Effects of Distiller’s Grains Biochar and Lactobacillus plantarum on the Remediation of Cd-Pb-Zn-Contaminated Soil and Growth of Sorghum-Sudangrass
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Experimental Setting
2.3. Samples Collection and Physicochemical Analysis
2.4. Statistical Analysis
3. Results and Analysis
3.1. The Physicochemical Properties of Soil
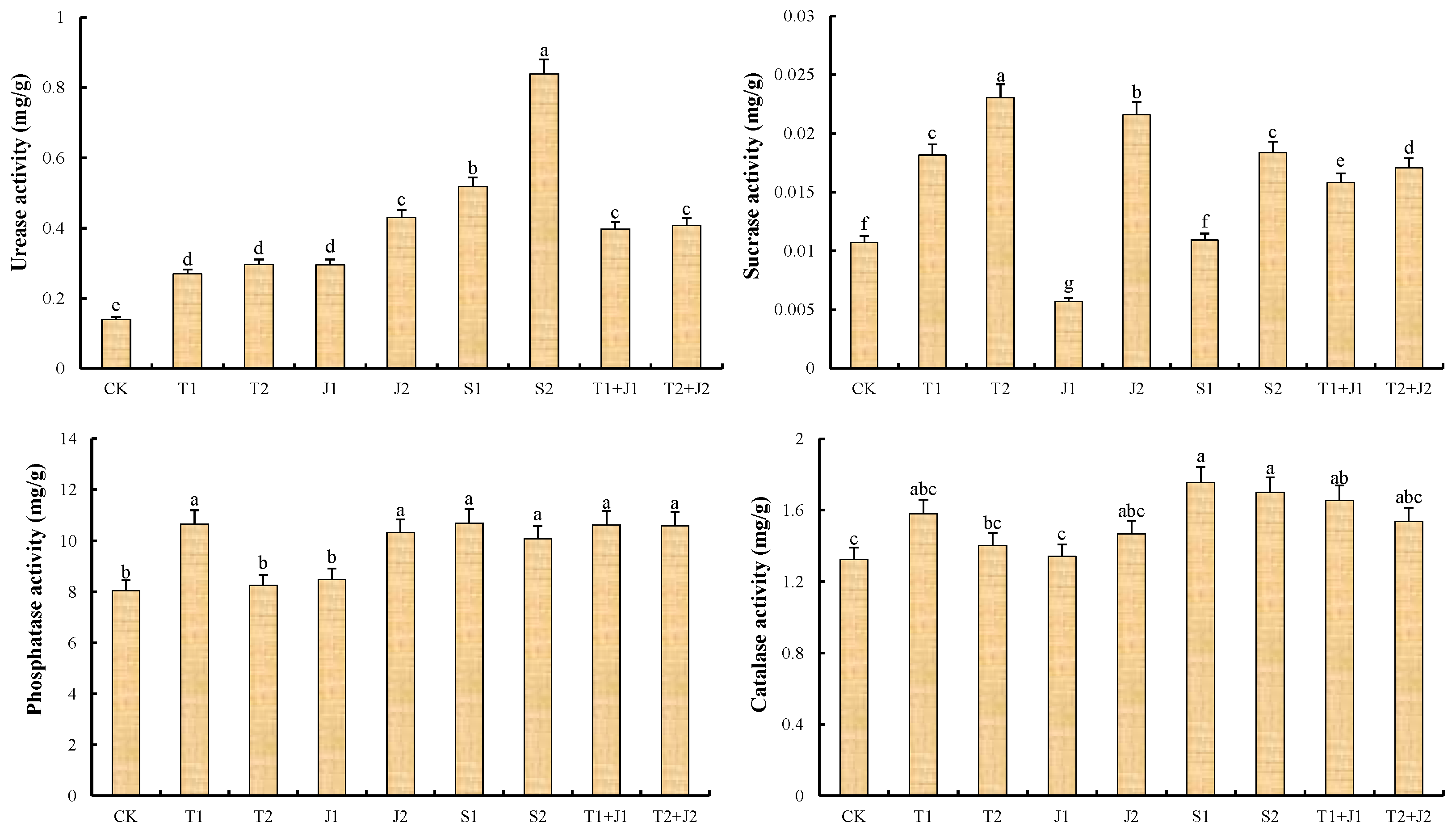
3.2. Soil Enzyme Activity
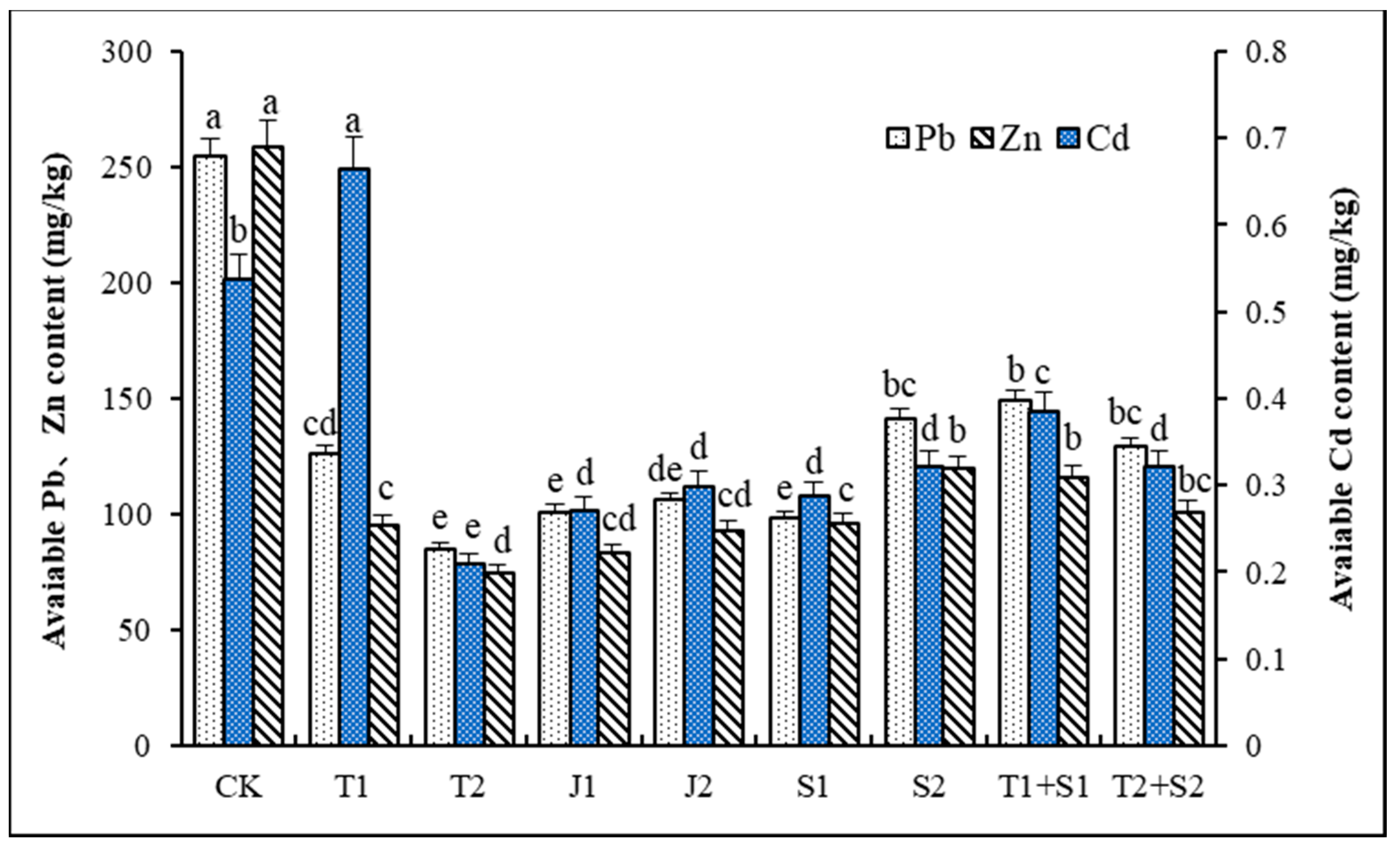
3.3. The Content of Available Heavy Metals in the Soil
3.4. Plant Growth and Biomass Production
3.5. Physio-Biochemical Indicators in Plants
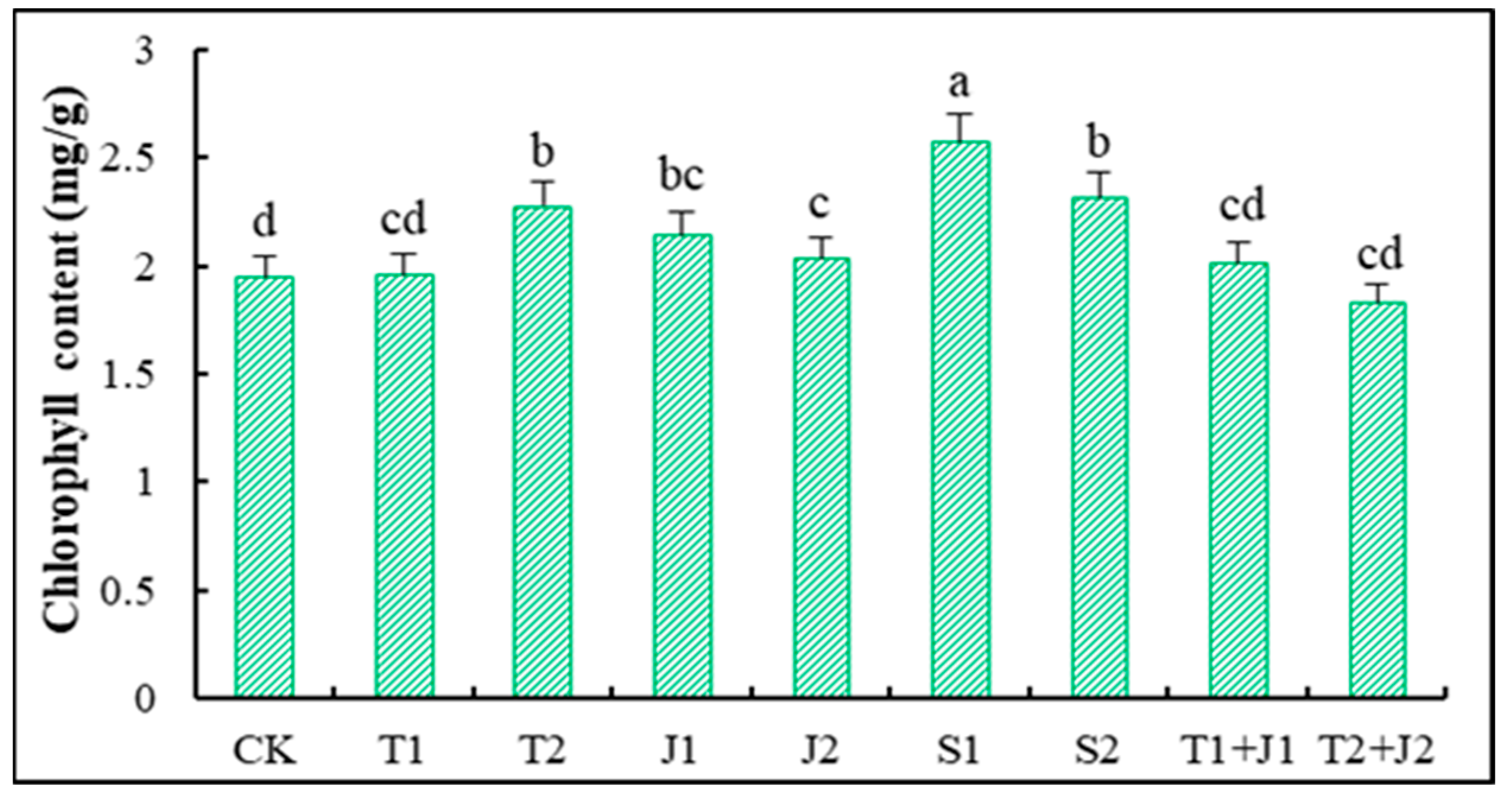
3.5.1. Chlorophyll Content
3.5.2. Soluble Sugar Content
3.5.3. Soluble Protein Content
3.5.4. Activity of Superoxide Dismutase (SOD)
3.5.5. Proline Content
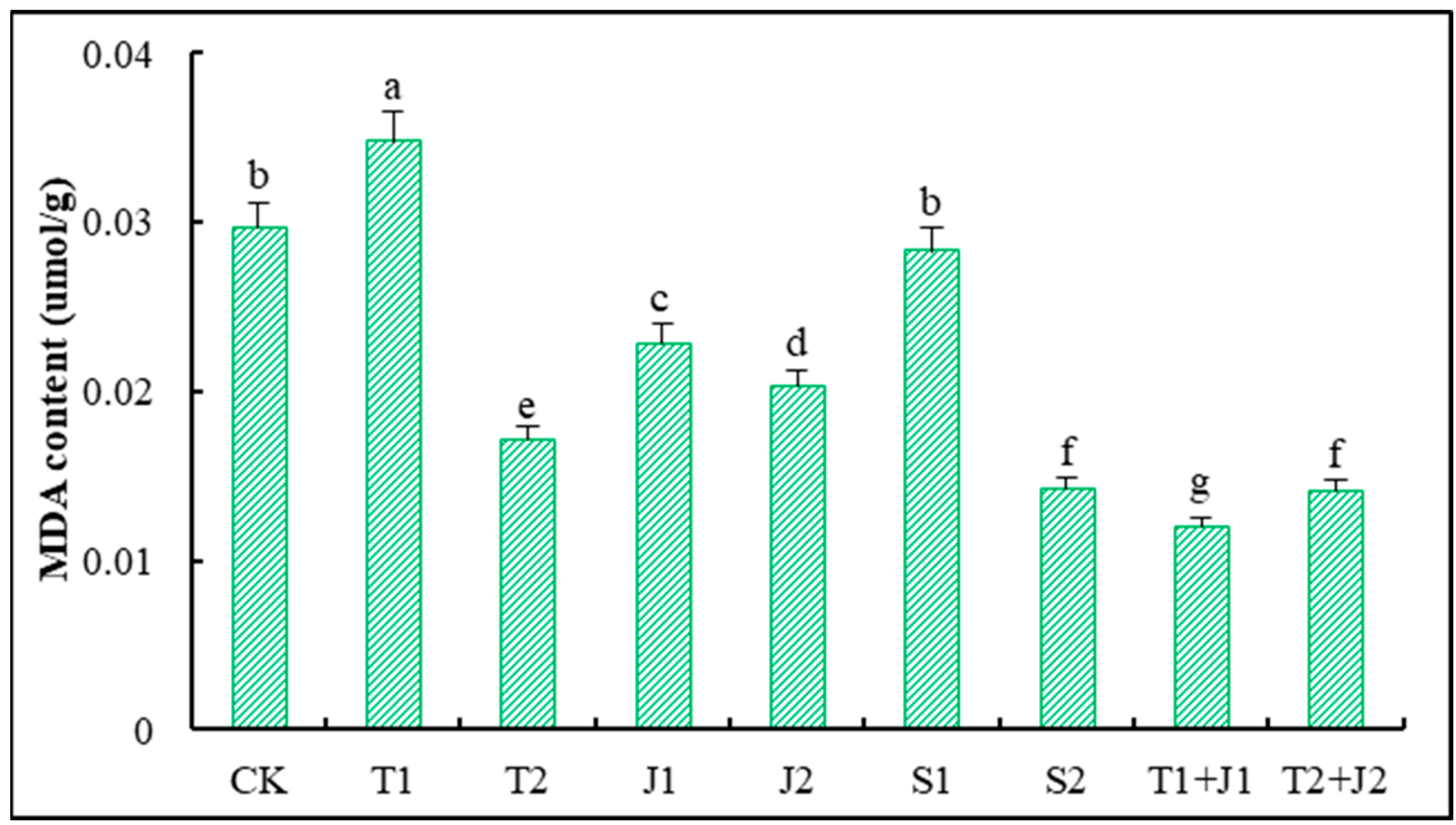
3.5.6. Malondialdehyde (MDA) Level
3.6. The Accumulation of Heavy Metals in Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.F.; Kumar, P.S.; Rozbu, M.R.; Chowdhury, A.T.; Nuzhat, S.; Rafa, N.; Mahlia, T.M.I.; Ong, H.C.; Mofijur, M. Heavy metal toxicity, sources, and remediation techniques for contaminated water and soil. Environ. Technol. Innov. 2022, 25, 102114. [Google Scholar] [CrossRef]
- Saddique, U.; Muhammad, S.; Tariq, M.; Zhang, H.; Arif, M. Potentially toxic elements in soil of the Khyber Pakhtunkhwa province and Tribal areas, Pakistan: Evaluation for human and ecological risk assessment. Environ. Geochem. Health 2018, 40, 2177–2190. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, X.; Liu, B. The characteristics of heavy metal pollution in surface dust in Tangshan, a heavily industrialized city in North China, and an assessment of associated health risks. J. Geochem. Explor. 2020, 210, 106432. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, S.; Shi, Y.; Wang, C.; Li, B.; Li, Y.; Wu, S. Heavy metals in food crops, soil, and water in the Lihe River Watershed of the Taihu Region and their potential health risks when ingested. Sci. Total Environ. 2018, 615, 141–149. [Google Scholar] [CrossRef]
