The Significance of the Cell-Mediated Host Immune Response in Syphilis
Abstract
1. Introduction
2. Characteristics of the Pathogen and Mechanisms of Immune Evasion by Treponema pallidum
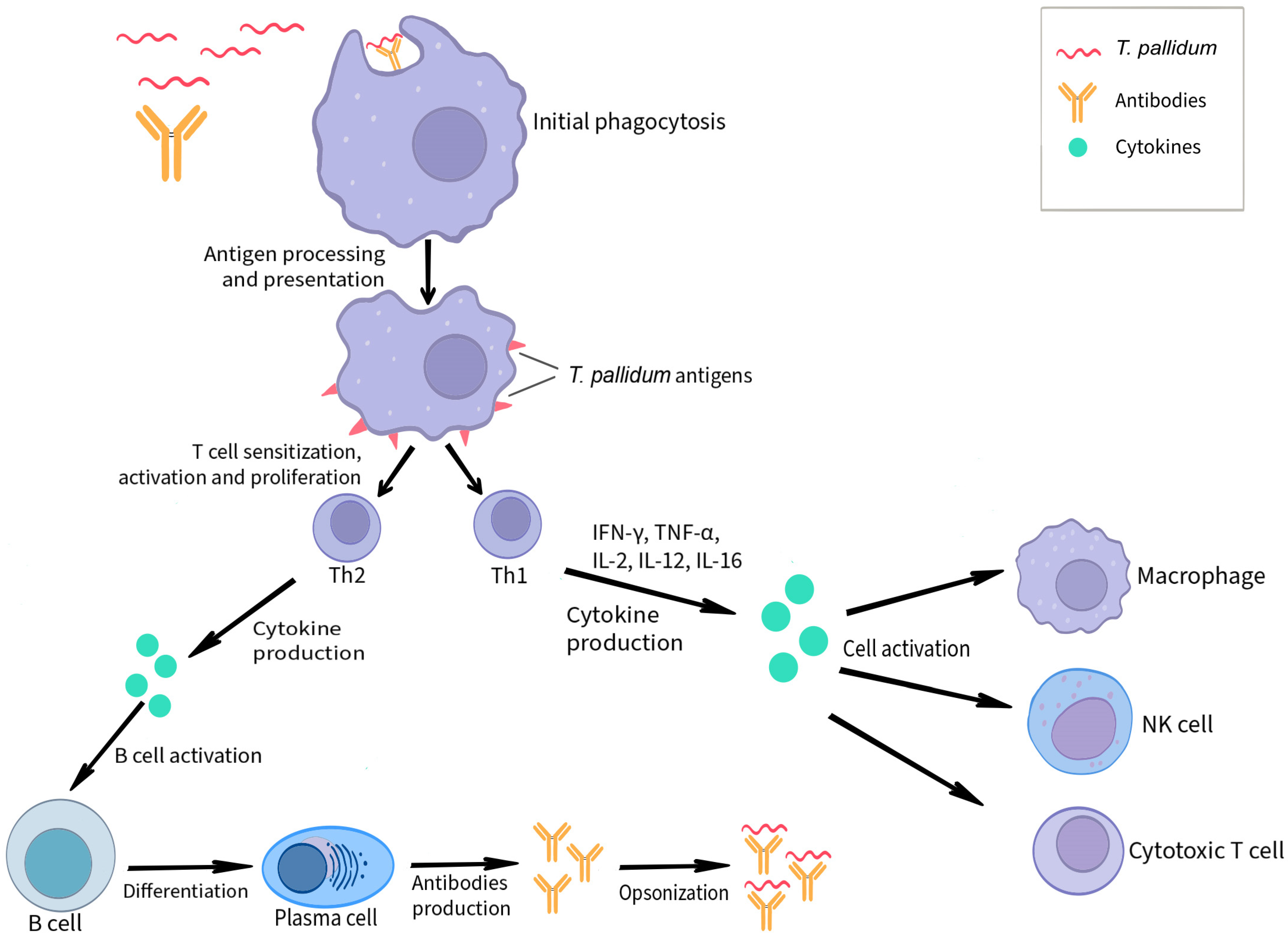
3. The Course of Infection and the Mechanisms of the Cell-Mediated Host Immune Response Accompanying It
3.1. Dendritic Cells
3.2. T Cells
3.3. Macrophages
3.4. NK Cells
3.5. Cytokines
3.6. The Role of the Vascular Endothelium in Cell-Mediated Immune Response to T. pallidum
3.7. Keratinocytes Involved in Immune Response Against T. pallidum
4. HIV/Syphilis Co-Infection
5. Humoral Response to T. pallidum Infection
6. Limitations of Research on T. pallidum
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stamm, L.V. Syphilis: Re-emergence of an old foe. Microb. Cell 2016, 3, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef]
- World Health Organization. Sexually Transmitted Infections (STIs). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 10 November 2024).
