Impact of Sample Storage Time and Temperature on the Stability of Respiratory Viruses and Enteric Viruses in Wastewater
Abstract
1. Introduction
2. Materials and Methods
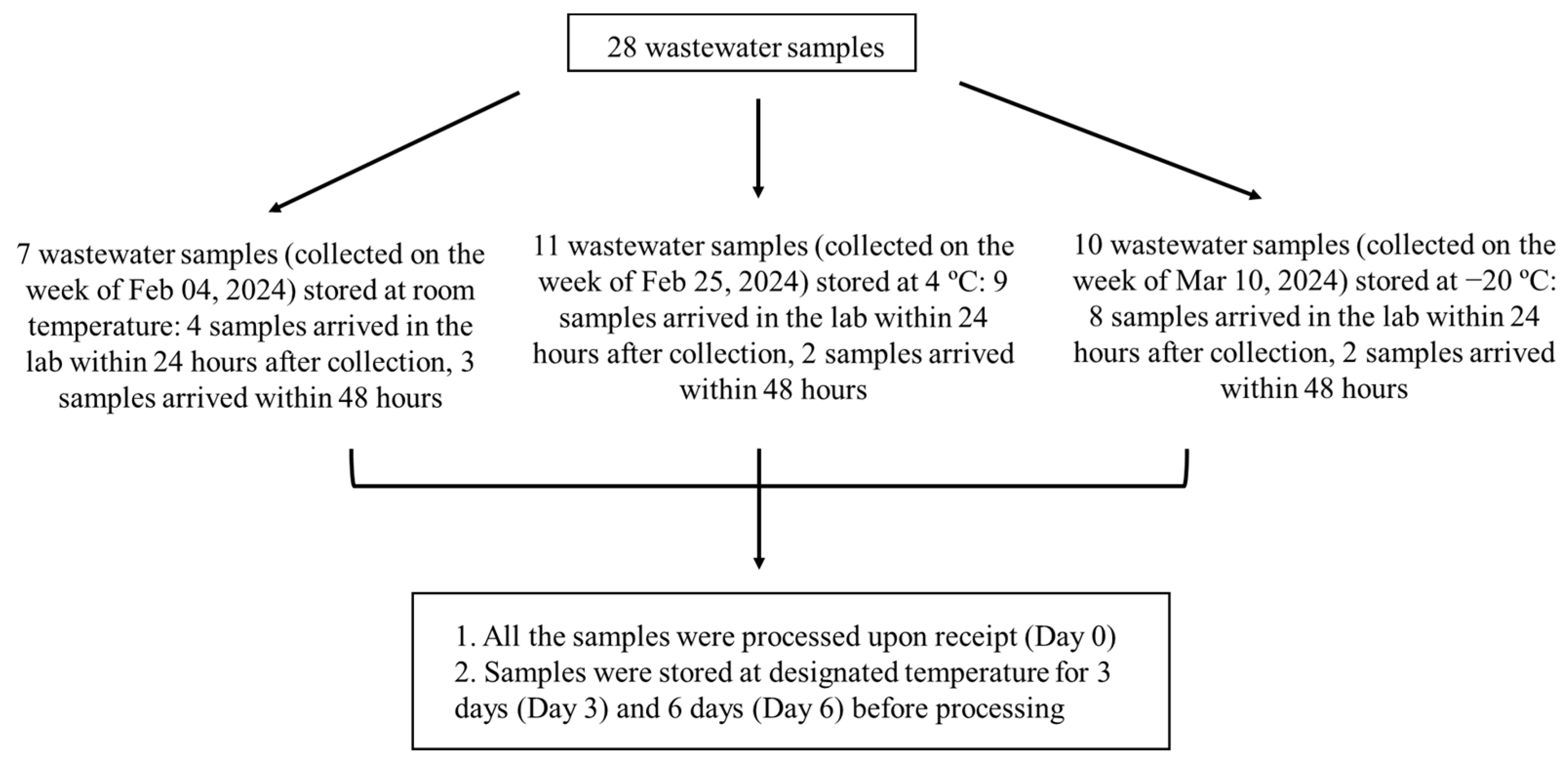
2.1. Wastewater Sample Collection and Storage
2.2. Virus Concentration and Viral Nucleic Acid Extraction
2.3. One-Step Digital PCR (RT-dPCR) for SARS-CoV-2 and PMMoV
2.4. Two-Step RT-qPCR for Enteric Viruses
2.5. One-Step RT-qPCR for IAV, IBV, and RSV
2.6. Statistical Analysis
3. Results
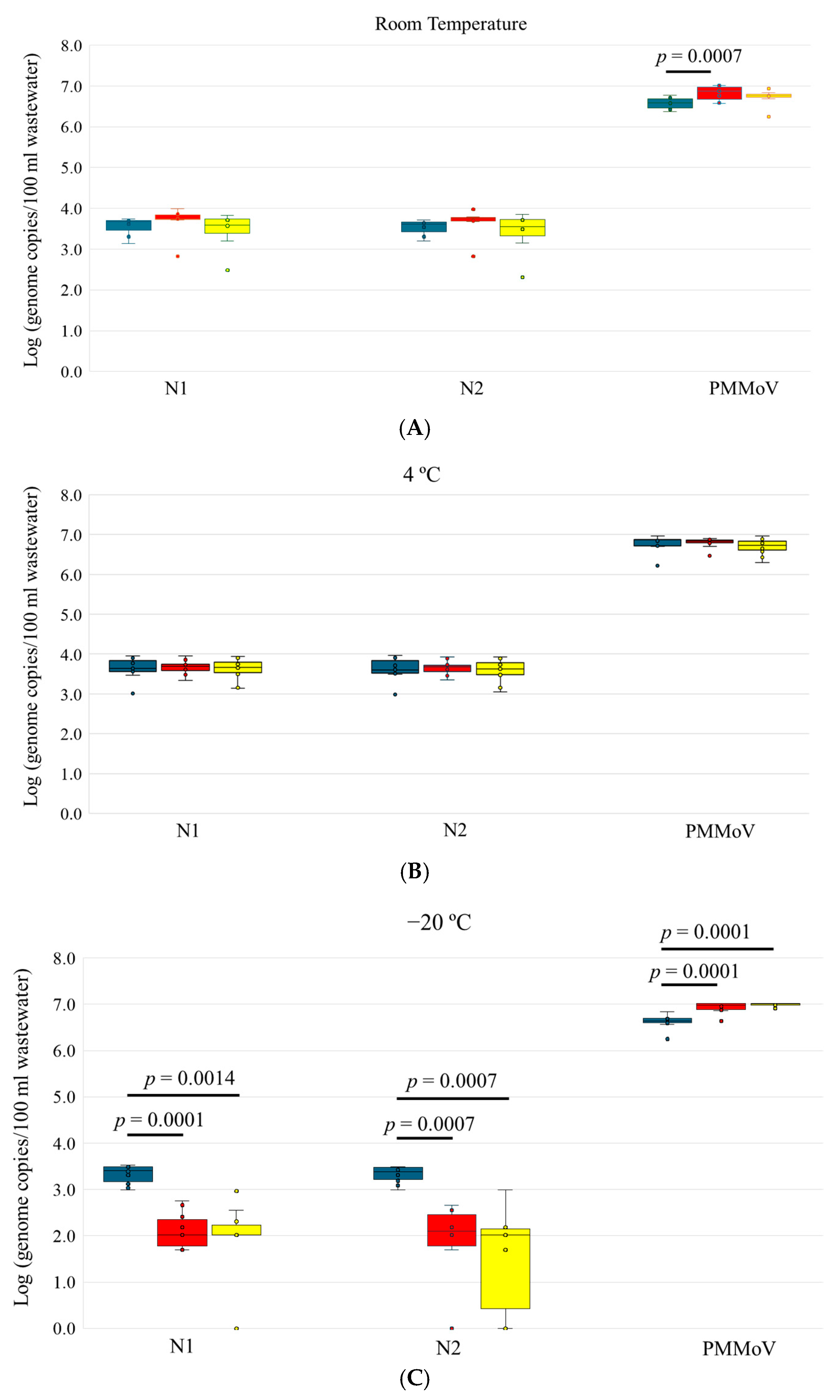
3.1. Stability of SARS-CoV-2 and PMMoV in Wastewater
3.2. Stability of IAV, IBV, and RSV in Wastewater
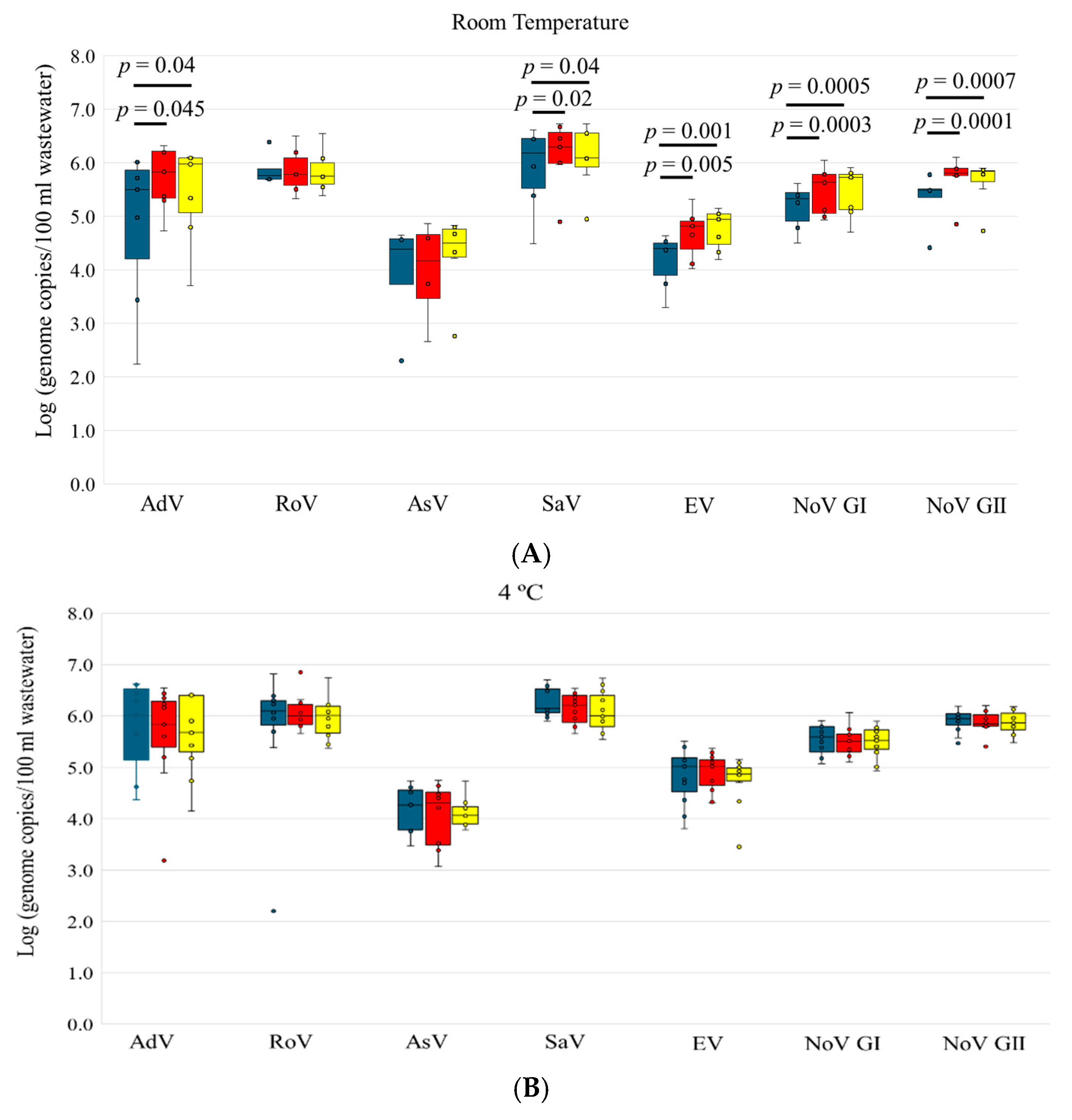
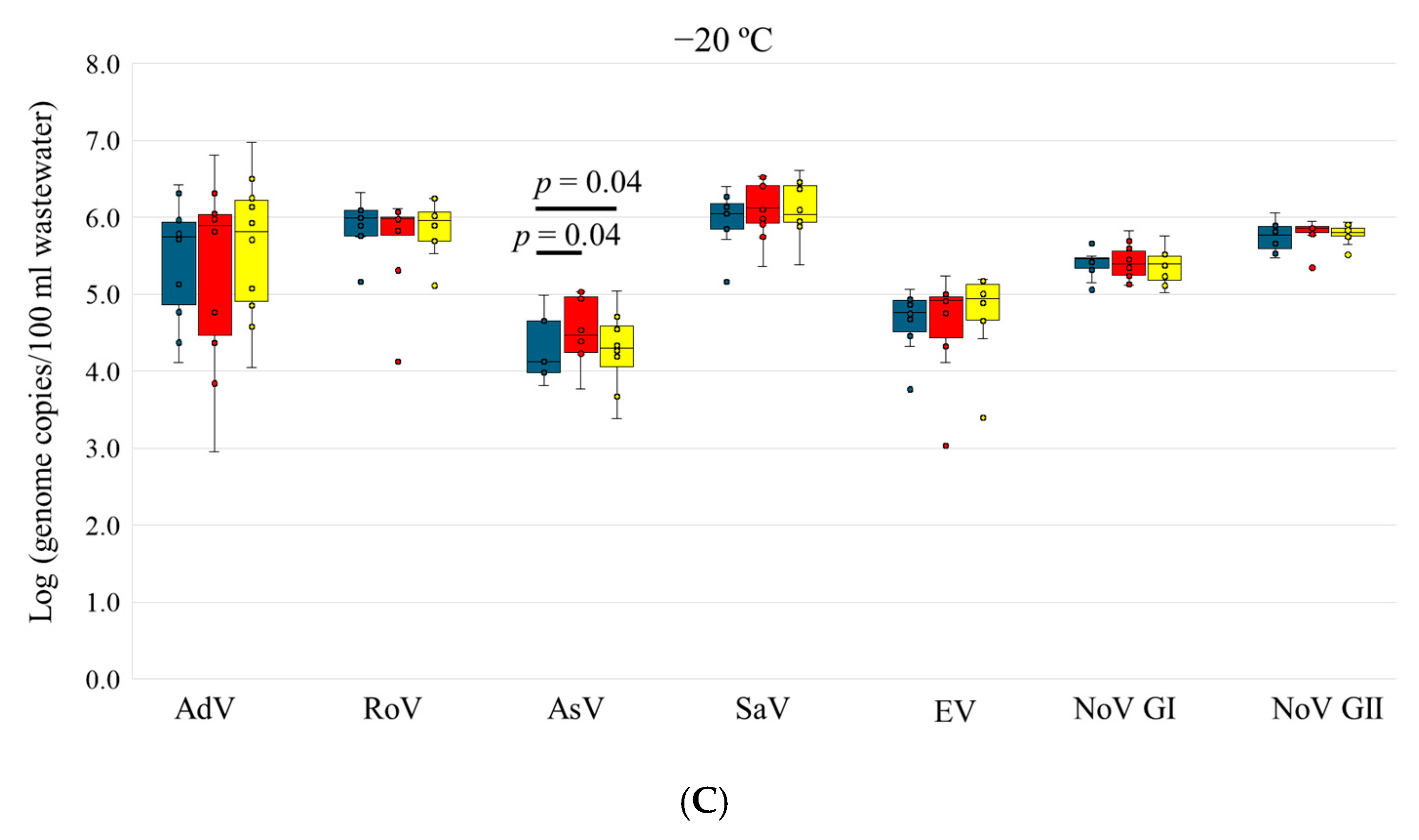
3.3. Stability of Enteric Viruses in Wastewater
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, X.; Gao, T.; Ellehoj, E.; Li, Q.; Qiu, Y.; Maal-Bared, R.; Sikora, C.; Tipples, G.; Diggle, M.; Hinshaw, D.; et al. Wastewater-Based Surveillance Is an Effective Tool for Trending COVID-19 Prevalence in Communities: A Study of 10 Major Communities for 17 Months in Alberta. ACS ES T Water 2022, 2, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xiao, A.; Zhang, J.; Moniz, K.; Endo, N.; Armas, F.; Bonneau, R.; Brown, M.A.; Bushman, M.; Chai, P.R.; et al. SARS-CoV-2 RNA concentrations in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. Sci. Total Environ. 2022, 805, 150121. [Google Scholar] [CrossRef]
- Weidhaas, J.; Aanderud, Z.T.; Roper, D.K.; VanDerslice, J.; Gaddis, E.B.; Ostermiller, J.; Hoffman, K.; Jamal, R.; Heck, P.; Zhang, Y.; et al. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021, 775, 145790. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Y.; Kaur, G.; Adiga, A.; Espinoza, B.; Venkatramanan, S.; Warren, A.; Lewis, B.; Crow, J.; Singh, R.; et al. Wastewater-based epidemiology for COVID-19 surveillance and beyond: A survey. Epidemics 2024, 49, 100793. [Google Scholar] [CrossRef]
- Maida, C.M.; Mazzucco, W.; Priano, W.; Palermo, R.; Graziano, G.; Costantino, C.; Russo, A.; Andolina, G.; Restivo, I.; Giangreco, V.; et al. Detection of influenza virus in urban wastewater during the season 2022/2023 in Sicily, Italy. Front. Public Health 2024, 12, 1383536. [Google Scholar] [CrossRef] [PubMed]
