The Gut Microbiome and Female Health
Abstract
Simple Summary
Abstract
1. Introduction
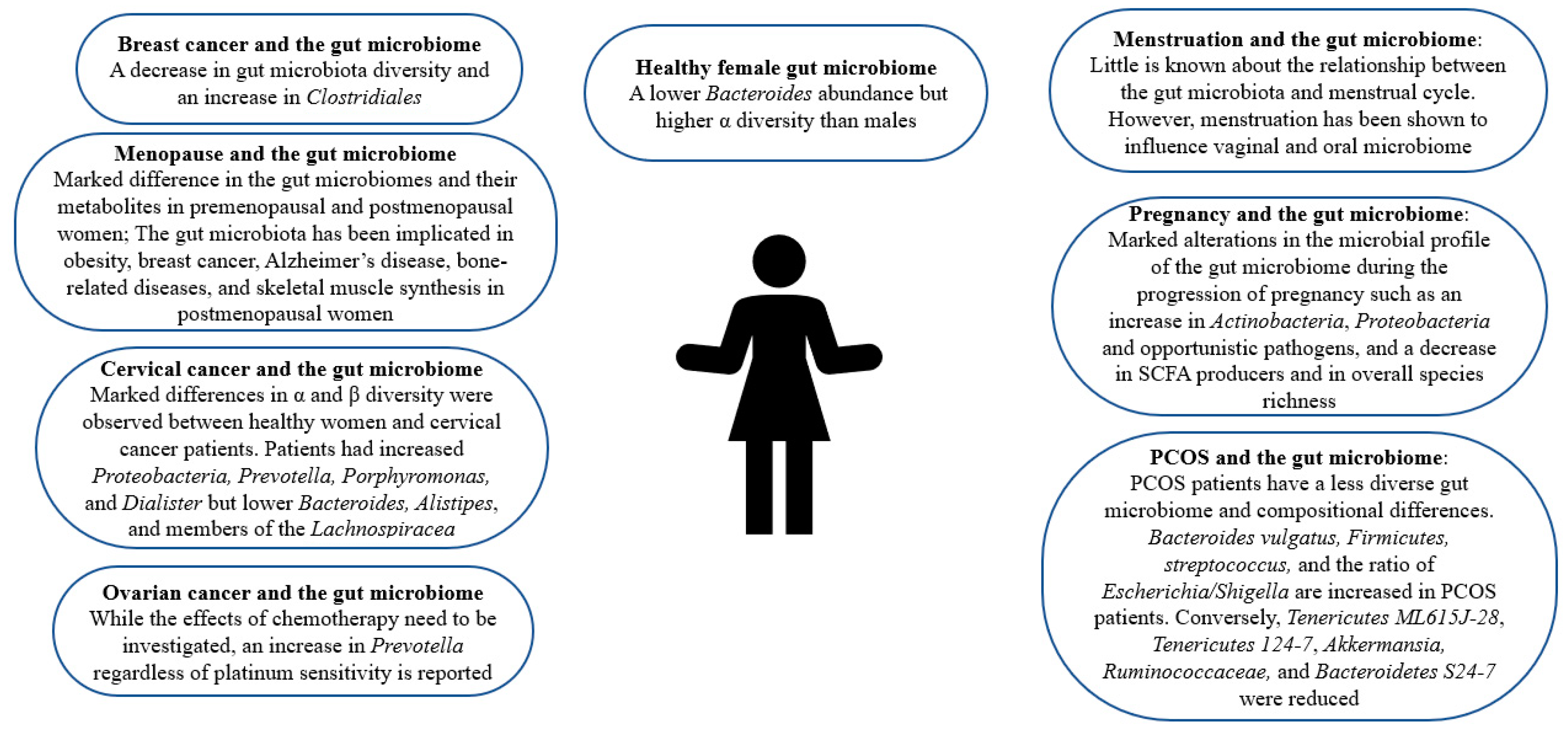
2. Gut Microbiome in Healthy Females
3. Gut Microbiome and PCOS
4. Gut Microbiome and Cancer
5. Gut Microbiome and Pregnancy
6. Changes in Gut Microbiome during the Menstrual Cycle
7. Gut Microbiota Composition Alterations Accompanying Menopause
8. The Role of the Gut Microbiome in Postmenopausal Female Health
9. The Role of the Gut Microbiome and Postmenopausal Bone Health
10. Therapeutic Strategies to Modulate the Gut Microbiome in Females
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed]
- What Health Issues or Conditions Affect Women Differently Than Men? Available online: https://www.nichd.nih.gov/health/topics/womenshealth/conditioninfo/howconditionsaffect (accessed on 1 February 2022).
