Differential Transcriptional Landscape of Vero Cells During Dengue Virus 2 Infection in the Presence of Sinococuline
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Immunofluorescence Microscopy
2.3. NS1 ELISA
2.4. Sample Preparation and RNA Extraction
2.5. Library Preparation and RNA Sequencing
2.6. Sequencing Analysis
2.7. Gene Expression Analysis
3. Results
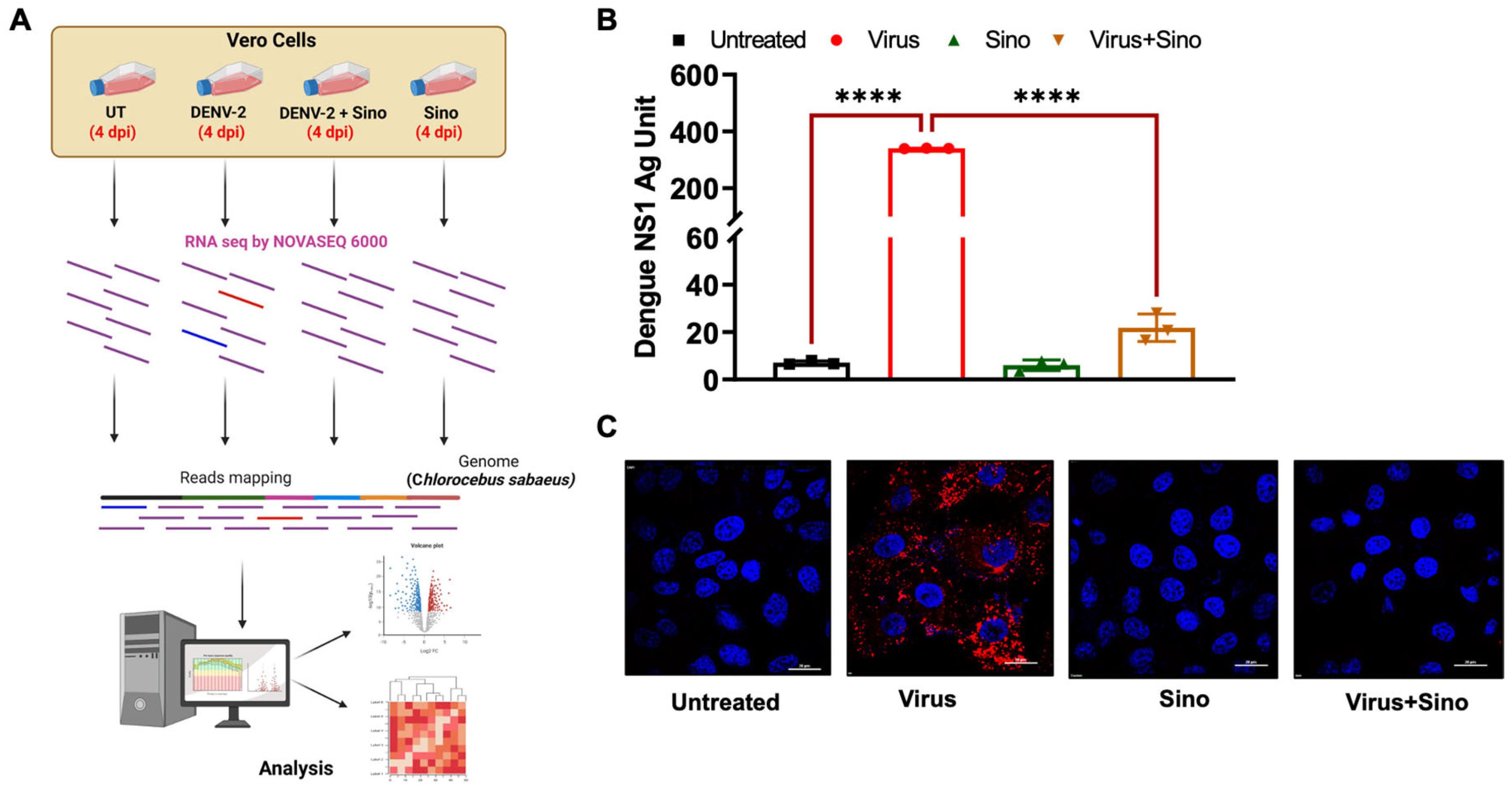
3.1. Infection of Vero Cells with DENV2
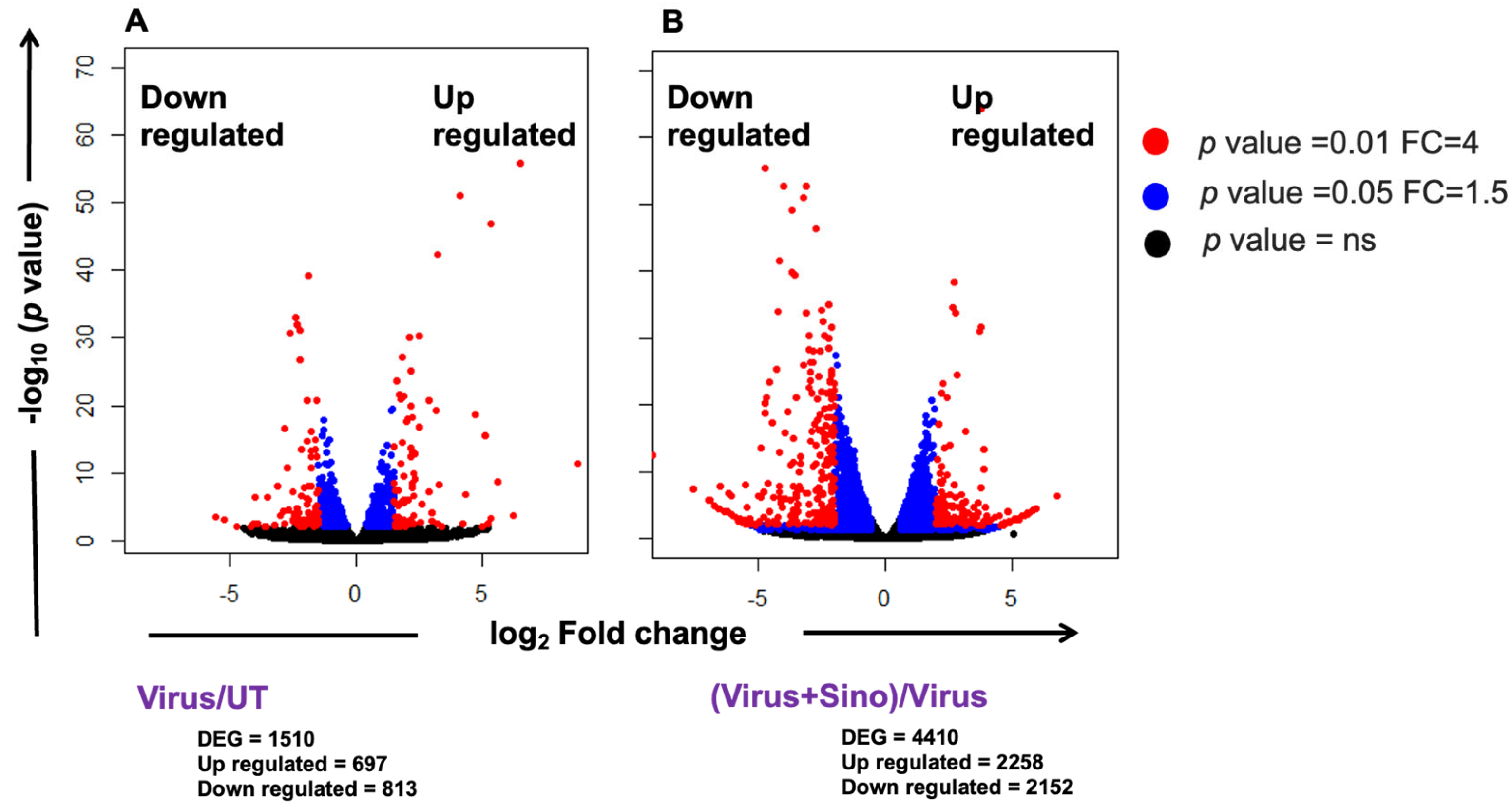
3.2. RNA-Seq Data and DEG Analysis
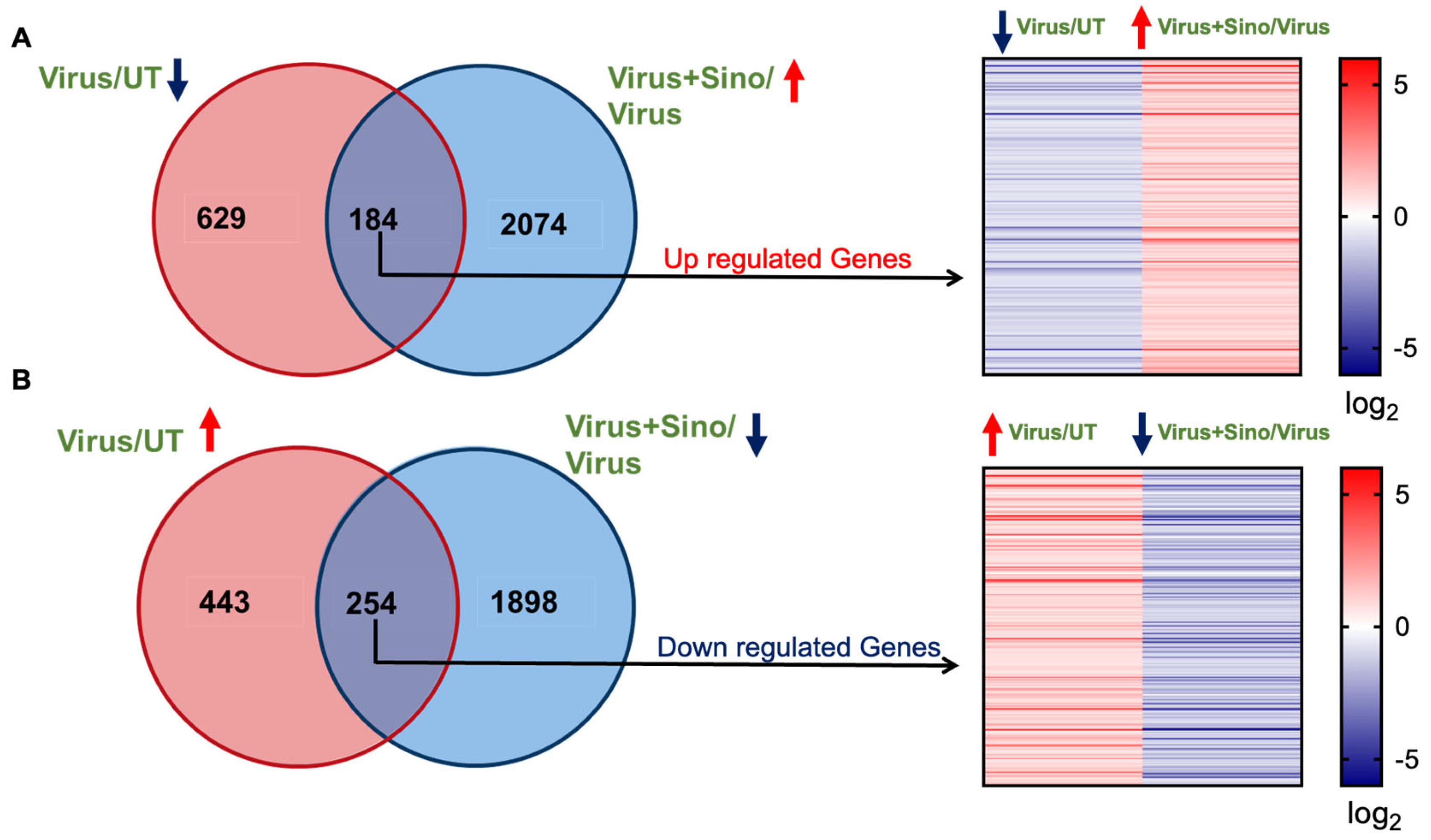
3.3. Comparison Between DENV2-Infected and Sinococuline-Treated Infected Group Transcriptomics
