The Gut Microbiota Is Involved in the Regulation of Cognitive Flexibility in Adolescent BALB/c Mice Exposed to Chronic Physical Stress and a High-Fat Diet
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Exposure to Chronic Physical Stress (MS and FS Groups)
2.3. Exposure to High-Fat Diet (MD and FD Groups)
2.4. Attentional Set-Shifting Test (ASST)
2.5. Microbiome Sample Collection and Metagenomic DNA Extraction
2.6. Sequencing and Bioinformatics Analysis
2.7. Taxonomic Identification and Prediction of Functional Gene Content
2.8. Statistical Analysis
3. Results
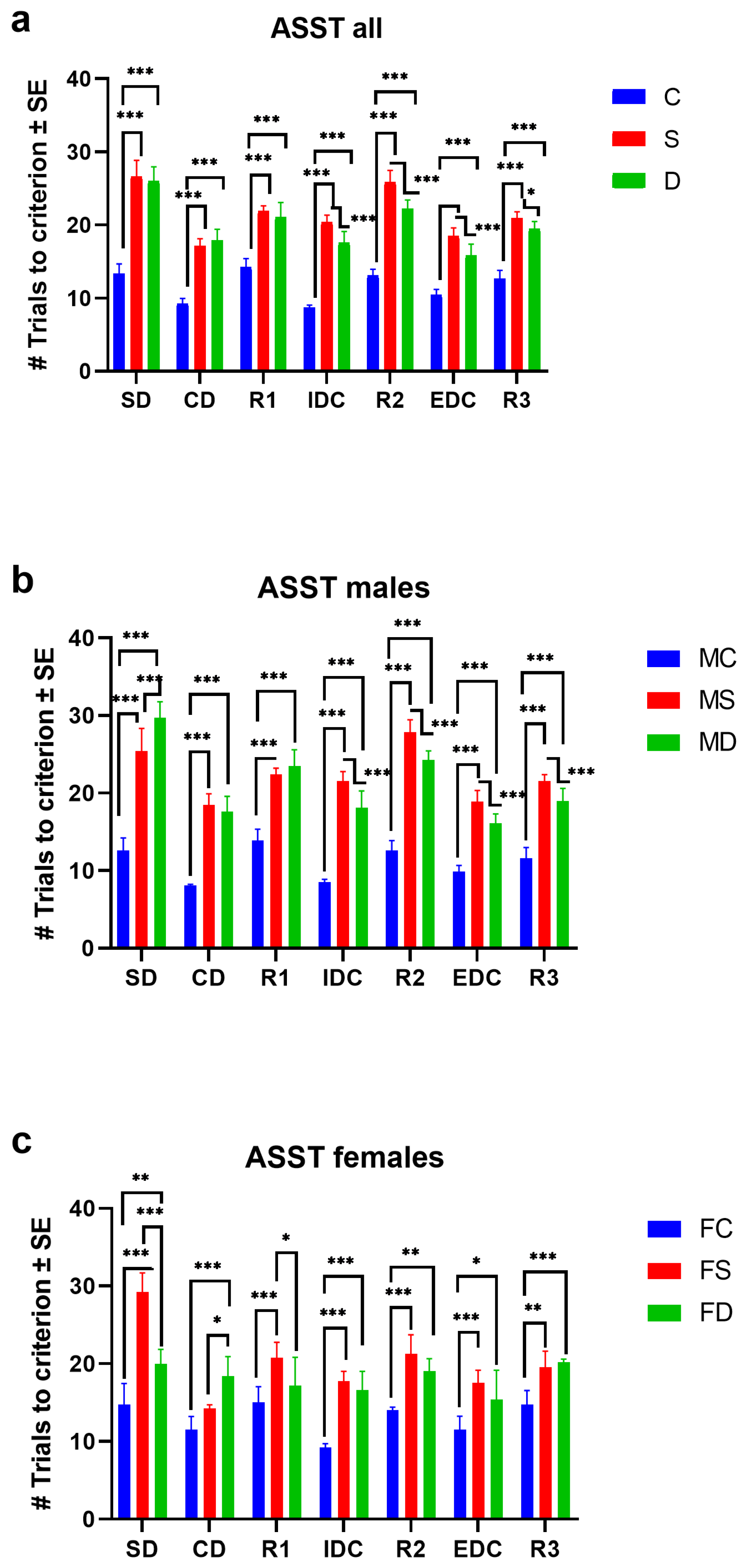
3.1. Attentional Set-Shifting Test in Groups Stress (MS/FS) and High-Fat Diet (MD/FD)
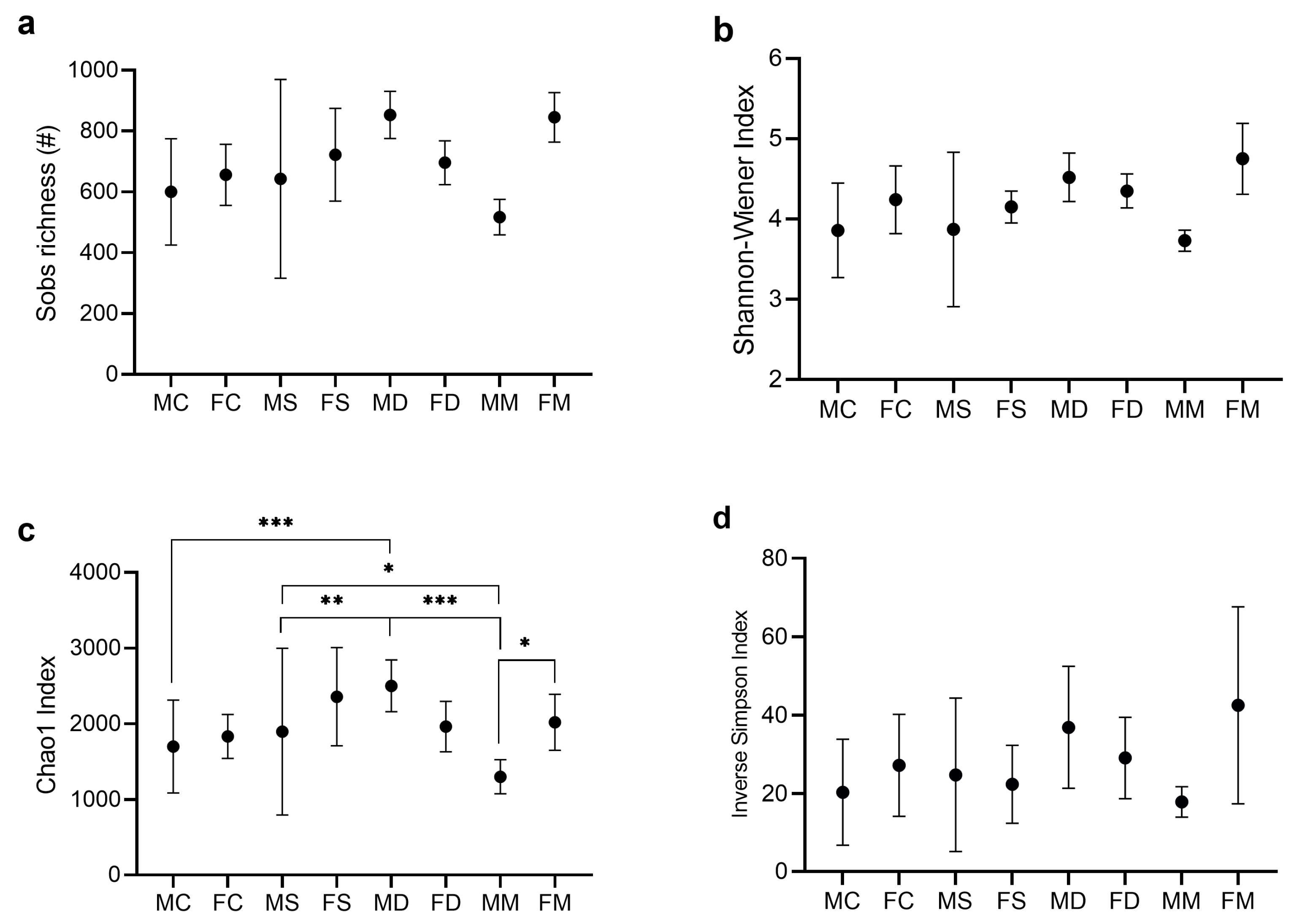
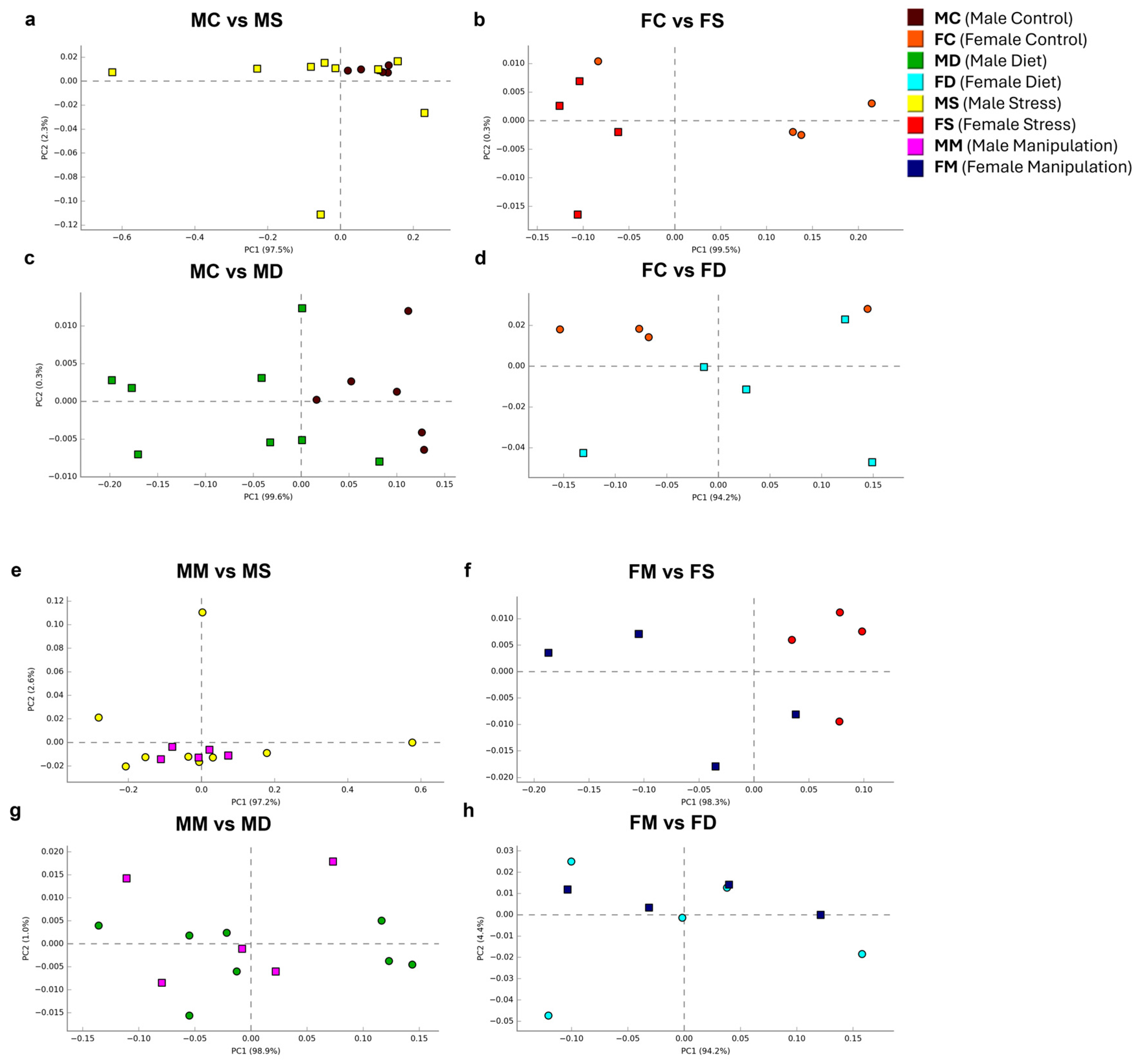
3.2. Analysis of Alpha and Beta Diversity Indices
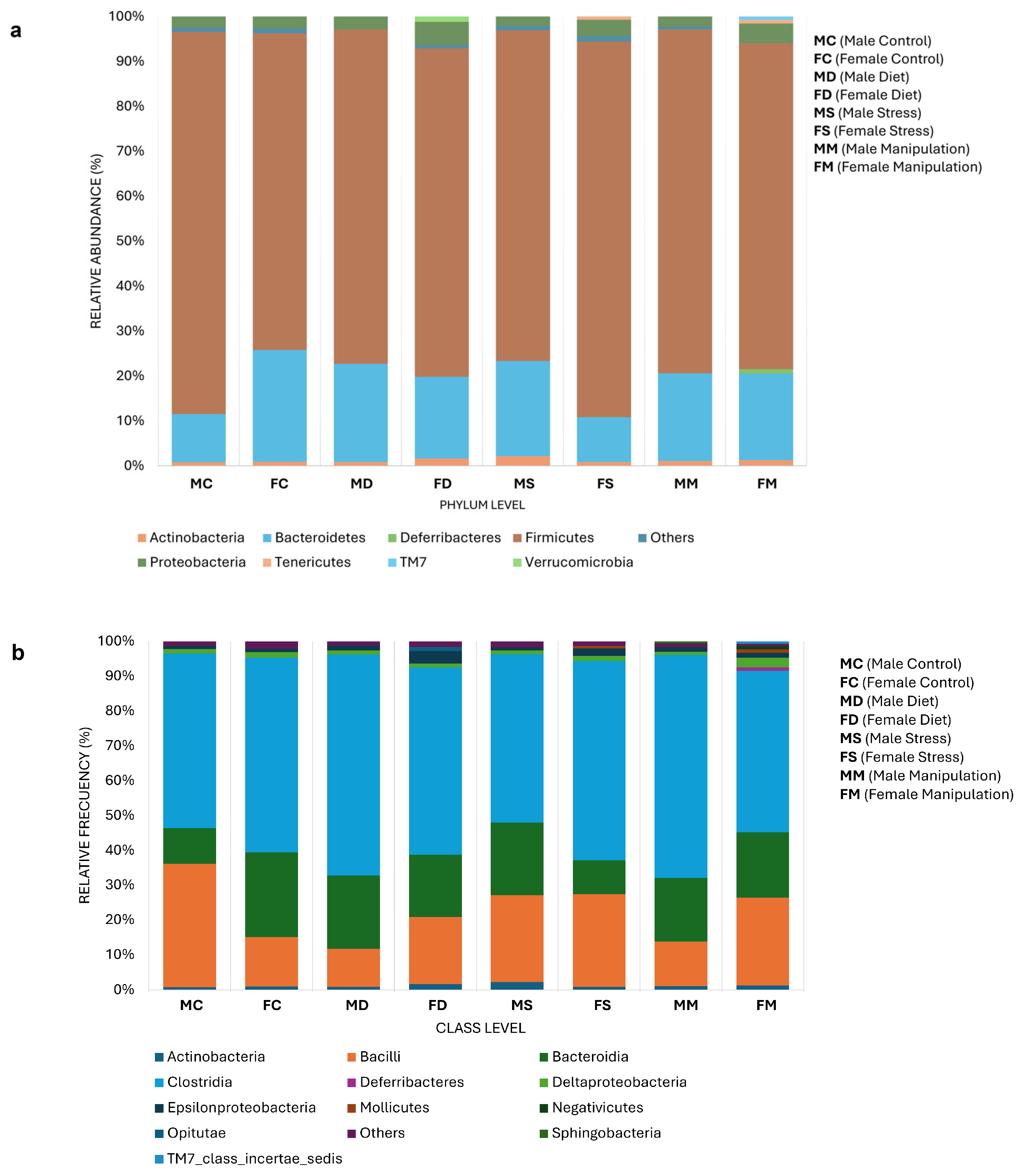
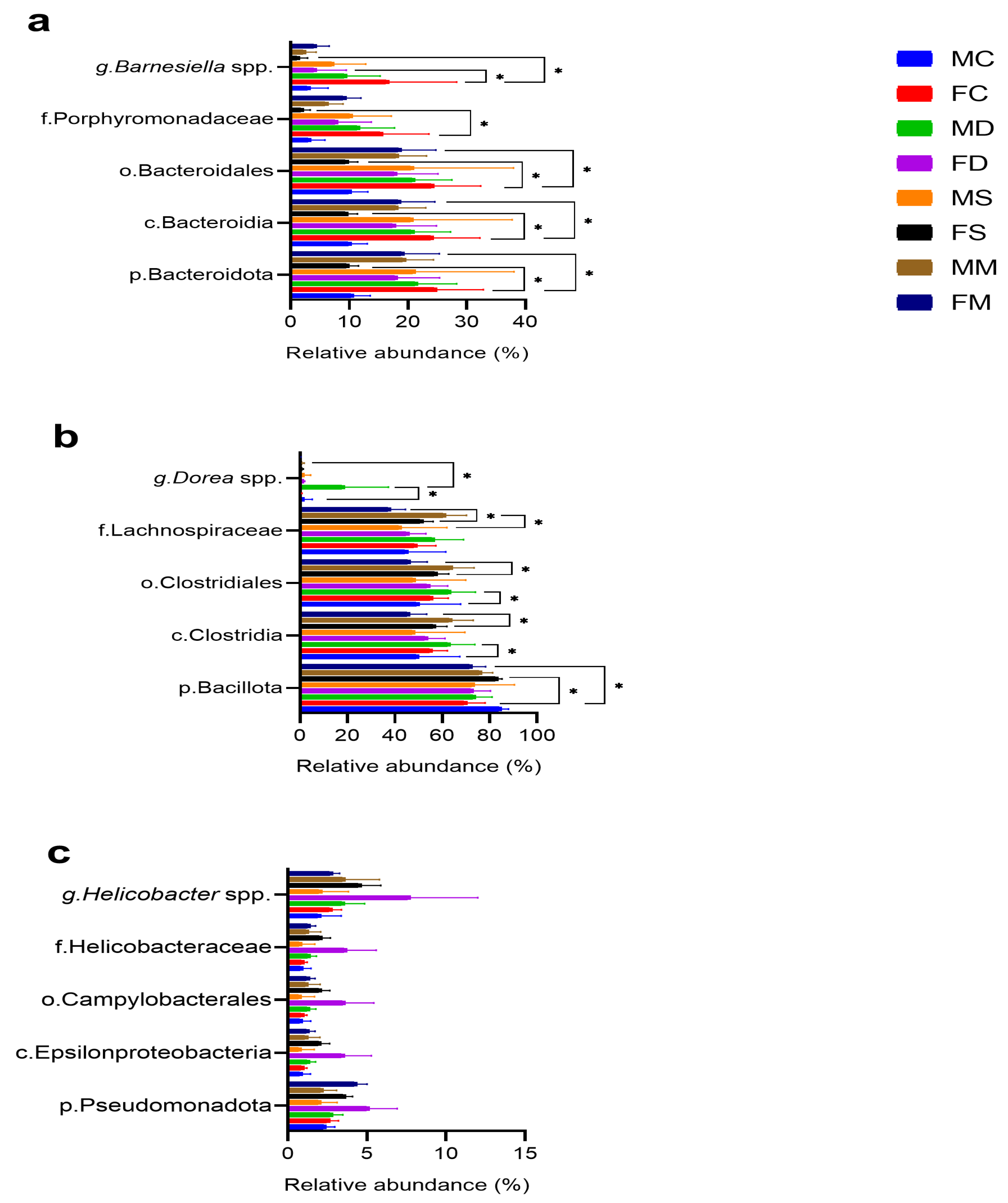
3.3. Microbial Composition
3.4. Comparative Microbial Analysis
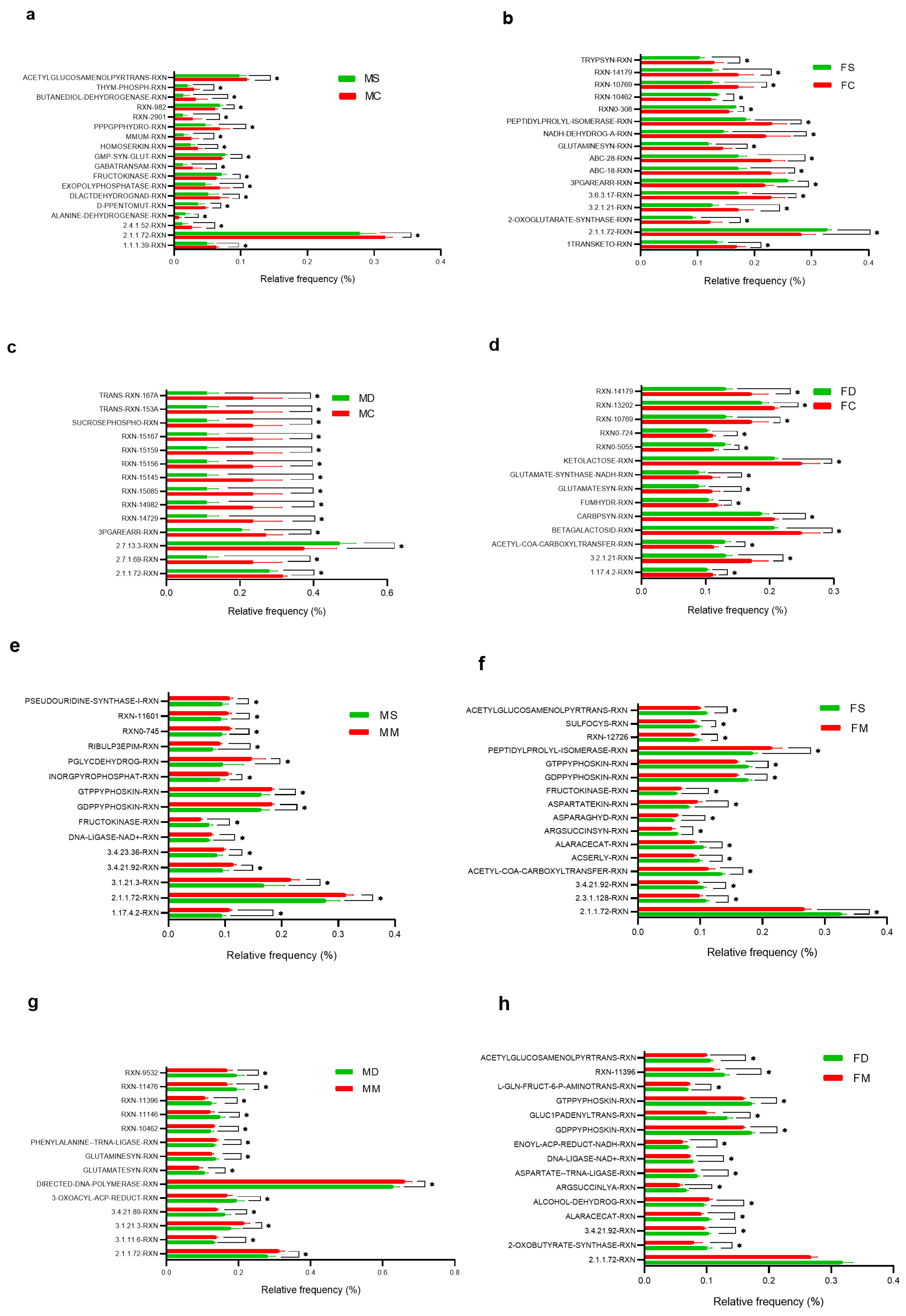
