Impact of Biochar Addition on Biofloc Nitrifying Bacteria and Inorganic Nitrogen Dynamics in an Intensive Aquaculture System of Shrimp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biofloc-Based System and Shrimp Stocking
2.2. Trial Design and Culture Management
2.3. Water Quality Monitoring and Biofloc Quantitative Evaluation
2.4. Quantification of Total Bacteria and Total Vibrio in Culture Water
2.5. Biofloc DNA Extraction, Metagenomic Sequencing, and Bioinformatics Analysis
2.6. Shrimp Harvest and Performance Determination
2.7. Statistical Analysis
3. Results
3.1. Biofloc Concentration and Bacterial Quantity Changes in Shrimp Culture Systems
3.2. Inorganic Nitrogen Dynamics and Shrimp Production Performance in Culture Systems
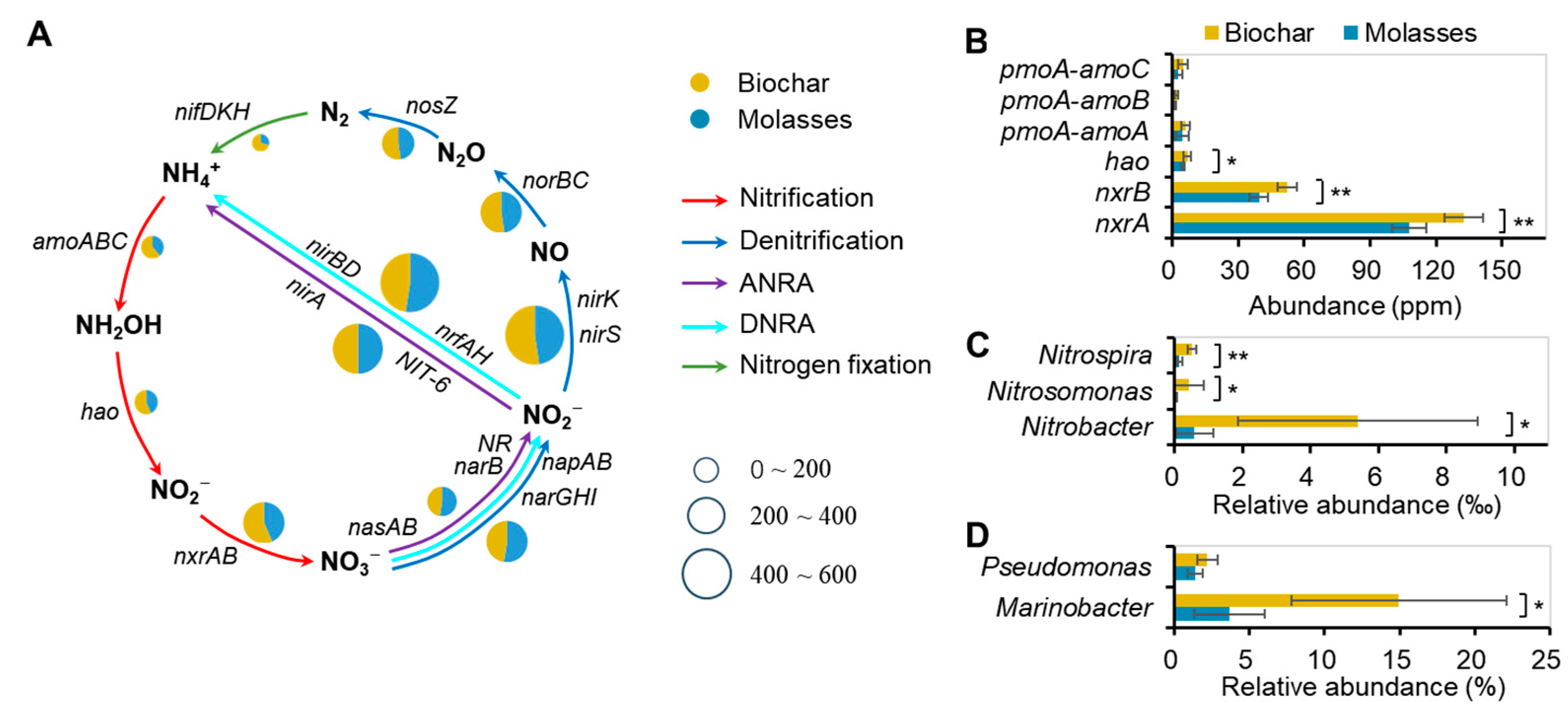
3.3. Bacterial Communities and N-Transformation Pathways of Bioflocs in Culture Systems
4. Discussion
4.1. Biochar Controls Biofloc Concentration and Vibrio Quantity in Shrimp Culture Systems
4.2. Biochar Improves Bacterial Community Diversity of Biofloc in Shrimp Culture Systems
4.3. Biochar Increases Nitrifying Bacteria and Gene of Biofloc in Shrimp Culture Systems
4.4. Biochar Promotes Nitrification to Control Harmful Nitrogen in Shrimp Culture Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture 2024: Towards Blue Transformation; FAO: Rome, Italy, 2024. [Google Scholar]
- Chatvijitkul, S.; Boyd, C.E.; Davis, D.A.; McNevin, A.A. Pollution potential indicators for feed-based fish and shrimp culture. Aquaculture 2017, 477, 43–49. [Google Scholar] [CrossRef]
- Emerenciano, M.G.C.; Rombenso, A.N.; Vieira, F.d.N.; Martins, M.A.; Coman, G.J.; Truong, H.H.; Noble, T.H.; Simon, C.J. Intensification of Penaeid Shrimp Culture: An Applied Review of Advances in Production Systems, Nutrition and Breeding. Animals 2022, 12, 236. [Google Scholar] [CrossRef]
- Han, S.; Wang, B.; Wang, M.; Liu, Q.; Zhao, W.; Wang, L. Effects of ammonia and nitrite accumulation on the survival and growth performance of white shrimp Litopenaeus vannamei. Invert. Surviv. J. 2017, 14, 221–232. [Google Scholar]
- Chen, Z.; Ge, H.X.; Chang, Z.Q.; Song, X.F.; Zhao, F.Z.; Li, J. Nitrogen Budget in Recirculating Aquaculture and Water Exchange Systems for Culturing Litopenaeus vannamei. J. Ocean Univ. China 2018, 17, 905–912. [Google Scholar] [CrossRef]
- Hatje, V.; De Souza, M.M.; Ribeiro, L.F.; Eca, G.F.; Barros, F. Detection of environmental impacts of shrimp farming through multiple lines of evidence. Environ. Pollut. 2016, 219, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Lai, D.Y.F.; Jin, B.S.; Bastviken, D.; Tan, L.S.; Tong, C. Dynamics of dissolved nutrients in the aquaculture shrimp ponds of the Min River estuary, China: Concentrations, fluxes and environmental loads. Sci. Total Environ. 2017, 603, 256–267. [Google Scholar] [CrossRef]
- Bossier, P.; Ekasari, J. Biofloc technology application in aquaculture to support sustainable development goals. Microb. Biotechnol. 2017, 10, 1012–1016. [Google Scholar] [CrossRef]
