Antibiotic Resistance in Vibrio Bacteria Associated with Red Spotting Disease in Sea Urchin Tripneustes gratilla (Echinodermata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Red-Spotting-Infected Sea Urchins
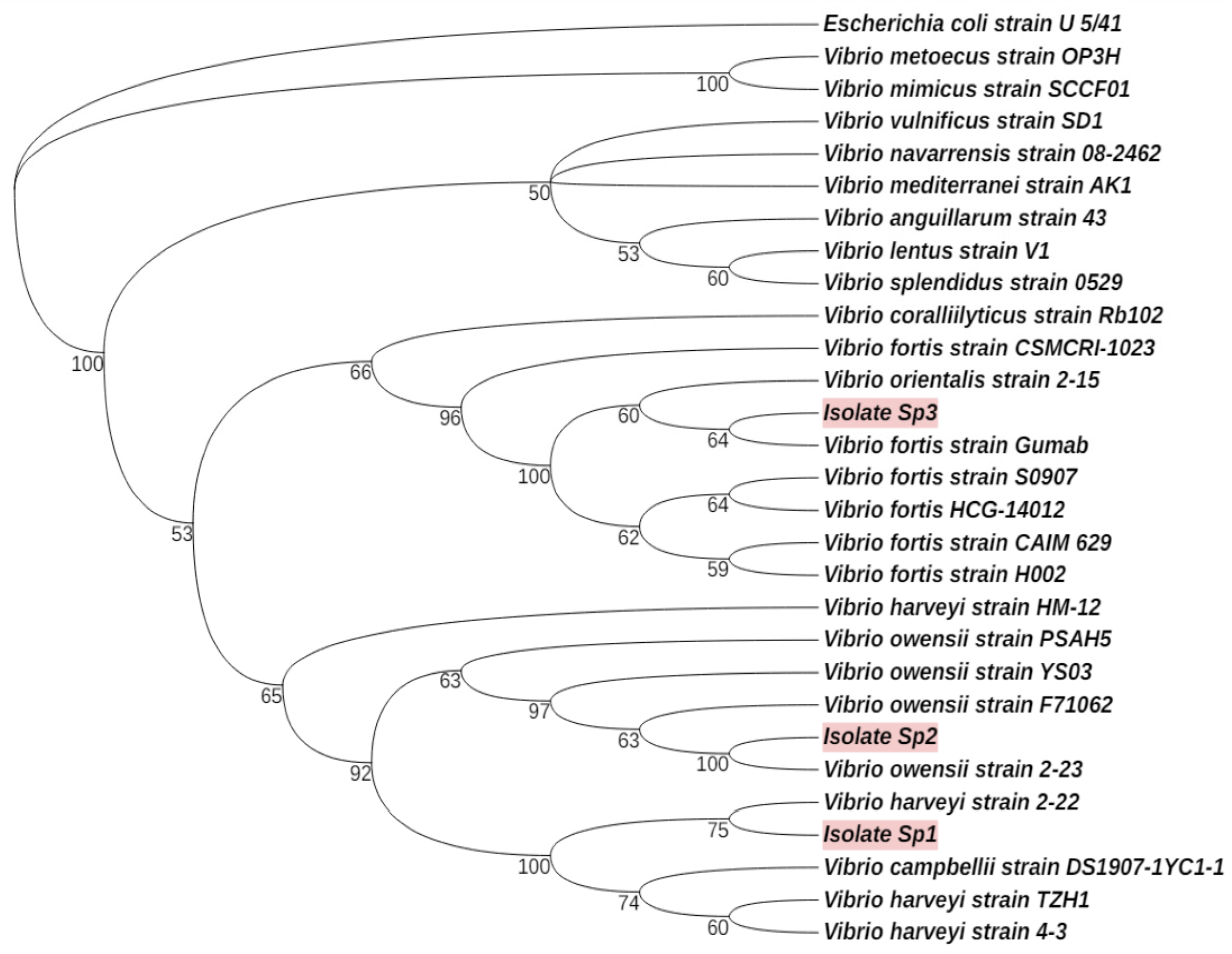
2.2. Bacterial Isolation and Identification
2.3. In Vitro Antibiotics Susceptibility Assay
2.4. In Silico Survey of Antibiotics Resistance in Vibrio spp.
3. Results
3.1. Potential Vibrio Pathogens Associated with Red Spotting Disease in T. gratilla
3.2. In Vitro Antibiotic Resistance Assay
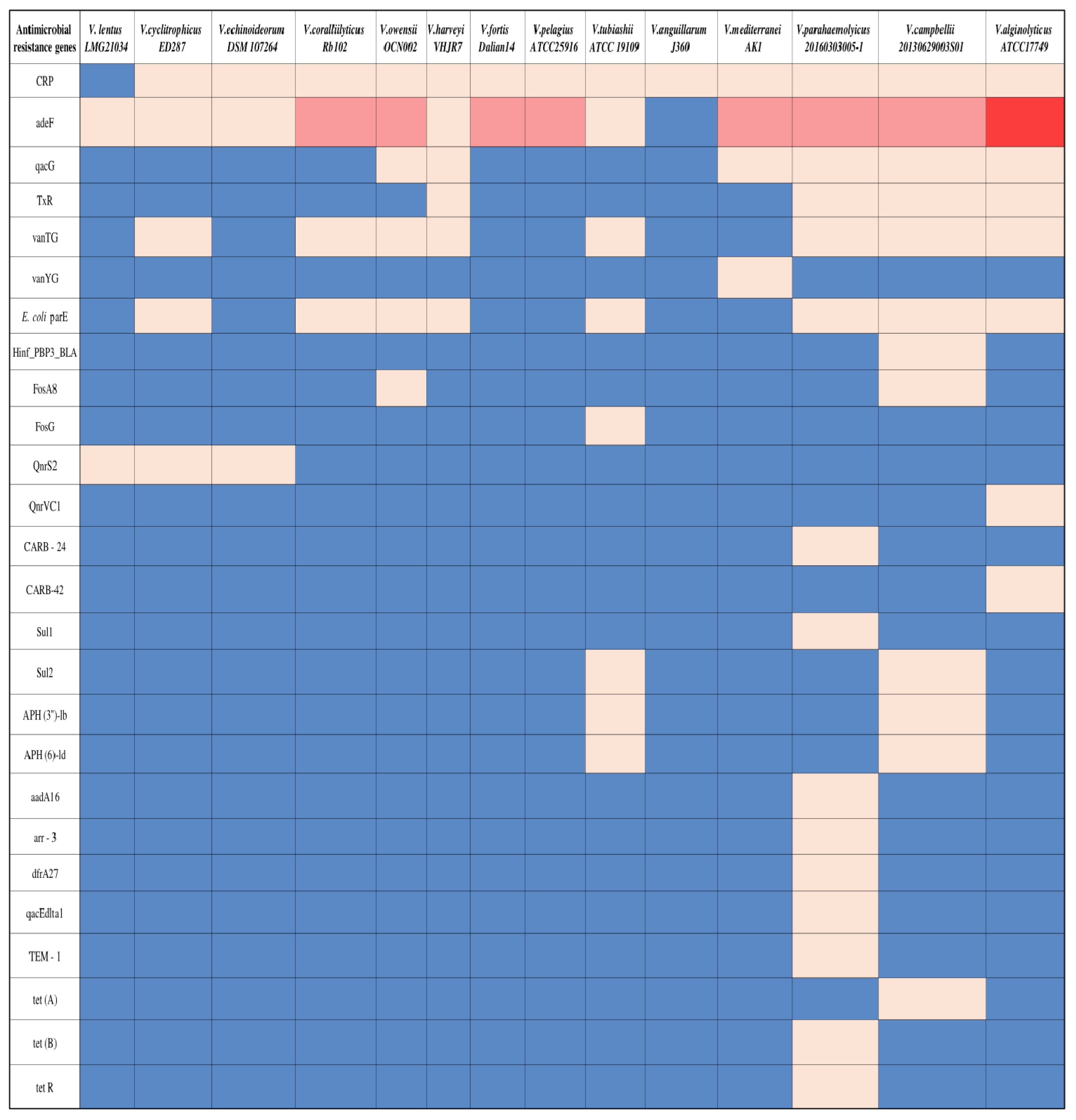
3.3. Antibiotic-Resistant Genes of Vibrio spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, R.; Dang, H.; Huang, Y.; Quan, Z.; Jiang, H.; Zhang, W.; Ding, J. Vibrio coralliilyticus as an agent of red spotting disease in the sea urchin Strongylocentrotus intermedius. Aquac. Rep. 2020, 16, 100244. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Li, X.; Qu, J.Y.; Zhao, X.M. Pathogenic mechanism of causative Vibrio found in “red spotting” diseased sea urchin Strongylocentrotus intermedius. J. Dalian Fish. Univ. 2005, 20, 11–15. (In Chinese) [Google Scholar]
- Wang, L.; He, B.; Chang, Y.; Ding, J. Characterization of the bacterial community associated with red spotting disease of the echinoid Strongylocentroyus intermedius. Aquaculture 2020, 529, 735606. [Google Scholar] [CrossRef]
- Zhang, X.H.; He, X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, W.; Yang, X.; Zhou, S.; Hu, L.; Li, X.; Qi, X.; Su, H.; Xie, L. A nonluminescent and highly virulent Vibrio harveyi strain is associated with “bacterial white tail disease” of Litopenaeus vannamei shrimp. PLoS ONE 2012, 7, e29961. [Google Scholar] [CrossRef]
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef]
- Stalder, T.; Barraud, O.; Casellas, M.; Dagot, C.; Ploy, M.C. Integron involvement in environmental spread of antibiotic resistance. Front. Microbiol. 2012, 3, 119. [Google Scholar] [CrossRef]
- Kumarage, P.M.; De Silva, L.A.D.S.; Heo, G.J. Aquatic environments: A potential source of antimicrobial-resistant Vibrio spp. J. Appl. Microbiol. 2022, 133, 2267–2279. [Google Scholar] [CrossRef]
- Kalantzi, I.; Rico, A.; Mylona, K.; Pergantis, S.A.; Tsapakis, M. Fish farming, metals and antibiotics in the eastern Mediterranean Sea: Is there a threat to sediment wildlife? Sci. Total Environ. 2021, 764, 142843. [Google Scholar] [CrossRef]
- Turk, J. Meeting projected food demands by 2050: Understanding and enhancing the role of grazing ruminants. J. Anim. Sci. 2016, 94 (Suppl. S6), 53–62. [Google Scholar] [CrossRef]
- Pepi, M.; Focardi, S. Antibiotic-resistant bacteria in aquaculture and climate change: A challenge for health in the Mediterranean area. Int. J. Environ. Res. Public Health 2021, 18, 5723. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Heuer, O.E.; Kruse, H.; Grave, K.; Collignon, P.; Karunasagar, I.; Angulo, F.J. Human health consequences of use of antimicrobial agents in aquaculture. Clin. Infect. Dis. 2009, 49, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Rico, A.; Phu, T.M.; Satapornvanit, K.; Min, J.; Shahabuddin, A.M.; Henriksson, P.J.; Murray, F.J.; Little, D.C.; Dalsgaard, A.; Brink, P.J.V.D. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 2013, 412, 231–243. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Hossain, A.; Habibullah-Al-Mamun, M.; Nagano, I.; Masunaga, S.; Kitazawa, D.; Matsuda, H. Antibiotics, antibiotic-resistant bacteria, and resistance genes in aquaculture: Risks, current concern, and future thinking. Environ. Sci. Pollut. Res. 2022, 29, 11054–11075. [Google Scholar] [CrossRef]