- Wolken, M.; Wang, M.; Schedler, J.; Campos, R.H., Jr.; Ensor, K.; Hopkins, L.; Treangen, T.; Stadler, L.B. PreK-12 school and citywide wastewater monitoring of the enteric viruses astrovirus, rotavirus, and sapovirus. Sci. Total Environ. 2024, 931, 172683. [Google Scholar] [CrossRef] [PubMed]
- Weil, M.; Sofer, D.; Shulman, L.M.; Weiss, L.; Levi, N.; Aguvaev, I.; Cohen, Z.; Kestin, K.; Vasserman, R.; Elul, M.; et al. Environmental surveillance detected type 3 vaccine-derived polioviruses in increasing frequency at multiple sites prior to detection of a poliomyelitis case. Sci. Total Environ. 2023, 871, 161985. [Google Scholar] [CrossRef]
- Mejia, E.M.; Hizon, N.A.; Dueck, C.E.; Lidder, R.; Daigle, J.; Wonitowy, Q.; Medina, N.G.; Mohammed, U.P.; Cox, G.W.; Safronetz, D.; et al. Detection of mpox virus in wastewater provides forewarning of clinical cases in Canadian cities. Sci. Total Environ. 2024, 933, 173108. [Google Scholar] [CrossRef]
- Islam, G.; Gedge, A.; Lara-Jacobo, L.; Kirkwood, A.; Simmons, D.; Desaulniers, J.P. Pasteurization, storage conditions and viral concentration methods influence RT-qPCR detection of SARS-CoV-2 RNA in wastewater. Sci. Total Environ. 2022, 821, 153228. [Google Scholar] [CrossRef]
- Beattie, R.E.; Blackwood, A.D.; Clerkin, T.; Dinga, C.; Noble, R.T. Evaluating the impact of sample storage, handling, and technical ability on the decay and recovery of SARS-CoV-2 in wastewater. PLoS ONE 2022, 17, e0270659. [Google Scholar] [CrossRef]
- Boogaerts, T.; Jacobs, L.; De Roeck, N.; Van den Bogaert, S.; Aertgeerts, B.; Lahousse, L.; van Nuijs, A.L.N.; Delputte, P. An alternative approach for bioanalytical assay optimization for wastewater-based epidemiology of SARS-CoV-2. Sci. Total Environ. 2021, 789, 148043. [Google Scholar] [CrossRef]
- Hokajarvi, A.M.; Rytkonen, A.; Tiwari, A.; Kauppinen, A.; Oikarinen, S.; Lehto, K.M.; Kankaanpaa, A.; Gunnar, T.; Al-Hello, H.; Blomqvist, S.; et al. The detection and stability of the SARS-CoV-2 RNA biomarkers in wastewater influent in Helsinki, Finland. Sci. Total Environ. 2021, 770, 145274. [Google Scholar] [CrossRef] [PubMed]
