Bacterial Diversity in the Different Ecological Niches Related to the Yonghwasil Pond (Republic of Korea)
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Site
2.2. Sample Collection
2.3. The Soil Chemical Properties
2.4. DNA Isolation
2.5. PCR Amplification and Sequencing
2.6. Sequencing Data Analyses
3. Results
3.1. Soil Physico-Chemical Properties
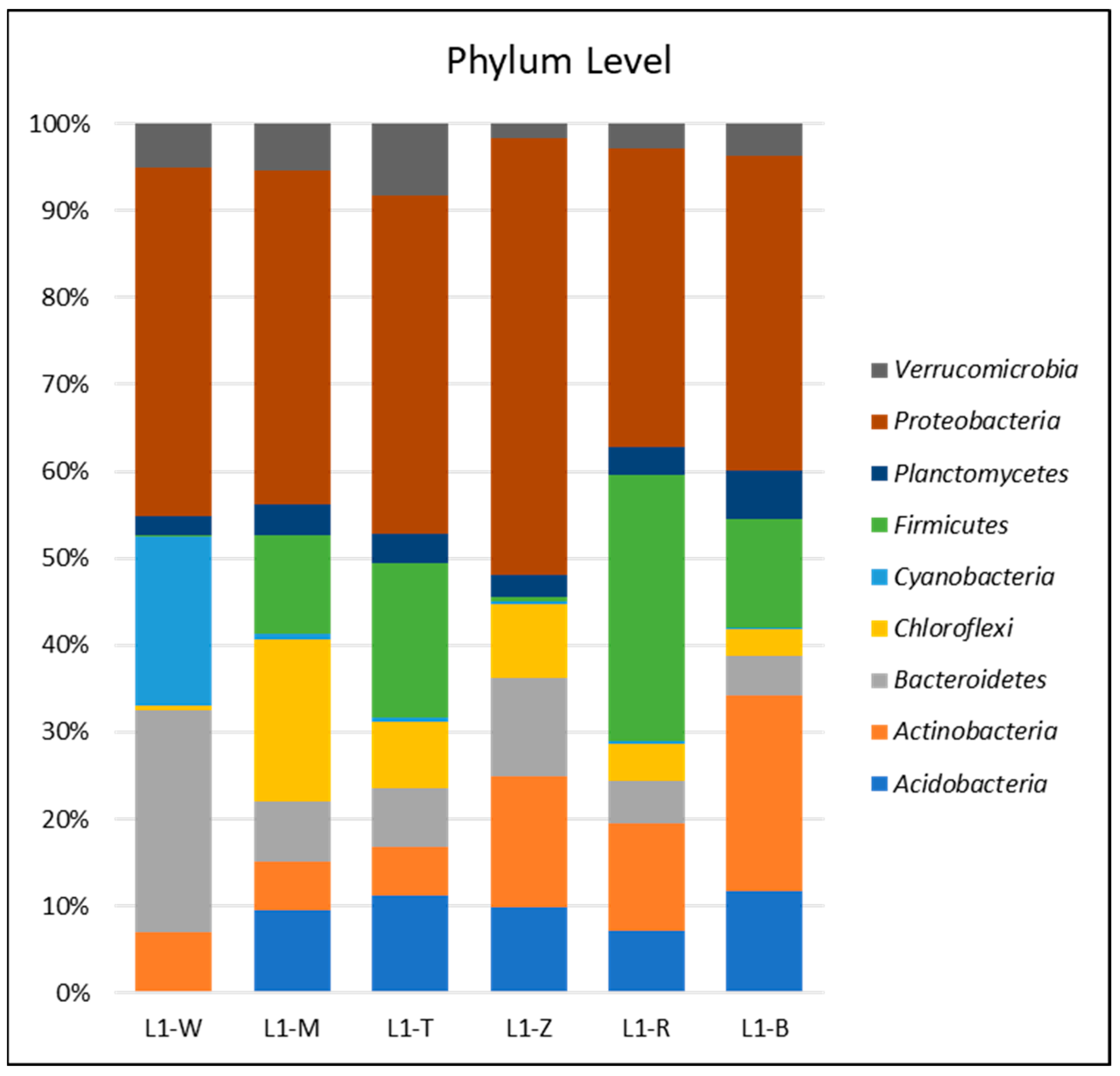
3.2. Bacterial Community Abundance and Composition
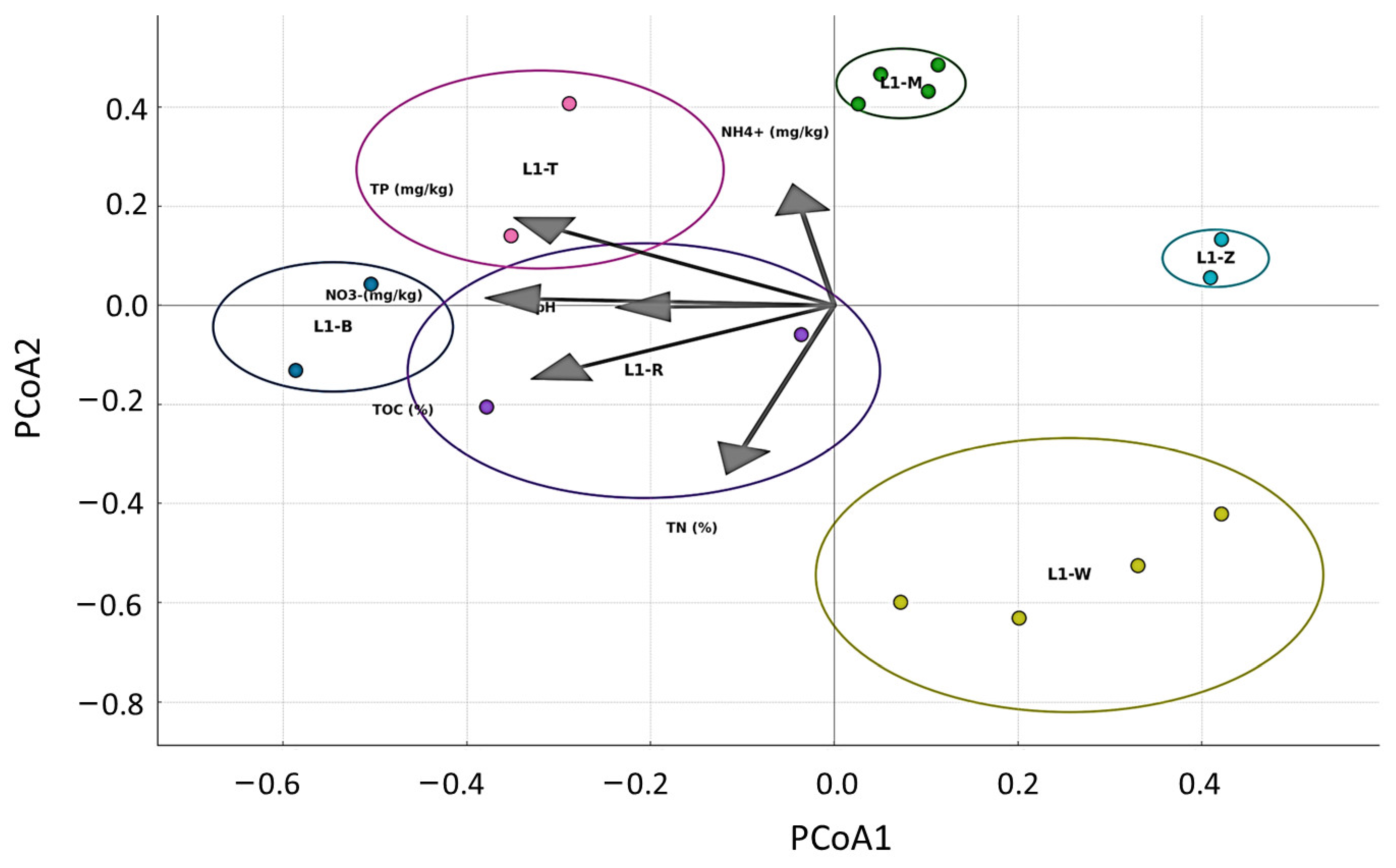
3.3. Correlation of Soil Properties and the Bacterial Community
4. Discussion
4.1. Proteobacteria: The Ecological Workhorse
4.2. Actinobacteria and Chloroflexi: Indicators of Environmental Gradients
4.3. Cyanobacteria and Bacteroidetes in Water Samples: More than Just Photosynthesis
4.4. Sediments: The Hidden Reservoir of Biodiversity
4.5. Aquatic Plants: Mediators of Microbial Interactions
4.6. Potential Anthropogenic Influences
4.7. Future Directions: From Observation to Manipulation
5. Conclusions
5.1. Recapitulating the Findings
5.2. Implications for Freshwater Ecology
5.3. Conservation and Public Health Perspectives
5.4. Charting the Future Trajectory
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wetzel, R.G. Freshwater Ecosystems. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2001; pp. 560–569. [Google Scholar]