- Dewi, R.R.; Hassan, L.; Daud, H.M.; Matori, M.F.; Nordin, F.; Ahmad, N.I.; Zakaria, Z. Prevalence and antimicrobial resistance of Escherichia coli, Salmonella and Vibrio derived from farm-raised Red Hybrid Tilapia (Oreochromis spp.) and Asian Sea Bass (Lates calcarifer, Bloch 1970) on the west coast of Peninsular Malaysia. Antibiotics 2022, 11, 136. [Google Scholar] [CrossRef]
- Hirshfeld, B.; Lavelle, K.; Lee, K.Y.; Atwill, E.R.; Kiang, D.; Bolkenov, B.; Gaa, M.; Li, Z.; Yu, A.; Li, X.; et al. Prevalence and antimicrobial resistance profiles of Vibrio spp. and Enterococcus spp. in retail shrimp in Northern California. Front. Microbiol. 2023, 14, 1192769. [Google Scholar] [CrossRef]
- Preena, P.G.; Swaminathan, T.R.; Kumar, V.J.R.; Singh, I.S.B. Antimicrobial resistance in aquaculture: A crisis for concern. Biologia 2020, 75, 1497–1517. [Google Scholar] [CrossRef]
- Vaiyapuri, M.; Pailla, S.; Rao Badireddy, M.; Pillai, D.; Chandragiri Nagarajarao, R.; Prasad Mothadaka, M. Antimicrobial resistance in Vibrios of shrimp aquaculture: Incidence, identification schemes, drivers and mitigation measures. Aquac. Res. 2021, 52, 2923–2941. [Google Scholar] [CrossRef]
- Dang, H.; Zhang, X.; Song, L.; Chang, Y.; Yang, G. Molecular determination of oxytetracycline-resistant bacteria and their resistance genes from mariculture environments of China. J. Appl. Microbiol. 2007, 103, 2580–2592. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, A.H.; Tomova, A.; López, A.; Maldonado, M.A.; Henríquez, L.A.; Ivanova, L. Salmon aquaculture and antimicrobial resistance in the marine environment. PLoS ONE 2012, 7, e42724. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.Q.; Cabello, F.C.; L’Abée-Lund, T.M.; Tomova, A.; Godfrey, H.P.; Buschmann, A.H.; Sørum, H. Antimicrobial resistance and antimicrobial resistance genes in marine bacteria from salmon aquaculture and non-aquaculture sites. Environ. Microbiol. 2014, 16, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Akinbowale, O.L.; Peng, H.; Barton, M.D. Antimicrobial resistance in bacteria isolated from aquaculture sources in Australia. J. Appl. Microbiol. 2006, 100, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Tendencia, E.A.; de la Peña, L.D. Antibiotic resistance of bacteria from shrimp ponds. Aquaculture 2001, 195, 193–204. [Google Scholar] [CrossRef]
- Sony, M.; Sumithra, T.G.; Anusree, V.N.; Amala, P.V.; Reshma, K.J.; Alex, S.; Sanil, N. Antimicrobial resistance and virulence characteristics of Vibrio vulnificus, Vibrio parahaemolyticus and Vibrio harveyi from natural disease outbreaks of marine/estuarine fishes. Aquaculture 2021, 539, 736608. [Google Scholar] [CrossRef]
- Dang, H.; Zhao, J.; Song, L.; Chen, M.; Chang, Y. Molecular characterizations of chloramphenicol-and oxytetracycline-resistant bacteria and resistance genes in mariculture waters of China. Mar. Pollut. Bull. 2009, 58, 987–994. [Google Scholar] [CrossRef]
- Ferrini, A.M.; Mannoni, V.; Suffredini, E.; Cozzi, L.; Croci, L. Evaluation of antibacterial resistance in Vibrio strains isolated from imported seafood and Italian aquaculture settings. Food Anal. Methods 2008, 1, 164–170. [Google Scholar] [CrossRef]