- Friedson-Ridenour, S.; Dutcher, T.V.; Calderon, C.; Brown, L.D.; Olsen, C.W. Gender analysis for one health: Theoretical perspectives and recommendations for practice. Ecohealth 2019, 16, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Fini, M.; Spoletini, I.; Lainscak, M.; Seferovic, P.; Rosano, G.M. Under-representation of elderly and women in clinical trials. Int. J. Cardiol. 2017, 232, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Mirin, A.A. Gender disparity in the funding of diseases by the US National Institutes of Health. J. Women’s Health 2021, 30, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Thackray, V.G. Sex, Microbes, and Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2019, 30, 54–65. [Google Scholar] [CrossRef]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016, 7, 313–322. [Google Scholar] [CrossRef]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Siddiqui, R.; Mungroo, M.R.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Use of Gut Microbial Modulation Strategies as Interventional Strategies for Ageing. Microorganisms 2022, 10, 1869. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Shanahan, F.; Ghosh, T.S.; O’Toole, P.W. The healthy microbiome—What is the definition of a healthy gut microbiome? Gastroenterology 2021, 160, 483–494. [Google Scholar] [CrossRef]
- Song, P.; Wang, Q.B.; Liang, B.; Jiang, S.J. Advances in research on the relationship between the gut microbiome and cancer. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5104–5112. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.N.; Lydon, K.; O’Donovan, C.M.; O’Sullivan, O.; Madigan, S.M. More than a gut feeling: What is the role of the gastrointestinal tract in female athlete health? Eur. J. Sport Sci. 2022, 22, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Hall, M.R.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.T.; Castelo, P.M.; Ribeiro, D.A.; Ferreira, C.M. Influence of Oral and Gut Microbiota in the Health of Menopausal Women. Front. Microbiol. 2017, 8, 1884. [Google Scholar] [CrossRef]
- Parida, S.; Sharma, D. The Microbiome-Estrogen Connection and Breast Cancer Risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J. Natl. Cancer Inst. 2016, 108, djw029. [Google Scholar] [CrossRef]
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williams, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019, 73, 24–37. [Google Scholar] [CrossRef]
- Mei, Z.; Li, D. The role of probiotics in vaginal health. Front. Cell. Infect. Microbiol. 2022, 12, 963868. [Google Scholar] [CrossRef]
- Falagas, M.E.; Betsi, G.I.; Tokas, T.; Athanasiou, S. Probiotics for prevention of recurrent urinary tract infections in women. Drugs 2006, 66, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. The development of probiotics for women’s health. Can. J. Microbiol. 2017, 63, 269–277. [Google Scholar] [CrossRef] [PubMed]
- He, F.F.; Li, Y.M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ni, Z.; Cheng, W.; Yu, J.; Sun, S.; Zhai, D.; Yu, C.; Cai, Z. Characteristic gut microbiota and predicted metabolic functions in women with PCOS. Endocr. Connect. 2020, 9, 63–73. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1459. [Google Scholar] [CrossRef]
- Lindheim, L.; Bashir, M.; Münzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V.; et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS ONE 2017, 12, e0168390. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Xi, H.; Chen, L.; Feng, X. Exploration of the Relationship Between Gut Microbiota and Polycystic Ovary Syndrome (PCOS): A Review. Geburtshilfe Frauenheilkd. 2020, 80, 161–171. [Google Scholar] [CrossRef]
- Rizk, M.G.; Thackray, V.G. Intersection of Polycystic Ovary Syndrome and the Gut Microbiome. J. Endocr. Soc. 2020, 5, bvaa177. [Google Scholar] [CrossRef]
- Torres, P.J.; Siakowska, M.; Banaszewska, B.; Pawelczyk, L.; Duleba, A.J.; Kelley, S.T.; Thackray, V.G. Gut Microbial Diversity in Women with Polycystic Ovary Syndrome Correlates with Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018, 103, 1502–1511. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- NIH. The Relationship of the Intestinal Microbiome and the Menstrual Cycle. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03581201 (accessed on 1 February 2022).