- Mai, X.; Tang, J.; Tang, J.; Zhu, X.; Yang, Z.; Liu, X.; Zhuang, X.; Feng, G.; Tang, L. Research progress on the environmental risk assessment and remediation technologies of heavy metal pollution in agricultural soil. J. Environ. Sci. 2024, 149, 1–20. [Google Scholar] [CrossRef]
- Alsafran, M.; Saleem, M.H.; Al Jabri, H.; Rizwan, M.; Usman, K. Principles and applicability of integrated remediation strategies for heavy metal removal/recovery from contaminated environments. J. Plant Growth Regul. 2023, 42, 3419–3440. [Google Scholar] [CrossRef]
- ur Rehman, Z.; Junaid, M.F.; Ijaz, N.; Khalid, U.; Ijaz, Z. Remediation methods of heavy metal contaminated soils from environmental and geotechnical standpoints. Sci. Total Environ. 2023, 867, 161468. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.; Ng, H.S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Duan, W.; Xie, M.; Dong, X. Heavy metal stabilization remediation in polluted soils with stabilizing materials: A review. Environ. Geochem. Health 2023, 45, 4127–4163. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Lin, H.; Du, D.; Li, G.; Alam, O.; Cheng, Z.; Liu, X.; Li, J. Remediation of heavy metals polluted soil environment: A critical review on biological approaches. Ecotoxicol. Environ. Saf. 2024, 284, 116883. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhang, X.; Zhu, Y.; Zhuo, R. Recent advances in phyto-combined remediation of heavy metal pollution in soil. Biotechnol. Adv. 2024, 72, 108337. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xiang, G.; Xiao, W.; Yang, Z.; Zhao, B. Microbial mediated remediation of heavy metals toxicity: Mechanisms and future prospects. Front. Plant Sci. 2024, 15, 1420408. [Google Scholar] [CrossRef]
- Verma, S.; Bhatt, P.; Verma, A.; Mudila, H.; Prasher, P.; Rene, E.R. Microbial technologies for heavy metal remediation: Effect of process conditions and current practices. Clean Technol. Environ. Policy 2023, 25, 1485–1507. [Google Scholar] [CrossRef]
- Tian, Z.; Li, G.; Tang, W.; Zhu, Q.; Li, X.; Du, C.; Li, C.; Li, J.; Zhao, C.; Zhang, L. Role of Sedum alfredii and soil microbes in the remediation of ultra-high content heavy metals contaminated soil. Agric. Ecosyst. Environ. 2022, 339, 108090. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Xiong, Y.; Zhang, L.; Cui, J.; Li, G.; Du, C.; Wen, K. Remediation mechanism of high concentrations of multiple heavy metals in contaminated soil by Sedum alfredii and native microorganisms. J. Environ. Sci. 2025, 147, 179–188. [Google Scholar] [CrossRef]