- Cutrupi, F.; Cadonna, M.; Manara, S.; Foladori, P. Surveillance of SARS-CoV-2 in extensive monitoring of municipal wastewater: Key issues to yield reliable results. Water Sci. Technol. 2021, 84, 3508–3514. [Google Scholar] [CrossRef] [PubMed]
- Markt, R.; Mayr, M.; Peer, E.; Wagner, A.O.; Lackner, N.; Insam, H. Detection and Stability of SARS-CoV-2 Fragments in Wastewater: Impact of Storage Temperature. Pathogens 2021, 10, 1215. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Scheidegger, A.; Banziger, C.; Cariti, F.; Corzon, A.T.; Ganesanandamoorthy, P.; Lemaitre, J.C.; Ort, C.; Julian, T.R.; Kohn, T. Wastewater monitoring outperforms case numbers as a tool to track COVID-19 incidence dynamics when test positivity rates are high. Water Res. 2021, 200, 117252. [Google Scholar] [PubMed]
- Qiu, Y.; Yu, J.; Pabbaraju, K.; Lee, B.E.; Gao, T.; Ashbolt, N.J.; Hrudey, S.E.; Diggle, M.; Tipples, G.; Maal-Bared, R.; et al. Validating and optimizing the method for molecular detection and quantification of SARS-CoV-2 in wastewater. Sci. Total Environ. 2022, 812, 151434. [Google Scholar] [CrossRef] [PubMed]
- Mercier, E.; D'Aoust, P.M.; Thakali, O.; Hegazy, N.; Jia, J.J.; Zhang, Z.; Eid, W.; Plaza-Diaz, J.; Kabir, M.P.; Fang, W.; et al. Municipal and neighbourhood level wastewater surveillance and subtyping of an influenza virus outbreak. Sci. Rep. 2022, 12, 15777. [Google Scholar] [CrossRef] [PubMed]
- Lehto, K.M.; Lansivaara, A.; Hyder, R.; Luomala, O.; Lipponen, A.; Hokajarvi, A.M.; Heikinheimo, A.; Pitkanen, T.; Oikarinen, S. WastPan Study, Wastewater-based surveillance is an efficient monitoring tool for tracking influenza A in the community. Water Res. 2024, 257, 121650. [Google Scholar] [CrossRef]
- Farkas, K.; Fletcher, J.; Oxley, J.; Ridding, N.; Williams, R.C.; Woodhall, N.; Weightman, A.J.; Cross, G.; Jones, D.L. Implications of long-term sample storage on the recovery of viruses from wastewater and biobanking. Water Res. 2024, 265, 122209. [Google Scholar] [CrossRef]
- Subroyen, S.; Pillay, L.; Bux, F.; Kumari, S. Evaluating storage conditions and enhancement strategies on viral biomarker recovery for WBE applications. Water Sci. Technol. 2024, 90, 213–224. [Google Scholar] [CrossRef]