- World Health Organization. Syphilis Cases Increase in the Americas. 2024. Available online: https://www.paho.org/en/news/22-5-2024-syphilis-cases-increase-americas (accessed on 10 November 2024).
- World Health Organization. New Report Flags Major Increase in Sexually Transmitted Infections, Amidst Challenges in HIV and Hepatitis. 2024. Available online: https://www.who.int/news/item/21-05-2024-new-report-flags-major-increase-in-sexually-transmitted-infections---amidst-challenges-in-hiv-and-hepatitis (accessed on 10 November 2024).
- European Centre for Disease Prevention and Control. Syphilis—Annual Epidemiological Report 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/syphilis-annual-epidemiological-report-2022 (accessed on 10 November 2024).
- Scurtu, L.G.; Jinga, V.; Simionescu, O. Fascinating Molecular and Immune Escape Mechanisms in the Treatment of STIs (Syphilis, Gonorrhea, Chlamydia, and Herpes Simplex). Int. J. Mol. Sci. 2022, 23, 3550. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.A.; Dabiri, G.; Cribier, B.; Sell, S. The immunopathobiology of syphilis: The manifestations and course of syphilis are determined by the level of delayed-type hypersensitivity. Am. J. Dermatopathol. 2011, 33, 433–460. [Google Scholar] [CrossRef]
- LaFond, R.E.; Lukehart, S.A. Biological basis for syphilis. Clin. Microbiol. Rev. 2006, 19, 29–49. [Google Scholar] [CrossRef]
- Radolf, J.D.; Hazlett, K.R.O.; Lukehart, S.A. Pathogenesis of Syphilis. In Pathogenic Treponemes: Cellular and Molecular Biology; Radolf, J.D., Lukehart, S.A., Eds.; Caister Academic Press: Norfolk, UK, 2006; pp. 197–236. [Google Scholar]
- Baughn, R.E.; Musher, D.M. Secondary syphilitic lesions. Clin. Microbiol. Rev. 2005, 18, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lin, S.; Wan, B.; Zhu, Y. A Systematic Literature Review of Syphilitic Hepatitis in Adults. J. Clin. Transl. Hepatol. 2018, 6, 306–309. [Google Scholar] [CrossRef]
- Hook, E.W., 3rd. Syphilis. Lancet 2019, 393, 986. [Google Scholar] [CrossRef]
- Salazar, J.C.; Cruz, A.R.; Pope, C.D.; Valderrama, L.; Trujillo, R.; Saravia, N.G.; Radolf, J.D. Treponema pallidum elicits innate and adaptive cellular immune responses in skin and blood during secondary syphilis: A flow-cytometric analysis. J. Infect. Dis. 2007, 195, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.L.; Radolf, J.D. Treponema pallidum: From pathogenesis to vaccines. Clin. Microbiol. Rev. 2016, 29, 207–228. [Google Scholar]
- Hook, E.W., 3rd; Marra, C.M. Acquired syphilis in adults. N. Engl. J. Med. 1992, 326, 1060–1069. [Google Scholar] [CrossRef]
- Wicher, K.; Wicher, V.; Abbruscato, F.; Baughn, R.E. Treponema pallidum subsp. pertenue displays pathogenic properties different from those of T. pallidum subsp. pallidum. Infect. Immun. 2000, 68, 3219–3225. [Google Scholar] [CrossRef]
- Centurion-Lara, A.; Arroll, T.; Castillo, R.; Shaffer, J.M.; Castro, C.; Van Voorhis, W.C.; Lukehart, S.A. Conservation of the 15-kilodalton lipoprotein among Treponema pallidum subspecies and strains and other pathogenic treponemes: Genetic and antigenic analyses. Infect. Immun. 1997, 65, 1440–1444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Centurion-Lara, A.; Castro, C.; Castillo, R.; Shaffer, J.M.; Van Voorhis, W.C.; Lukehart, S.A. The flanking region sequences of the 15-kDa lipoprotein gene differentiate pathogenic treponemes. J. Infect. Dis. 1998, 177, 1036–1040. [Google Scholar] [CrossRef]
- Cameron, C.E.; Castro, C.; Lukehart, S.A.; Van Voorhis, W.C. Sequence conservation of glycerophosphodiester phosphodiesterase among Treponema pallidum strains. Infect. Immun. 1999, 67, 3168–3170. [Google Scholar] [CrossRef]
- Wicher, K.; Baughn, R.E.; Wicher, V.; Nakeeb, S. Experimental congenital syphilis: Guinea pig model. Infect. Immun. 1992, 60, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Wicher, K.; Baughn, R.E.; Abbruscato, F.; Wicher, V. Vertical transmission of Treponema pallidum to various litters and generations of guinea pigs. J. Infect. Dis. 1999, 179, 1206–1212. [Google Scholar] [CrossRef]