- Imran, I.; Khan, A.A.; Kamal, A.; Alrefaei, A.F.; Zaman, W. Utilization and characterization of microbes for heavy metal remediation. Pol. J. Environ. Stud. 2024, 33, 1179–1190. [Google Scholar] [CrossRef]
- Sarker, A.; Al Masud, M.A.; Deepo, D.M.; Das, K.; Nandi, R.; Ansary, M.W.R.; Islam, A.R.M.T.; Islam, T. Biological and green remediation of heavy metal contaminated water and soils: A state-of-the-art review. Chemosphere 2023, 332, 138861. [Google Scholar] [CrossRef]
- Fakhar, A.; Gul, B.; Gurmani, A.R.; Khan, S.M.; Ali, S.; Sultan, T.; Chaudhary, H.J.; Rafique, M.; Rizwan, M. Heavy metal remediation and resistance mechanism of Aeromonas, Bacillus, and Pseudomonas: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1868–1914. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, Q.; Li, B.; Zhou, T.; Cen, Q.; Liu, Z.; Zhou, Y. Insights into remediation of cadmium and lead contaminated-soil by Fe-Mn modified biochar. J. Environ. Chem. Eng. 2024, 12, 112771. [Google Scholar] [CrossRef]
- Maqbool, Z.; Farooq, M.S.; Rafiq, A.; Uzair, M.; Yousuf, M.; Khan, M.R.; Huo, S. Unlocking the potential of biochar in the remediation of soils contaminated with heavy metals for sustainable agriculture. Funct. Plant Biol. 2024, 51, FP23257. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Gou, J.L.; Chen, X.M.; Xiao, S.Q.; Ali, I.; Shang, R.; Wang, D.; Wu, Y.W.; Han, M.W.; Luo, X.G. Application of mixed bacteria-loaded biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J. Hazard. Mater. 2021, 401, 123823. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.J.; Khan, A.; Zhang, K.; Sun, P.; Akther, S.; Zhang, Y. Biochar remediation of soil: Linking biochar production with function in heavy metal contaminated soils. Plant Soil Environ. 2021, 67, 183–201. [Google Scholar]
- Gholizadeh, M.; Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, X.; Du, R.; Wen, S.; Du, L.; Tu, Q. Adsorption of Cd2+ by Lactobacillus Plantarum immobilized on distiller’s grains biochar: Mechanism and action. Microorganisms 2024, 12, 1406. [Google Scholar] [CrossRef]