- Ji, Y.J.; Li, H.; Xie, P.F.; Li, Z.H.; Li, H.W.; Yin, Y.L.; Blachier, F.; Kong, X.F. Stages of pregnancy and weaning influence the gut microbiota diversity and function in sows. J. Appl. Microbiol. 2019, 127, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Mallott, E.K.; Borries, C.; Koenig, A.; Amato, K.R.; Lu, A. Reproductive hormones mediate changes in the gut microbiome during pregnancy and lactation in Phayre’s leaf monkeys. Sci. Rep. 2020, 10, 9961. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Ziv, O.; Belogolovski, A.; Barsheshet, Y.; Bloch, N.; Uzan, A.; Lahav, R.; Peretz, A.; Frishman, S.; et al. Progesterone increases Bifidobacterium relative abundance during late pregnancy. Cell Rep. 2019, 27, 730–736.e3. [Google Scholar] [CrossRef]
- Jin, M.; Li, D.; Ji, R.; Liu, W.; Xu, X.; Feng, X. Changes in Gut Microorganism in Patients with Positive Immune Antibody-Associated Recurrent Abortion. BioMed Res. Int. 2020, 2020, 4673250. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Ma, M.; Zhang, W.; Bi, Y.; Cheng, P.; Yu, X.; Fu, Y.; Chao, Y.; Ji, T.; Li, J.; et al. The gut microbiota during the progression of atherosclerosis in the perimenopausal period shows specific compositional changes and significant correlations with circulating lipid metabolites. Gut Microbes 2021, 13, 1–27. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Arnoriaga-Rodríguez, M.; Luque-Córdoba, D.; Priego-Capote, F.; Pérez-Brocal, V.; Moya, A.; Burokas, A.; Maldonado, R.; Fernández-Real, J.M. Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: Influences of obesity and menopausal status. Microbiome 2020, 8, 136. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Rangel-Zuñiga, O.A.; Jimenez-Lucena, R.; Quintana-Navarro, G.M.; Garcia-Carpintero, S.; Malagon, M.M.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; Lopez-Miranda, J.; et al. Influence of gender and menopausal status on gut microbiota. Maturitas 2018, 116, 43–53. [Google Scholar] [CrossRef]
- Rettedal, E.A.; Ilesanmi-Oyelere, B.L.; Roy, N.C.; Coad, J.; Kruger, M.C. The gut microbiome is altered in postmenopausal women with osteoporosis and osteopenia. JBMR Plus 2021, 5, e10452. [Google Scholar] [CrossRef]
- He, J.; Xu, S.; Zhang, B.; Xiao, C.; Chen, Z.; Si, F.; Fu, J.; Lin, X.; Zheng, G.; Yu, G.; et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging 2020, 12, 8583–8604. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, K.; Wu, C.; Chen, J.; Pan, H.; Liu, Y.; Wu, P.; Yuan, J.; Huang, F.; Lang, J.; et al. An emerging role of Prevotella histicola on estrogen deficiency–induced bone loss through the gut microbiota–bone axis in postmenopausal women and in ovariectomized mice. Am. J. Clin. Nutr. 2021, 114, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, D.; Kubota, R.; Maeno, T.; Abdelhakim, M.; Hitosugi, N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos. Int. 2021, 32, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Mikó, E.; Kovács, T.; Sebő, É.; Tóth, J.; Csonka, T.; Ujlaki, G.; Sipos, A.; Szabó, J.; Méhes, G.; Bai, P. Microbiome-Microbial Metabolome-Cancer Cell Interactions in Breast Cancer-Familiar, but Unexplored. Cells 2019, 8, 293. [Google Scholar] [CrossRef]
