M.; Eerikäinen, T. Optimization of Streptomyces peucetius Var. Caesius N47 Cultivation and ε-Rhodomycinone Production Using Experimental Designs and Response Surface Methods. J. Ind. Microbiol. Biotechnol. 2004, 31, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Méndez, C.; Salas, J.A. Engineering Glycosylation in Bioactive Compounds by Combinatorial Biosynthesis. In Biocombinatorial Approaches for Drug Finding; Wohlleben, W., Spellig, T., Müller-Tiemann, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 127–146. [Google Scholar] [CrossRef]
- Nielsen, J. The Role of Metabolic Engineering in the Production of Secondary Metabolites. Curr. Opin. Microbiol. 1998, 1, 330–336. [Google Scholar] [CrossRef]
- Tanaka, Y.; Izawa, M.; Hiraga, Y.; Misaki, Y.; Watanabe, T.; Ochi, K. Metabolic Perturbation to Enhance Polyketide and Nonribosomal Peptide Antibiotic Production Using Triclosan and Ribosome-Targeting Drugs. Appl. Microbiol. Biotechnol. 2017, 101, 4417–4431. [Google Scholar] [CrossRef]
- Olano, C.; Lombó, F.; Méndez, C.; Salas, J.A. Improving Production of Bioactive Secondary Metabolites in Actinomycetes by Metabolic Engineering. Metab. Eng. 2008, 10, 281–292. [Google Scholar] [CrossRef]
- Craney, A.; Ozimok, C.; Pimentel-Elardo, S.M.; Capretta, A.; Nodwell, J.R. Chemical Perturbation of Secondary Metabolism Demonstrates Important Links to Primary Metabolism. Chem. Biol. 2012, 19, 1020–1027. [Google Scholar] [CrossRef]
- Yukinori, T.; Ken, K.; Yutaka, H.; Kiriko, M.; Rie, K.; Kozo, O. Activation and Products of the Cryptic Secondary Metabolite Biosynthetic Gene Clusters by Rifampin Resistance (RpoB) Mutations in Actinomycetes. J. Bacteriol. 2013, 195, 2959–2970. [Google Scholar] [CrossRef]
- Norimasa, T.; Takeshi, H.; Jun, X.; Haifeng, H.; Noboru, O.; Kozo, O. Innovative Approach for Improvement of an Antibiotic-Overproducing Industrial Strain of Streptomyces Albus. Appl. Environ. Microbiol. 2003, 69, 6412–6417. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J. Nanobiotechnology 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Pudhuvai, B.; Koul, B.; Das, R.; Shah, M.P. Nano-Fertilizers (NFs) for Resurgence in Nutrient Use Efficiency (NUE): A Sustainable Agricultural Strategy. Curr. Pollut. Rep. 2024, 11, 1. [Google Scholar] [CrossRef]
- Graf, E.; Eaton, J.W. Antioxidant Functions of Phytic Acid. Free Radic. Biol. Med. 1990, 8, 61–69. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of Phytic Acid and Enhancement of Bioavailable Micronutrients in Food Grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Juillerat, M.A.; Reddy, M.B.; Lynch, S.R.; Dassenko, S.A.; Cook, J.D. Soy Protein, Phytate, and Iron Absorption in Humans. Am. J. Clin. Nutr. 1992, 56, 573–578. [Google Scholar] [CrossRef]
- Hamedi, J.; Malekzadeh, F.; Saghafi-nia, A.E. Enhancing of Erythromycin Production by Saccharopolyspora erythraea with Common and Uncommon Oils. J. Ind. Microbiol. Biotechnol. 2004, 31, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Efthimiou, G.; Thumser, A.E.; Avignone-Rossa, C.A. A Novel Finding That Streptomyces clavuligerus Can Produce the Antibiotic Clavulanic Acid Using Olive Oil as a Sole Carbon Source. J. Appl. Microbiol. 