- Baughn, R.E.; Wicher, V.; Jakubowski, A.; Wicher, K. Humoral response in Treponema pallidum-infected guinea pigs. II. Circulating immune complexes and autoimmune responses. J. Immunol. 1987, 138, 4435–4440. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.R.; Ramirez, L.G.; Zuluaga, A.V.; Pillay, A.; Abreu, C.; Valencia, C.A.; La Vake, C.; Cervantes, J.L.; Dunham-Ems, S.; Cartun, R.; et al. Immune evasion and recognition of the syphilis spirochete in blood and skin of secondary syphilis patients: Two immunologically distinct compartments. PLoS Neglected Trop. Dis. 2012, 6, e1717. [Google Scholar] [CrossRef] [PubMed]
- Norgard, M.V.; Arndt, L.L.; Akins, D.R.; Curetty, L.L.; Harrich, D.A.; Radolf, J.D. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NF-kappa B. Infect. Immun. 1996, 64, 3845–3852. [Google Scholar] [CrossRef]
- Sellati, T.J.; Bouis, D.A.; Kitchens, R.L.; Darveau, R.P.; Pugin, J.; Ulevitch, R.J.; Gangloff, S.C.; Goyert, S.M.; Norgard, M.V.; Radolf, J.D. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J. Immunol. 1998, 160, 5455–5464. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.E. The T. pallidum outer membrane and outer membrane proteins. In Pathogenic Treponema: Molecular and Cellular Biology; Radolf, J.D., Lukehart, S.A., Eds.; Caister Academic Press: Norwich, UK, 2006; pp. 237–266. [Google Scholar]
- Bourell, K.W.; Schulz, W.; Norgard, M.V.; Radolf, J.D. Treponema pallidum rare outer membrane proteins: Analysis of mobility by freeze-fracture electron microscopy. J. Bacteriol. 1994, 176, 1598–1608. [Google Scholar] [CrossRef]
- Jones, J.D.; Bourell, K.W.; Norgard, M.V.; Radolf, J.D. Membrane topology of Borrelia burgdorferi and Treponema pallidum lipoproteins. Infect. Immun. 1995, 63, 2424–2434. [Google Scholar] [CrossRef]
- Desrosiers, D.C.; Anand, A.; Luthra, A.; Dunham-Ems, S.M.; LeDoyt, M.; Cummings, M.A.; Eshghi, A.; Cameron, C.E.; Cruz, A.R.; Salazar, J.C.; et al. TP0326, a Treponema pallidum β-barrel assembly machinery A (BamA) orthologue and rare outer membrane protein. Mol. Microbiol. 2011, 80, 1496–1515. [Google Scholar] [CrossRef]
- Cox, D.L.; Luthra, A.; Dunham-Ems, S.; Desrosiers, D.C.; Salazar, J.C.; Caimano, M.J.; Radolf, J.D. Surface immunolabeling and consensus computational framework to identify candidate rare outer membrane proteins of Treponema pallidum. Infect. Immun. 2010, 78, 5178–5194. [Google Scholar] [CrossRef] [PubMed]
- Radolf, J.D.; Tramont, E.C.; Salazar, J.C. Treponema pallidum: New insights into pathogenesis and immunity. Nat. Rev. Microbiol. 2016, 14, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Lukehart, S.A.; Shaffer, J.M.; Baker-Zander, S.A. A subpopulation of Treponema pallidum is resistant to phagocytosis: Possible mechanism of persistence. J. Infect. Dis. 1992, 166, 1449–1453. [Google Scholar] [CrossRef]
- Moore, M.W.; Cruz, A.R.; LaVake, C.J.; Marzo, A.L.; Eggers, C.H.; Salazar, J.C.; Radolf, J.D. Phagocytosis of Borrelia burgdorferi and Treponema pallidum potentiates innate immune activation and induces gamma interferon production. Infect. Immun. 2007, 75, 2046–2062. [Google Scholar] [CrossRef]
- LaFond, R.E.; Molini, B.J.; Van Voorhis, W.C.; Lukehart, S.A. Antigenic variation of TprK V regions abrogates specific antibody binding in syphilis. Infect. Immun. 2006, 74, 6244–6251. [Google Scholar] [CrossRef]
- Sell, S.; Salman, J.; Norris, S.J. Reinfection of chancre-immune rabbits with Treponema pallidum. I. Light and immunofluorescence studies. Am. J. Pathol. 1985, 118, 248–255. [Google Scholar] [PubMed]
- Ho, E.L.; Lukehart, S.A. Syphilis: Using modern approaches to understand an old disease. J. Clin. Investig. 2011, 121, 4584–4592. [Google Scholar] [CrossRef]
- Xia, W.; Zhao, J.; Su, B.; Jiao, Y.; Weng, W.; Zhang, M.; Wang, X.; Guo, C.; Wu, H.; Zhang, T.; et al. Syphilitic infection impairs immunity by inducing both apoptosis and pyroptosis of CD4+ and CD8+ T lymphocytes. Innate Immun. 2021, 27, 99–106. [Google Scholar] [CrossRef]
- Babolin, C.; Amedei, A.; Ozolins, D.; Zilevica, A.; D’Elios, M.M.; de Bernard, M. TpF1 from Treponema pallidum activates inflammasome and promotes the development of regulatory T cells. J. Immunol. 2011, 187, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Pozzobon, T.; Facchinello, N.; Bossi, F.; Capitani, N.; Benagiano, M.; Di Benedetto, G.; Zennaro, C.; West, N.; Codolo, G.; Bernardini, M.; et al. Treponema pallidum (syphilis) antigen TpF1 induces angiogenesis through the activation of the IL-8 pathway. Sci. Rep. 2016, 6, 18785. [Google Scholar] [CrossRef] [PubMed]