- Jacobson, D.; Moore, K.; Gunderson, C.; Rowland, M.; Austin, R.; Honap, T.P.; Xu, J.; Warinner, C.; Sankaranarayanan, K.; Lewis, C.M., Jr. Shifts in gut and vaginal microbiomes are associated with cancer recurrence time in women with ovarian cancer. PeerJ 2021, 9, e11574. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.T.; Colbert, L.E.; Zheng, J.; Delgado Medrano, A.Y.; Hoffman, K.L.; Ramondetta, L.; Jazaeri, A.; Jhingran, A.; Schmeler, K.M.; Daniel, C.R.; et al. Gut microbial diversity and genus-level differences identified in cervical cancer patients versus healthy controls. Gynecol. Oncol. 2019, 155, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Lindheim, L.; Manti, M.; Fornes, R.; Bashir, M.; Czarnewski, P.; Diaz, O.E.; Seifert, M.; Engstrand, L.; Villablanca, E.J.; Obermayer-Pietsch, B.; et al. Reproductive and Behavior Dysfunction Induced by Maternal Androgen Exposure and Obesity Is Likely Not Gut Microbiome-Mediated. J. Endocr. Soc. 2018, 2, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Jiao, J.; Jia, S.; Li, G.; Zhang, W.; Yang, K.; Wang, Z.; Liu, C.; Li, D.; Wang, X. 16S rDNA Full-Length Assembly Sequencing Technology Analysis of Intestinal Microbiome in Polycystic Ovary Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 634981. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Han, Q.; Xu, J.; Wang, J.; Sun, Y.; Li, W.; Chen, Z.J.; Du, Y. Metagenomic analysis identified microbiome alterations and pathological association between intestinal microbiota and polycystic ovary syndrome. Fertil. Steril. 2020, 113, 1286–1298.e4. [Google Scholar] [CrossRef]
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterol 2018, 155, 33–37.e6. [Google Scholar] [CrossRef]
- Wong, S.H.; Kwong, T.; Wu, C.Y.; Yu, J. Clinical applications of gut microbiota in cancer biology. Semin. Cancer Biol. 2019, 55, 28–36. [Google Scholar] [CrossRef]
- Akbar, N.; Khan, N.A.; Muhammad, J.S.; Siddiqui, R. The role of gut microbiome in cancer genesis and cancer prevention. Health Sci. Rev. 2022, 2, 100010. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, M.; Yao, Z.; Liang, W.; Li, Q.; Liu, J.; Yang, H.; Ji, Y.; Wei, W.; Tan, A.; et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Yi, M.; Yu, S.; Qin, S.; Liu, Q.; Xu, H.; Zhao, W.; Chu, Q.; Wu, K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Alpuim Costa, D.; Nobre, J.G.; Batista, M.V.; Ribeiro, C.; Calle, C.; Cortes, A.; Marhold, M.; Negreiros, I.; Borralho, P.; Brito, M.; et al. Human Microbiota and Breast Cancer-Is There Any Relevant Link?—A Literature Review and New Horizons Toward Personalised Medicine. Front. Microbiol. 2021, 12, 584332. [Google Scholar] [CrossRef] [PubMed]
- Muccee, F.; Ghazanfar, S.; Ajmal, W.; Al-Zahrani, M. In-Silico Characterization of Estrogen Reactivating β-Glucuronidase Enzyme in GIT Associated Microbiota of Normal Human and Breast Cancer Patients. Genes 2022, 13, 1545. [Google Scholar] [CrossRef]
- Lumachi, F.; Santeufemia, D.A.; Basso, S.M. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Suraya, R.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Microbiome as a Target for Cancer Therapy. Integr. Cancer Ther. 2020, 19, 1534735420920721. [Google Scholar] [CrossRef]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming endocrine resistance in breast cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Scheidemann, E.R.; Shajahan-Haq, A.N. Resistance to CDK4/6 Inhibitors in Estrogen Receptor-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 12292. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, Z.; Cui, L.; Wen, Y.; Chen, X.; Gong, F.; Yi, H. Opportunities and Challenges of the Human Microbiome in Ovarian Cancer. Front. Oncol. 2020, 10, 163. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Zhao, J.; Gong, L.; Zhang, Y.; Wang, X.; Yuan, Z. Altered diversity and composition of the gut microbiome in patients with cervical cancer. AMB Express 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Mutic, A.D.; Jordan, S.; Edwards, S.M.; Ferranti, E.P.; Thul, T.A.; Yang, I. The postpartum maternal and newborn microbiomes. MCN. Am. J. Matern. Child Nurs. 2017, 42, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cai, M.; Li, L.; Zhang, X.; Xu, Y.; Xiao, J.; Huang, Q.; Luo, G.; Zeng, Z.; Jin, C.; et al. Gut microbiota changes in preeclampsia, abnormal placental growth and healthy pregnant women. BMC Microbiol. 2021, 21, 265. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Xing, J.W.; Zheng, Q.Q.; Gao, P.F. 919 Syrup Alleviates Postpartum Depression by Modulating the Structure and Metabolism of Gut Microbes and Affecting the Function of the Hippocampal GABA/Glutamate System. Front. Cell. Infect. Microbiol. 2021, 11, 694443. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; Liu, Y.; Borghuis, T.; van Loo-Bouwman, C.A.; Harmsen, H.; De Vos, P. Microbiota induced changes in the immune response in pregnant mice. Front. Immunol. 2020, 10, 2976. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, E.S.; Allan, J.A.; Theodoropoulos, C.; Ross, T.; Beagley, K.W.; Knox, C.L. Hormone-dependent bacterial growth, persistence and biofilm formation–a pilot study investigating human follicular fluid collected during IVF cycles. PLoS ONE 2012, 7, e49965. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, J.; Zhao, Y.; He, M.; Ke, S.; Wu, J.; Zhou, Y.; Fu, H.; Yang, H.; Chen, C.; et al. Dramatic remodeling of the gut microbiome around parturition and its relationship with host serum metabolic changes in sows. Front. Microbiol. 2019, 10, 2123. [Google Scholar] [CrossRef]
- Smid, M.C.; Ricks, N.M.; Panzer, A.; Mccoy, A.N.; Azcarate-Peril, M.A.; Keku, T.O.; Boggess, K.A. Maternal gut microbiome biodiversity in pregnancy. Am. J. Perinatol. 2018, 35, 24–30. [Google Scholar] [CrossRef]
- Di Simone, N.; Santamaria Ortiz, A.; Specchia, M.; Tersigni, C.; Villa, P.; Gasbarrini, A.; Scambia, G.; D’Ippolito, S. Recent insights on the maternal microbiota: Impact on pregnancy outcomes. Front. Immunol. 2020, 11, 528202. [Google Scholar] [CrossRef]
- Sakurai, K.; Kato, T.; Tanabe, H.; Taguchi-Atarashi, N.; Sato, Y.; Eguchi, A.; Watanabe, M.; Ohno, H.; Mori, C. Association between gut microbiota composition and glycoalbumin level during pregnancy in Japanese women: Pilot study from Chiba Study of Mother and Child Health. J. Diabetes Investig. 2020, 11, 699–706. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef] [PubMed]