- Ottaviani, D.; Bacchiocchi, I.; Masini, L.; Leoni, F.; Carraturo, A.; Giammarioli, M.; Sbaraglia, G. Antimicrobial susceptibility of potentially pathogenic halophilic Vibrios isolated from seafood. Int. J. Antimicrob. Agents 2001, 18, 135–140. [Google Scholar] [CrossRef]
- Fukuda, A.; Tsunashima, R.; Usui, M. Antimicrobial Resistant Bacteria Monitoring in Raw Seafood Retailed: A Pilot Study Focused on Vibrio and Aeromonas. Food Saf. 2023, 11, 65–77. [Google Scholar] [CrossRef]
- Narayanan, S.V.; Joseph, T.C.; Peeralil, S.; Mothadaka, M.P.; Lalitha, K.V. Prevalence, virulence characterization, AMR pattern and genetic relatedness of Vibrio parahaemolyticus isolates from retail seafood of Kerala, India. Front. Microbiol. 2020, 11, 525327. [Google Scholar] [CrossRef] [PubMed]
- Neetoo, H.; Reega, K.; Manoga, Z.S.; Nazurally, N.; Bhoyroo, V.; Allam, M.; Jaufeerally-Fakim, Y.; Ghoorah, A.W.; Jaumdally, W.; Hossen, A.M.; et al. Prevalence, genomic characterization, and risk assessment of human pathogenic Vibrio species in seafood. J. Food Prot. 2022, 85, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liu, L.; Li, C.; Zou, Y.; Yan, H.; Zhan, Y.; Chang, Y. Isolation and characterization of four pathogenic strains of Vibrio spp. from farmed sea urchins, Strongylocentrotus intermedius, in China. Microb. Pathog. 2022, 169, 105651. [Google Scholar] [CrossRef] [PubMed]
- Hira, J.; Stensvåg, K. Evidence for association of Vibrio echinoideorum with tissue necrosis on test of the green sea urchin Strongylocentrotus droebachiensis. Sci. Rep. 2022, 12, 4859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Liu, Y.P.; Chang, Y.Q. Comparative analysis of bacterial community composition in coelomic fluid between sick and healthy Strongylocentrotus intermedius by PCR-DGGE. J. Agric. Sci. Technol. 2011, 13, 111–116. [Google Scholar]
- Gilles, K.W.; Pearse, J.S. Disease in sea urchins Strogylocentrotus purpuratus: Experimental infection and bacterial virulence. Dis. Aquat. Org. 1986, 1, 105–114. [Google Scholar] [CrossRef]
- Grech, D.; Mandas, D.; Farina, S.; Guala, I.; Brundu, R.; Cristo, B.; Panzalis, P.A.; Salati, F.; Carella, F. Vibrio splendidus clade associated with a disease affecting Paracentrotus lividus (Lamarck, 1816) in Sardinia (Western Mediterranean). J. Invertebr. Pathol. 2022, 192, 107783. [Google Scholar] [CrossRef]
- Sweet, M.; Bulling, M.; Williamson, J.E. New disease outbreak affects two dominant sea urchin species associated with Australian temperate reefs. Mar. Ecol. Prog. Ser. 2016, 551, 171–183. [Google Scholar] [CrossRef]
- Bauer, J.C.; Young, C.M. Epidermal lesions and mortality caused by vibriosis in deep-sea Bahamian echinoids: A laboratory study. Dis. Aquat. Org. 2000, 39, 193–199. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, N.; Li, Q.; Ding, J.; Zhan, Y.; Chang, Y. Isolation and characterization of bacteria associated with a syndrome disease of sea urchin Strongylocentrotus intermedius in North China. Aquac. Res. 2013, 44, 691–700. [Google Scholar] [CrossRef]
- Sun, J.; Liu, L.; Song, J.; Zhan, Y.; Zhang, W.; Wang, B.; Zhang, B.; Chang, Y. Characterization of two strains of Vibrio sp. from cultured sea urchin, Strongylocentrotus intermedius, in China. Aquac. Rep. 2021, 20, 100667. [Google Scholar] [CrossRef]
- Shpigel, M.; Shauli, L.; Odintsov, V.; Ben-Ezra, D.; Neori, A.; Guttman, L. The sea urchin, Paracentrotus lividus, in an Integrated Multi-Trophic Aquaculture (IMTA) system with fish (Sparus aurata) and seaweed (Ulva lactuca): Nitrogen partitioning and proportional configurations. Aquaculture 2018, 490, 260–269. [Google Scholar] [CrossRef]