- Leader, B.T.; Godornes, C.; VanVoorhis, W.C.; Lukehart, S.A. CD4+ lymphocytes and gamma interferon predominate in local immune responses in early experimental syphilis. Infect. Immun. 2007, 75, 3021–3026. [Google Scholar] [CrossRef]
- Chung, K.Y.; Kim, K.S.; Lee, M.G.; Chang, N.S.; Lee, J.B. Treponema pallidum induces up-regulation of interstitial collagenase in human dermal fibroblasts. Acta Derm. Venereol. 2002, 82, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Riley, B.S.; Oppenheimer-Marks, N.; Hansen, E.J.; Radolf, J.D.; Norgard, M.V. Virulent Treponema pallidum activates human vascular endothelial cells. J. Infect. Dis. 1992, 165, 484–493. [Google Scholar] [CrossRef]
- Tomson, F.L.; Conley, P.G.; Norgard, M.V.; Hagman, K.E. Assessment of cell-surface exposure and vaccinogenic potentials of Treponema pallidum candidate outer membrane proteins. Microbes Infect. 2007, 9, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.G.; Norris, S.J. In Vitro Cultivation of the Syphilis Spirochete Treponema pallidum. Curr. Protoc. 2021, 1, e44. [Google Scholar] [CrossRef] [PubMed]
- Lukehart, S.A.; Baker-Zander, S.A.; Sell, S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J. Immunol. 1980, 124, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Lukehart, S.A.; Baker-Zander, S.A.; Lloyd, R.M.; Sell, S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J. Immunol. 1980, 124, 461–467. [Google Scholar] [CrossRef]
- Van Voorhis, W.C.; Barrett, L.K.; Koelle, D.M.; Nasio, J.M.; Plummer, F.A.; Lukehart, S.A. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J. Infect. Dis. 1996, 173, 491–495. [Google Scholar] [CrossRef]
- Arroll, T.W.; Centurion-Lara, A.; Lukehart, S.A.; Van Voorhis, W.C. T-Cell responses to Treponema pallidum subsp. pallidum antigens during the course of experimental syphilis infection. Infect. Immun. 1999, 67, 4757–4763. [Google Scholar] [CrossRef]
- Cruz, A.R.; Pillay, A.; Zuluaga, A.V.; Ramirez, L.G.; Duque, J.E.; Aristizabal, G.E.; Fiel-Gan, M.D.; Jaramillo, R.; Trujillo, R.; Valencia, C.; et al. Secondary syphilis in cali, Colombia: New concepts in disease pathogenesis. PLoS Neglected Trop. Dis. 2010, 4, e690. [Google Scholar] [CrossRef] [PubMed]
- Radolf, J.D.; Deka, R.K.; Anand, A.; Šmajs, D.; Norgard, M.V.; Yang, X.F. Treponema pallidum, the syphilis spirochete: Making a living as a stealth pathogen. Nat. Rev. Microbiol. 2016, 14, 744–759. [Google Scholar] [CrossRef]
- Shin, J.L.; Chung, K.Y.; Kang, J.M.; Lee, T.H.; Lee, M.G. The effects of Treponema pallidum on human dendritic cells. Yonsei Med. J. 2004, 45, 515–522. [Google Scholar] [CrossRef][Green Version]
- Bouis, D.A.; Popova, T.G.; Takashima, A.; Norgard, M.V. Dendritic cells phagocytose and are activated by Treponema pallidum. Infect. Immun. 2001, 69, 518–528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Engelkens, H.J.; ten Kate, F.J.; Judanarso, J.; Vuzevski, V.D.; van Lier, J.B.H.; Godschalk, J.C.; van der Sluis, J.J.; Stolz, E. The localisation of treponemes and characterisation of the inflammatory infiltrate in skin biopsies from patients with primary or secondary syphilis, or early infectious yaws. Sex. Transm. Infect. 1993, 69, 102–107. [Google Scholar] [CrossRef]
- Stary, G.; Klein, I.; Bruggen, M.C.; Kohlhofer, S.; Brunner, P.M.; Spazierer, D.; Müllauer, L.; Petzelbauer, P.; Stingl, G. Host defense mechanisms in secondary syphilitic lesions: A role for IFN-gamma-/IL-17-producing CD8+ T cells? Am. J. Pathol. 2010, 177, 2421–2432. [Google Scholar] [CrossRef]
- Van Voorhis, W.C.; Barrett, L.K.; Nasio, J.M.; Plummer, F.A.; Lukehart, S.A. Lesions of primary and secondary syphilis contain activated cytolytic T cells. Infect. Immun. 1996, 64, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L. A new foreign policy: MHC class I molecules monitor the outside world. Immunol. Today 1996, 17, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Lin, Y.H. Immunological aspects of syphilis. J. Formos. Med. Assoc. 2020, 119, 581–587. [Google Scholar]