- Gohir, W.; Whelan, F.J.; Surette, M.G.; Moore, C.; Schertzer, J.D.; Sloboda, D.M. Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother’s periconceptional diet. Gut Microbes 2015, 6, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.S.; Pierdon, M.K.; Misic, A.M.; Sullivan, M.C.; O’Brien, K.; Chen, Y.; Murray, S.J.; Ramharack, L.A.; Baldassano, R.N.; Parsons, T.D.; et al. Remodeling of the maternal gut microbiome during pregnancy is shaped by parity. Microbiome 2021, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gosalbes, M.J.; Compte, J.; Moriano-Gutierrez, S.; Vallès, Y.; Jiménez-Hernández, N.; Pons, X.; Artacho, A.; Francino, M.P. Metabolic adaptation in the human gut microbiota during pregnancy and the first year of life. EbioMedicine 2019, 39, 497–509. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Chen, W.; Lan, T.; Wang, Y.; Wu, Y.; Liao, X.; Mi, J. The Dynamic Changes of Gut Microbiota during the Perinatal Period in Sows. Animals 2020, 10, 2254. [Google Scholar] [CrossRef]
- Fu, H.; He, M.; Wu, J.; Zhou, Y.; Ke, S.; Chen, Z.; Liu, Q.; Liu, M.; Jiang, H.; Huang, L.; et al. Deep Investigating the Changes of Gut Microbiome and Its Correlation with the Shifts of Host Serum Metabolome Around Parturition in Sows. Front. Microbiol. 2021, 12, 729039. [Google Scholar] [CrossRef]
- Sun, B.; Xu, X.; Xia, Y.; Cheng, Y.; Mao, S.; Xiang, X.; Xia, D.; Wang, X.; Li, J. Variation of gut microbiome in free-ranging female tibetan macaques (Macaca thibetana) across different reproductive states. Animals 2021, 11, 39. [Google Scholar] [CrossRef]
- Mulak, A.; Taché, Y.; Larauche, M. Sex hormones in the modulation of irritable bowel syndrome. WJG 2014, 20, 2433–2448. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Barber, M.D.; Graff, L.A.; Shen, B. Symptomatology of irritable bowel syndrome and inflammatory bowel disease during the menstrual cycle. Gastroenterol. Rep. 2015, 3, 185–193. [Google Scholar] [CrossRef]
- Simmons, L.; Heitkemper, M.; Shaver, J. Gastrointestinal function during the menstrual cycle. Health Care Women Int. 1988, 9, 201–209. [Google Scholar] [CrossRef]
- Wald, A.; Van Thiel, D.H.; Hoechstetter, L.; Gavaler, J.S.; Egler, K.M.; Verm, R.; Scott, L.; Lester, R. Gastrointestinal transit: The effect of the menstrual cycle. Gastroenterology 1981, 80, 1497–1500. [Google Scholar] [CrossRef]
- Sovijit, W.N.; Sovijit, W.E.; Pu, S.; Usuda, K.; Inoue, R.; Watanabe, G.; Yamaguchi, H.; Nagaoka, K. Ovarian progesterone suppresses depression and anxiety-like behaviors by increasing the Lactobacillus population of gut microbiota in ovariectomized mice. Neurosci. Res. 2021, 168, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Krog, M.C.; Hugerth, L.W.; Bashir, Z.; Fransson, E.; Boulund, F.; Belibasakis, G.N.; Wannerberger, K.; Engstrand, L.; Nielsen, H.S.; et al. Dysbiosis of the human oral microbiome during the menstrual cycle and vulnerability to the external exposures of smoking and dietary sugar. Front. Cell. Infect. Microbiol. 2021, 11, 625229. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Madak-Erdogan, Z. Estrogen and microbiota crosstalk: Should we pay attention? Trends Endocrinol. Metab. 2016, 27, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T. Stress and Disease-Related Alterations of the Intestinal Microflora across the Life Span of Rhesus Monkeys; The University of Wisconsin-Madison: Madison, WI, USA, 2002. [Google Scholar]
- Tilt, L. The Microbiome and the Menstrual Cycle–Is There a Link. 2022. Symprove for Professionals. Available online: https://www.symprove.com/blogs/symprove-for-professionals/the-microbiome-and-the-menstrual-cycle-is-there-a-link (accessed on 15 August 2022).