3.5. Comparative Prediction of Functional Gene Content Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flores-Reséndiz, C.; Soto-Piña, A.E.; Valdés-Ramos, R.; Benítez-Arciniega, A.D.; Tlatempa-Sotelo, P.; Guadarrama-López, A.L.; Martínez-Carrillo, B.E.; Pulido-Alvarado, C.C. Association Between Cardiovascular Risk Factors and Stress Hormones with Cognitive Performance in Mexican Adolescents. J. Pediatr. Psychol. 2019, 44, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Heisler, J.M.; Morales, J.; Donegan, J.J.; Jett, J.D.; Redus, L.; O’Connor, J.C. The Attentional Set Shifting Task: A Measure of Cognitive Flexibility in Mice. J. Vis. Exp. JoVE 2015, 4, 51944. [Google Scholar] [CrossRef]
- Birrell, J.M.; Brown, V.J. Medial Frontal Cortex Mediates Perceptual Attentional Set Shifting in the Rat. J. Neurosci. 2000, 20, 4320–4324. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; McGaughy, J. Adolescent Rats Show Cognitive Rigidity in a Test of Attentional Set Shifting. Dev. Psychobiol. 2011, 53, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liang, J.; Hong, C.; Liang, R.; Luo, Y. Cardiometabolic Multimorbidity, Lifestyle Behaviours, and Cognitive Function: A Multicohort Study. Lancet Healthy Longev. 