- Ghanem, K.G. The changing epidemiology of syphilis. JAMA 2018, 320, 1294–1304. [Google Scholar]
- Miossec, P.; Korn, T.; Kuchroo, V.K. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009, 361, 888–898. [Google Scholar] [CrossRef]
- Bedoya, S.K.; Lam, B.; Lau, K.; Larkin, J. Th17 Cells in Immunity and Autoimmunity. Clin. Dev. Immunol. 2013, 2013, 986789. [Google Scholar] [CrossRef]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, J.; Zhang, X.; Li, Q.; Yang, X. Equilibrium of Treg/Th17 cells of peripheral blood in syphilitic patients with sero-resistance. Exp. Ther. Med. 2016, 11, 2300–2304. [Google Scholar] [CrossRef] [PubMed]
- Ishigame, H.; Kakuta, S.; Nagai, T.; Kadoki, M.; Nambu, A.; Komiyama, Y.; Fujikado, N.; Tanahashi, Y.; Akitsu, A.; Kotaki, H.; et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 2009, 30, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Goodall, J.C.; Hill Gaston, J.S. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009, 60, 1647–1656. [Google Scholar] [CrossRef]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Lit, L.C.; Tam, L.S.; Li, E.K.; Wong, P.T.; Lam, C.W. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: Implications for Th17-mediated inflammation in auto-immunity. Clin. Immunol. 2008, 127, 385–393. [Google Scholar] [CrossRef]
- Kagami, S.; Rizzo, H.L.; Lee, J.J.; Koguchi, Y.; Blauvelt, A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J. Investig. Dermatol. 2010, 130, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pliego, A.; Vergara-Ortega, D.N.; Herrera-Ortíz, A.; Toledano-Jaimes, C.; Esquivel-Guadarrama, F.R.; Sánchez-Alemán, M.Á. IL-10 and IL-17 as Progression Markers of Syphilis in People Living with HIV: A Systematic Review. Biomolecules 2022, 12, 1472. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Gao, Z.; Guan, Z.; Lu, H.; Shi, M.; Gao, Y.; Xu, H.; Yang, X.F.; Zhou, P. Increased interleukin-17 in peripheral blood and cerebrospinal fluid of neurosyphilis patients. PLoS Neglected Trop. Dis. 2014, 8, e3004. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dwivedi, V.P.; Singh, Y.; Siddiqui, I.; Sharma, P.; Van Kaer, L.; Chattopadhyay, D.; Das, G. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog. 2011, 7, e1002378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curtis, M.M.; Way, S.S. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 2009, 126, 177–185. [Google Scholar] [CrossRef]
- Rudner, X.L.; Happel, K.I.; Young, E.A.; Shellito, J.E. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect. Immun. 2007, 75, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Saijo, S.; Ikeda, S.; Yamabe, K.; Kakuta, S.; Ishigame, H.; Akitsu, A.; Fujikado, N.; Kusaka, T.; Kubo, S.; Chung, S.-H.; et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010, 32, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Luo, H. Investigation of the role of interleukin 27 in the immune regulation of Treg and Th17 cells in neurosyphilis patients. Folia Neuropathol. 2023, 61, 387–395. [Google Scholar] [CrossRef]
- Afzali, B.; Lombardi, G.; Lechler, R.I.; Lord, G.M. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin. Exp. Immunol. 2007, 148, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Wei, S.; Zou, L.; Altuwaijri, S.; Szeliga, W.; Kolls, J.; Chang, A.; Zou, W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J. Immunol. 2007, 178, 6730–6733. [Google Scholar] [CrossRef] [PubMed]
- Pastuszczak, M.; Jakiela, B.; Jaworek, A.K.; Wypasek, E.; Zeman, J.; Wojas-Pelc, A. Association of Interleukin-10 promoter polymorphisms with neurosyphilis. Hum. Immunol. 2015, 76, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Neufert, C.; Becker, C.; Wirtz, S.; Fantini, M.C.; Weigmann, B.; Galle, P.R.; Neurath, M.F. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur. J. Immunol. 2007, 37, 1809–1816. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J. Immunol. 2008, 180, 6325–6333. [Google Scholar] [CrossRef]