- Krog, M.C.; Hugerth, L.W.; Fransson, E.; Bashir, Z.; Andersen, A.N.; Edfeldt, G.; Engstrand, L.; Schuppe-Koistinen, I.; Nielsen, H.S. The Healthy Female Microbiome Across Body Sites: Effect of Hormonal Contraceptives and the Menstrual Cycle. Hum. Reprod. 2021, 37, 1525–1543. [Google Scholar] [CrossRef]
- Song, S.D.; Acharya, K.D.; Zhu, J.E.; Deveney, C.M.; Walther-Antonio, M.R.; Tetel, M.J.; Chia, N. Daily vaginal microbiota fluctuations associated with natural hormonal cycle, contraceptives, diet, and exercise. Msphere 2020, 5, e00593-20. [Google Scholar] [CrossRef]
- Dupuit, M.; Rance, M.; Morel, C.; Bouillon, P.; Boscaro, A.; Boisseau, N.; Vincent, M.; Vazeille, E.; Barnich, N.; Chassaing, B. Impact of concurrent training on body composition and gut microbiota in postmenopausal women with overweight or obesity. Med. Sci. Sport. Exerc. 2021, 54, 517–529. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Li, X.; Sun, Q.; Qin, P.; Wang, Q. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Lett. 2019, 593, 2655–2664. [Google Scholar] [CrossRef]
- Miller, L.M.; Lampe, J.W.; Newton, K.M.; Gundersen, G.; Fuller, S.; Reed, S.D.; Frankenfeld, C.L. Being overweight or obese is associated with harboring a gut microbial community not capable of metabolizing the soy isoflavone daidzein to O-desmethylangolensin in peri-and post-menopausal women. Maturitas 2017, 99, 37–42. [Google Scholar] [CrossRef]
- Lim, E.Y.; Song, E.J.; Kim, J.G.; Jung, S.Y.; Lee, S.Y.; Shin, H.S.; Nam, Y.D.; Kim, Y.T. Lactobacillus intestinalis YT2 restores the gut microbiota and improves menopausal symptoms in ovariectomized rats. Benef. Microbes 2021, 12, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Hwang, Y.J.; Shin, M.J.; Yi, H. Difference in the gut microbiome between ovariectomy-induced obesity and diet-induced obesity. J. Microbiol. Biotechnol. 2017, 27, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.Q.; Lin, X.; Shen, H.; Liu, H.M.; Qiu, X.; Li, B.Y.; Shen, W.D.; Ge, C.L.; Lv, F.Y.; Shen, J.; et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J. Cachexia Sarcopenia Muscle 2021, 12, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Cox-York, K.A.; Sheflin, A.M.; Foster, M.T.; Gentile, C.L.; Kahl, A.; Koch, L.G.; Britton, S.L.; Weir, T.L. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol. Rep. 2015, 3, e12488. [Google Scholar] [CrossRef]
- Goedert, J.J.; Jones, G.; Hua, X.; Xu, X.; Yu, G.; Flores, R.; Falk, R.T.; Gail, M.H.; Shi, J.; Ravel, J.; et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: A population-based case-control pilot study. JNCI J. Natl. Cancer Inst. 2015, 107, djv147. [Google Scholar] [CrossRef]
- Fuhrman, B.J.; Feigelson, H.S.; Flores, R.; Gail, M.H.; Xu, X.; Ravel, J.; Goedert, J.J. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J. Clin. Endocrinol. Metab. 2014, 99, 4632–4640. [Google Scholar] [CrossRef]
- He, C.; Liu, Y.; Ye, S.; Yin, S.; Gu, J. Changes of intestinal microflora of breast cancer in premenopausal women. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 503–513. [Google Scholar] [CrossRef]
- Jett, S.; Malviya, N.; Schelbaum, E.; Jang, G.; Jahan, E.; Clancy, K.; Hristov, H.; Pahlajani, S.; Niotis, K.; Loeb-Zeitlin, S.; et al. Endogenous and Exogenous Estrogen Exposures: How Women’s Reproductive Health Can Drive Brain Aging and Inform Alzheimer's Prevention. Front. Aging Neurosci. 2022, 14, 831807. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef]
- Shieh, A.; Epeldegui, M.; Karlamangla, A.S.; Greendale, G.A. Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight 2020, 5, e134092. [Google Scholar] [CrossRef]