2008, 105, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Huang, D.; Jin, L.; Wang, C.; Wen, J. Comparative Proteomic and Metabolomic Analysis of Streptomyces tsukubaensis Reveals the Metabolic Mechanism of FK506 Overproduction by Feeding Soybean Oil. Appl. Microbiol. Biotechnol. 2017, 101, 2447–2465. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, J.; Dong, J.; Li, Y.; Li, Y.; Chen, Y.; Guan, W. Enhanced Triacylglycerol Metabolism Contributes to Efficient Oil Utilization and High-Level Production of Salinomycin in Streptomyces albus ZD11. Appl. Environ. Microbiol. 2020, 86, e00763-20. [Google Scholar] [CrossRef] [PubMed]
- Eiki, H.; Gushima, H.; Saito, T.; Ishida, H.; Oka, Y.; Osono, T. Product Inhibition and Its Removal on Josamycin Fermentation by Streptomyces narbonensis Var. josamyceticus. J. Ferment. Technol. 1988, 66, 559–565. [Google Scholar] [CrossRef]
- Young, T.; Li, Y.; Efthimiou, G. Olive Pomace Oil Can Be Used as an Alternative Carbon Source for Clavulanic Acid Production by Streptomyces clavuligerus. Waste Biomass Valorization 2020, 11, 3965–3970. [Google Scholar] [CrossRef]
- Seke, M.; Petrovic, D.; Labudovic Borovic, M.; Borisev, I.; Novakovic, M.; Rakocevic, Z.; Djordjevic, A. Fullerenol/Iron Nanocomposite Diminishes Doxorubicin-Induced Toxicity. J. Nanoparticle Res. 2019, 21, 239. [Google Scholar] [CrossRef]
- Calendi, E.; Di Marco, A.; Reggiani, M.; Scarpinato, B.; Valentini, L. On Physico-Chemical Interactions between Daunomycin and Nucleic Acids. Biochim. Biophys. Acta Nucleic Acids Protein Synth. 1965, 103, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yang, R.; Lyu, M.; Wang, S.; Liu, X.; Wen, Y.; Song, Y.; Li, J.; Chen, Z. IdeR, a DtxR Family Iron Response Regulator, Controls Iron Homeostasis, Morphological Differentiation, Secondary Metabolism, and the Oxidative Stress Response in Streptomyces avermitilis. Appl. Environ. Microbiol. 2018, 84, e01503-18. [Google Scholar] [CrossRef]
- Samuni, A.; Chong, P.L.-G.; Barenholz, Y.; Thompson, T.E. Physical and Chemical Modifications of Adriamycin:Iron Complex by Phospholipid Bilayers1. Cancer Res. 1986, 46, 594–599. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudhuvai, B.; Beneš, K.; Čurn, V.; Bohata, A.; Lencova, J.; Vrzalova, R.; Barta, J.; Matha, V. The Daunomycin: Biosynthesis, Actions, and the Search for New Solutions to Enhance Production. Microorganisms 2024, 12, 2639. https://doi.org/10.3390/microorganisms12122639
Pudhuvai B, Beneš K, Čurn V, Bohata A, Lencova J, Vrzalova R, Barta J, Matha V. The Daunomycin: Biosynthesis, Actions, and the Search for New Solutions to Enhance Production. Microorganisms. 2024; 12(12):2639. https://doi.org/10.3390/microorganisms12122639
Chicago/Turabian StylePudhuvai, Baveesh, Karel Beneš, Vladislav Čurn, Andrea Bohata, Jana Lencova, Radka Vrzalova, Jan Barta, and Vladimir Matha. 2024. "The Daunomycin: Biosynthesis, Actions, and the Search for New Solutions to Enhance Production" Microorganisms 12, no. 12: 2639. https://doi.org/10.3390/microorganisms12122639
APA StylePudhuvai, B., Beneš, K., Čurn, V., Bohata, A., Lencova, J., Vrzalova, R., Barta, J., & Matha, V. (2024). The Daunomycin: Biosynthesis, Actions, and the Search for New Solutions to Enhance Production. Microorganisms, 12(12), 2639. https://doi.org/10.3390/microorganisms12122639