- Neori, A.; Shpigel, M.; Ben-Ezra, D. A sustainable integrated system for culture of fish, seaweed and abalone. Aquaculture 2000, 186, 279–291. [Google Scholar] [CrossRef]
- Biemer, J.J. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Ann. Clin. Lab. Sci. 1973, 3, 135–140. [Google Scholar] [PubMed]
- Schlossberg, D.L.; Samuel, R. Antibiotics Manual: A Guide to Commonly Used Antimicrobials, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Etebu, E.; Arikekpar, I. Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives. Int. J. Appl. Microbiol. Biotechnol. Res. 2016, 4, 90–101. [Google Scholar]
- M 100-S22; Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2012.
- Aljahani, A.H.; Alarjani, K.M.; Hassan, Z.K.; Elkhadragy, M.F.; Ismail, E.A.; Al-Masoud, A.H.; Yehia, H.M. Molecular detection of methicillin heat-resistant Staphylococcus aureus strains in pasteurized camel milk in Saudi Arabia. Biosci. Rep. 2020, 40, BSR20193470. [Google Scholar] [CrossRef]
- Ding, J.; Dou, Y.; Wang, Y.; Chang, Y. Draft genome sequence of Vibrio fortis Dalian14 isolated from diseased sea urchin (Strongylocentrotus intermedius). Genome Announc. 2014, 2, 10–1128. [Google Scholar] [CrossRef]
- Hira, J.; Bentdal, S.; Devold, H.; Stensvåg, K.; Landfald, B. Vibrio echinoideorum sp. nov., isolated from an epidermal lesion on the test of a green sea urchin (Strongylocentrotus droebachiensis). Int. J. Syst. Evol. Microbiol. 2019, 69, 2277–2282. [Google Scholar] [CrossRef]
- Wang, Y.N.; Chang, Y.Q.; Lawrence, J.M. Disease in sea urchins. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 179–186. [Google Scholar] [CrossRef]
- Schuh, N.W.; Carrier, T.J.; Schrankel, C.S.; Reitzel, A.M.; Heyland, A.; Rast, J.P. Bacterial exposure mediates developmental plasticity and resistance to lethal Vibrio lentus infection in purple sea urchin (Strongylocentrotus purpuratus) larvae. Front. Immunol. 2020, 10, 491364. [Google Scholar] [CrossRef]
- Ransangan, J.; Lal, T.M.; Al-Harbi, A.H. Characterization and experimental infection of Vibrio harveyi isolated from diseased Asian seabass (Lates calcarifer). Malays. J. Microbiol. 2012, 8, 104–115. [Google Scholar] [CrossRef]
- Ushijima, B.; Smith, A.; Aeby, G.S.; Callahan, S.M. Vibrio owensii induces the tissue loss disease Montipora white syndrome in the Hawaiian reef coral Montipora capitata. PLoS ONE 2012, 7, e46717. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, N.M. Isolation and characterization of Vibrio alginolyticus strain HAT3 causing skin ulceration disease in cultured sea cucumber Holothuria atra (Jaeger, 1833). Egypt. J. Aquat. Res. 2022, 48, 75–81. [Google Scholar] [CrossRef]
- Tarazona, E.; Lucena, T.; Arahal, D.R.; Macián, M.C.; Ruvira, M.A.; Pujalte, M.J. Multilocus sequence analysis of putative Vibrio mediterranei strains and description of Vibrio thalassae sp. nov. Syst. Appl. Microbiol. 2014, 37, 320–328. [Google Scholar] [CrossRef]
- Yang, F.; Xu, L.; Huang, W.; Li, F. Highly lethal Vibrio parahaemolyticus strains cause acute mortality in Penaeus vannamei post-larvae. Aquaculture 2022, 548, 737605. [Google Scholar] [CrossRef]
- Dong, X.; Wang, H.; Zou, P.; Chen, J.; Liu, Z.; Wang, X.; Huang, J. Complete genome sequence of Vibrio campbellii strain 20130629003S01 isolated from shrimp with acute hepatopancreatic necrosis disease. Gut Pathog. 2017, 9, 31. [Google Scholar] [CrossRef]