- Joffre, O.M.; Klerkx, L.; Khoa, T.N.D. Aquaculture innovation system analysis of transition to sustainable intensification in shrimp farming. Agron. Sustain. Dev. 2018, 38, 34. [Google Scholar] [CrossRef]
- Nguyen, T.A.T.; Nguyen, K.A.T.; Jolly, C. Is Super-Intensification the Solution to Shrimp Production and Export Sustainability? Sustainability 2019, 11, 5277. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Rev. Aquacult. 2021, 13, 676–705. [Google Scholar] [CrossRef]
- Javourez, U.; O‘Donhue, M.; Hamelin, L. Waste-to-nutrition: A review of current and emerging conversion pathways. Biotechnol. Adv. 2021, 53, 107857. [Google Scholar] [CrossRef]
- Yu, Y.B.; Choi, J.H.; Lee, J.H.; Jo, A.H.; Lee, J.W.; Choi, H.J.; Kang, Y.J.; Choi, C.Y.; Kang, J.C.; Lee, K.M.; et al. The use, application and efficacy of biofloc technology (BFT) in shrimp aquaculture industry: A review. Environ. Technol. Innov. 2024, 33, 103345. [Google Scholar] [CrossRef]
- Abakari, G.; Wu, X.; He, X.; Fan, L.P.; Luo, G.Z. Bacteria in biofloc technology aquaculture systems: Roles and mediating factors. Rev. Aquacult. 2022, 14, 1260–1284. [Google Scholar] [CrossRef]
- Robles-Porchas, G.R.; Gollas-Galván, T.; Martínez-Porchas, M.; Martínez-Cordova, L.R.; Miranda-Baeza, A.; Vargas-Albores, F. The nitrification process for nitrogen removal in biofloc system aquaculture. Rev. Aquacult. 2020, 12, 2228–2249. [Google Scholar] [CrossRef]
- Xu, W.J.; Huang, F.; Zhao, Y.Z.; Su, H.C.; Hu, X.J.; Xu, Y.; Wen, G.L.; Cao, Y.C. Carbohydrate addition strategy affects nitrogen dynamics, budget and utilization, and its microbial mechanisms in biofloc-based Penaeus vannamei culture. Aquaculture 2024, 589, 740907. [Google Scholar] [CrossRef]
- Xu, W.J.; Morris, T.C.; Samocha, T.M. Effects of C/N ratio on biofloc development, water quality, and performance of Litopenaeus vannamei juveniles in a biofloc-based, high-density, zero-exchange, outdoor tank system. Aquaculture 2016, 453, 169–175. [Google Scholar] [CrossRef]
- Schveitzer, R.; Baccarat, R.F.C.; Gaona, C.A.P.; Wasielesky, W., Jr.; Arantes, R. Concentration of suspended solids in superintensive culture of the Pacific white shrimp Litopenaeus vannamei with biofloc technology (BFT): A review. Rev. Aquacult. 2024, 16, 785–795. [Google Scholar] [CrossRef]
- Xu, W.J.; Xu, Y.; Su, H.C.; Hu, X.J.; Xu, Y.N.; Li, Z.J.; Wen, G.L.; Cao, Y.C. Production performance, inorganic nitrogen control and bacterial community characteristics in a controlled biofloc-based system for indoor and outdoor super-intensive culture of Litopenaeus vannamei. Aquaculture 2021, 531, 735749. [Google Scholar] [CrossRef]
- Abakari, G.; Luo, G.Z.; Kombat, E.O.; Alhassan, E.H. Supplemental carbon sources applied in biofloc technology aquaculture systems: Types, effects and future research. Rev. Aquacult. 2021, 13, 1193–1222. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology—A Practical Guide Book, 3rd ed.; The World Aquaculture Society: Baton Rouge, LA, USA, 2015. [Google Scholar]