- Cantonati, M.; Poikane, S.; Pringle, C.M.; Stevens, L.E.; Turak, E.; Heino, J.; Richardson, J.S.; Bolpagni, R.; Borrini, A.; Cid, N.; et al. Characteristics, Main Impacts, and Stewardship of Natural and Artificial Freshwater Environments: Consequences for Biodiversity Conservation. Water 2020, 12, 260. [Google Scholar] [CrossRef]
- Stoffers, T.; Buijse, A.D.; Geerling, G.W.; Jans, L.H.; Schoor, M.M.; Poos, J.J.; Verreth, J.A.J.; Nagelkerke, L.A.J. Freshwater fish biodiversity restoration in floodplain rivers requires connectivity and habitat heterogeneity at multiple spatial scales. Sci. Total Environ. 2022, 838, 156509. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.T.; Sivaraj, N.; Kamala, V.; Pandravada, S.R.; Sunil, N.; Dikshit, N. Classification, Characterization and Comparison of Aquatic Ecosystems in the Landscape of Adilabad District, Telangana, Deccan Region, India. Open Access Libr. J. 2018, 5, 49. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 315, 344–358. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Gulf Professional Publishing: Houston, TX, USA, 2001. [Google Scholar]
- Straile, D.; Livingstone, D.M.; Weyhenmeyer, G.A.; George, D.G. The response of freshwater ecosystems to climate variability associated with the North Atlantic Oscillation. In The North Atlantic Oscillation: Climatic Significance and Environmental Impact; American Geophysical Union: Washington, DC, USA, 2003. [Google Scholar]
