Planted Citrus Regulates the Community and Networks of phoD-Harboring Bacteria to Drive Phosphorus Availability Between Karst and Non-Karst Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling
2.3. General Soil Parameters
2.4. DNA Extraction and Illumina Sequencing
2.5. Statistical Analyses
3. Results
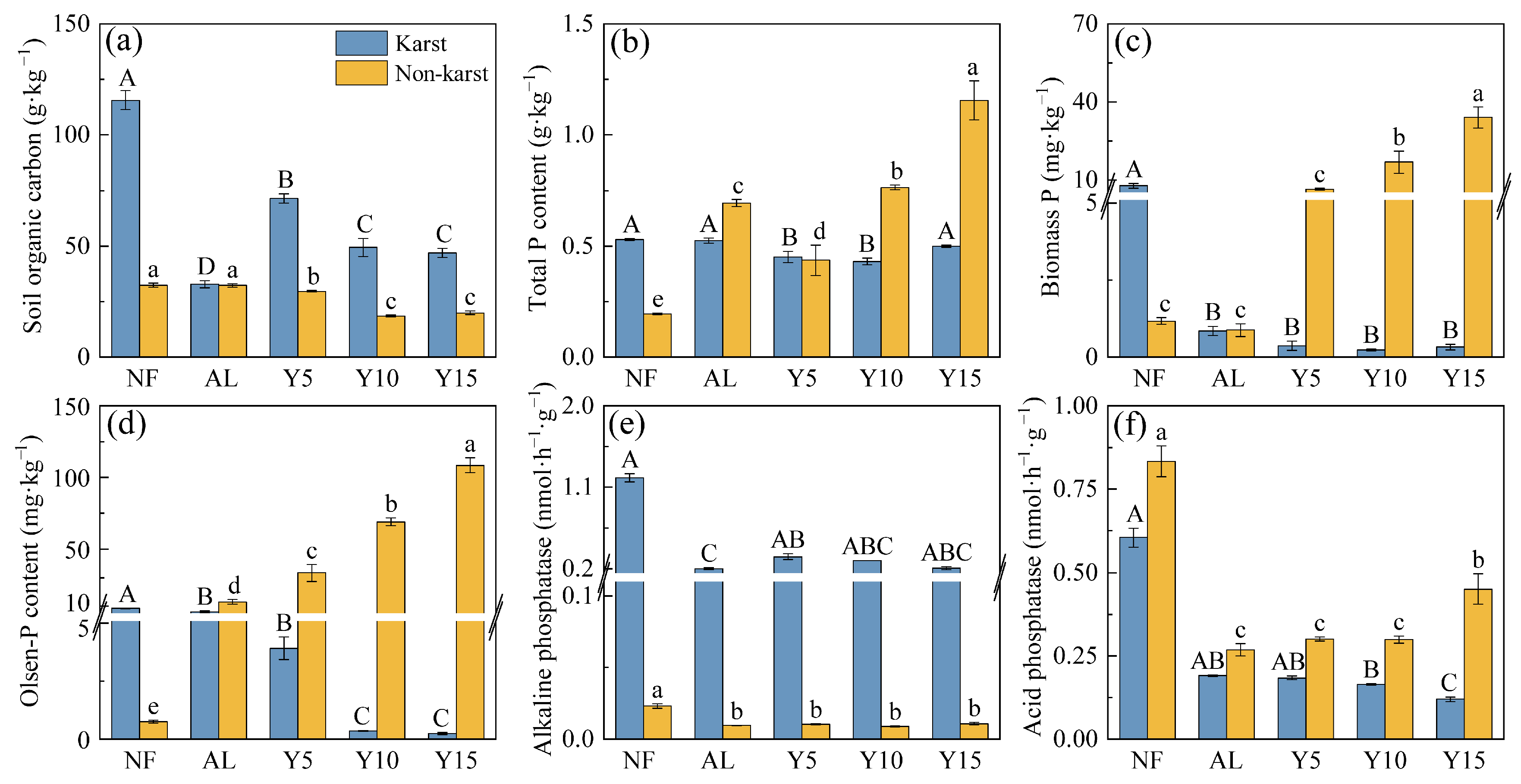
3.1. Soil P Contents and Enzyme Activities in Karst and Non-Karst Citrus Cultivations
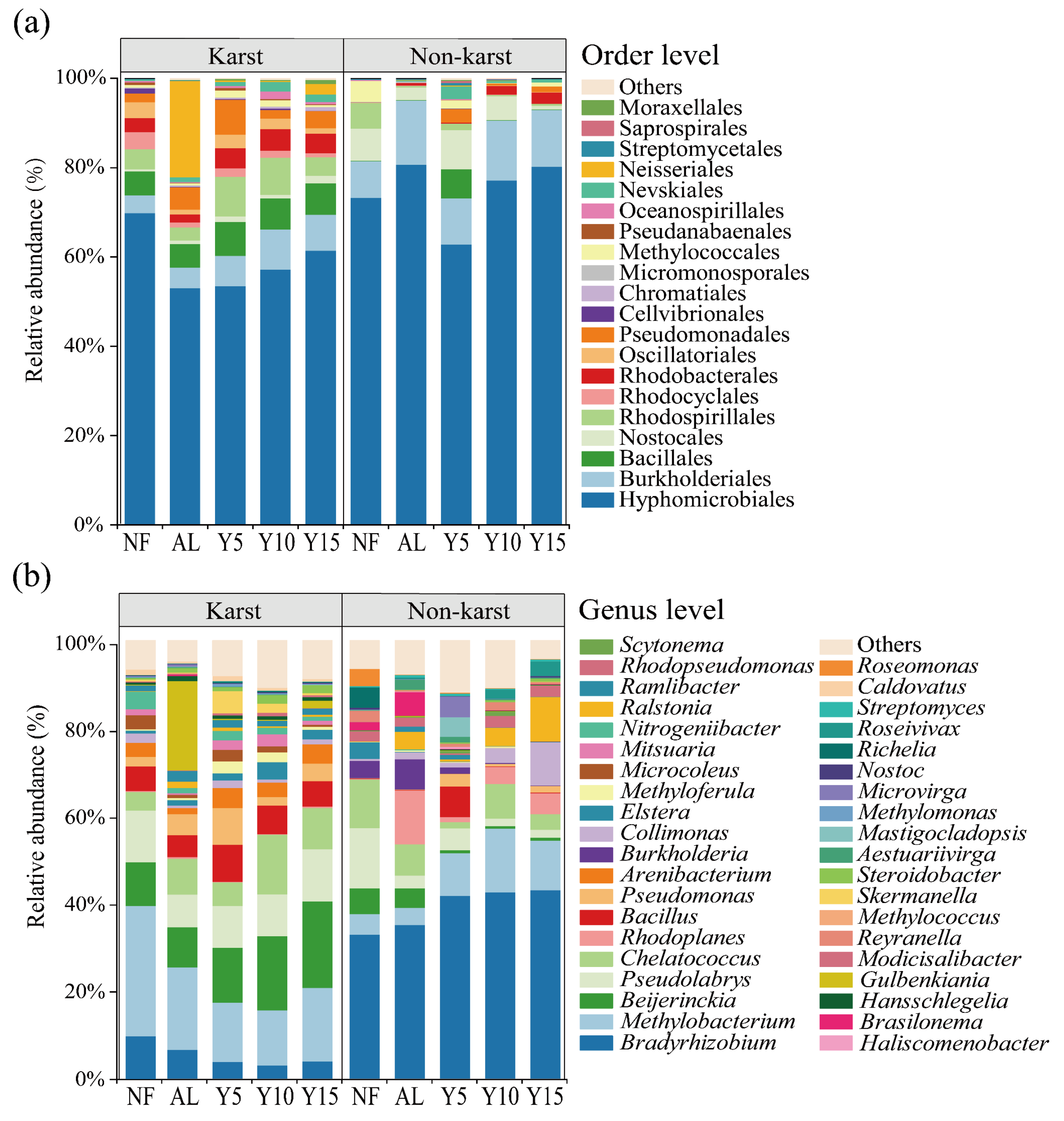
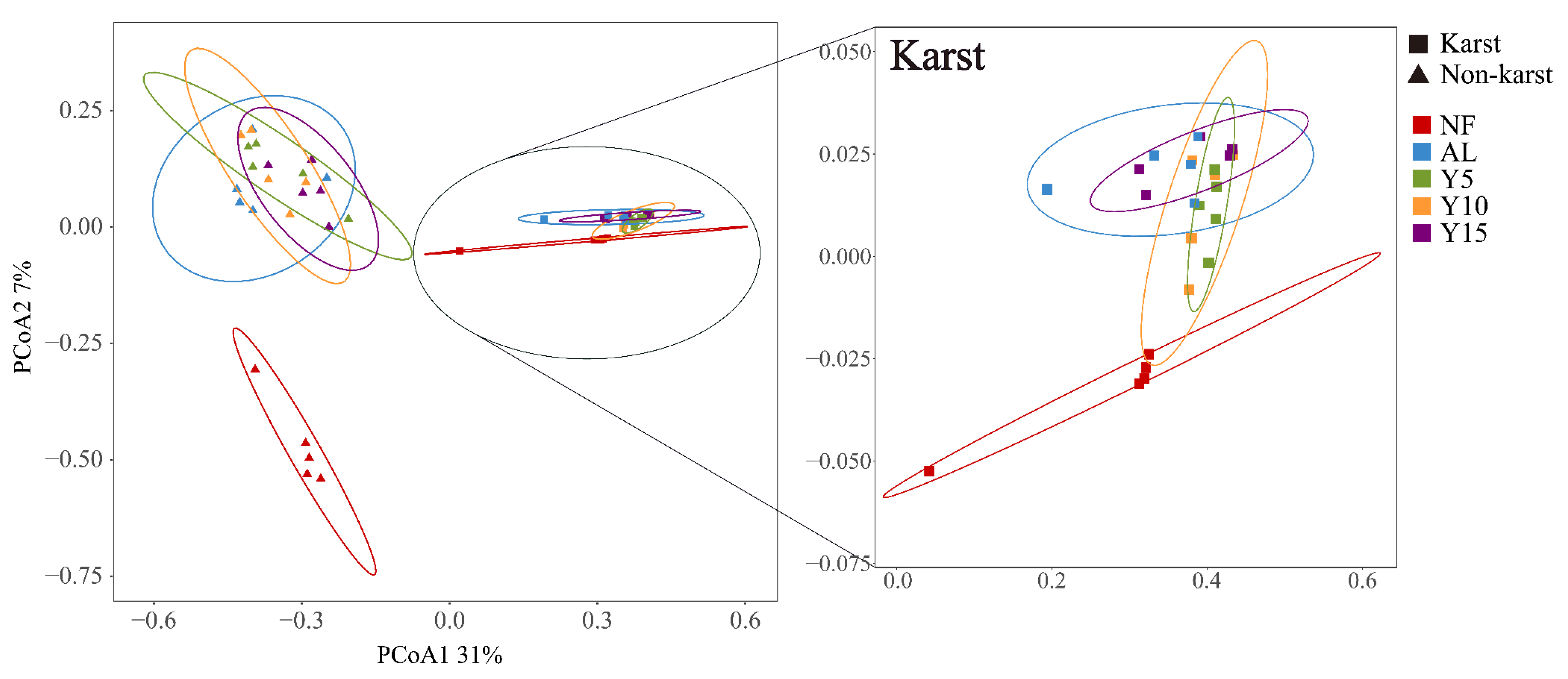
3.2. Community Structure, Diversity, and Co-Occurrence Networks of phoD-Harboring Bacteria
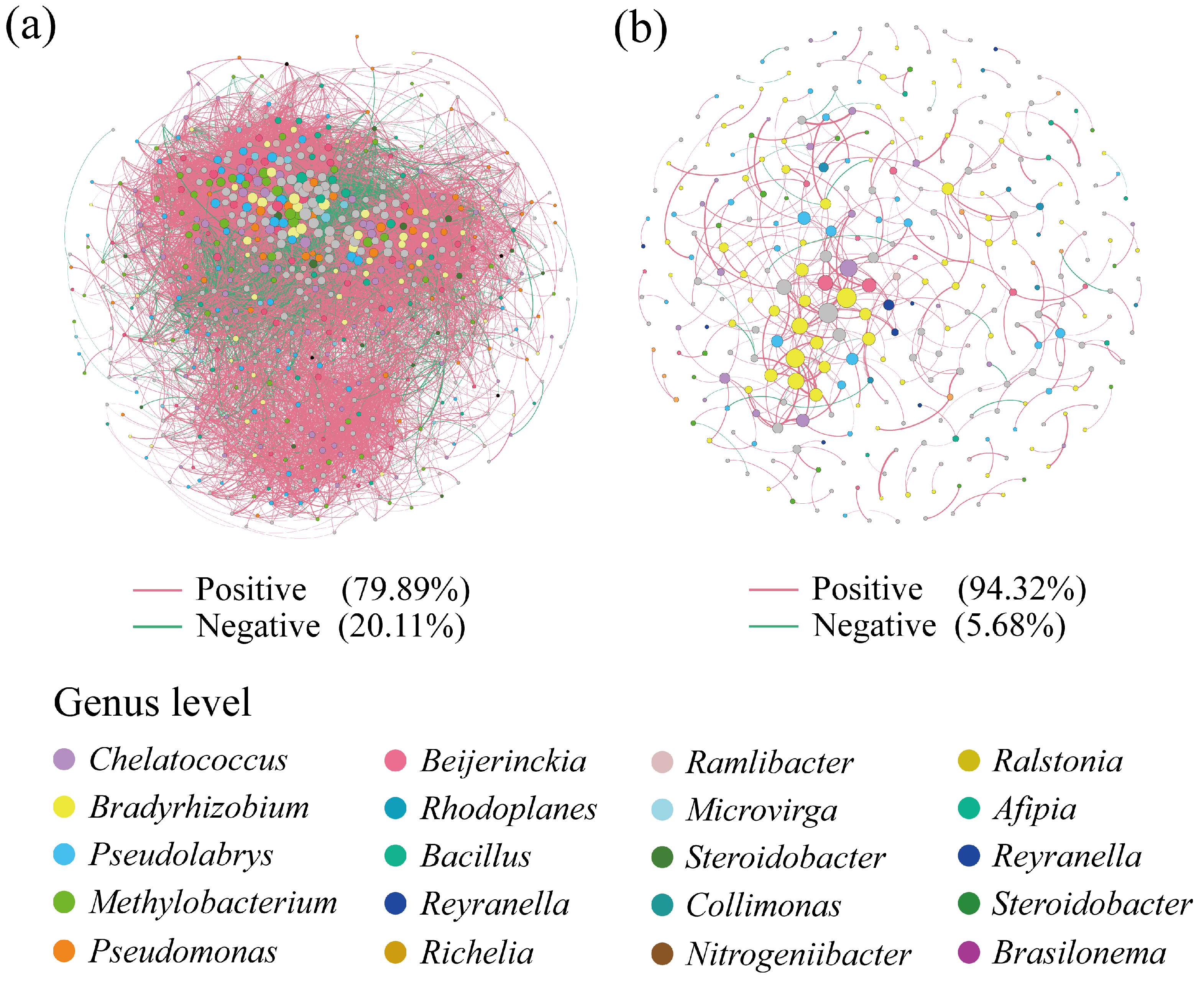
3.3. Co-Occurrence Networks of phoD-Harboring Bacteria
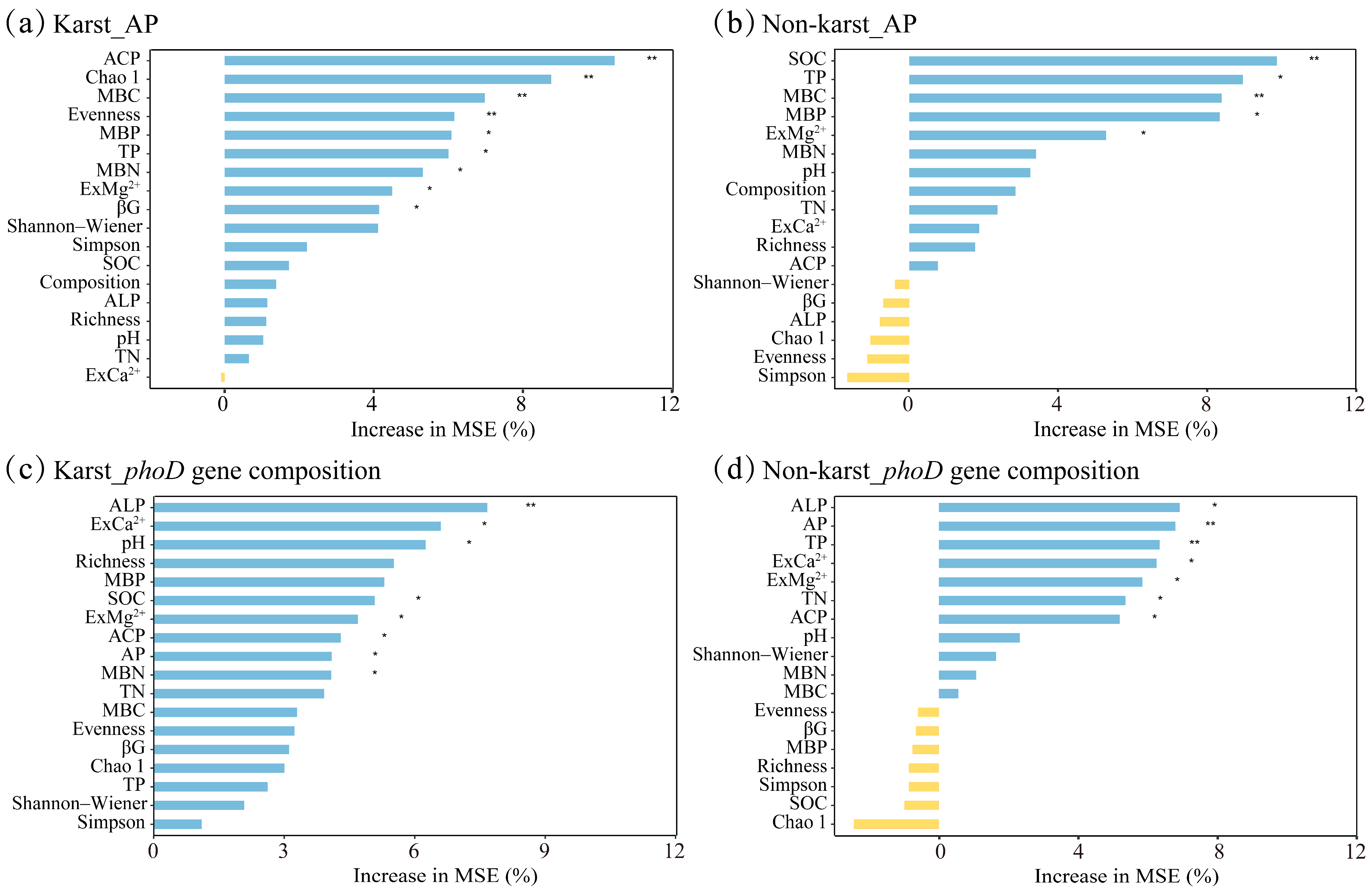
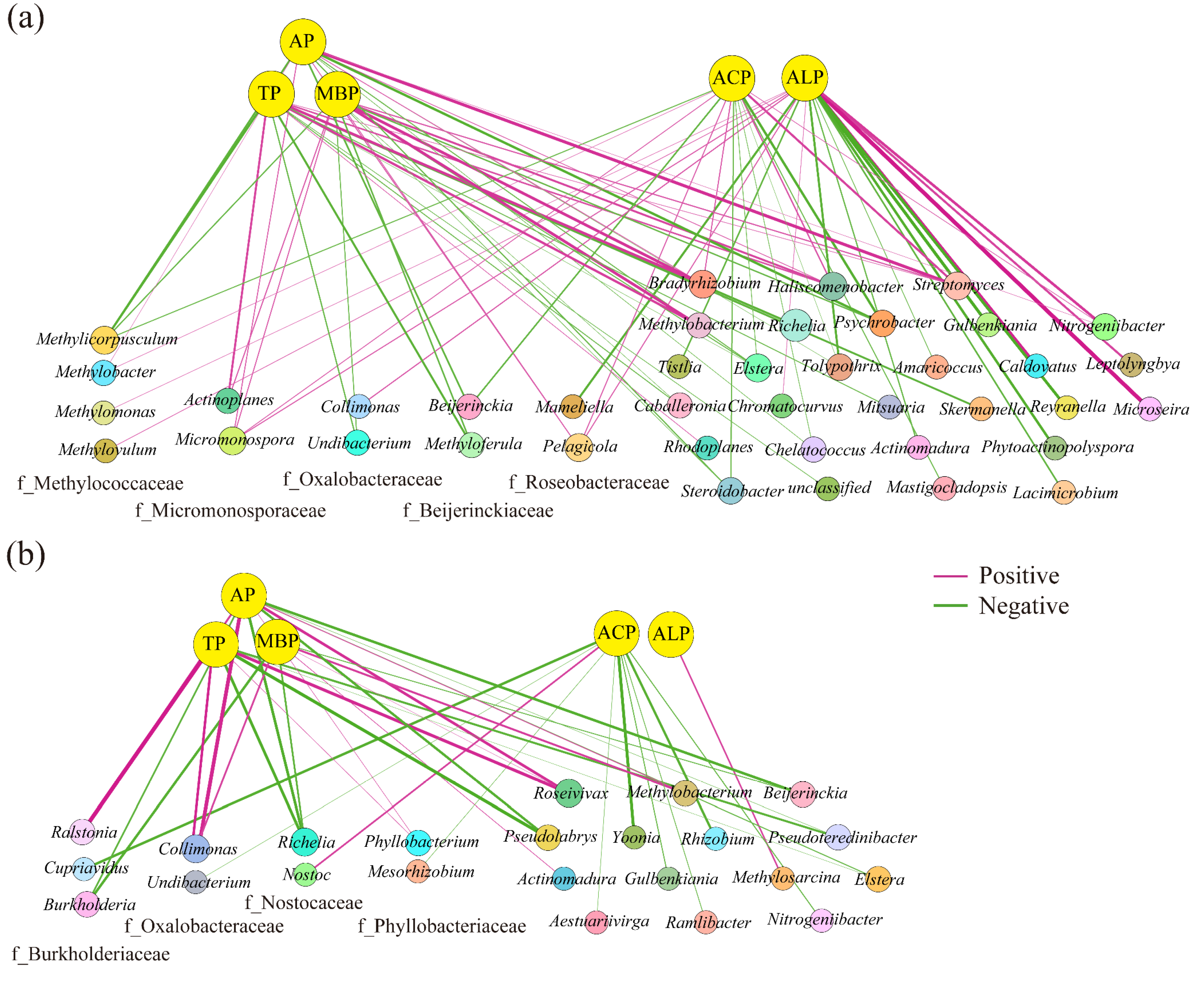
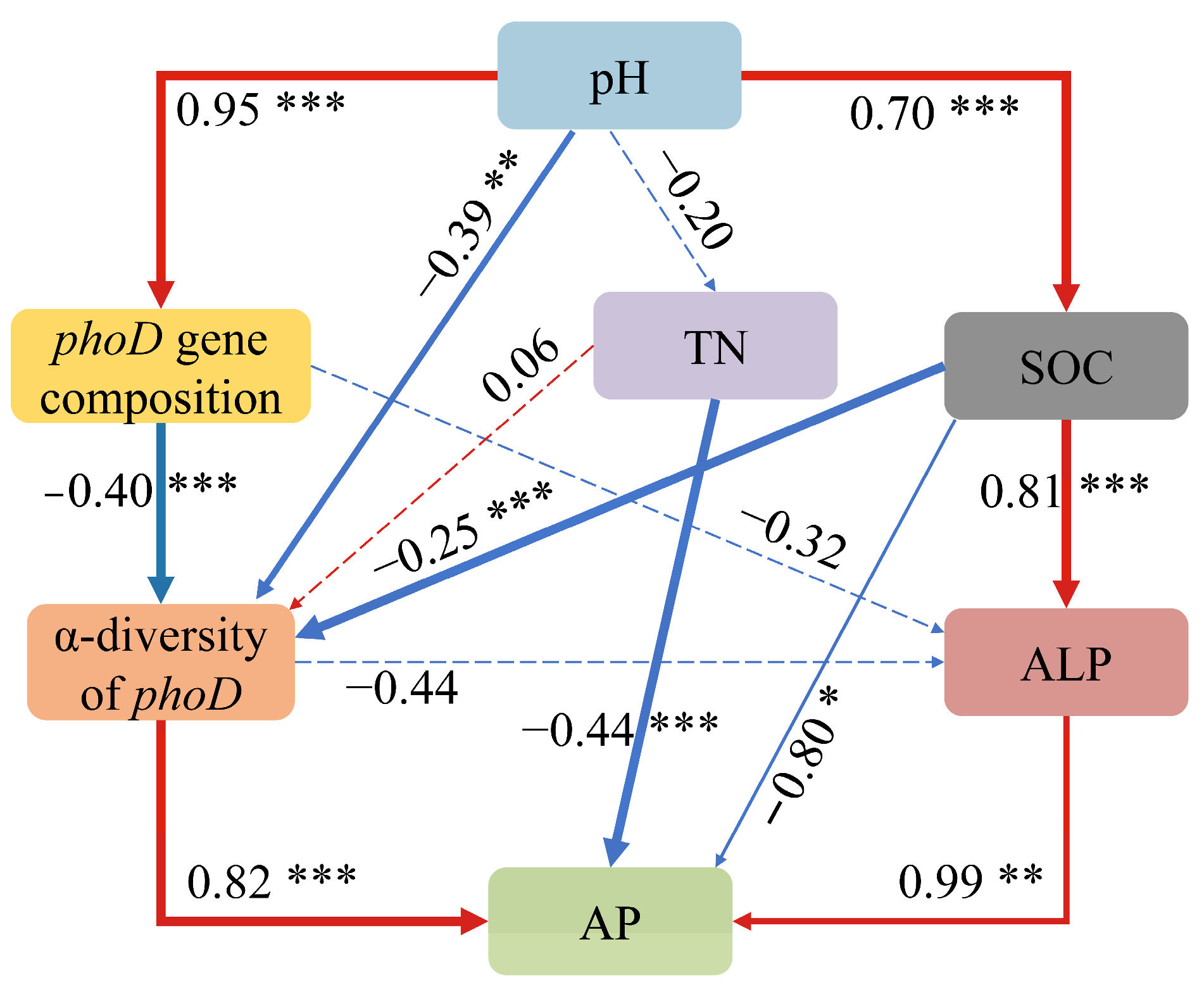
3.4. Soil phoD-Harboring Bacteria Affected P Availability
4. Discussion
4.1. Soil P Availability and Influencing Factors
4.2. phoD-Harboring Microbial Community and Co-Occurrence Networks and Influencing Factors
4.3. Mechanism by Which phoD-Harboring Bacteria Influenced P Availability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and Characterization of a Phosphate-Solubilizing Halophilic Bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid. Based Complement. Altern. Med. 2011, 2011, 615032. [Google Scholar] [CrossRef]
- Ma, X.; Li, F.; Chen, Y.; Chang, Y.; Lian, X.; Li, Y.; Ye, L.; Yin, T.; Lu, X. Effects of Fertilization Approaches on Plant Development and Fertilizer Use of Citrus. Plants 2022, 11, 2547. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Yang, M.; Zhang, Z.-Z.; Li, W.-L.; Guo, C.-Y.; Chen, X.-P.; Shi, X.-J.; Zhou, P.; Tang, X.