- Samocha, T.M.; Prangnell, D.I.; Hanson, T.R.; Treece, G.D.; Morris, T.C.; Castro, L.F.; Staresinic, N. Design and Operation of Super Intensive, Biofloc-Dominated Systems for Indoor Production of the Pacific White Shrimp, Litopenaeus vannamei—The Texas A&M AgriLife Research Experience; The World Aquaculture Society: Baton Rouge, LA, USA, 2017. [Google Scholar]
- Wang, J.L.; Wang, S.Z. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Abakari, G.; Luo, G.Z.; Shao, L.N.; Abdullateef, Y.; Cobbina, S.J. Effects of biochar on microbial community in bioflocs and gut of reared in a biofloc system. Aquacult. Int. 2021, 29, 1295–1315. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, L.; Li, Y.; Brookes, P.C.; Xu, J.; Luo, Y. The effects of combinations of biochar, lime, and organic fertilizer on nitrification and nitrifiers. Biol. Fertil. Soils 2016, 53, 77–87. [Google Scholar] [CrossRef]
- Xu, W.J.; Wen, G.L.; Su, H.C.; Xu, Y.; Hu, X.J.; Cao, Y.C. Effect of Input C/N Ratio on Bacterial Community of Water Biofloc and Shrimp Gut in a Commercial Zero-Exchange System with Intensive Production of Penaeus vannamei. Microorganisms 2022, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of the Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Hu, X.; Cao, Y.; Zhao, X.; Su, H.; Wen, G.; Yang, Y. Effect of bacterial community succession on environmental factors during litter decomposition of the seaweed Gracilaria lemaneiformis. Mar. Pollut. Bull. 2023, 197, 115797. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Won, K.M.; Lee, E.S.; Cho, M.; Jung, S.H.; Kim, M.S. Detection of Vibrio and ten Vibrio species in cage-cultured fish by multiplex polymerase chain reaction using house-keeping genes. Aquaculture 2019, 506, 417–423. [Google Scholar] [CrossRef]
- Wang, Q.; Fish, J.A.; Gilman, M.; Sun, Y.N.; Brown, C.T.; Tiedje, J.M.; Cole, J.R. Xander: Employing a novel method for efficient gene-targeted metagenomic assembly. Microbiome 2015, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- De Souza Valente, C.; Wan, A. Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J. Invertebr. Pathol. 2021, 181, 107527. [Google Scholar] [CrossRef] [PubMed]
- Prangnell, D.I.; Castro, L.F.; Ali, A.S.; Browdy, C.L.; Zimba, P.V.; Laramore, S.E.; Samocha, T.M. Some Limiting Factors in Superintensive Production of Juvenile Pacific White Shrimp, Litopenaeus vannamei, in No-water-exchange, Biofloc-dominated Systems. J. World Aquacult Soc. 2016, 47, 396–413. [Google Scholar] [CrossRef]
- Copetti, F.; Gregoracci, G.B.; Vadstein, O.; Schveitzer, R. Management of biofloc concentrations as an ecological strategy for microbial control in intensive shrimp culture. Aquaculture 2021, 543, 736969. [Google Scholar] [CrossRef]