- Vasquez, I.; Cao, T.; Chakraborty, S.; Gnanagobal, H.; O’Brien, N.; Monk, J.; Boyce, D.; Westcott, J.D.; Santander, J. Comparative genomics analysis of vibrio anguillarum isolated from lumpfish (Cyclopterus lumpus) in newfoundland reveal novel chromosomal organizations. Microorganisms 2020, 8, 1666. [Google Scholar] [CrossRef]
- Richards, G.P.; Watson, M.A.; Needleman, D.S.; Church, K.M.; Häse, C.C. Mortalities of Eastern and Pacific oyster larvae caused by the pathogens Vibrio coralliilyticus and Vibrio tubiashii. Appl. Environ. Microbiol. 2015, 81, 292–297. [Google Scholar] [CrossRef]
- Villamil, L.; Figueras, A.; Toranzo, A.E.; Planas, M.; Novoa, B. Isolation of a highly pathogenic Vibrio pelagius strain associated with mass mortalities of turbot, Scophthalmus maximus (L.), larvae. J. Fish Dis. 2003, 26, 293–303. [Google Scholar] [CrossRef]
- Poey, M.E.; Azpiroz, M.F.; Laviña, M. On sulfonamide resistance, sul genes, class 1 integrons and their horizontal transfer in Escherichia coli. Microb. Pathog. 2019, 135, 103611. [Google Scholar] [CrossRef]
- Wang, Y.; Batra, A.; Schulenburg, H.; Dagan, T. Gene sharing among plasmids and chromosomes reveals barriers for antibiotic resistance gene transfer. Philos. Trans. R. Soc. B 2022, 377, 20200467. [Google Scholar] [CrossRef]
- Agboola, T.D.; Nmema, E.E.; Odetoyin, B.W. Distribution and antibiogram of Vibrio species from hospital wastewater in Southwest Nigeria. Pan Afr. Med. J. 2023, 45, 80. [Google Scholar] [CrossRef]
- Isnansetyo, A.; Istiqomah, I.; Anshary, H.; Sriwulan, S.; Yudiati, E.; Subagiyo, S.; Arif, A.; Kartikasari, D.W. Identification and antibiotic-resistant properties of Vibrio owensii and V. alginolyticus isolated from the Spermonde Islands, Indonesia. Biodiversitas J. Biol. Divers. 2022, 23. [Google Scholar] [CrossRef]
- Mohamad, N.; Amal, M.N.A.; Saad, M.Z.; Yasin, I.S.M.; Zulkiply, N.A.; Mustafa, M.; Nasruddin, N.S. Virulence-associated genes and antibiotic resistance patterns of Vibrio spp. isolated from cultured marine fishes in Malaysia. BMC Vet. Res. 2019, 15, 176. [Google Scholar] [CrossRef]
- Adeyemi, A.; Enyinnia, V.; Nwanze, R.; Smith, S.; Omonigbehin, E. Antimicrobial susceptibility of potentially pathogenic halophilic Vibrio species isolated from seafoods in Lagos, Nigeria. Afr. J. Biotechnol. 2008, 7, 3791–3794. [Google Scholar]
- Kang, H.; Yu, Y.; Liao, M.; Wang, Y.; Yang, G.; Zhang, Z.; Li, B.; Rong, X.; Wang, C. Physiology, metabolism, antibiotic resistance, and genetic diversity of Harveyi clade bacteria isolated from coastal mariculture system in China in the last two decades. Front. Mar. Sci. 2022, 9, 932255. [Google Scholar] [CrossRef]
- Badhai, J.; Kumari, P.; Krishnan, P.; Ramamurthy, T.; Das, S.K. Presence of SXT integrating conjugative element in marine bacteria isolated from the mucus of the coral Fungia echinata from Andaman Sea. FEMS Microbiol. Lett. 2013, 338, 118–123. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, X.; Wang, Z.; Chen, H.; Luo, Y.; Wu, B.; Qi, S.; Lin, M.; Tian, J.; Qiao, Y.; et al. Seasonal variation, virulence gene and antibiotic resistance of Vibrio in a semi-enclosed bay with mariculture (Dongshan Bay, Southern China). Mar. Pollut. Bull. 2022, 184, 114112. [Google Scholar] [CrossRef]
- You, K.G.; Bong, C.W.; Lee, C.W. Antibiotic resistance and plasmid profiling of Vibrio spp. in tropical waters of Peninsular Malaysia. Environ. Monit. Assess. 2016, 188, 171. [Google Scholar] [CrossRef]