-D.; Zhang, Y.-Q.; et al. An Ecological Research on Potential for Zero-growth of Chemical Fertilizer Use in Citrus Production in China. Ekoloji 2019, 28, 1049–1059. [Google Scholar]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Zancanaro, L.E.; Nunes, R.; Sousa, D.; Busato, J.; Figueiredo, C. Response of Maize to Different Soil Residual Phosphorus Conditions. Agron. J. 2019, 111, 3291–3300. [Google Scholar] [CrossRef]
- Carpenter, S. Eutrophication of Aquatic Ecosystems: Bistability and Soil Phosphorus. Proc. Natl. Acad. Sci. USA 2005, 102, 10002–10005. [Google Scholar] [CrossRef]
- Chen, X.; Condron, L.M.; Dunfield, K.E.; Wakelin, S.A.; Chen, L. Impact of grassland afforestation with contrasting tree species on soil phosphorus fractions and alkaline phosphatase gene communities. Soil Biol. Biochem. 2021, 159, 108274. [Google Scholar] [CrossRef]
- Zeng, Q.; Mei, T.; Delgado-Baquerizo, M.; Wang, M.; Tan, W. Suppressed phosphorus-mineralizing bacteria after three decades of fertilization. Agric. Ecosyst. Environ. 2022, 323, 107679. [Google Scholar] [CrossRef]
- Bibi, S.; Irshad, M.; Ullah, F.; Mahmood, Q.; Shahzad, M.; Tariq, M.A.U.R.; Hussain, Z.; Mohiuddin, M.; An, P.; Ng, A.W.M.; et al. Phosphorus extractability in relation to soil properties in different fields of fruit orchards under similar ecological conditions of Pakistan. Front. Ecol. Evol. 2023, 10, 1077270. [Google Scholar] [CrossRef]
- MacDonald, G.K.; Bennett, E.M.; Taranu, Z.E. The influence of time, soil characteristics, and land-use history on soil phosphorus legacies: A global meta-analysis. Glob. Change Biol. 2012, 18, 1904–1917. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, X.; Ni, K.; Ma, L.; Shi, Y.; Wang, Y.; Cai, Y.; Ma, Q.; Ruan, J. Nitrogen addition reduces phosphorus availability and induces a shift in soil phosphorus cycling microbial community in a tea (Camellia sinensis L.) plantation. J. Environ. Manag. 2023, 342, 118207. [Google Scholar] [CrossRef] [PubMed]
- Fraser, T.D.; Lynch, D.H.; Bent, E.; Entz, M.H.; Dunfield, K.E. Soil bacterial phoD gene abundance and expression in response to applied phosphorus and long-term management. Soil Biol. Biochem. 2015, 88, 137–147. [Google Scholar] [CrossRef]
- Tan, H.; Barret, M.; Mooij, M.J.; Rice, O.; Morrissey, J.P.; Dobson, A.; Griffiths, B.; O’Gara, F. Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol. Fertil. Soils 2013, 49, 661–672. [Google Scholar] [CrossRef]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Lynch, M.D.J.; Neufeld, J.D. Ecology and exploration of the rare biosphere. Nat. Rev. Microbiol. 2015, 13, 217–229. [Google Scholar] [CrossRef]
- Wei, X.; Hu, Y.; Razavi, B.S.; Zhou, J.; Shen, J.; Nannipieri, P.; Wu, J.; Ge, T. Rare taxa of alkaline phosphomonoesterase-harboring microorganisms mediate soil phosphorus mineralization. Soil Biol. Biochem. 2019, 131, 62–70. [Google Scholar] [CrossRef]
- Tong, X.; Brandt, M.; Yue, Y.; Horion, S.; Wang, K.; Keersmaecker, W.D.; Tian, F.; Schurgers, G.; Xiao, X.; Luo, Y.; et al. Increased vegetation growth and carbon stock in China karst via ecological engineering. Nat. Sustain. 2018, 1, 44–50. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Huang, Y.; Augusto, L.; Goll, D.S.; Helfenstein, J.; Hou, E. Toward a Global Model for Soil Inorganic Phosphorus Dynamics: Dependence of Exchange Kinetics and Soil Bioavailability on Soil Physicochemical Properties. Glob. Biogeochem. Cycles 2022, 36, e2021GB007061. [Google Scholar] [CrossRef]