- Schommer, V.A.; Vanin, A.P.; Nazari, M.T.; Ferrari, V.; Dettmer, A.; Colla, L.M.; Piccin, J.S. Biochar-immobilized Bacillus spp. for heavy metals bioremediation: A review on immobilization techniques, bioremediation mechanisms and effects on soil. Sci. Total Environ. 2023, 881, 163385. [Google Scholar] [CrossRef]
- Abbas, H.M.M.; Rais, U.; Altaf, M.M.; Rasul, F.; Shah, A.; Tahir, A.; Nafees-Ur-Rehman, M.; Shaukat, M.; Sultan, H.; Zou, R.; et al. Microbial-inoculated biochar for remediation of salt and heavy metal contaminated soils. Sci. Total Environ. 2024, 954, 176104. [Google Scholar] [CrossRef]
- Enan, G.; Mamdouh, M.; Negm, S.; Ismaiel, A.A.; Abdel-Shafi, S. Classification, antimicrobial potential, industrial applications and probiotic capability of lactic acid bacteria: A review article. Res. J. Appl. Sci. 2018, 13, 742–757. [Google Scholar] [CrossRef]
- Kumar, N.; Kumari, V.; Ram, C.; Thakur, K.; Tomar, S.K. Bio-prospectus of cadmium bioadsorption by lactic acid bacteria to mitigate health and environmental impacts. Appl. Microbiol. Biotechnol. 2018, 102, 1599–1615. [Google Scholar] [CrossRef]
- Zhai, Q.; Wang, G.; Zhao, J.; Narbad, A.; Chen, Y.Q.; Zhang, H.; Tian, F.; Chen, W. Protective effects of Lactobacillus plantarum CCFM8610 against chronic cadmium toxicity in mice indicate routes of protection besides intestinal sequestration. Appl. Environ. Microbiol. 2014, 80, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.A.; Hamdan, A.M.; El-Naggar, M.Y. Assessment of the heavy metal bioremediation efficiency of the novel marine lactic acid bacterium, Lactobacillus plantarum MF042018. Sci. Rep. 2020, 10, 314. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, J.N.; Ohnishi, K.; Munekage, Y.; Iwasaki, K.; Wei, M.Q. Characterization of lactic acid bacteria-based probiotics as potential heavy metal sorbents. J. Appl. Microbiol. 2012, 112, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Ma, X. Heavy metals remediation through lactic acid bacteria: Current status and future prospects. Sci. Total Environ. 2024, 946, 174455. [Google Scholar] [CrossRef]