- Postel, S.; Carpenter, S. Freshwater ecosystem services. In Nature’s Services: Societal Dependence on Natural Ecosystems; Island Press: Washington, DC, USA, 1997; p. 195. [Google Scholar]
- Mitsch, W.J.; Gosselink, J.G. Wetlands; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Molden, D. Water for Food Water for Life: A Comprehensive Assessment of Water Management in Agriculture; Routledge: England, UK, 2013. [Google Scholar]
- Halwart, M.; Funge-Smith, S.; Moehl, J. The role of aquaculture in rural development. In Review of the State of World Aquaculture; FAO: Rome, Italy, 2003; pp. 47–58. [Google Scholar]
- Kiparsky, M.; Owen, D.; Green Nylen, N.; Doremus, H.; Christian-Smith, J.; Cosens, B.; Fisher, A.; Milman, A. Designing Effective Groundwater Sustainability Agencies: Criteria for Evaluation of Local Governance Options. Center for Law, Energy & the Environment. 2016. Available online: https://escholarship.org/uc/item/5043w5fm (accessed on 10 December 2023).
- Devito, K.; Mendoza, C.; Qualizza, C. Conceptualizing Water Movement in the Boreal Plains. Implications for Watershed Reconstruction. 2012. Available online: https://era.library.ualberta.ca/items/d934019b-141c-4da4-8495-cb634e6f75cf (accessed on 10 December 2023).