- Boehm, A.B.; Shelden, B.; Duong, D.; Banaei, N.; White, B.J.; Wolfe, M.K. A retrospective longitudinal study of adenovirus group F, norovirus GI and GII, rotavirus, and enterovirus nucleic acids in wastewater solids at two wastewater treatment plants: Solid-liquid partitioning and relation to clinical testing data. mSphere 2024, 9, e0073623. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Figueroa, A.; Rosales, R.E.; Fernandez, R.; Ramirez, V.; Bastardo, M.; Farias, A.; Vizzi, E. Detection and diversity of gastrointestinal viruses in wastewater from Caracas, Venezuela, 2021–2022. Virology 2024, 589, 109913. [Google Scholar] [CrossRef] [PubMed]
- Dzinamarira, T.; Pierre, G.; Iradukunda, P.G.; Tungwarara, N.; Mukwenha, S.; Mpabuka, E.; Mataruka, K.; Chitungo, I.; Musuka, G.; Murewanhema, G. Epidemiological surveillance of enteric viral diseases using wastewater in Africa—A rapid review. J. Infect. Public Health 2022, 15, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.L.; Lee, B.E.; Pabbaraju, K.; Gabos, S.; Craik, S.; Payment, P.; Neumann, N. Pre-analytical and analytical procedures for the detection of enteric viruses and enterovirus in water samples. J. Virol. Methods 2012, 184, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.L.; JPreiksaitis, K.; Lee, B.E. Enhanced enteric virus detection in sporadic gastroenteritis using a multi-target real-time PCR panel: A one-year study. J. Med. Virol. 2014, 86, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lee, B.E.; Ruecker, N.J.; Neumann, N.; Ashbolt, N.; Pang, X. A one-step centrifugal ultrafiltration method to concentrate enteric viruses from wastewater. J. Virol. Methods 2016, 237, 150–153. [Google Scholar] [CrossRef]
- Selvaraju, S.B.; Selvarangan, R. Evaluation of three influenza A and B real-time reverse transcription-PCR assays and a new 2009 H1N1 assay for detection of influenza viruses. J. Clin. Microbiol. 2010, 48, 3870–3875. [Google Scholar] [CrossRef]