- de Andrade Bulos, R.B.; da Gama Paz, F.; Machado, C.G.; Tavares, P.P.L.G.; de Souza, C.O.; Umsza-Guez, M.A. Scientific and technological research on the use of wine lees. Food Prod. Process. Nutr. 2023, 5, 25. [Google Scholar] [CrossRef]
- Zhu, G.X.; Cheng, D.D.; Wang, X.F.; Guo, Q.J.; Zhang, Q.; Zhang, J.; Tu, Q.; Li, W.J. Free amino acids, carbon and nitrogen isotopic compositions responses to cadmium stress in two castor (Ricinus communis L.) species. Plant Physiol. Biochem. 2022, 184, 40–46. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Hakeem, K.R.; Alharby, H.F.; Rehman, R.U. Lead toxicity alters the antioxidant defense machinery and modulate the biomarkers in Tartary buckwheat plants. Int. Biodeterior. Biodegrad. 2020, 151, 104992. [Google Scholar] [CrossRef]
- Zhou, F.; Cui, J.; Zhou, J.; Yang, J.; Li, Y.; Leng, Q.; Wang, Y.; He, D.; Song, L.; Gao, M.; et al. Increasing atmospheric deposition nitrogen and ammonium reduced microbial activity and changed the bacterial community composition of red paddy soil. Sci. Total Environ. 2018, 633, 776–784. [Google Scholar] [CrossRef]
- Xie, J.; Hu, W.; Pei, H.; Dun, M.; Qi, F. Detection of amount and activity of living algae in fresh water by dehydrogenase activity (DHA). Environ. Monit. Assess. 2008, 146, 473–478. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef]
- Liang, J.; Yang, Z.; Tang, L.; Zeng, G.; Yu, M.; Li, X.; Wu, H.; Qian, Y.; Li, X.; Luo, Y. Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 2017, 181, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Deng, S.; Tan, D.; Long, J.; Lei, M. Heavy metal distribution, translocation, and human health risk assessment in the soil-rice system around Dongting Lake area, China. Environ. Sci. Pollut. R. 2019, 26, 17655–17665. [Google Scholar] [CrossRef] [PubMed]
- Fryzova, R.; Pohanka, M.; Martinkova, P.; Cihlarova, H.; Brtnicky, M.; Hladky, J.; Kynicky, J. Oxidative stress and heavy metals in plants. Rev. Environ. Contam. Toxicol. 2018, 245, 129–156. [Google Scholar]