- Green, S.M.; Dungait, J.A.J.; Tu, C.; Buss, H.L.; Sanderson, N.; Hawkes, S.J.; Xing, K.; Yue, F.; Hussey, V.L.; Peng, J.; et al. Soil functions and ecosystem services research in the Chinese karst Critical Zone. Chem. Geol. 2019, 527, 119107. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, H.; Zhu, T.; Zhang, C.; Zhu, D. Preliminary Research on Agricultural Cultivation Decreasing Amino Sugar Accumulation in Calcareous Soils in Subtropical Karst Region of China. Land 2022, 11, 1684. [Google Scholar] [CrossRef]
- Sun, M.; Yang, R.; Tang, Y.; Xiao, D.; Zhang, W.; Xu, Z.; Shi, Z.; Hu, P.; Wu, H.; Wang, K. Lithologic control of soil C:N:P stoichiometry across a climatic gradient in southwest China. J. Soil Sediment. 2023, 23, 1662–1673. [Google Scholar] [CrossRef]
- Pan, F.; Yu, X.; Chen, M.; Liang, Y. Vegetation recovery reshapes the composition and enhances the network connectivity of phoD-harboring microorganisms to promote P availability in a karst ecosystem. Sci. Total Environ. 2024, 918, 170561. [Google Scholar] [CrossRef] [PubMed]
- Finzi, A.C.; Sinsabaugh, R.L.; Long, T.M.; Osgood, M.P. Microbial Community Responses to Atmospheric Carbon Dioxide Enrichment in a Warm-Temperate Forest. Ecosystems 2006, 9, 215–226. [Google Scholar] [CrossRef]
- Sakurai, M.; Wasaki, J.; Tomizawa, Y.; Shinano, T.; Osaki, M. Analysis of bacterial communities on alkaline phosphatase genes in soil supplied with organic matter. Soil Sci. Plant Nutr. 2008, 54, 62–71. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Fish, J.A.; Chai, B.; Wang, Q.; Sun, Y.; Brown, C.T.; Tiedje, J.M.; Cole, J.R. FunGene: The functional gene pipeline and repository. Front. Microbiol. 2013, 4, 291. [Google Scholar] [CrossRef]