- Papillard-Marechal, S.; Enouf, V.; Schnuriger, A.; Vabret, A.; Macheras, E.; Rameix-Welti, M.A.; Page, B.; Freymuth, F.; van der Werf, S.; Garbarg-Chenon, A.; et al. Monitoring epidemic viral respiratory infections using one-step real-time triplex RT-PCR targeting influenza A and B viruses and respiratory syncytial virus. J. Med. Virol. 2011, 83, 695–701. [Google Scholar] [CrossRef]
- Tavazzi, S.; Cacciatori, C.; Comero, S.; Fatta-Kassinos, D.; Karaolia, P.; Iakovides, I.C.; Loutsiou, P.; Gutierrez-Aguirre, I.; Lengar, Z.; Bajde, I.; et al. Short-term stability of wastewater samples for storage and shipment in the context of the EU Sewage Sentinel System for SARS-CoV-2. J. Environ. Chem. Eng. 2023, 11, 109623. [Google Scholar] [CrossRef]
- Burnet, J.B.; Cauchie, H.M.; Walczak, C.; Goeders, N.; Ogorzaly, L. Persistence of endogenous RNA biomarkers of SARS-CoV-2 and PMMoV in raw wastewater: Impact of temperature and implications for wastewater-based epidemiology. Sci. Total Environ. 2023, 857 Pt 2, 159401. [Google Scholar] [CrossRef]
- Williams, R.C.; Perry, W.B.; Lambert-Slosarska, K.; Futcher, B.; Pellett, C.; Richardson-O, I.; Paterson, S.; Grimsley, J.M.S.; Wade, M.J.; Weightman, A.J.; et al. Examining the stability of viral RNA and DNA in wastewater: Effects of storage time, temperature, and freeze-thaw cycles. Water Res. 2024, 259, 121879. [Google Scholar] [CrossRef] [PubMed]
- Maal-Bared, R.; Qiu, Y.; Li, Q.; Gao, T.; Hrudey, S.E.; Bhavanam, S.; Ruecker, N.J.; Ellehoj, E.; Lee, B.E.; Pang, X. Does normalization of SARS-CoV-2 concentrations by Pepper Mild Mottle Virus improve correlations and lead time between wastewater surveillance and clinical data in Alberta (Canada): Comparing twelve SARS-CoV-2 normalization approaches. Sci. Total Environ. 2023, 856 Pt 1, 158964. [Google Scholar] [CrossRef] [PubMed]
- D'Aoust, P.M.; Mercier, E.; Montpetit, D.; Jia, J.J.; Alexandrov, I.; Neault, N.; Baig, A.T.; Mayne, J.; Zhang, X.; Alain, T.; et al. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities with low COVID-19 incidence and prevalence. Water Res. 2021, 188, 116560. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ash, K.T.; Joyner, D.C.; Williams, D.E.; Alamilla, I.; McKay, P.J.; Iler, C.; Green, B.M.; Kara-Murdoch, F.; Swift, C.M.; et al. Decay of enveloped SARS-CoV-2 and non-enveloped PMMoV RNA in raw sewage from university dormitories. Front. Microbiol. 