- Thillaichidambaram, M.; Narayanan, K.; Selvaraj, S.; Sundararaju, S.; Muthiah, R.C.; Figge, M.J. Isolation and characterization of Vibrio owensii from Palk Bay and its infection study against post larvae of Litopenaeus vannamei. Microb. Pathog. 2022, 172, 105751. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Chen, D.; Li, Y. Genomic characterization and comparative genomic analysis of pathogenic Vibrio isolated from aquaculture-grown white-leg shrimp (Penaeus vannamei) in Guangdong and Jiangsu, China. Aquaculture 2024, 580, 740302. [Google Scholar] [CrossRef]
- Misol Jr, G.N.; Kokkari, C.; Katharios, P. Biological and genomic characterization of a novel jumbo bacteriophage, vB_VhaM_pir03 with broad host lytic activity against Vibrio harveyi. Pathogens 2020, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Adesiyan, I.M.; Bisi-Johnson, M.A.; Okoh, A.I. Incidence of antibiotic resistance genotypes of Vibrio species recovered from selected freshwaters in Southwest Nigeria. Sci. Rep. 2022, 12, 18912. [Google Scholar] [CrossRef] [PubMed]
- Okoh, A.I.; Igbinosa, E.O. Antibiotic susceptibility profiles of some Vibrio strains isolated from wastewater final effluents in a rural community of the Eastern Cape Province of South Africa. BMC Microbiol. 2010, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Dubert, J.; Osorio, C.R.; Prado, S.; Barja, J.L. Persistence of antibiotic resistant Vibrio spp. in shellfish hatchery environment. Microb. Ecol. 2016, 72, 851–860. [Google Scholar] [CrossRef]
- Zupičić, I.G.; Oraić, D.; Križanović, K.; Zrnčić, S. Whole genome sequencing of Vibrio harveyi from different sites in the Mediterranean Sea providing data on virulence and antimicrobial resistance genes. Aquaculture 2024, 581, 740439. [Google Scholar] [CrossRef]
- Igbinosa, E.O. Detection and antimicrobial resistance of Vibrio isolates in aquaculture environments: Implications for public health. Microb. Drug Resist. 2016, 22, 238–245. [Google Scholar] [CrossRef]
- Ferri, G.; Olivieri, V.; Olivastri, A.; Pennisi, L.; Vergara, A. Multi-drug resistant Vibrio spp. identified from mussels farmed for human consumption in Central Italy. J. Appl. Microbiol. 2024, 135, lxae098. [Google Scholar] [CrossRef]
- Banerjee, R.; Das, B.; Nair, G.B.; Basak, S. Dynamics in genome evolution of Vibrio cholerae. Infect. Genet. Evol. 2014, 23, 32–41. [Google Scholar] [CrossRef]
- Bian, S.; Zeng, W.; Li, Q.; Li, Y.; Wong, N.K.; Jiang, M.; Zuo, L.; Hu, Q.; Li, L. Genetic structure, function, and evolution of capsule biosynthesis loci in Vibrio parahaemolyticus. Front. Microbiol. 2021, 11, 546150. [Google Scholar] [CrossRef]
- Darmon, E.; Leach, D.R. Bacterial genome instability. Microbiol. Mol. Biol. Rev. 2014, 78, 1–39. [Google Scholar] [CrossRef]
- Deng, Y.; Mao, C.; Lin, Z.; Su, W.; Cheng, C.; Li, Y.; Gu, Q.; Gao, R.; Su, Y.; Feng, J. Nutrients, temperature, and oxygen mediate microbial antibiotic resistance in sea bass (Lateolabrax maculatus) ponds. Sci. Total Environ. 2022, 819, 153120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ellabaan, M.M.H.; Charusanti, P.; Munck, C.; Blin, K.; Tong, Y.; Weber, T.; Sommer, M.O.A.; Lee, S.Y. Dissemination of antibiotic resistance genes from antibiotic producers to pathogens. Nat. Commun. 2017, 8, 15784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guo, W.; Luo, H.; Xing, C.; Wang, H.; Liu, B.; Si, Q.; Ren, N. Deciphering the transfers of antibiotic resistance genes under antibiotic exposure conditions: Driven by functional modules and bacterial community. Water Res. 2021, 205, 117672. [Google Scholar] [CrossRef] [PubMed]