- Faust, K. Open challenges for microbial network construction and analysis. ISME J. 2021, 15, 3111–3118. [Google Scholar] [CrossRef]
- Berry, D.; Widder, S. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 2014, 5, 219. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Cao, N.; Duan, C.; Ding, C.; Huang, Y.; Wang, J. Biodegradable microplastics enhance soil microbial network complexity and ecological stochasticity. J. Hazard. Mater. 2022, 439, 129610. [Google Scholar] [CrossRef]
- Guimerà, R.; Nunes Amaral, L.A. Functional cartography of complex metabolic networks. Nature 2005, 433, 895–900. [Google Scholar] [CrossRef]
- Xiao, X.; Fan, M.; Wang, E.; Chen, W.; Wei, G. Interactions of plant growth-promoting rhizobacteria and soil factors in two leguminous plants. Appl. Microbiol. Biotechnol. 2017, 101, 8485–8497. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Yang, R.; Li, J.; Long, J.; Liao, H.; Wang, X.; Li, Y. Structural characteristics of bacterial community in rhizosphere soil of Zanthoxylum bungeamun in different planting years in Karst Areas of Guizhou. Ecol. Environ. Sci. 2021, 30, 81–91. (In Chinese) [Google Scholar] [CrossRef]
- Liao, L.; Shi, F.; Zhang, N.; Chen, X.; Bu, H.; Sun, F. Effects of Different Planting Years on Rhizosphere Soil Physiochemical Properties and Microbial Community of Zanthoxylum bungeanum. Bull. Bot. Res. 2022, 42, 466–474. (In Chinese) [Google Scholar] [CrossRef]