2023, 14, 1144026. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, S.; Parambil, A.M.; Jayaram, A.; Varamballi, P.; Mukhopadhyay, C.; Jagadesh, A. Surveillance of influenza A and B viruses from community and hospital wastewater treatment plants. Environ. Microbiol. Rep. 2024, 16, e13317. [Google Scholar] [CrossRef]
- Dumke, R.; Geissler, M.; Skupin, A.; Helm, B.; Mayer, R.; Schubert, S.; Oertel, R.; Renner, B.; Dalpke, A.H. Simultaneous Detection of SARS-CoV-2 and Influenza Virus in Wastewater of Two Cities in Southeastern Germany, January to May 2022. Int. J. Environ. Res. Public Health 2022, 19, 13374. [Google Scholar] [CrossRef]
- Stobnicka-Kupiec, A.; Golofit-Szymczak, M.; Cyprowski, M.; Gorny, R.L. Detection and identification of potentially infectious gastrointestinal and respiratory viruses at workplaces of wastewater treatment plants with viability qPCR/RT-qPCR. Sci. Rep. 2022, 12, 4517. [Google Scholar] [CrossRef] [PubMed]
- Calgua, B.; Carratala, A.; Guerrero-Latorre, L.; de Abreu Correa, A.; Kohn, T.; Sommer, R.; Girones, R. UVC Inactivation of dsDNA and ssRNA Viruses in Water: UV Fluences and a qPCR-Based Approach to Evaluate Decay on Viral Infectivity. Food Environ. Virol. 2014, 6, 260–268. [Google Scholar] [CrossRef]
- Qiu, Y.; Lee, B.E.; Neumann, N.; Ashbolt, N.; Craik, S.; Maal-Bared, R.; Pang, X.L. Assessment of human virus removal during municipal wastewater treatment in Edmonton, Canada. J. Appl. Microbiol. 2015, 119, 1729–1739. [Google Scholar] [CrossRef] [PubMed]


| N1 | N2 | PMMoV | |||||
|---|---|---|---|---|---|---|---|
| Detected Sample, n | Viral Load (Mean ± SD) | Detected Sample, n | Viral Load (Mean ± SD) | Detected Sample, n | Viral Load (Mean ± SD) | ||
| R.T storage | Day 0 | 7/7 | 3.56 ± 0.24 | 7/7 | 3.53 ± 0.2 | 7/7 | 6.57 ± 0.15 |
| Day 3 | 7/7 | 3.67 ± 0.39 | 7/7 | 3.64 ± 0.37 | 7/7 | 6.82 ± 0.19 | |
| Day 6 | 7/7 | 3.45 ± 0.47 | 7/7 | 3.4 ± 0.53 | 7/7 | 6.71 ± 0.22 | |
| 4 °C storage | Day 0 | 11/11 | 3.64 ± 0.27 | 11/11 | 3.63 ± 0.28 | 11/11 | 6.78 ± 0.20 |