- Arnold, J.G.; Srinivasan, R.; Muttiah, R.S.; Williams, J.R. Large area hydrologic modeling and assessment part I: Model development 1. JAWRA J. Am. Water Resour. Assoc. 1998, 34, 73–89. [Google Scholar] [CrossRef]
- Ficke, A.D.; Myrick, C.A.; Hansen, L.J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007, 17, 581–613. [Google Scholar] [CrossRef]
- Rouse, W.R.; Douglas, M.S.; Hecky, R.E.; Hershey, A.E.; Kling, G.W.; Lesack, L.; Marsh, P.; McDonald, M.; Nicholson, B.J.; Roulet, N.T. Effects of climate change on the freshwaters of arctic and subarctic North America. Hydrol. Process. 1997, 11, 873–902. [Google Scholar] [CrossRef]
- Ramachandra, T. Ecology, Biodiversity and Hydrology: Linkages; Researchgate: Berlin, Germany, 2010. [Google Scholar]
- Eckert, E.M.; Anicic, N.; Fontaneto, D. Freshwater zooplankton microbiome composition is highly flexible and strongly influenced by the environment. Mol. Ecol. 2021, 30, 1545–1558. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Ji, S.; Chang, M.; Wang, L.; Gan, Y.; Liu, J. The ecology of the plastisphere: Microbial composition, function, assembly, and network in the freshwater and seawater ecosystems. Water Res. 2021, 202, 117428. [Google Scholar] [CrossRef]
- Philippot, L.; Griffiths, B.S.; Langenheder, S. Microbial Community Resilience across Ecosystems and Multiple Disturbances. Microbiol. Mol. Biol. Rev. 2021, 85, 10–1128. [Google Scholar] [CrossRef]
- Ferreira, V.; Elosegi, A.; Tiegs, S.D.; von Schiller, D.; Young, R. Organic Matter Decomposition and Ecosystem Metabolism as Tools to Assess the Functional Integrity of Streams and Rivers—A Systematic Review. Water 2020, 12, 3523. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Telo da Gama, J. The Role of Soils in Sustainability, Climate Change, and Ecosystem Services: Challenges and Opportunities. Ecologies 2023, 4, 552–567. [Google Scholar] [CrossRef]
- Sehnal, L.; Brammer-Robbins, E.; Wormington, A.M.; Blaha, L.; Bisesi, J.; Larkin, I.; Martyniuk, C.J.; Simonin, M.; Adamovsky, O. Microbiome Composition and Function in Aquatic Vertebrates: Small Organisms Making Big Impacts on Aquatic Animal Health. Front. Microbiol. 2021, 12, 567408. [Google Scholar] [CrossRef]
- Rhak Yeon, C. Paleoecological Perspectives of Anthropogenic Impact in Seocheon, on the West Coast of the Korean Peninsula; Seoul National University Graduate School: Seoul, Republic of Korea, 2015. [Google Scholar]
- Djurhuus, A.; Port, J.; Closek, C.J.; Yamahara, K.M.; Romero-Maraccini, O.; Walz, K.R.; Goldsmith, D.B.; Michisaki, R.; Breitbart, M.; Boehm, A.B.; et al. Evaluation of Filtration and DNA Extraction Methods for Environmental DNA Biodiversity Assessments across Multiple Trophic Levels. Front. Mar. Sci. 2017, 4, 314. [Google Scholar] [CrossRef]
- Kalra, Y.P. Determination of pH of Soils by Different Methods: Collaborative Study. J. Aoac Int. 2020, 78, 310–324. [Google Scholar] [CrossRef]
- Bremner, J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Rowell, M.J.; Florence, L.Z. Characteristics associated with differences between undisturbed and industrially-disturbed soils. Soil Biol. Biochem. 1993, 25, 1499–1511. [Google Scholar] [CrossRef]
- Rowell, M.; Coetzee, M. The measurement of low organic matter contents in soils. S. Afr. J. Plant Soil 2003, 20, 49–53. [Google Scholar] [CrossRef]
- Mehra, O.; Jackson, M. Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate. In Clays and Clay Minerals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 317–327. [Google Scholar]