- Shpigel, M.; Erez, J. Effect of diets and light regimes on calcification and somatic growth of the sea urchin Tripneustes gratilla elatensis. Aquaculture 2020, 529, 735547. [Google Scholar] [CrossRef]
- Becker, P.; Gillan, D.C.; Eeckhaut, I. Microbiological study of the body wall lesions of the echinoid Tripneustes gratilla. Dis. Aquat. Org. 2007, 77, 73–82. [Google Scholar] [CrossRef]
- Brink, M.; Rhode, C.; Macey, B.M.; Christison, K.W.; Roodt-Wilding, R. Metagenomic assessment of body surface bacterial communities of the sea urchin, Tripneustes gratilla. Mar. Genom. 2019, 47, 100675. [Google Scholar] [CrossRef]
- Das, B.; Verma, J.; Kumar, P.; Ghosh, A.; Ramamurthy, T. Antibiotic resistance in Vibrio cholerae: Understanding the ecology of resistance genes and mechanisms. Vaccine 2020, 38, A83–A92. [Google Scholar] [CrossRef]
- Osei Sekyere, J. Genomic insights into nitrofurantoin resistance mechanisms and epidemiology in clinical Enterobacteriaceae. Future Sci. OA 2018, 4, FSO293. [Google Scholar] [CrossRef]
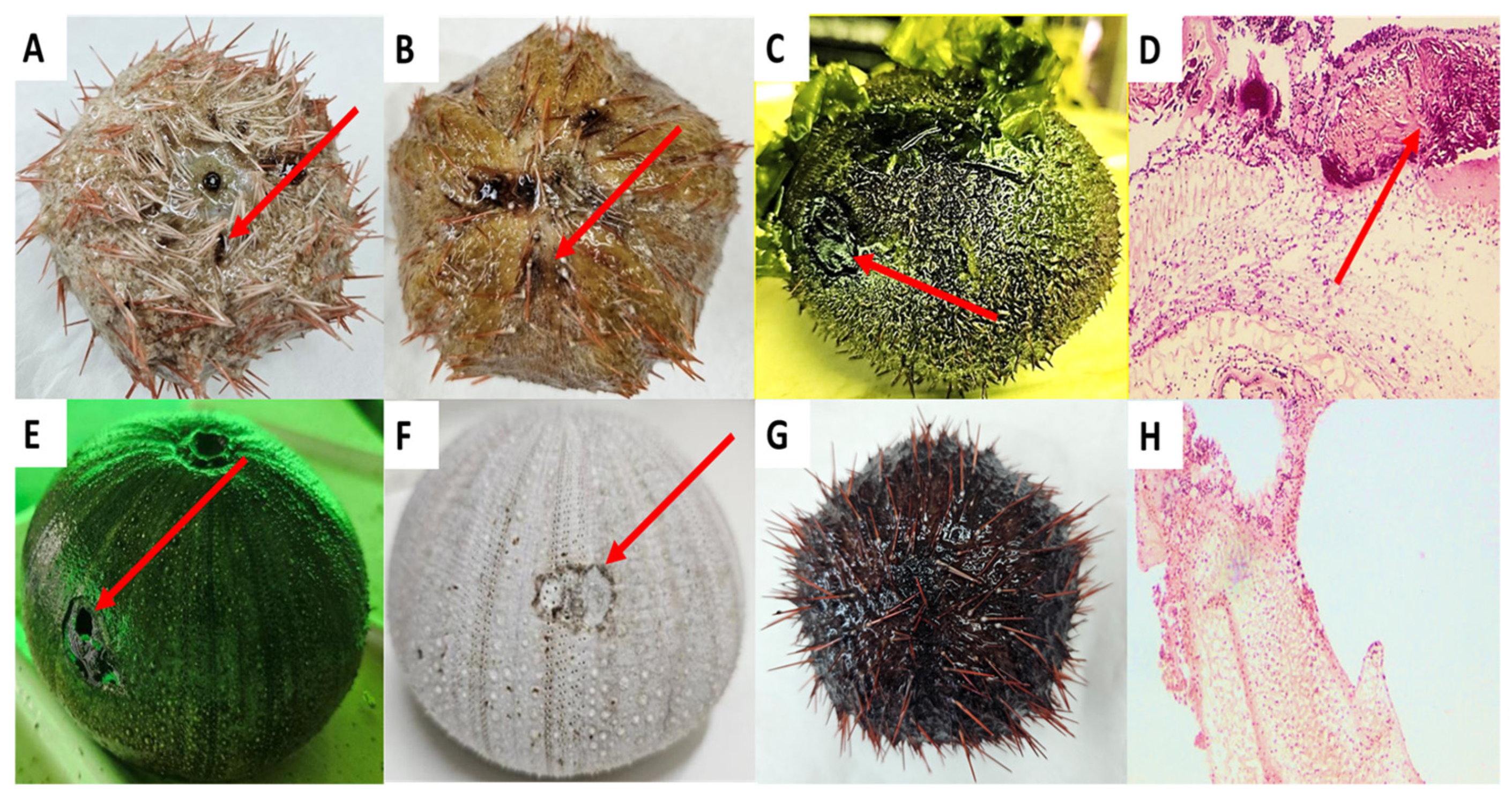

| Disease Name | Infected Sea Urchin | Proposed Pathogen | References |
|---|---|---|---|
| Black peristomial disease | Strongylocentrotus intermedius | Vibrio splendidus Vibrio lentus Vibrio atlanticus Vibrio echinoideorum | [33] |
| Lesion syndrome/Tissue necrosis | Strongylocentrotus droebachiensis | Vibrio echinoideorum | [34] |
| Strongylocentrotus intermedius | Vibrio splendidus Vibrio fortis Vibrio shilonii Vibrio harveyi | [35] | |
| Bald sea urchin disease | Strongylocentrotus purpuratus | Vibrio anguillarum | [36] |
| Paracentrotus lividus | Vibrio splendidus | [37] | |
| Vibriosis | Holopneustes purpurascens | Vibrio anguillarum | [38] |
| Heliocidaris erythrogramma | |||
| Archaeopneustes hystrix | Vibrio alginolyticus | [39] | |
| Paleopneustes cristatus | |||
| Spotting disease | Strongylocentrotus intermedius | Vibrio shilonii Vibrio splendidus Vibrio harveyi Vibrio fortis | [40] |
| Vibrio owensii Vibrio aquaticus | [41] | ||
| Red spotting disease | Strongylocentrotus intermedius | Vibrio coralliilyticus | [1] |
| Tripneustes gratilla | Vibrio harveyi Vibrio fortis Vibrio owensii | Current study |
| Bacterial Strain and Sequence Accession Number in the Genbank Database | Infected Animal | Associated Disease | References |
|---|---|---|---|
| Vibrio fortis Dalian 14 Accession no. GCF_000695685.1 | Sea urchin Strongylocentrotus intermedius | Lesion syndrome | [41,49] |
| Vibrio coralliilyticus Rb102 Accession no. GCF_029541605.1 | Sea urchin Strongylocentrotus intermedius | Red spotting disease | [1] |
| Vibrio echinoideorum DSM 107264 Accession no. GCF_024347455.1 | Sea urchin Strongylocentrotus droebachiensis | Lesion infection | [50] |
| Vibrio cyclitrophicus ED287 Accession no. GCF_023206055.1 | Sea urchin Strongylocentrotus intermedius | Red spotting disease | [51] |
| Vibrio lentus LMG21034 Accession no. GCF_024347555.1 | Sea urchin Strongylocentrotus purpuraus | Vibriosis | [52] |
| Vibrio harveyi VHJR7 Accession no. GCF_000442925.1 | Fishes Seabass Lates calcarifer Humpback grouper Cromileptis altivelis Black tiger shrimp Penaeus monodon | Vibriosis | [53] |
| Vibrio owensii OCN002 Accession no. GCF_000818275.1 | Coral Montipora capitat | White Syndrome | [54] |