- Stumhofer, J.S.; Hunter, C.A. Advances in understanding the anti-inflammatory properties of IL-27. Immunol. Lett. 2008, 117, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.; Baker-Zander, S.; Powell, H.C. Experimental syphilitic orchitis in rabbits: Ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab. Investig. 1982, 46, 355–364. [Google Scholar]
- Lukehart, S.A.; Miller, J.N. Demonstration of the in vivo phagocytosis of Treponema pallidum by Rabbit peritoneal macrophages. J. Immunol. 1978, 121, 2014–2024. [Google Scholar] [CrossRef]
- Baker-Zander, S.A.; Lukehart, S.A. Macrophage-mediated killing of opsonized Treponema pallidum. J. Infect. Dis. 1992, 165, 69–74. [Google Scholar] [CrossRef]
- Cameron, C.E.; Lukehart, S.A. Treponema pallidum: Surface and subsurface proteins in the pathogenesis of syphilis. Microbes Infect. 2004, 6, 1263–1276. [Google Scholar]
- Katz, Y.; Nadiv, O.; Beer, Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: A possible role as a “fine-tuning cytokine” in inflammation processes. Arthritis Rheum. 2001, 44, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- LeGrand, A.; Fermor, B.; Fink, C.; Pisetsky, D.S.; Weinberg, J.B.; Vail, T.P.; Guilak, F. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001, 44, 2078–2083. [Google Scholar] [CrossRef] [PubMed]
- Pastuszczak, M.; Jakiela, B.; Wielowieyska-Szybinska, D.; Jaworek, A.K.; Zeman, J.; Wojas-Pelc, A. Elevated cerebrospinal fluid interleukin-17A and interferon-γ levels in early asymptomatic neurosyphilis. Sex. Transm. Dis. 2013, 40, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.; Closhen, D.; Croxford, A.; White, R.; Kulig, P.; Pietrowski, E.; Bechmann, I.; Becher, B.; Luhmann, H.J.; Waisman, A.; et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010, 24, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Tabor, D.R.; Kiel, D.P.; Jacobs, R.F. Cyclophosphamide-sensitive activity of suppressor T-cells during treponemal infection. Immunology 1987, 62, 127–132. [Google Scholar]
- Borish, L. IL-10: Evolving concepts. J. Allergy Clin. Immunol. 1998, 101, 293–297. [Google Scholar] [CrossRef]
- Groux, H.; Bigler, M.; de Vries, J.E.; Roncarolo, M.G. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J. Immunol. 1998, 160, 3188–3193. [Google Scholar] [CrossRef] [PubMed]
- Chatila, T. Role of regulatory T cells in human diseases. J. Allergy Clin. Immunol. 2005, 116, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Medina, T.S.; Costa, S.P.; Oliveira, M.D.; Ventura, A.M.; Souza, J.M.; Gomes, T.F.; Vallinoto, A.C.; Póvoa, M.M.; Silva, J.S.; Cunha, M.G. Increased interleukin-10 and interferon-γ levels in Plasmodium vivax malaria suggest a reciprocal regulation which is not altered by IL-10 gene promoter polymorphism. Malar. J. 2011, 10, 264. [Google Scholar] [CrossRef]
- Reed, S.G.; Brownell, C.E.; Russo, D.M.; Silva, J.S.; Grabstein, K.H.; Morrissey, P.J. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J. Immunol. 1994, 153, 3135–3140. [Google Scholar] [CrossRef] [PubMed]
- Roque, S.; Nobrega, C.; Appelberg, R.; Correia-Neves, M. IL-10 underlies distinct susceptibility of BALB/c and C57BL/6 mice to Mycobacterium avium infection and influences efficacy of antibiotic therapy. J. Immunol. 2007, 178, 8028–8035. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.; Wynn, T.A.; Berzofsky, J.A.; Blatt, S.P.; Hendrix, C.W.; Sher, A.; Coffman, R.L.; Shearer, G.M. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J. Clin. Investig. 1994, 93, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Accapezzato, D.; Francavilla, V.; Paroli, M.; Casciaro, M.; Chircu, L.V.; Cividini, A.; Abrignani, S.; Mondelli, M.U.; Barnaba, V. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J. Clin. Investig. 2004, 113, 963–972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waugh, S.; Ranasinghe, A.; Gomez, A.; Houston, S.; Lithgow, K.V.; Eshghi, A.; Fleetwood, J.; Conway, K.M.E.; Reynolds, L.A.; Cameron, C.E. Syphilis and the host: Multi-omic analysis of host cellular responses to Treponema pallidum provides novel insight into syphilis pathogenesis. Front. Microbiol. 2023, 14, 1254342. [Google Scholar] [CrossRef]