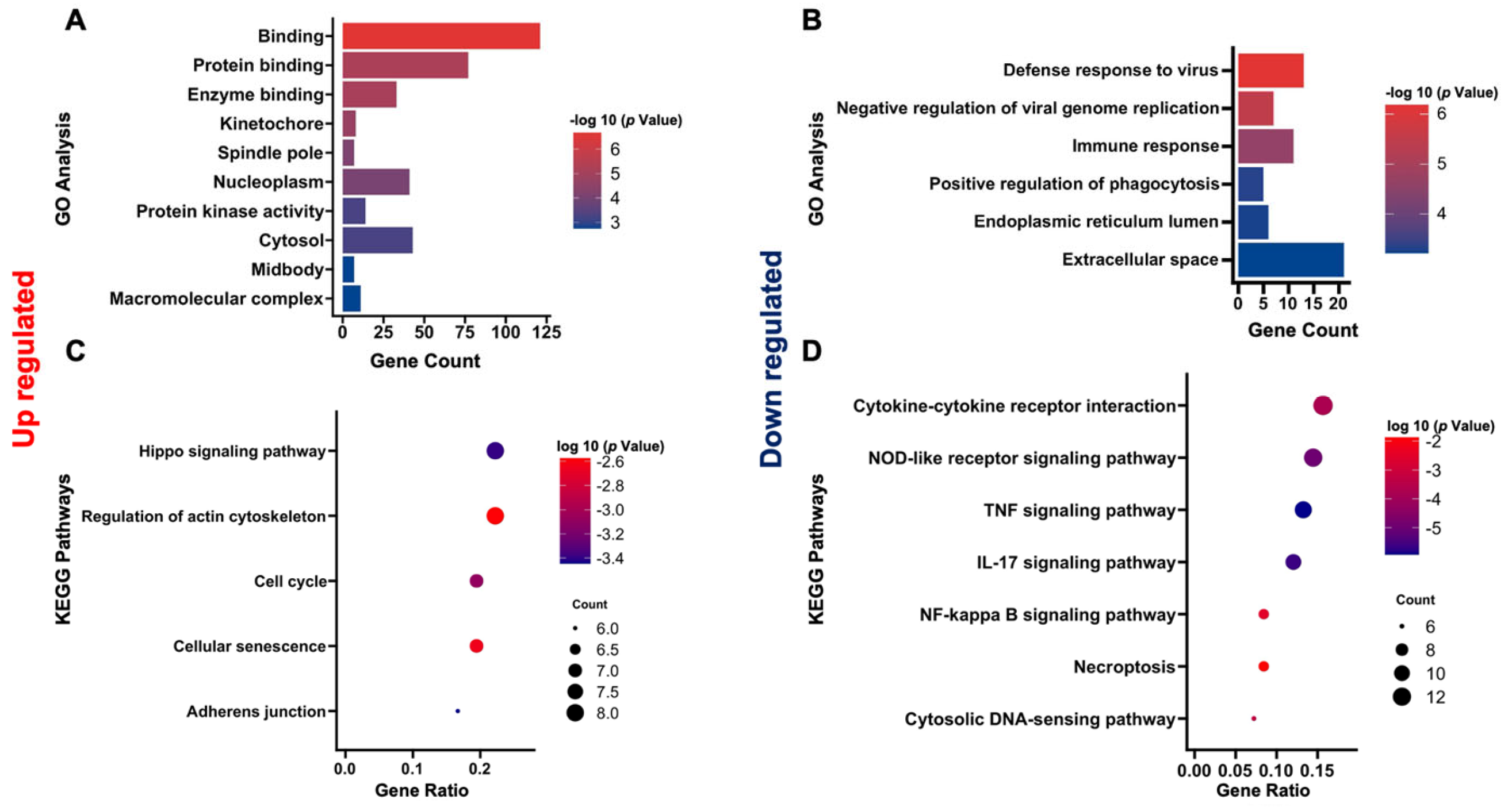
3.4. GO Enrichment Analysis of DEGs
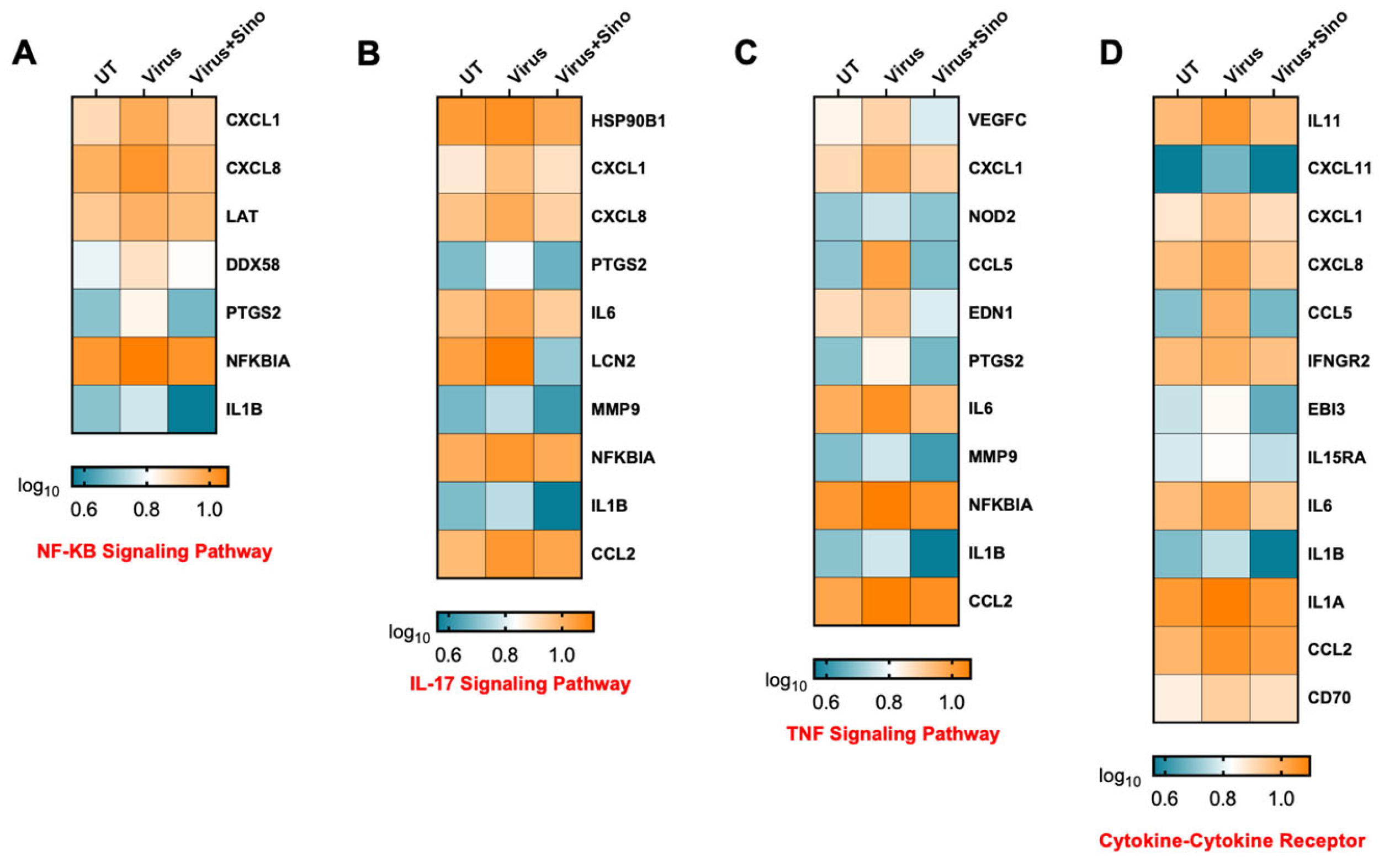
3.5. KEGG Enrichment Analysis of DEGs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castaneda-Orjuela, C.A.; et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Back, A.T.; Lundkvist, A. Dengue viruses—An overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Fonville, J.M.; Gromowski, G.D.; Bustos Arriaga, J.; Green, A.; James, S.L.; Lau, L.; Montoya, M.; Wang, C.; VanBlargan, L.A.; et al. Dengue viruses cluster antigenically but not as discrete serotypes. Science 2015, 349, 1338–1343. [Google Scholar] [CrossRef]
- Sabir, M.J.; Al-Saud, N.B.S.; Hassan, S.M. Dengue and human health: A global scenario of its occurrence, diagnosis and therapeutics. Saudi J. Biol. Sci. 2021, 28, 5074–5080. [Google Scholar] [CrossRef] [PubMed]
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; New Edition; WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2009.