- Widiyanto, T.; Rusmana, I.; Febrianti, D.; Shohihah, H.; Triana, A.; Mardiati, Y. Profiles of Vibrio and heterotrophic bacteria in the intensive Vanamei shrimp culture using bioremediation technique in Karawang. IOP Conf. Ser. Earth Environ. Sci. 2020, 535, 012019. [Google Scholar] [CrossRef]
- Deng, M.; Chen, J.Y.; Gou, J.W.; Hou, J.; Li, D.P.; He, X.G. The effect of different carbon sources on water quality, microbial community and structure of biofloc systems. Aquaculture 2018, 482, 103–110. [Google Scholar] [CrossRef]
- Addo, F.G.; Zhang, S.H.; Manirakiza, B.; Ohore, O.E.; Shudong, Y. The impacts of straw substrate on biofloc formation, bacterial community and nutrient removal in shrimp ponds. Bioresour. Technol. 2021, 326, 124727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Ma, T.; Wang, L.L.; Yu, X.L.; Zhao, X.R.; Gao, W.D.; Van Zwieten, L.; Singh, B.P.; Li, G.T.; Lin, Q.M.; et al. Distinct biophysical and chemical mechanisms governing sucrose mineralization and soil organic carbon priming in biochar amended soils: Evidence from 10 years of field studies. Biochar 2024, 6, 52. [Google Scholar] [CrossRef]
- Fang, J.; Weng, Y.N.; Li, B.E.; Liu, H.J.; Liu, L.J.; Tian, Z.L.; Du, S.T. Graphene oxide decreases the abundance of nitrogen cycling microbes and slows nitrogen transformation in soils. Chemosphere 2022, 309, 136642. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, Y.X.; Marotta, F.; Xamxidin, M.; Li, H.; Xu, J.Q.; Hu, B.L.; Wu, M. The microbial community structure and nitrogen cycle of high-altitude pristine saline lakes on the Qinghai-Tibetan plateau. Front. Microbiol. 2024, 15, 1424368. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, X.; Cao, X.; Qi, W.; Peng, J.; Liu, H.; Qu, J. The influence of wet-to-dry season shifts on the microbial community stability and nitrogen cycle in the Poyang Lake sediment. Sci. Total Environ. 2023, 903, 166036. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.S.; Kim, D.H.; Kim, J.G.; Kim, Y.S.; Yoon, H.S. The microbial communities (bacteria, algae, zooplankton, and fungi) improved biofloc technology including the nitrogen-related material cycle in Litopenaeus vannamei farms. Front. Bioeng. Biotechnol. 2022, 10, 883522. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, Y.; Su, H.; Hu, X.; Yang, K.; Wen, G.; Cao, Y. Characteristics of Ammonia Removal and Nitrifying Microbial Communities in a Hybrid Biofloc-RAS for Intensive Litopenaeus vannamei Culture: A Pilot-Scale Study. Water 2020, 12, 3000. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Kindaichi, T.; Ito, T.; Okabe, S. Ecophysiological Interaction between Nitrifying Bacteria and Heterotrophic Bacteria in Autotrophic Nitrifying Biofilms as Determined by Microautoradiography-Fluorescence in Situ Hybridization. Appl. Environ. Microbiol. 2004, 70, 3. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-Hernández, J.J.; Sánchez-Fernández, L.P.; Villa-Vargas, L.A.; Carrasco-Ochoa, J.A.; Martínez-Trinidad, J.F. Water quality assessment in shrimp culture using an analytical hierarchical process. Ecol. Indic. 2013, 29, 148–158. [Google Scholar] [CrossRef]