- Peng, S.; Kuang, X.; Cheng, H.; Wei, K.; Cai, K.; Tian, J. Post-agricultural succession affects the accumulation and enzymatic transformation of organic phosphorus in a karst area, southwest China. Plant Soil 2024, 498, 5–20. [Google Scholar] [CrossRef]
- Kumar, M.; Kour, D.; Yadav, A.N.; Saxena, R.; Rai, P.K.; Jyoti, A.; Tomar, R.S. Biodiversity of methylotrophic microbial communities and their potential role in mitigation of abiotic stresses in plants. Biologia 2019, 74, 287–308. [Google Scholar] [CrossRef]
- Wielbo, J.; Kidaj, D.; Koper, P.; Kubik-Komar, A.; Skorupska, A. The effect of biotic and physical factors on the competitive ability of Rhizobium leguminosarum. Cent. Eur. J. Biol. 2012, 7, 13–24. [Google Scholar] [CrossRef]
- Luo, G.; Ling, N.; Nannipieri, P.; Chen, H.; Raza, W.; Wang, M.; Guo, S.; Shen, Q. Long-term fertilisation regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol. Fertil. Soils 2017, 53, 375–388. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, X.; Lin, Q.; Li, G. Distribution Characteristics of phoD-Harbouring Bacterial Community Structure and Its Roles in Phosphorus Transformation in Steppe Soils in Northern China. J. Soil Sci. Plant Nutr. 2021, 21, 1531–1541. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, S.; Liu, S.; Guo, H.; Wang, T.; Zhou, J.; Peng, X. Changes in Plant Rhizosphere Microbial Communities under Different Vegetation Restoration Patterns in Karst and Non-karst Ecosystems. Sci. Rep. 2019, 9, 8761. [Google Scholar] [CrossRef]
- Barber, J.N.; Nicholson, L.C.; Woods, L.C.; Judd, L.M.; Sezmis, A.L.; Hawkey, J.; Holt, K.E.; McDonald, M.J. Species interactions constrain adaptation and preserve ecological stability in an experimental microbial community. ISME J. 2022, 16, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Yuan, J.; Tang, Z.; Wang, J.; Zhang, Y. Long-Term Organic Fertilization Strengthens the Soil Phosphorus Cycle and Phosphorus Availability by Regulating the pqqC- and phoD-Harboring Bacterial Communities. Microb. Ecol. 2023, 86, 2716–2732. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; He, X.; Zhang, W.; Cheng, M.; Hu, P.; Wang, K. Diazotroph and arbuscular mycorrhizal fungal diversity and community composition responses to karst and non-karst soils. Appl. Soil Ecol. 2022, 170, 104227. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Xiao, D.; He, X.; Zhang, W.; Hu, P.; Sun, M.; Wang, K. Comparison of bacterial and fungal diversity and network connectivity in karst and non-karst forests in southwest China. Sci. Total Environ. 2022, 822, 153179. [Google Scholar] [CrossRef]
- Xu, L.; Cao, H.; Li, C.; Wang, C.; He, N.; Hu, S.; Yao, M.; Wang, C.; Wang, J.; Zhou, S.; et al. The importance of rare versus abundant phoD-harboring subcommunities in driving soil alkaline phosphatase activity and available P content in Chinese steppe ecosystems. Soil Biol. Biochem. 2022, 164, 108491. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef]
- Xun, W.; Liu, Y.; Li, W.; Ren, Y.; Xiong, W.; Xu, Z.; Zhang, N.; Miao, Y.; Shen, Q.; Zhang, R. Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome 2021, 9, 35. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.-J.; Banerjee, S.; Zhou, N.; Zhao, Z.-Y.; Zhang, K.; Tian, C.-Y. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Xu, J.; Ma, L.; Olk, D.C.; Zhao, B.; Zhang, J.; Xin, X. Chemical nature of soil organic carbon under different long-term fertilization regimes is coupled with changes in the bacterial community composition in a Calcaric Fluvisol. Biol. Fertil. Soils 2018, 54, 999–1012. [Google Scholar] [CrossRef]