2023, 4, e265–e273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Liu, L.; Li, Z.; Zhou, Y.; Wei, J.; Xu, Y.; Zhou, Y.; Tang, Y. Cognitive Function and Cardiovascular Health in the Elderly: Network Analysis Based on Hypertension, Diabetes, Cerebrovascular Disease, and Coronary Heart Disease. Front. Aging Neurosci. 2023, 15, 1229559. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D. The Gut Microbiota in Obesity and Weight Management: Microbes as Friends or Foe? Nat. Rev. Endocrinol. 2023, 19, 258–271. [Google Scholar] [CrossRef]
- Karlsson, F.; Tremaroli, V.; Nielsen, J.; Bäckhed, F. Assessing the Human Gut Microbiota in Metabolic Diseases. Diabetes 2013, 62, 3341–3349. [Google Scholar] [CrossRef]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.K.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced Diversity of the Intestinal Microbiota during Infancy Is Associated with Increased Risk of Allergic Disease at School Age. J. Allergy Clin. Immunol. 2011, 128, 646–652.e5. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Liu, X. The Microbiota–Gut–Brain Axis and Neurodevelopmental Disorders. Protein Cell 2023, 14, 762–775. [Google Scholar] [CrossRef]
- Kim, G.-H.; Shim, J.-O. Gut Microbiota Affects Brain Development and Behavior. Clin. Exp. Pediatr. 2022, 66, 274–280. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Xia, B.; Jin, X.; Zou, Q.; Zeng, Z.; Zhao, W.; Yan, S.; Li, L.; Yuan, S.; et al. High-Fiber Diet Mitigates Maternal Obesity-Induced Cognitive and Social Dysfunction in the Offspring via Gut-Brain Axis. Cell Metab. 2021, 33, 923–938.e6. [Google Scholar] [CrossRef]
- Lapiz-Bluhm, M.D.S.; Soto-Piña, A.E.; Hensler, J.G.; Morilak, D.A. Chronic Intermittent Cold Stress and Serotonin Depletion Induce Deficits of Reversal Learning in an Attentional Set-Shifting Test in Rats. Psychopharmacology 2009, 202, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-S.; Cha, J.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.-M.; Cha, L. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.-J.; Morris, M.J. Diet, Inflammation and the Gut Microbiome: Mechanisms for Obesity-Associated Cognitive Impairment. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2020, 1866, 165767. [Google Scholar] [CrossRef] [PubMed]
- McLean, F.H.; Grant, C.; Morris, A.C.; Horgan, G.W.; Polanski, A.J.; Allan, K.; Campbell, F.M.; Langston, R.F.; Williams, L.M. Rapid and Reversible Impairment of Episodic Memory by a High-Fat Diet in Mice. Sci. Rep. 2018, 8, 11976. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Ward, L.C.; Brown, L. Inulin Oligofructose Attenuates Metabolic Syndrome in High-Carbohydrate, High-Fat Diet-Fed Rats. Br. J. Nutr. 2016, 116, 1502–1511. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, W.; Ge, Q.; Zhou, X. Effect of Different Types of Oil Intake on the Blood Index and the Intestinal Flora of Rats. AMB Express 2022, 12, 49. [Google Scholar] [CrossRef]
- Diet-Microbiota Interactions and Their Implications for Healthy Living. In Health and the Gut; Apple Academic Press: Palm Bay, FL, USA, 2014; ISBN 978-0-429-18343-0.