| Day 3 | 11/11 | 3.66 ± 0.17 | 11/11 | 3.65 ± 0.17 | 11/11 | 6.80 ± 0.12 | |
| Day 6 | 11/11 | 3.62 ± 0.27 | 11/11 | 3.60 ± 0.28 | 11/11 | 6.69 ± 0.20 | |
| −20 °C storage | Day 0 | 10/10 | 3.32 ± 0.20 | 10/10 | 3.32 ± 0.18 | 10/10 | 6.62 ± 0.16 |
| Day 3 | 10/10 | 2.22 ± 0.39 | 8/10 | 1.79 ± 0.99 | 10/10 | 6.93 ± 0.12 | |
| Day 6 | 8/10 | 1.79 ± 0.99 | 7/10 | 1.51 ± 1.09 | 10/10 | 6.98 ± 0.04 | |
| IAV | IBV | RSV | |||||
|---|---|---|---|---|---|---|---|
| Detected Sample, n | Viral Load (Mean ± SD) | Detected Sample, n | Viral Load (Mean ± SD) | Detected Sample, n | Viral Load (Mean ± SD) | ||
| R.T storage | Day 0 | 2/7 | 1.51 ± 0.44 | 0/7 | N/A | 2/7 | 1.23 ± 0.22 |
| Day 3 | 3/7 | 1.60 ± 0.65 | 0/7 | N/A | 3/7 | 0.93 ± 0.26 | |
| Day 6 | 4/7 | 1.58 ± 0.18 | 0/7 | N/A | 2/7 | 1.02 ± 0.3 | |
| 4 °C storage | Day 0 | 6/11 | 1.85 ± 0.54 | 2/11 | 2.22 ± 0.001 | 10/11 | 1.54 ± 0.45 |
| Day 3 | 6/11 | 1.59 ± 0.92 | 1/11 | 2.29 | 9/11 | 1.34 ± 0.43 | |
| Day 6 | 7/11 | 1.56 ± 0.76 | 1/11 | 2.17 | 7/11 | 1.29 ± 0.34 | |
| −20 °C storage | Day 0 | 2/10 | 1.54 ± 0.70 | 0/10 | N/A | 6/10 | 1.27 ± 0.36 |
| Day 3 | 0/10 | N/A | 0/10 | N/A | 2/10 | 1.1 ± 0.37 | |
| Day 6 | 0/10 | N/A | 0/10 | N/A | 1/10 | 1.13 | |
| AdV | RoV | AsV | SaV | EV | NoV GI | NoV GII | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detected Sample, n | Viral Load (Mean ± SD) | Detected Sample, n | Viral Load (Mean ± SD) | Detected Sample, n | Viral Load (Mean ± SD) | Detected Sample, n | Viral Load (Mean ± SD) | Detected Sample, n | Viral Load (Mean ± SD) | Detected Sample, n | Viral Load (Mean ± SD) | Detected Sample, n | Viral Load (Mean ± SD) | ||
| R.T | Day 0 | 7/7 | 4.84 ± 1.46 | 5/7 | 5.88 ± 0.29 | 4/7 | 3.93 ± 1.1 | 6/7 | 5.89 ± 0.82 | 6/7 | 4.17 ± 0.53 | 6/7 | 5.17 ± 0.43 | 6/7 | 5.35 ± 0.48 |
| Day 3 | 7/7 | 5.71 ± 0.60 | 6/7 | 5.85 ± 0.43 | 4/7 | 3.96 ± 0.99 | 7/7 | 6.15 ± 0.62 | 7/7 | 4.68 ± 0.46 | 7/7 | 5.47 ± 0.45 | 7/7 | 5.72 ± 0.40 | |
| Day 6 | 7/7 | 5.44 ± 0.92 | 6/7 | 5.84 ± 0.41 | 6/7 | 4.27 ± 0.78 | 7/7 | 6.11 ± 0.61 | 7/7 | 4.76 ± 0.38 | 7/7 | 5.45 ± 0.46 | 7/7 | 5.64 ± 0.42 | |