- Saravanan, A.; Swaminaathan, P.; Kumar, P.S.; Yaashikaa, P.R.; Kamalesh, R.; Rangasamy, G. A comprehensive review on immobilized microbes-biochar and their environmental remediation: Mechanism, challenges and future perspectives. Environ. Res. 2023, 236, 116723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Feng, Y. The effects of biochar addition on soil physicochemical properties: A review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- de Sousa Lima, J.R.; de Moraes Silva, W.; de Medeiros, E.V.; Duda, G.P.; Corrêa, M.M.; Martins Filho, A.P.; Clermont-Dauphin, C.; Antonino, A.C.D.; Hammecker, C. Effect of biochar on physicochemical properties of a sandy soil and maize growth in a greenhouse experiment. Geoderma 2018, 319, 14–23. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar increases soil microbial biomass with changes in extra-and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Teutscherova, N.; Lojka, B.; Houška, J.; Masaguer, A.; Benito, M.; Vazquez, E. Application of holm oak biochar alters dynamics of enzymatic and microbial activity in two contrasting mediterranean soils. Eur. J. Soil Biol. 2018, 88, 15–26. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debskam, B.; Haddadm, S.A. Soil enzyme activity response under the amendment of different types of biochar. Agronomy 2022, 12, 569. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Xiong, J.; Xu, J.; Zhou, M.; Zhao, W.; Chen, C.; Wang, M.; Tan, W.; Koopal, L. Quantitative characterization of the site density and the charged state of functional groups on biochar. ACS Sustain. Chem. Eng. 2021, 9, 2600–2608. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Abdullah, R.; Ling, T.C.; Ismail, S.; Lau, B.F.; Ong, H.C.; Chew, K.W.; Show, P.L.; Chang, J.S. Bioformulation of biochar as a potential inoculant carrier for sustainable agriculture. Environ. Technol. Innov. 2020, 20, 101168. [Google Scholar] [CrossRef]
- Gupta, R.; Khan, F.; Alqahtani, F.M.; Hashem, M.; Ahmad, F. Plant growth–promoting Rhizobacteria (PGPR) assisted bioremediation of heavy metal toxicity. Appl. Biochem. Biotechnol. 2024, 196, 2928–2956. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Yang, L.L.; Yang, L.; Yang, X.; Zhang, T.; Lan, Y.M.; Zhao, Y.; Han, M.; Yang, L.M. Drought stress induces biosynthesis of flavonoids in leaves and saikosaponins in roots of Bupleurum chinense DC. Phytochemistry 2020, 177, 112434. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Ahmad, Z.; Khan, S.M.; Page, S.E.; Balzter, H.; Ullah, A.; Ali, S.; Jehangir, S.; Ejaz, U.; Afza, R.; Mukhamezhanova, A.S. Environmental sustainability and resilience in a polluted ecosystem via phytoremediation of heavy metals and plant physiological adaptations. J. Clean. Prod. 2023, 385, 135733. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, S.; Liu, Z.; Yang, F.; Yin, G. Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with enhanced antioxidative protection and declined lipid peroxidation. J. Plant Physiol. 2019, 232, 226–240. [Google Scholar] [CrossRef] [PubMed]





| Lables | Treatments |
|---|---|
| CK | Control group |
| T1 | 2.2% distiller’s grains biochar |
| T2 | 4.4% distiller’s grains biochar |
| J1 | 0.0125% Lactobacillus plantarum thallus |
| J2 | 0.025% Lactobacillus plantarum thallus |
| S1 | 0.055 mL·g−1 Lactobacillus plantarum supernatant |
| S2 | 0.11 mL·g−1 Lactobacillus plantarum supernatant |
| T1 + J1 | 2.2% distiller’s grains biochar + 0.0125% Lactobacillus plantarum thallus |
| T2 + J2 | 4.4% distiller’s grains biochar + 0.025% Lactobacillus plantarum thallus |
| Treatment | pH | Total Nitrogen (g/kg) | Alkaline Nitrogen (mg/kg) | Total Phosphorus (g/kg) | Available Phosphorus (mg/kg) | Total Potassium (g/kg) | Available Potassium (mg/kg) | Organic Matter (g/kg) |
|---|---|---|---|---|---|---|---|---|
| CK | 7.63 ± 0.38 a | 1.16 ± 0.06 c | 85.18 ± 4.26 ef | 0.43 ± 0.02 e | 23.84 ± 1.19 de | 6.95 ± 0.35 a | 112.58 ± 5.63 d | 42.53 ± 2.13 b |
| T1 | 7.61 ± 0.38 a | 1.81 ± 0.09 b | 89.18 ± 4.46 def | 0.52 ± 0.03 de | 21.02 ± 1.05 e | 7.6 ± 0.38 a | 137.1 ± 6.86 c | 60.26 ± 3.01 a |
| T2 | 7.73 ± 0.39 a | 2.36 ± 0.12 a | 80.81 ± 4.04 f | 0.91 ± 0.05 a | 35.45 ± 1.77 a | 7.18 ± 0.36 a | 181 ± 9.05 b | 64.47 ± 3.22 a |
| J1 | 7.62 ± 0.38 a | 1.29 ± 0.06 c | 105.93 ± 5.3 bc | 0.61 ± 0.03 cd | 28.07 ± 1.4 bc | 6.86 ± 0.34 a | 112.82 ± 5.64 d | 47.72 ± 2.39 b |
| J2 | 7.74 ± 0.39 a | 1.35 ± 0.07 c | 127.69 ± 6.38 a | 0.66 ± 0.03 bc | 25.92 ± 1.3 bcd | 7.07 ± 0.35 a | 131.44 ± 6.57 cd | 48.3 ± 2.42 b |
| S1 | 7.71 ± 0.39 a | 1.29 ± 0.06 c | 101.11 ± 5.06 bcd | 0.64 ± 0.03 c | 25.38 ± 1.27 bcd | 7.15 ± 0.36 a | 134.77 ± 6.74 cd | 45.46 ± 2.27 b |