- Zhu, X.; Cai, J.; Wang, Y.; Liu, X.; Chen, X.; Wang, H.; Wu, Z.; Bao, W.; Fan, H.; Wu, S. A High-Fat Diet Increases the Characteristics of Gut Microbial Composition and the Intestinal Damage Associated with Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 16733. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, Depression, Diet, and the Gut Microbiota: Human-Bacteria Interactions at the Core of Psychoneuroimmunology and Nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Arnoriaga-Rodríguez, M.; Fernández-Real, J.M. Microbiota Impacts on Chronic Inflammation and Metabolic Syndrome—Related Cognitive Dysfunction. Rev. Endocr. Metab. Disord. 2019, 20, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Lapiz-Bluhm, M.D.S.; Bondi, C.O.; Doyen, J.; Rodriguez, G.A.; Bédard-Arana, T.; Morilak, D.A. Behavioural Assays to Model Cognitive and Affective Dimensions of Depression and Anxiety in Rats. J. Neuroendocrinol. 2008, 20, 1115–1137. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.; Marston, H.M.; McQuade, R.; Gartside, S.E. Evidence That the Attentional Set Shifting Test in Rats Can Be Applied in Repeated Testing Paradigms. J. Psychopharmacol. Oxf. Engl. 2014, 28, 691–696. [Google Scholar] [CrossRef]
- Young, J.W.; Powell, S.B.; Geyer, M.A.; Jeste, D.V.; Risbrough, V.B. The Mouse Attentional-Set-Shifting Task: A Method for Assaying Successful Cognitive Aging? Cogn. Affect. Behav. Neurosci. 2010, 10, 243–251. [Google Scholar] [CrossRef]
- Colacicco, G.; Welzl, H.; Lipp, H.-P.; Würbel, H. Attentional Set-Shifting in Mice: Modification of a Rat Paradigm, and Evidence for Strain-Dependent Variation. Behav. Brain Res. 2002, 132, 95–102. [Google Scholar] [CrossRef]
- Bissonette, G.B.; Powell, E.M. Reversal Learning and Attentional Set-Shifting in Mice. Neuropharmacology 2012, 62, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.J.; Tait, D.S. Attentional Set-Shifting Across Species. Curr. Top. Behav. Neurosci. 2016, 28, 363–395. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Especificaciones Tecnicas Para La Produccion, Cuidado Y Uso De Los Animales De Laboratorio. Norma Oficial Mexicana: Fort Myers, FL, USA, 1999.
- Oros-Pantoja, R.; Román-Aguilar, V.; Martínez-Carrillo, B.E.; Valdés-Ramos, R.; Rivera-Hernández, D.M. SFLT1/PIGF Angiogenic Relationship in the Experimental Model of Preeclampsia Induced by Sympathetic Activation in the Mouse (679.5). FASEB J. 2014, 28, 679.5. [Google Scholar] [CrossRef]
- Repositorio de Tesis DGBSDI: Inducción Por Dieta Del Síndrome Metabólico En El Hámster Dorado (Mesocricetus Auratus) y Efecto de La Gobernadora (Larrea Tridentata). Available online: https://ru.dgb.unam.mx/handle/20.500.14330/TES01000754979 (accessed on 21 September 2024).
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and Tools for High Throughput rRNA Analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef]
- Schloss, P.D. Reintroducing Mothur: 10 Years Later. Appl. Environ. Microbiol. 2020, 86, e02343-19. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical Analysis of Taxonomic and Functional Profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Parks, D.H.; Beiko, R.G. Identifying Biologically Relevant Differences between Metagenomic Communities. Bioinformatics 2010, 26, 715–721. [Google Scholar] [CrossRef]
- Keefe, C.R.; Dillon, M.R.; Gehret, E.; Herman, C.; Jewell, M.; Wood, C.V.; Bolyen, E.; Caporaso, J.G. Facilitating Bioinformatics Reproducibility with QIIME 2 Provenance Replay. PLOS Comput. Biol. 2023, 19, e1011676. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An Improved Greengenes Taxonomy with Explicit Ranks for Ecological and Evolutionary Analyses of Bacteria and Archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- PICRUSt2 Tutorial (v2.3.0 Beta). Available online: https://github.com/picrust/picrust2 (accessed on 20 September 2024).
- Reichelt, A.C.; Gibson, G.D.; Abbott, K.N.; Hare, D.J. A High-Fat High-Sugar Diet in Adolescent Rats Impairs Social Memory and Alters Chemical Markers Characteristic of Atypical Neuroplasticity and Parvalbumin Interneuron Depletion in the Medial Prefrontal Cortex. Food Funct. 2019, 10, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.Y.; Kim, E.; Seo, K.; Kang, Y.J.; Kim, J.Y.; Kim, C.-H.; Song, H.T.; Saksida, L.M.; Lee, J.E. Assessment of Cognitive Impairment in a Mouse Model of High-Fat Diet-Induced Metabolic Stress with Touchscreen-Based Automated Battery System. Exp. Neurobiol. 2018, 27, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Dalley, J.; Dutcher, E.; Lopez-Cruz, L.; Pama, E.; Lynall, M.-E.; Bevers, I.; Jones, J.; Khan, S.; Sawiak, S.; Milton, A.; et al. Early-Life Stress Biases Responding to Negatively Valenced Stimuli and Increases Amygdala Volume and Vulnerability to Later-Life Stress 2022. Transl. Psychiatry 2023, 13, 81. [Google Scholar]