- Kameoka, S.; Motooka, D.; Watanabe, S.; Kubo, R.; Jung, N.; Midorikawa, Y.; Shinozaki, N.O.; Sawai, Y.; Takeda, A.K.; Nakamura, S. Benchmark of 16S rRNA gene amplicon sequencing using Japanese gut microbiome data from the V1–V2 and V3–V4 primer sets. BMC Genom. 2021, 22, 527. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, E.; Cardozo-Mino, M.G.; Rapp, J.Z.; Bienhold, C.; Salter, I.; Salman-Carvalho, V.; Molari, M.; Tegetmeyer, H.E.; Buttigieg, P.L.; Boetius, A. Comparison of Two 16S rRNA Primers (V3–V4 and V4–V5) for Studies of Arctic Microbial Communities. Front. Microbiol. 2021, 12, 637526. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 March 2023).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 28. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Anderson, I.C.; Singh, B.K. Response of soil properties and microbial communities to agriculture: Implications for primary productivity and soil health indicators. Front. Plant Sci. 2016, 7, 990. [Google Scholar] [CrossRef] [PubMed]
- Naess, A. From ecology to ecosophy, from science to wisdom. World Futures J. Gen. Evol. 1989, 27, 185–190. [Google Scholar] [CrossRef]
- Naeem, S.; Chazdon, R.; Duffy, J.E.; Prager, C.; Worm, B. Biodiversity and human well-being: An essential link for sustainable development. Proc. R. Soc. B Biol. Sci. 2016, 283, 20162091. [Google Scholar] [CrossRef] [PubMed]
- Rocchini, D.; Salvatori, N.; Beierkuhnlein, C.; Chiarucci, A.; De Boissieu, F.; Förster, M.; Garzon-Lopez, C.X.; Gillespie, T.W.; Hauffe, H.C.; He, K.S. From local spectral species to global spectral communities: A benchmark for ecosystem diversity estimate by remote sensing. Ecol. Inform. 2021, 61, 101195. [Google Scholar] [CrossRef]
- Loke, L.H.; Chisholm, R.A. Measuring habitat complexity and spatial heterogeneity in ecology. Ecol. Lett. 2022, 25, 2269–2288. [Google Scholar] [CrossRef]
- Tukiainen, H.; Toivanen, M.; Maliniemi, T. Geodiversity and biodiversity. Geol. Soc. Lond. Spéc. Publ. 2022, 530, 31–47. [Google Scholar] [CrossRef]
- Niesenbaum, R.A. The integration of conservation, biodiversity, and sustainability. Sustainability 2019, 11, 4676. [Google Scholar] [CrossRef]
- Lemoine-Rodríguez, R.; García-Arroyo, M.; Gómez-Martínez, M.A.; Back, M.; Lindeman, T.; MacGregor-Fors, I. Unveiling urban ecological integrity: Spatially explicit assessment in contrasting environments. Urban Ecosyst. 2024, 27, 1167–1174. [Google Scholar] [CrossRef]
- Zhang, N.; Nunan, N.; Hirsch, P.R.; Sun, B.; Zhou, J.; Liang, Y. Theory of microbial coexistence in promoting soil–plant ecosystem health. Biol. Fertil. Soils 2021, 57, 897–911. [Google Scholar] [CrossRef]
- Tilak, K.; Ranganayaki, N.; Pal, K.; De, R.; Saxena, A.; Nautiyal, C.S.; Mittal, S.; Tripathi, A.; Johri, B. Diversity of plant growth and soil health supporting bacteria. Curr. Sci. 2005, 89, 136–150. [Google Scholar]
- Oertli, B.; Parris, K.M. Toward management of urban ponds for freshwater biodiversity. Ecosphere 2019, 10, e02810. [Google Scholar] [CrossRef]
- Van Der Valk, A.G. Succession in wetlands: A gleasonian appraoch. Ecology 1981, 62, 688–696. [Google Scholar] [CrossRef]
- Ahn, C.; Dee, S. Early development of plant community in a created mitigation wetland as affected by introduced hydrologic design elements. Ecol. Eng. 2011, 37, 1324–1333. [Google Scholar] [CrossRef]
- Bélanger, L.; Maisonneuve, C.; Rodrigue, J. Avian use of dairy farm ponds and landowners’ perceptions of their management for wildlife conservation. Birds 2021, 2, 476–491. [Google Scholar] [CrossRef]
- An, Y.; Gao, Y.; Tong, S. Emergence and growth performance of Bolboschoenus planiculmis varied in response to water level and soil planting depth: Implications for wetland restoration using tuber transplantation. Aquat. Bot. 2018, 148, 10–14. [Google Scholar] [CrossRef]
- Choi, H.; Kim, H.T.; Nam, B.E.; Bae, Y.J.; Kim, J.G. Effect of initial planting on vegetation establishment in different depth zones of constructed farm ponds. Restor. Ecol. 2022, 30, e13488. [Google Scholar] [CrossRef]
- Kim, S.H.; Nam, J.M.; Kim, J.G. Establishment strategy of a rare wetland species Sparganium erectum in Korea. J. Ecol. Environ. 2017, 41, 27. [Google Scholar] [CrossRef]