- Pan, F.J.; Yang, Q.; Liang, Y.M.; Yu, X.; Hu, P.L.; Zhang, W.; Pang, Y.L. Lithology and elevated temperature impact phoD-harboring bacteria on soil available P enhancing in subtropical forests. Sci. Total Environ. 2024, 948, 174815. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Feng, L.; Huang, Y.; Liang, Y.; Pan, F.; Zhang, W.; Zhao, Y.; Xiao, Y. Planted Citrus Regulates the Community and Networks of phoD-Harboring Bacteria to Drive Phosphorus Availability Between Karst and Non-Karst Soils. Microorganisms 2024, 12, 2582. https://doi.org/10.3390/microorganisms12122582
Yu X, Feng L, Huang Y, Liang Y, Pan F, Zhang W, Zhao Y, Xiao Y. Planted Citrus Regulates the Community and Networks of phoD-Harboring Bacteria to Drive Phosphorus Availability Between Karst and Non-Karst Soils. Microorganisms. 2024; 12(12):2582. https://doi.org/10.3390/microorganisms12122582
Chicago/Turabian StyleYu, Xuan, Lulu Feng, Yuan Huang, Yueming Liang, Fujing Pan, Wei Zhang, Yuan Zhao, and Yuexin Xiao. 2024. "Planted Citrus Regulates the Community and Networks of phoD-Harboring Bacteria to Drive Phosphorus Availability Between Karst and Non-Karst Soils" Microorganisms 12, no. 12: 2582. https://doi.org/10.3390/microorganisms12122582
APA StyleYu, X., Feng, L., Huang, Y., Liang, Y., Pan, F., Zhang, W., Zhao, Y., & Xiao, Y. (2024). Planted Citrus Regulates the Community and Networks of phoD-Harboring Bacteria to Drive Phosphorus Availability Between Karst and Non-Karst Soils. Microorganisms, 12(12), 2582. https://doi.org/10.3390/microorganisms12122582