| Vibrio alginolyticus ATCC17749 Accession no. GCF_000354175.2 | Sea cucumber Holothuria atra | Skin ulceration disease | [55] |
| Vibrio mediterranei AK1 Accession no. GCF_000181535.1 | Coral Oculina patagonica | Bleaching | [56] |
| Vibrio parahaemolyticus 20160303005-1 Accession no. GCF_009883875.1 | Shrimp (larvae) Penaeus vannamei | Glass post-larval disease | [57] |
| Vibrio campbellii 20130629003S01 Accession no. GCF_002140055.1 | Shrimp Litopenaeus vannamei | Acute hepatopancreatic necrosis disease (AHPND) | [58] |
| Vibrio anguillarum strain J360 Accession no. GCF_003399575.2 | Lumpfish Cyclopterus lumpus | Vibriosis | [59] |
| Vibrio tubiashii ATCC 19109 Accession no. GCF_000772105.1 | Larval shellfish Crassostrea virginica and Crassostrea gigas | Bacillary necrosis | [60] |
| Vibrio pelagius ATCC25916 Accession no. GCF_024347575.1 | Turbot fish (larvae) Scophthalmus maximus | Swelling and necrosis of gill secondary lamellae, intestinal mucosa, and tissue necrosis | [61] |
| Antimicrobial Resistance Genes | Drug Class | Resistance Mechanism |
|---|---|---|
| CRP | Macrolides, fluoroquinolones, penams | Antibiotic efflux-Intracellular pump expels antibiotics. |
| adeF | Fluoroquinolones, tetracyclines | |
| qacG | Disinfecting agents and antiseptics | |
| TxR | Tetracyclines | |
| FosA8 | Phosphonic acids | |
| qacEdlta1 | Disinfecting agents and antiseptics | |
| tet (A) | Tetracyclines | |
| tet (B) | Tetracyclines | |
| tet R | Tetracyclines | |
| tet R | Tetracyclines | Antibiotic target alteration-Genetic modification disrupts antibiotic target sites. |
| vanTG | Glycopeptides | |
| vanYG | Glycopeptides | |
| E. coli parE | Fluoroquinolones | |
| Hinf_PBP3_BLA | Cephalosporin, cephamycin, penams | |
| dfrA27 | Diaminopyrimidines | Antibiotic target replacement-Substitution of antibiotic targets occurs. |
| Sul1 | Sulfonamides | |
| Sul2 | Sulfonamides | Antibiotic target protection-Molecular shielding safeguards antibiotic target sites. |
| QnrVC1 | Fluoroquinolones | |
| CARB-24 | Penams | Antibiotic inactivation-Chemical alteration disabling antibiotics’ effectiveness. |
| CARB-42 | Penams | |
| APH (6)-ld | Aminoglycosides | |
| aadA16 | Aminoglycosides | |
| arr-3 | Rifamycins | |
| TEM-1 | Monobactams, cephalosporins, penams | |
| FosG | Phosphonic acids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Natan, M.; Masasa, M.; Shashar, N.; Guttman, L. Antibiotic Resistance in Vibrio Bacteria Associated with Red Spotting Disease in Sea Urchin Tripneustes gratilla (Echinodermata). Microorganisms 2024, 12, 2460. https://doi.org/10.3390/microorganisms12122460
Ben Natan M, Masasa M, Shashar N, Guttman L. Antibiotic Resistance in Vibrio Bacteria Associated with Red Spotting Disease in Sea Urchin Tripneustes gratilla (Echinodermata). Microorganisms. 2024; 12(12):2460. https://doi.org/10.3390/microorganisms12122460
Chicago/Turabian StyleBen Natan, Mayan, Matan Masasa, Nadav Shashar, and Lior Guttman. 2024. "Antibiotic Resistance in Vibrio Bacteria Associated with Red Spotting Disease in Sea Urchin Tripneustes gratilla (Echinodermata)" Microorganisms 12, no. 12: 2460. https://doi.org/10.3390/microorganisms12122460
APA StyleBen Natan, M., Masasa, M., Shashar, N., & Guttman, L. (2024). Antibiotic Resistance in Vibrio Bacteria Associated with Red Spotting Disease in Sea Urchin Tripneustes gratilla (Echinodermata). Microorganisms, 12(12), 2460. https://doi.org/10.3390/microorganisms12122460