- Newton, R.J.; Jones, S.E.; Eiler, A.; McMahon, K.D.; Bertilsson, S. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 14–49. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T. Distribution characteristics of phosphorus in the sediments and overlying water of Poyang lake. PLoS ONE 2015, 10, e0125859. [Google Scholar] [CrossRef]
- Bryant, D.A. Phototrophy and Phototrophs. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: England, UK, 2019; pp. 527–537. [Google Scholar]
- Naylor, D.; McClure, R.; Jansson, J. Trends in Microbial Community Composition and Function by Soil Depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.d.C.; Kitano, I.T.; Ribeiro, I.A.d.F.; Lacava, P.T. The Potential Use of Actinomycetes as Microbial Inoculants and Biopesticides in Agriculture. Front. Soil Sci. 2022, 2, 833181. [Google Scholar] [CrossRef]
- Allgaier, M.; Grossart, H.P. Diversity and seasonal dynamics of Actinobacteria populations in four lakes in northeastern Germany. Appl. Environ. Microbiol. 2006, 72, 3489–3497. [Google Scholar] [CrossRef]
- Zothanpuia; Passari, A.K.; Leo, V.V.; Singh, B.P. Chapter 4—Freshwater Actinobacteria: Potential Source for Natural Product Search and Discovery. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, B.P., Gupta, V.K., Passari, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–77. [Google Scholar]
- Siro, G.; Pipite, A.; Christi, K.; Srinivasan, S.; Subramani, R. Marine actinomycetes associated with stony corals: A potential hotspot for specialized metabolites. Microorganisms 2022, 10, 1349. [Google Scholar] [CrossRef]
- Selim, M.S.M.; Abdelhamid, S.A.; Mohamed, S.S. Secondary metabolites and biodiversity of actinomycetes. J. Genet. Eng. Biotechnol. 2021, 19, 72. [Google Scholar] [CrossRef]
- Qin, Y.; Hou, J.; Deng, M.; Liu, Q.; Wu, C.; Ji, Y.; He, X. Bacterial abundance and diversity in pond water supplied with different feeds. Sci. Rep. 2016, 6, 35232. [Google Scholar] [CrossRef]
- Tang, X.; Xie, G.; Shao, K.; Dai, J.; Chen, Y.; Xu, Q.; Gao, G. Bacterial Community Composition in Oligosaline Lake Bosten: Low Overlap of Betaproteobacteria and Bacteroidetes with Freshwater Ecosystems. Microbes Environ. 2015, 30, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Tsedeke, A.; Degefu, T.; Wolde-Meskel, E.; Davis, J. Effect of pond depth and lining plastic color on growth and nitrogen fixing capacity of the cyanobacteria, Anabaena sp. E3. Afr. J. Biotechnol. 2016, 15, 1442–1451. [Google Scholar]
- Cottingham, K.L.; Ewing, H.A.; Greer, M.L.; Carey, C.C.; Weathers, K.C. Cyanobacteria as biological drivers of lake nitrogen and phosphorus cycling. Ecosphere 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Xiao, Y.; Zhang, Y.; Yu, Y.; Zheng, Z.; Liu, Y.; Li, Q. The Impact of Cyanobacteria Blooms on the Aquatic Environment and Human Health. Toxins 2022, 14, 658. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.J.; Quadra, G.R.; Resende, N.d.S.; Roland, F. The role of sediments in the carbon and pollutant cycles in aquatic ecosystems. Acta Limnol. Bras. 2019, 31, e201. [Google Scholar] [CrossRef]
- Kuang, B.; Xiao, R.; Hu, Y.; Wang, Y.; Zhang, L.; Wei, Z.; Bai, J.; Zhang, K.; Acuña, J.J.; Jorquera, M.A.; et al. Metagenomics reveals biogeochemical processes carried out by sediment microbial communities in a shallow eutrophic freshwater lake. Front. Microbiol. 2023, 13, 1112669. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, R.; Singh, R.L. Microbes and Environment. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; eCollection 2017; Springer: Singapore, 2016; pp. 43–84. [Google Scholar] [CrossRef]
- Mulder, R.; Schmidt, C. Groundwater, Surface Water, and Sediment Monitoring for Pesticides and Nitrate in Billings, Montana; Montana Department of Agriculture: Helena, MT, USA, 2011.