- Jadhav, M.P. High-throughput screening (HTS) for the identification of novel antiviral scaffolds. Clin. Pharmacol. Drug Dev. 2014, 3, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.M.; Lim, S.P.; Yue, K.; Busby, S.A.; Arora, R.; Seh, C.C.; Wright, S.K.; Nutiu, R.; Niyomrattanakit, P.; Wan, K.F.; et al. Identifying initiation and elongation inhibitors of dengue virus RNA polymerase in a high-throughput lead-finding campaign. J. Biomol. Screen. 2015, 20, 153–163. [Google Scholar] [CrossRef]
- Yokokawa, F.; Nilar, S.; Noble, C.G.; Lim, S.P.; Rao, R.; Tania, S.; Wang, G.; Lee, G.; Hunziker, J.; Karuna, R.; et al. Discovery of Potent Non-Nucleoside Inhibitors of Dengue Viral RNA-Dependent RNA Polymerase from a Fragment Hit Using Structure-Based Drug Design. J. Med. Chem. 2016, 59, 3935–3952. [Google Scholar] [CrossRef]
- Anusuya, S.; Velmurugan, D.; Gromiha, M.M. Identification of dengue viral RNA-dependent RNA polymerase inhibitor using computational fragment-based approaches and molecular dynamics study. J. Biomol. Struct. Dyn. 2016, 34, 1512–1532. [Google Scholar] [CrossRef]
- Anusuya, S.; Gromiha, M.M. Quercetin derivatives as non-nucleoside inhibitors for dengue polymerase: Molecular docking, molecular dynamics simulation, and binding free energy calculation. J. Biomol. Struct. Dyn. 2017, 35, 2895–2909. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Tran, C.N.; Phung, L.K.; Duong, K.T.; Huynh Hle, A.; Farrar, J.; Nguyen, Q.T.; Tran, H.T.; Nguyen, C.V.; Merson, L.; et al. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J. Infect. Dis. 2013, 207, 1442–1450. [Google Scholar] [CrossRef]
- Low, J.G.; Sung, C.; Wijaya, L.; Wei, Y.; Rathore, A.P.S.; Watanabe, S.; Tan, B.H.; Toh, L.; Chua, L.T.; Hou, Y.; et al. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): A phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect. Dis. 2014, 14, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Tricou, V.; Minh, N.N.; Van, T.P.; Lee, S.J.; Farrar, J.; Wills, B.; Tran, H.T.; Simmons, C.P. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl. Trop. Dis. 2010, 4, e785. [Google Scholar] [CrossRef]
- Hitotsuyanagi, Y.; Ikuta, H.; Nishimura, K.; Takeya, K.; Itokawa, H. Synthesis of an Antitumour Alkaloid Sinococuline from Sinomenine. J. Chem. Soc. Chem. Commun. 1994, 23, 2707–2708. [Google Scholar] [CrossRef]
- Shukla, R.; Rajpoot, R.K.; Poddar, A.; Ahuja, R.; Beesetti, H.; Shanmugam, R.K.; Chaturvedi, S.; Nayyar, K.; Singh, D.; Singamaneni, V.; et al. Cocculus hirsutus-Derived Phytopharmaceutical Drug Has Potent Anti-dengue Activity. Front. Microbiol. 2021, 12, 746110. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.K.; Wang, X.K.; Che, C.T. Cytotoxic effects of sinococuline. Cancer Lett. 1996, 99, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Tsuruoka, S.; Takeya, K.; Mori, N.; Sonobe, T.; Kosemura, S.; Hamanaka, T. An antitumor morphinane alkaloid, sinococuline, from Cocculus trilobus. Chem. Pharm. Bull. 1987, 35, 1660–1662. [Google Scholar] [CrossRef]
- Kraus, A.A.; Messer, W.; Haymore, L.B.; de Silva, A.M. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J. Clin. Microbiol. 2007, 45, 3777–3780. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.T.; Lai, M.M.C.; Yu, C.Y. How Dengue Virus Circumvents Innate Immunity. Front. Immunol. 2018, 9, 2860. [Google Scholar] [CrossRef]