- Lemichez, E.; Lecuit, M.; Nassif, X.; Bourdoulous, S. Breaking the wall: Targeting of the endothelium by pathogenic bacteria. Nat. Rev. Microbiol. 2010, 8, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Tomlin, H.; Piccinini, A.M. A complex interplay between the extracellular matrix and the innate immune response to microbial pathogens. Immunology 2018, 155, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.C.; Rathi, A.; Michael, N.L.; Radolf, J.D.; Jagodzinski, L.L. Assessment of the kinetics of Treponema pallidum dissemination into blood and tissues in experimental syphilis by real-time quantitative PCR. Infect. Immun. 2007, 75, 2954–2958. [Google Scholar] [CrossRef]
- Gao, Z.X.; Liu, L.L.; Lin, L.R.; Tong, M.L.; Liu, F.; Yang, T.C. Treponema pallidum induces the secretion of HDVSMC inflammatory cytokines to promote the migration and adhesion of THP-1 cells. Front. Cell. Infect. Microbiol. 2019, 9, 220. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, J.; Qu, B.; Zhang, Y.; Wei, Y.; Liu, H.; Wu, C. CXCL13 and TH1/Th2 cytokines in the serum and cerebrospinal fluid of neurosyphilis patients. Medicine 2017, 96, e8850. [Google Scholar] [CrossRef]
- Paul, R.; Koedel, U.; Winkler, F.; Kieseier, B.C.; Fontana, A.; Kopf, M.; Hartung, H.P.; Pfister, H.W. Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain 2003, 126 Pt 8, 1873–1882. [Google Scholar] [CrossRef]
- Blecharz-Lang, K.G.; Wagner, J.; Fries, A.; Nieminen-Kelhä, M.; Rösner, J.; Schneider, U.C.; Vajkoczy, P. Interleukin 6-Mediated Endothelial Barrier Disturbances Can Be Attenuated by Blockade of the IL6 Receptor Expressed in Brain Microvascular Endothelial Cells. Transl. Stroke Res. 2018, 9, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Aoki, C.; Yoshio-Hoshino, N.; Takayama, K.; Curiel, D.T.; Nishimoto, N. Interleukin-6 induces both cell growth and VEGF production in malignant mesotheliomas. Int. J. Cancer 2006, 119, 1303–1311. [Google Scholar] [CrossRef]
- Macaron, N.C.; Cohen, C.; Chen, S.C.; Arbiser, J.L. Cutaneous lesions of secondary syphilis are highly angiogenic. J. Am. Acad. Dermatol. 2003, 48, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Houston, S.; Schovanek, E.; Conway, K.M.E.; Mustafa, S.; Gomez, A.; Ramaswamy, R.; Haimour, A.; Boulanger, M.J.; Reynolds, L.A.; Cameron, C.E. Identification and Functional Characterization of Peptides with Antimicrobial Activity from the Syphilis Spirochete, Treponema pallidum. Front. Microbiol. 2022, 13, 888525. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Sehgal, A.; Irvine, K.M.; Hume, D.A. Functions of macrophage colony-stimulating factor (CSF1) in development, homeostasis, and tissue repair. Semin. Immunol. 2021, 54, 101509. [Google Scholar] [CrossRef]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The diverse contribution of keratinocytes to immune responses in skin. JCI Insight 2020, 5, e142067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, X.; Gao, Z.X.; Lin, S.W.; Tong, M.L.; Liu, L.L.; Lin, L.R.; Ke, W.J.; Yang, T.C. Recombinant Treponema pallidum protein Tp0136 promotes fibroblast migration by modulating MCP-1/CCR2 through TLR4. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xie, Y.; Jiang, C.; Xiao, Y.; Kuang, X.; Wen, Y.; Tan, Y.; Tan, M.; Zhao, F.; Zeng, T.; et al. Treponema pallidum flagellins elicit proinflammatory cytokines from human monocytes via TLR5 signaling pathway. Immunobiology 2017, 222, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xu, M.; Kuang, X.; Xiao, J.; Tan, M.; Xie, Y.; Xiao, Y.; Zhao, F.; Wu, Y. Treponema pallidum flagellins stimulate MMP-9 and MMP-13 expression via TLR5 and MAPK/NF-κB signaling pathways in human epidermal keratinocytes. Exp. Cell Res. 2017, 361, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Yang, J.; Zhang, J.; Ke, W.; Zou, F.; Wan, C.; Wang, L.; Zhang, X.; Liang, F.; Mei, S.; et al. MicroRNA-101-3p Downregulates TLR2 Expression, Leading to Reduction in Cytokine Production by Treponema pallidum-Stimulated Macrophages. J Investig. Dermatol. 2020, 140, 1566–1575.e1. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, M.; Xiao, Y.; Liu, Z.; Jiang, C.; Kuang, X.; Wang, C.; Wu, H.; Peng, J.; Li, C.; et al. Treponema pallidum flagellin FlaA2 induces IL-6 secretion in THP-1 cells via the Toll-like receptor 2 signaling pathway. Mol. Immunol. 