- Chen, L.; Yan, S.; Yang, M.; Yu, F.; Wang, J.; Wang, X.; Xu, H.; Shi, J.; Pan, L.; Zeng, Y.; et al. The gut microbiome is associated with bone turnover markers in postmenopausal women. Am. J. Transl. Res. 2021, 13, 12601–12613. [Google Scholar] [PubMed]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V.; Jamilian, M.; Bahmani, F.; Asemi, Z. Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J. Ovarian Res. 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Mansury, S.; Bahmani, F.; Heidar, Z.; Amirani, E.; Asemi, Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J. Ovarian Res. 2018, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Dominguez-Recio, M.E.; Jimenez-Rodriguez, B.; Lavado, R.; Comino-Méndez, I.; Alba, E.; Queipo-Ortuño, M.I. Breast and gut microbiota action mechanisms in breast cancer pathogenesis and treatment. Cancers 2020, 12, 2465. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; Skrypnik, K.; Sobieska, M.; Korybalska, K.; Suliburska, J.; Bogdański, P. Multispecies probiotic supplementation favorably affects vascular function and reduces arterial stiffness in obese postmenopausal women—A 12-week placebo-controlled and randomized clinical study. Nutrients 2018, 10, 1672. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. Ser. A 2021, 76, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, C.; Song, J.; Li, J. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Mild Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis. Front. Nutr. 2021, 8, 757673. [Google Scholar] [CrossRef]
- Bermingham, K.M.; Linenberg, I.; Hall, W.L.; Kadé, K.; Franks, P.W.; Davies, R.; Wolf, J.; Hadjigeorgiou, G.; Asnicar, F.; Segata, N.; et al. Menopause is associated with postprandial metabolism, metabolic health and lifestyle: The ZOE PREDICT study. EBioMedicine 2022, 85, 104303. [Google Scholar] [CrossRef]
| Condition | Dysbiosis | Strains Increased | Strains Decreased | References |
|---|---|---|---|---|
| Menstruation | Little is known about the relationship between menstruation and the gut microbiome. | [32] | ||
| Pregnancy | Gut microbial changes mediated by gestational hormonal changes | Actinobacteria, Proteobacteria, and opportunistic pathogens | SCFA producers and in overall species richness | [33,34,35,36] |
| positive immune antibody-associated miscarriage | Unreported | Blautia and Bacteroides | None reported | [37] |
| Perimenopause | Present | Enterobacter | Lactobacillus and Bifidobacteria | [38] |
| Postmenopause | Grows in resemblance to male gut microbiome | Conflicting results | Conflicting results | [38,39,40] |
| Postmenopause-associated bone diseases | decreased bacterial richness and diversity | unclassified Clostridia and methanogenic archaea | PrevotellaStudies report conflicting results on Bacteroides | [41,42,43,44] |
| PCOS | Present with decreased diversity | Bacteroides vulgatus, Firmicutes, Streptococcus, and the ratio of Escherichia/Shigella | Tenericutes ML615J-28, Tenericutes 124-7, Akkermansia, Ruminococcaceae, and Bacteroidetes S24-7 | [25,26,27,28,30,31] |
| Breast cancer | Present with a decrease in diversity | Clostridiales | None reported | [45] |
| Ovarian cancer | Present | Prevotella, yet the effects of chemotherapy have not been accounted for. | None reported | [46] |
| Cervical cancer | Present with changes in diversity | Proteobacteria, Prevotella, Porphyromonas, and Dialister | Bacteroides, Alistipes, and members of the Lachnospiracea | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, R.; Makhlouf, Z.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Gut Microbiome and Female Health. Biology 2022, 11, 1683. https://doi.org/10.3390/biology11111683
Siddiqui R, Makhlouf Z, Alharbi AM, Alfahemi H, Khan NA. The Gut Microbiome and Female Health. Biology. 2022; 11(11):1683. https://doi.org/10.3390/biology11111683
Chicago/Turabian StyleSiddiqui, Ruqaiyyah, Zinb Makhlouf, Ahmad M. Alharbi, Hasan Alfahemi, and Naveed Ahmed Khan. 2022. "The Gut Microbiome and Female Health" Biology 11, no. 11: 1683. https://doi.org/10.3390/biology11111683
APA StyleSiddiqui, R., Makhlouf, Z., Alharbi, A. M., Alfahemi, H., & Khan, N. A. (2022). The Gut Microbiome and Female Health. Biology, 11(11), 1683. https://doi.org/10.3390/biology11111683