- Greenbaum, D.; Colangelo, C.; Williams, K.; Gerstein, M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003, 4, 117. [Google Scholar] [CrossRef]
- Marcotte, E.M.; Pellegrini, M.; Thompson, M.J.; Yeates, T.O.; Eisenberg, D. A combined algorithm for genome-wide prediction of protein function. Nature 1999, 402, 83–86. [Google Scholar] [CrossRef]
- Morin, R.; Bainbridge, M.; Fejes, A.; Hirst, M.; Krzywinski, M.; Pugh, T.; McDonald, H.; Varhol, R.; Jones, S.; Marra, M. Profiling the HeLa S3 transcriptome using randomly primed cDNA and massively parallel short-read sequencing. Biotechniques 2008, 45, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.; Cole, C.; Volden, R.; Vollmers, C. Realizing the potential of full-length transcriptome sequencing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190097. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Raut, R.; Tyagi, P.; Pareek, P.K.; Barman, T.K.; Singhal, S.; Shirumalla, R.K.; Kanoje, V.; Subbarayan, R.; Rajerethinam, R.; et al. Cissampelos pareira Linn: Natural Source of Potent Antiviral Activity against All Four Dengue Virus Serotypes. PLoS Negl. Trop. Dis. 2015, 9, e0004255. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Li, J.; Miao, B.; Wang, S.; Dong, W.; Xu, H.; Si, C.; Wang, W.; Duan, S.; Lou, J.; Bao, Z.; et al. Hiplot: A comprehensive and easy-to-use web service for boosting publication-ready biomedical data visualization. Brief. Bioinform. 2022, 23, bbac261. [Google Scholar] [CrossRef]
- Shukla, R.; Ahuja, R.; Beesetti, H.; Garg, A.; Aggarwal, C.; Chaturvedi, S.; Nayyar, K.; Arora, U.; Lal, A.A.; Khanna, N. Sinococuline, a bioactive compound of Cocculus hirsutus has potent anti-dengue activity. Sci. Rep. 2023, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Khetarpal, N.; Khanna, I. Dengue Fever: Causes, Complications, and Vaccine Strategies. J. Immunol. Res. 2016, 2016, 6803098. [Google Scholar] [CrossRef]
- Green, A.M.; Beatty, P.R.; Hadjilaou, A.; Harris, E. Innate immunity to dengue virus infection and subversion of antiviral responses. J. Mol. Biol. 2014, 426, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Li, G.; Shen, M.; Yu, Z.; Ge, W.; Lao, Z.; Fan, Y.; Chen, K.; Ding, Z.; Wang, W.; et al. DENV NS1 and MMP-9 cooperate to induce vascular leakage by altering endothelial cell adhesion and tight junction. PLoS Pathog. 2021, 17, e1008603. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Chan, K.W.; Wang, J.; Rivino, L.; Lok, S.M.; Vasudevan, S.G. Dengue Virus Infection with Highly Neutralizing Levels of Cross-Reactive Antibodies Causes Acute Lethal Small Intestinal Pathology without a High Level of Viremia in Mice. J. Virol. 2015, 89, 5847–5861. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Pandey, N.; Garg, R.K.; Kumar, R. IL-17 level in patients with Dengue virus infection & its association with severity of illness. J. Clin. Immunol. 2013, 33, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.T.; Chen, B.H.; Ma, S.H.; Liu, C.I.; Tsai, H.P.; Wu, H.C.; Jiang, S.Y.; Yang, K.D.; Shaio, M.F. Potential dengue virus-triggered apoptotic pathway in human neuroblastoma cells: Arachidonic acid, superoxide anion, and NF-kappaB are sequentially involved. J. Virol. 2000, 74, 8680–8691. [Google Scholar] [CrossRef] [PubMed]