2017, 81, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.R.; Shang, S.X.; Zhao, L.S.; Long, F.Q. MiR-216a-5p-containing exosomes suppress rTp17-induced inflammatory response by targeting TLR4. Biosci. Rep. 2019, 39, BSR20190686. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.; Chen, J.; Zhou, J.; Zhao, F.; Liu, S.; Zeng, T.; Xu, M.; Xiao, Y. Treponema pallidum FlaA2 inducing the release of pro-inflammatory cytokines is mediated via TLR2 in keratinocytes. Microb. Pathog. 2022, 173 Pt A, 105879. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, H.; Kim, S.; Kim, K.H.; Chung, J.H. Activation of toll-like receptors 2, 3 or 5 induces matrix metalloproteinase-1 and -9 expression with the involvement of MAPKs and NF-kappaB in human epidermal keratinocytes. Exp. Dermatol. 2010, 19, e44–e49. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.; Benfield, T.; Kofoed, K. Cytokine expression during syphilis infection in HIV-1-infected individuals. Sex. Transm. Dis. 2009, 36, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, X.; Hu, Z.; Luo, Z.; Jiang, W.; Wu, H.; Gao, Y.; Yan, J.; Zhang, Q.; Song, A.; et al. Syphilis Infection Differentially Regulates the Phenotype and Function of γδ T Cells in HIV-1-Infected Patients Depends on the HIV-1 Disease Stage. Front. Immunol. 2017, 8, 991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kenyon, C.; Osbak, K.K.; Crucitti, T.; Kestens, L. The immunological response to syphilis differs by HIV status; a prospective observational cohort study. BMC Infect. Dis. 2017, 17, 111. [Google Scholar] [CrossRef]
- Campo, J.J.; Romeis, E.; Oberai, A.; Pablo, J.V.; Hung, C.; Teng, A.A.; Shandling, A.D.; Phan, A.; Haynes, A.M.; Giacani, L. A novel pan-proteome array for high-throughput profiling of the humoral response to Treponema pallidum. iScience 2024, 27, 110618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blanco, D.R.; Champion, C.I.; Dooley, A.; Cox, D.L.; Whitelegge, J.P.; Faull, K.; Lovett, M.A. A monoclonal antibody that conveys in vitro killing and partial protection in experimental syphilis binds a phosphorylcholine surface epitope of Treponema pallidum. Infect. Immun. 2005, 73, 3083–3095. [Google Scholar] [CrossRef]
- Blanco, D.R.; Miller, J.N.; Lovett, M.A. Surface antigens of the syphilis spirochete and their potential as virulence determinants. Emerg. Infect. Dis. 1997, 3, 11–20. [Google Scholar]
- Blanco, D.R.; Miller, J.N.; Hanff, P.A. Humoral immunity in experimental syphilis: The demonstration of IgG as a treponemicidal factor in immune rabbit serum. J. Immunol. 1984, 133, 2693–2697. [Google Scholar] [CrossRef]
- Salazar, J.C.; Hazlett, K.R.; Radolf, J.D. The immune response to infection with Treponema pallidum, the stealth pathogen. Microbes Infect. 2002, 4, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Belkum, A.V. Pathogenic Treponema: Molecular and cellular biology. J. Microbiol. Methods 2007, 69, 421–422. [Google Scholar] [CrossRef]
- Folds, J.D.; Rauchbach, A.S.; Shores, E.; Saunders, J.M. Evaluation of the inbred mouse as a model for experimental Treponema pallidum infection. Scand. J. Immunol. 1983, 18, 201–206. [Google Scholar] [CrossRef]
- Lu, S.; Zheng, K.; Wang, J.; Xu, M.; Xie, Y.; Yuan, S.; Wang, C.; Wu, Y. Characterization of Treponema pallidum Dissemination in C57BL/6 Mice. Front. Immunol. 2021, 11, 577129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaminiów, K.; Kiołbasa, M.; Pastuszczak, M. The Significance of the Cell-Mediated Host Immune Response in Syphilis. Microorganisms 2024, 12, 2580. https://doi.org/10.3390/microorganisms12122580
Kaminiów K, Kiołbasa M, Pastuszczak M. The Significance of the Cell-Mediated Host Immune Response in Syphilis. Microorganisms. 2024; 12(12):2580. https://doi.org/10.3390/microorganisms12122580
Chicago/Turabian StyleKaminiów, Konrad, Martyna Kiołbasa, and Maciej Pastuszczak. 2024. "The Significance of the Cell-Mediated Host Immune Response in Syphilis" Microorganisms 12, no. 12: 2580. https://doi.org/10.3390/microorganisms12122580
APA StyleKaminiów, K., Kiołbasa, M., & Pastuszczak, M. (2024). The Significance of the Cell-Mediated Host Immune Response in Syphilis. Microorganisms, 12(12), 2580. https://doi.org/10.3390/microorganisms12122580