| 4 °C | Day 0 | 11/11 | 5.78 ± 0.87 | 11/11 | 5.76 ± 1.24 | 7/11 | 4.16 ± 0.49 | 11/11 | 6.19 ± 0.36 | 11/11 | 4.83 ± 0.56 | 11/11 | 5.54 ± 0.29 | 11/11 | 5.90 ± 0.22 |
| Day 3 | 11/11 | 5.64 ± 0.97 | 10/11 | 6.07 ± 0.34 | 8/11 | 4.06 ± 0.64 | 11/11 | 6.15 ± 0.31 | 11/11 | 4.89 ± 0.37 | 11/11 | 5.50 ± 0.27 | 11/11 | 5.88 ± 0.21 | |
| Day 6 | 11/11 | 5.65 ± 0.76 | 10/11 | 5.96 ± 0.42 | 8/11 | 4.12 ± 0.30 | 11/11 | 6.09 ± 0.40 | 11/11 | 4.63 ± 0.58 | 11/11 | 5.49 ± 0.31 | 11/11 | 5.87 ± 0.23 | |
| −20 °C | Day 0 | 10/10 | 5.44 ± 0.80 | 9/10 | 5.90 ± 0.33 | 5/10 | 4.31 ± 0.49 | 10/10 | 5.99 ± 0.36 | 10/10 | 4.66 ± 0.39 | 10/10 | 5.40 ± 0.18 | 10/10 | 5.75 ± 0.19 |
| Day 3 | 10/10 | 5.29 ± 1.24 | 9/10 | 5.69 ± 0.63 | 8/10 | 4.52 ± 0.45 | 10/10 | 6.11 ± 0.38 | 10/10 | 4.62 ± 0.65 | 10/10 | 5.41 ± 0.24 | 10/10 | 5.81 ± 0.17 | |
| Day 6 | 10/10 | 5.60 ± 0.93 | 9/10 | 5.86 ± 0.37 | 8/10 | 4.27 ± 0.54 | 10/10 | 6.11 ± 0.37 | 10/10 | 4.76 ± 0.54 | 10/10 | 5.36 ± 0.23 | 10/10 | 5.78 ± 0.13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.Y.; Mah, R.; Brand, L.A.; Pang, X.; Barnett, M.; Diggle, M.; Tipples, G. Impact of Sample Storage Time and Temperature on the Stability of Respiratory Viruses and Enteric Viruses in Wastewater. Microorganisms 2024, 12, 2459. https://doi.org/10.3390/microorganisms12122459
Qiu JY, Mah R, Brand LA, Pang X, Barnett M, Diggle M, Tipples G. Impact of Sample Storage Time and Temperature on the Stability of Respiratory Viruses and Enteric Viruses in Wastewater. Microorganisms. 2024; 12(12):2459. https://doi.org/10.3390/microorganisms12122459
Chicago/Turabian StyleQiu, Judy Y., Richardson Mah, Logan A. Brand, Xiaoli Pang, Melodie Barnett, Mathew Diggle, and Graham Tipples. 2024. "Impact of Sample Storage Time and Temperature on the Stability of Respiratory Viruses and Enteric Viruses in Wastewater" Microorganisms 12, no. 12: 2459. https://doi.org/10.3390/microorganisms12122459
APA StyleQiu, J. Y., Mah, R., Brand, L. A., Pang, X., Barnett, M., Diggle, M., & Tipples, G. (2024). Impact of Sample Storage Time and Temperature on the Stability of Respiratory Viruses and Enteric Viruses in Wastewater. Microorganisms, 12(12), 2459. https://doi.org/10.3390/microorganisms12122459