- Marianneau, P.; Cardona, A.; Edelman, L.; Deubel, V.; Despres, P. Dengue virus replication in human hepatoma cells activates NF-kappaB which in turn induces apoptotic cell death. J. Virol. 1997, 71, 3244–3249. [Google Scholar] [CrossRef] [PubMed]
- Martina, B.E.; Koraka, P.; Osterhaus, A.D. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef]
- Fernandez-Mestre, M.T.; Gendzekhadze, K.; Rivas-Vetencourt, P.; Layrisse, Z. TNF-alpha-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens 2004, 64, 469–472. [Google Scholar] [CrossRef]
- Meena, A.A.; Murugesan, A.; Sopnajothi, S.; Yong, Y.K.; Ganesh, P.S.; Vimali, I.J.; Vignesh, R.; Elanchezhiyan, M.; Kannan, M.; Dash, A.P.; et al. Increase of Plasma TNF-alpha Is Associated with Decreased Levels of Blood Platelets in Clinical Dengue Infection. Viral Immunol. 2020, 33, 54–60. [Google Scholar] [CrossRef]
- Yen, Y.T.; Chen, H.C.; Lin, Y.D.; Shieh, C.C.; Wu-Hsieh, B.A. Enhancement by tumor necrosis factor alpha of dengue virus-induced endothelial cell production of reactive nitrogen and oxygen species is key to hemorrhage development. J. Virol. 2008, 82, 12312–12324. [Google Scholar] [CrossRef]
- Magoro, T.; Dandekar, A.; Jennelle, L.T.; Bajaj, R.; Lipkowitz, G.; Angelucci, A.R.; Bessong, P.O.; Hahn, Y.S. IL-1beta/TNF-alpha/IL-6 inflammatory cytokines promote STAT1-dependent induction of CH25H in Zika virus-infected human macrophages. J. Biol. Chem. 2019, 294, 14591–14602. [Google Scholar] [CrossRef] [PubMed]
- Zidovec-Lepej, S.; Vilibic-Cavlek, T.; Barbic, L.; Ilic, M.; Savic, V.; Tabain, I.; Ferenc, T.; Grgic, I.; Gorenec, L.; Bogdanic, M.; et al. Antiviral Cytokine Response in Neuroinvasive and Non-Neuroinvasive West Nile Virus Infection. Viruses 2021, 13, 342. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, P.; Du, H.; Wang, T.; Li, M.; Hu, H.; Ye, C.; Zheng, X.; Zhang, Y.; Lei, Y.; et al. The Comparison of Inflammatory Cytokines (IL-6 and IL-18) and Immune Cells in Japanese Encephalitis Patients with Different Progression. Front. Cell. Infect. Microbiol. 2022, 12, 826603. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, A.; Shukla, R.; Kumar, A.; Aggarwal, C.; Mukhopadhyay, A.; Khanna, N. Differential Transcriptional Landscape of Vero Cells During Dengue Virus 2 Infection in the Presence of Sinococuline. Microorganisms 2024, 12, 2529. https://doi.org/10.3390/microorganisms12122529
Garg A, Shukla R, Kumar A, Aggarwal C, Mukhopadhyay A, Khanna N. Differential Transcriptional Landscape of Vero Cells During Dengue Virus 2 Infection in the Presence of Sinococuline. Microorganisms. 2024; 12(12):2529. https://doi.org/10.3390/microorganisms12122529
Chicago/Turabian StyleGarg, Amit, Rahul Shukla, Amit Kumar, Charu Aggarwal, Arnab Mukhopadhyay, and Navin Khanna. 2024. "Differential Transcriptional Landscape of Vero Cells During Dengue Virus 2 Infection in the Presence of Sinococuline" Microorganisms 12, no. 12: 2529. https://doi.org/10.3390/microorganisms12122529
APA StyleGarg, A., Shukla, R., Kumar, A., Aggarwal, C., Mukhopadhyay, A., & Khanna, N. (2024). Differential Transcriptional Landscape of Vero Cells During Dengue Virus 2 Infection in the Presence of Sinococuline. Microorganisms, 12(12), 2529. https://doi.org/10.3390/microorganisms12122529