- Kong, Z.; Liu, H. Modification of Rhizosphere Microbial Communities: A Possible Mechanism of Plant Growth Promoting Rhizobacteria Enhancing Plant Growth and Fitness. Front. Plant Sci. 2022, 13, 920813. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y. The Function of Root Exudates in the Root Colonization by Beneficial Soil Rhizobacteria. Biology 2024, 13, 95. [Google Scholar] [CrossRef]
- Qu, X.; Ren, Z.; Zhang, H.; Zhang, M.; Zhang, Y.; Liu, X.; Peng, W. Influences of anthropogenic land use on microbial community structure and functional potentials of stream benthic biofilms. Sci. Rep. 2017, 7, 15117. [Google Scholar] [CrossRef]
- Hartman, W.H.; Richardson, C.J.; Vilgalys, R.; Bruland, G.L. Environmental and anthropogenic controls over bacterial communities in wetland soils. Proc. Natl. Acad. Sci. USA 2008, 105, 17842–17847. [Google Scholar] [CrossRef]
- Ballesteros, N.; Páez, L.; Luna, N.; Reina, A.; Urrea, V.; Sánchez, C.; Ramírez, A.; Ramirez, J.D.; Muñoz, M. Characterization of microbial communities in seven wetlands with different anthropogenic burden using Next Generation Sequencing in Bogotá, Colombia. Sci. Rep. 2023, 13, 16973. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lim, B.S.; Seol, J.; Lee, C.S. Principle of restoration ecology reflected in the process creating the National Institute of Ecology. J. Ecol. Environ. 2021, 45, 12. [Google Scholar] [CrossRef]



| Sample ID | Texture | pH | TN (%) | TP (mg/kg) | TOC (%) | NH4+ (mg/kg) | NO3−(mg/kg) |
|---|---|---|---|---|---|---|---|
| L1-W | Water | 4.9 | 0.96 | 0.02 | 8.20 | 0.10 | 0.70 |
| L1-M | Mud | 5.9 | 0.26 | 1091.29 | 6.42 | 125.74 | ND |
| L1-T | Mud | 5.7 | 0.12 | 496.01 | 5.24 | 3.12 | ND |
| L1-Z | Mud | 5.6 | 0.05 | 480.03 | 2.59 | ND | ND |
| L1-R | Mud | 5.7 | 0.43 | 907.58 | 10.68 | 20.19 | ND |
| L1-B | Soil | 5.6 | 0.35 | 850.21 | 6.70 | 3.91 | 21.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.K.; Lim, B.-S.; Lee, C.S.; Srinivasan, S. Bacterial Diversity in the Different Ecological Niches Related to the Yonghwasil Pond (Republic of Korea). Microorganisms 2024, 12, 2547. https://doi.org/10.3390/microorganisms12122547
Kim MK, Lim B-S, Lee CS, Srinivasan S. Bacterial Diversity in the Different Ecological Niches Related to the Yonghwasil Pond (Republic of Korea). Microorganisms. 2024; 12(12):2547. https://doi.org/10.3390/microorganisms12122547
Chicago/Turabian StyleKim, Myung Kyum, Bong-Soon Lim, Chang Seok Lee, and Sathiyaraj Srinivasan. 2024. "Bacterial Diversity in the Different Ecological Niches Related to the Yonghwasil Pond (Republic of Korea)" Microorganisms 12, no. 12: 2547. https://doi.org/10.3390/microorganisms12122547
APA StyleKim, M. K., Lim, B.-S., Lee, C. S., & Srinivasan, S. (2024). Bacterial Diversity in the Different Ecological Niches Related to the Yonghwasil Pond (Republic of Korea). Microorganisms, 12(12), 2547. https://doi.org/10.3390/microorganisms12122547