- Yu, H.; Qin, X.; Yu, Z.; Chen, Y.; Tang, L.; Shan, W. Effects of High-Fat Diet on the Formation of Depressive-like Behavior in Mice. Food Funct. 2021, 12, 6416–6431. [Google Scholar] [CrossRef]
- Tait, D.S.; Bowman, E.M.; Neuwirth, L.S.; Brown, V.J. Assessment of Intradimensional/Extradimensional Attentional Set-Shifting in Rats. Neurosci. Biobehav. Rev. 2018, 89, 72–84. [Google Scholar] [CrossRef]
- Bremner, J.D.; Moazzami, K.; Wittbrodt, M.T.; Nye, J.A.; Lima, B.B.; Gillespie, C.F.; Rapaport, M.H.; Pearce, B.D.; Shah, A.J.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef]
- Watson, L.S.; Stone, T.D.; Williams, D.; Williams, A.S.; Sims-Robinson, C. High-Fat Diet Impairs Tactile Discrimination Memory in the Mouse. Behav. Brain Res. 2020, 382, 112454. [Google Scholar] [CrossRef]
- de Noronha, S.I.S.R.; de Moraes, L.A.G.; Hassell, J.E.; Stamper, C.E.; Arnold, M.R.; Heinze, J.D.; Foxx, C.L.; Lieb, M.M.; Cler, K.E.; Karns, B.L.; et al. High-Fat Diet, Microbiome-Gut-Brain Axis Signaling, and Anxiety-like Behavior in Male Rats. Biol. Res. 2024, 57, 23. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Li, Z.; Mao, Z. Association between Social Activities and Cognitive Function among the Elderly in China: A Cross-Sectional Study. Int. J. Environ. Res. Public. Health 2018, 15, 231. [Google Scholar] [CrossRef]
- Badji, A.; Youwakim, J.; Cooper, A.; Westman, E.; Marseglia, A. Vascular Cognitive Impairment—Past, Present, and Future Challenges. Ageing Res. Rev. 2023, 90, 102042. [Google Scholar] [CrossRef] [PubMed]
- Feola, B.; Dougherty, L.R.; Riggins, T.; Bolger, D.J. Prefrontal Cortical Thickness Mediates the Association between Cortisol Reactivity and Executive Function in Childhood. Neuropsychologia 2020, 148, 107636. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vargas, J. Estrés, Atención y Memoria 1. In Las Claves del Mobbing; Editorial EOS: Madrid, Spain, 2005; pp. 35–65. ISBN 978-84-9727-187-5. [Google Scholar]
- Miralbell, J.; López-Cancio, E.; López-Oloriz, J.; Arenillas, J.F.; Barrios, M.; Soriano-Raya, J.J.; Galán, A.; Cáceres, C.; Alzamora, M.; Pera, G.; et al. Cognitive Patterns in Relation to Biomarkers of Cerebrovascular Disease and Vascular Risk Factors. Cerebrovasc. Dis. 2013, 36, 98–105. [Google Scholar] [CrossRef]
- Beck, D.; de Lange, A.G.; Pedersen, M.L.; Alnæs, D.; Maximov, I.I.; Voldsbekk, I.; Richard, G.; Sanders, A.; Ulrichsen, K.M.; Dørum, E.S.; et al. Cardiometabolic Risk Factors Associated with Brain Age and Accelerate Brain Ageing. Hum. Brain Mapp. 2021, 43, 700–720. [Google Scholar] [CrossRef]
- Yanguas-Casás, N.; Torres-Fuentes, C.; Crespo-Castrillo, A.; Diaz-Pacheco, S.; Healy, K.; Stanton, C.; Chowen, J.A.; Garcia-Segura, L.M.; Arevalo, M.A.; Cryan, J.F.; et al. High-Fat Diet Alters Stress Behavior, Inflammatory Parameters and Gut Microbiota in Tg APP Mice in a Sex-Specific Manner. Neurobiol. Dis. 2021, 159, 105495. [Google Scholar] [CrossRef]
- Imdad, S.; So, B.; Jang, J.; Park, J.; Lee, S.-J.; Kim, J.-H.; Kang, C. Temporal Variations in the Gut Microbial Diversity in Response to High-Fat Diet and Exercise. Sci. Rep. 2024, 14, 3282. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; Meijer, B.; Hugenholtz, F.; van der Gaast-de Jongh, C.; Savelkoul, H.F.; de Jonge, M.I.; Faas, M.M.; Boekschoten, M.V.; et al. The Impact of Gut Microbiota on Gender-Specific Differences in Immunity. Front. Immunol. 2017, 8, 754. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.-Y.; Park, M.-S. Sex Differences in Gut Microbiota. World J. Mens Health 2020, 38, 48–60. [Google Scholar] [CrossRef]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex Differences and Hormonal Effects on Gut Microbiota Composition in Mice. Gut Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef]
- Shobeiri, P.; Kalantari, A.; Teixeira, A.L.; Rezaei, N. Shedding Light on Biological Sex Differences and Microbiota–Gut–Brain Axis: A Comprehensive Review of Its Roles in Neuropsychiatric Disorders. Biol. Sex Differ. 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of High-Fat-Diet on Gut Microbiota: A Driving Force for Chronic Disease Risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Kropp, D.R.; Rainville, J.R.; Glover, M.E.; Tsyglakova, M.; Samanta, R.; Hage, T.R.; Carlson, A.E.; Clinton, S.M.; Hodes, G.E. Chronic Variable Stress Leads to Sex Specific Gut Microbiome Alterations in Mice. Brain Behav. Immun.—Health 2024, 37, 100755. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E. Stress in the Microbiome-Immune Crosstalk. Gut Microbes 2024, 16, 2327409. [Google Scholar] [CrossRef] [PubMed]
- The Gut Microbiome and Female Health. Available online: https://www.mdpi.com/2079-7737/11/11/1683 (accessed on 29 September 2024).