| S2 | 7.63 ± 0.38 a | 1.24 ± 0.06 c | 108.73 ± 5.44 b | 0.88 ± 0.04 a | 28.92 ± 1.45 b | 7.08 ± 0.35 a | 143.93 ± 7.2 c | 46.72 ± 2.34 b |
| T1 + J1 | 7.71 ± 0.39 a | 1.37 ± 0.07 c | 91.68 ± 4.58 cdef | 0.56 ± 0.03 cd | 24.4 ± 1.22 cde | 7.03 ± 0.35 a | 133.78 ± 6.69 cd | 49.02 ± 2.45 b |
| T2 + J2 | 7.77 ± 0.39 a | 2.16 ± 0.11 a | 96.5 ± 4.82 bcde | 0.75 ± 0.04 b | 28.54 ± 1.43 bc | 6.55 ± 0.33 a | 216.12 ± 10.81 a | 65.14 ± 3.26 a |
| Treatments | Average Plant Height (cm) | Dry Weight (g/plant) |
|---|---|---|
| CK | 79.00 ± 3.95 bcd | 3.65 ± 0.18 e |
| T1 | 82.40 ± 4.12 bc | 5.72 ± 0.29 b |
| T2 | 89.80 ± 4.49 a | 6.28 ± 0.31 a |
| J1 | 73.60 ± 3.68 d | 3.43 ± 0.17 e |
| J2 | 74.40 ± 3.72 d | 5.09 ± 0.25 cd |
| S1 | 76.10 ± 3.80 cd | 3.72 ± 0.24 e |
| S2 | 80.00 ± 4.00 bcd | 5.43 ± 0.27 bc |
| T1 + J1 | 82.10 ± 4.10 bc | 4.68 ± 0.23 d |
| T2 + J2 | 86.60 ± 4.33 ab | 6.42 ± 0.32 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Li, Y.; Cheng, D.; Chen, R.; Wang, Y.; Tu, Q. Effects of Distiller’s Grains Biochar and Lactobacillus plantarum on the Remediation of Cd-Pb-Zn-Contaminated Soil and Growth of Sorghum-Sudangrass. Microorganisms 2024, 12, 2592. https://doi.org/10.3390/microorganisms12122592
Zhu G, Li Y, Cheng D, Chen R, Wang Y, Tu Q. Effects of Distiller’s Grains Biochar and Lactobacillus plantarum on the Remediation of Cd-Pb-Zn-Contaminated Soil and Growth of Sorghum-Sudangrass. Microorganisms. 2024; 12(12):2592. https://doi.org/10.3390/microorganisms12122592
Chicago/Turabian StyleZhu, Guangxu, Yufeng Li, Dandan Cheng, Rongkun Chen, Yunyan Wang, and Qiang Tu. 2024. "Effects of Distiller’s Grains Biochar and Lactobacillus plantarum on the Remediation of Cd-Pb-Zn-Contaminated Soil and Growth of Sorghum-Sudangrass" Microorganisms 12, no. 12: 2592. https://doi.org/10.3390/microorganisms12122592
APA StyleZhu, G., Li, Y., Cheng, D., Chen, R., Wang, Y., & Tu, Q. (2024). Effects of Distiller’s Grains Biochar and Lactobacillus plantarum on the Remediation of Cd-Pb-Zn-Contaminated Soil and Growth of Sorghum-Sudangrass. Microorganisms, 12(12), 2592. https://doi.org/10.3390/microorganisms12122592