- Ray, A.J.; Lotz, J.M. Comparing salinities of 10, 20, and 30% in intensive, commercial-scale biofloc shrimp (Litopenaeus vannamei) production systems. Aquaculture 2017, 476, 29–36. [Google Scholar] [CrossRef]
- Suantika, G.; Situmorang, M.L.; Kurniawan, J.B.; Pratiwi, S.A.; Aditiawati, P.; Astuti, D.I.; Azizah, F.F.N.; Djohan, Y.A.; Zuhri, U.; Simatupang, T.M. Development of a zero water discharge (ZWD)-Recirculating aquaculture system (RAS) hybrid system for super intensive white shrimp (Litopenaeus vannamei) culture under low salinity conditions and its industrial trial in commercial shrimp urban farming in Gresik, East Java, Indonesia. Aquacult. Eng. 2018, 82, 12–24. [Google Scholar]
- Khoa, T.N.D.; Tao, C.T.; Khanh, L.V.; Hai, T.N. Super-intensive culture of white leg shrimp (Litopenaeus vannamei) in outdoor biofloc systems with different sunlight exposure levels: Emphasis on commercial applications. Aquaculture 2020, 524, 735277. [Google Scholar] [CrossRef]





| Indicator | Biochar | Molasses | p Value |
|---|---|---|---|
| Harvest weight (g) | 17.6 ± 0.4 | 17.7 ± 0.6 | 0.68 |
| Growth rate (g week−1) | 1.90 ± 0.05 | 1.92 ± 0.08 | 0.68 |
| Survival rate (%) | 87.3 ± 2.6 | 83.3 ± 4.4 | 0.09 |
| Yield (kg m−3) | 7.97 ± 0.29 | 7.66 ± 0.29 | 0.08 |
| Feed conversion ratio | 1.21 ± 0.04 | 1.19 ± 0.03 | 0.45 |
| Carbon source usage # (kg kg−1 shrimp) | 0.07 ± 0.01 | 0.17 ± 0.02 | 0.00 |
| Sodium carbonate usage (kg kg−1 shrimp) | 0.04 ± 0.00 | 0.23 ± 0.01 | 0.00 |
| Water usage (L kg−1 shrimp) | 355 ± 18 | 417 ± 25 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Zhang, D.; Su, H.; Xu, Y.; Hu, X.; Wen, G.; Cao, Y. Impact of Biochar Addition on Biofloc Nitrifying Bacteria and Inorganic Nitrogen Dynamics in an Intensive Aquaculture System of Shrimp. Microorganisms 2024, 12, 2581. https://doi.org/10.3390/microorganisms12122581
Xu W, Zhang D, Su H, Xu Y, Hu X, Wen G, Cao Y. Impact of Biochar Addition on Biofloc Nitrifying Bacteria and Inorganic Nitrogen Dynamics in an Intensive Aquaculture System of Shrimp. Microorganisms. 2024; 12(12):2581. https://doi.org/10.3390/microorganisms12122581
Chicago/Turabian StyleXu, Wujie, Demin Zhang, Haochang Su, Yu Xu, Xiaojuan Hu, Guoliang Wen, and Yucheng Cao. 2024. "Impact of Biochar Addition on Biofloc Nitrifying Bacteria and Inorganic Nitrogen Dynamics in an Intensive Aquaculture System of Shrimp" Microorganisms 12, no. 12: 2581. https://doi.org/10.3390/microorganisms12122581
APA StyleXu, W., Zhang, D., Su, H., Xu, Y., Hu, X., Wen, G., & Cao, Y. (2024). Impact of Biochar Addition on Biofloc Nitrifying Bacteria and Inorganic Nitrogen Dynamics in an Intensive Aquaculture System of Shrimp. Microorganisms, 12(12), 2581. https://doi.org/10.3390/microorganisms12122581