- Marano, G.; Traversi, G.; Gaetani, E.; Gasbarrini, A.; Mazza, M. Gut Microbiota in Women: The Secret of Psychological and Physical Well-Being. World J. Gastroenterol. 2023, 29, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wen, Z.; Zhao, L.; Yu, D.; Meng, K.; Yang, P. Lactobacillus Plantarum FRT4 Alleviated Obesity by Modulating Gut Microbiota and Liver Metabolome in High-Fat Diet-Induced Obese Mice. Food Nutr. Res. 2022, 66, 10.29219/fnr.v66.7974. [Google Scholar] [CrossRef]
- Quesada-Vázquez, S.; Castells-Nobau, A.; Latorre, J.; Oliveras-Cañellas, N.; Puig-Parnau, I.; Tejera, N.; Tobajas, Y.; Baudin, J.; Hildebrand, F.; Beraza, N.; et al. Potential Therapeutic Implications of Histidine Catabolism by the Gut Microbiota in NAFLD Patients with Morbid Obesity. Cell Rep. Med. 2023, 4, 101341. [Google Scholar] [CrossRef]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Marzo, V.D.; Cristino, L. Obesity Affects the Microbiota–Gut–Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef]
- Warmbrunn, M.V.; Attaye, I.; Aron-Wisnewsky, J.; Rampanelli, E.; van der Vossen, E.W.J.; Hao, Y.; Koopen, A.; Bergh, P.-O.; Stols-Gonçalves, D.; Mohamed, N.; et al. Oral Histidine Affects Gut Microbiota and MAIT Cells Improving Glycemic Control in Type 2 Diabetes Patients. Gut Microbes 2024, 16, 2370616. [Google Scholar] [CrossRef]
- Herculano, B.; Tamura, M.; Ohba, A.; Shimatani, M.; Kutsuna, N.; Hisatsune, T. β-Alanyl-L-Histidine Rescues Cognitive Deficits Caused by Feeding a High Fat Diet in a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. JAD 2013, 33, 983–997. [Google Scholar] [CrossRef]
- Hamamah, S.; Amin, A.; Al-Kassir, A.L.; Chuang, J.; Covasa, M. Dietary Fat Modulation of Gut Microbiota and Impact on Regulatory Pathways Controlling Food Intake. Nutrients 2023, 15, 3365. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Shen, J.; Fan, R.; Xiao, R.; Ma, W. High-Fat Diets Containing Different Types of Fatty Acids Modulate Gut-Brain Axis in Obese Mice. Nutr. Metab. 2022, 19, 40. [Google Scholar] [CrossRef]
- May, A.A.; Liu, M.; Woods, S.C.; Begg, D.P. CCK Increases the Transport of Insulin into the Brain. Physiol. Behav. 2016, 165, 392–397. [Google Scholar] [CrossRef]
- Romaní-Pérez, M.; Bullich-Vilarrubias, C.; López-Almela, I.; Liébana-García, R.; Olivares, M.; Sanz, Y. The Microbiota and the Gut–Brain Axis in Controlling Food Intake and Energy Homeostasis. Int. J. Mol. Sci. 2021, 22, 5830. [Google Scholar] [CrossRef]
- Ratsika, A.; Codagnone, M.G.; Bastiaanssen, T.F.S.; Hoffmann Sarda, F.A.; Lynch, C.M.K.; Ventura-Silva, A.P.; Rosell-Cardona, C.; Caputi, V.; Stanton, C.; Fülling, C.; et al. Maternal High-Fat Diet-Induced Microbiota Changes Are Associated with Alterations in Embryonic Brain Metabolites and Adolescent Behaviour. Brain. Behav. Immun. 2024, 121, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the Gut-Brain Axis: Regulation by the Microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Morys, J.; Malecki, A.; Nowacka-Chmielewska, M.M. Stress and the Gut-Brain Axis: An Inflammatory Perspective. Front. Mol. Neurosci. 2024, 17, 1415567. [Google Scholar] [CrossRef]
- Queiroz, S.A.L.; Ton, A.M.M.; Pereira, T.M.C.; Campagnaro, B.P.; Martinelli, L.; Picos, A.; Campos-Toimil, M.; Vasquez, E.C. The Gut Microbiota-Brain Axis: A New Frontier on Neuropsychiatric Disorders. Front. Psychiatry 2022, 13, 872594. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the Microbiota-Gut-Brain Axis in Chronic Restraint Stress: Disturbances of the Kynurenine Metabolic Pathway in Both the Gut and Brain. Gut Microbes 2021, 13, 1869501. [Google Scholar] [CrossRef]
- Schwarcz, R.; Pellicciari, R. Manipulation of Brain Kynurenines: Glial Targets, Neuronal Effects, and Clinical Opportunities. J. Pharmacol. Exp. Ther. 2002, 303, 1–10. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s Metabolites in Exercise, Inflammation, and Mental Health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, C.Y.; Estrada, J.A.; Oros-Pantoja, R.; Colín-Ferreyra, M.d.C.; Benitez-Arciniega, A.D.; Soto Piña, A.E.; Aguirre-Garrido, J.F. The Gut Microbiota Is Involved in the Regulation of Cognitive Flexibility in Adolescent BALB/c Mice Exposed to Chronic Physical Stress and a High-Fat Diet. Microorganisms 2024, 12, 2542. https://doi.org/10.3390/microorganisms12122542
González CY, Estrada JA, Oros-Pantoja R, Colín-Ferreyra MdC, Benitez-Arciniega AD, Soto Piña AE, Aguirre-Garrido JF. The Gut Microbiota Is Involved in the Regulation of Cognitive Flexibility in Adolescent BALB/c Mice Exposed to Chronic Physical Stress and a High-Fat Diet. Microorganisms. 2024; 12(12):2542. https://doi.org/10.3390/microorganisms12122542
Chicago/Turabian StyleGonzález, Cristian Yuriana, José Antonio Estrada, Rigoberto Oros-Pantoja, María del Carmen Colín-Ferreyra, Alejandra Donaji Benitez-Arciniega, Alexandra Estela Soto Piña, and José Félix Aguirre-Garrido. 2024. "The Gut Microbiota Is Involved in the Regulation of Cognitive Flexibility in Adolescent BALB/c Mice Exposed to Chronic Physical Stress and a High-Fat Diet" Microorganisms 12, no. 12: 2542. https://doi.org/10.3390/microorganisms12122542
APA StyleGonzález, C. Y., Estrada, J. A., Oros-Pantoja, R., Colín-Ferreyra, M. d. C., Benitez-Arciniega, A. D., Soto Piña, A. E., & Aguirre-Garrido, J. F. (2024). The Gut Microbiota Is Involved in the Regulation of Cognitive Flexibility in Adolescent BALB/c Mice Exposed to Chronic Physical Stress and a High-Fat Diet. Microorganisms, 12(12), 2542. https://doi.org/10.3390/microorganisms12122542





