Genomic Insights into the Bactericidal and Fungicidal Potential of Bacillus mycoides b12.3 Isolated in the Soil of Olkhon Island in Lake Baikal, Russia
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of the Strain from a Soil Sample
2.2. Characterization of the Strain by Microscopy and Colony Morphology
2.3. Identification of Isolates Based on the gyrB Locus Analysis
2.4. Assessing the Bactericidal and Fungicidal Activity of the Strain Against Plant Pathogens of Agricultural Crops
2.5. DNA Extraction and Quality Control
2.6. Genome Assembly and Annotation
2.7. Descriptive Analysis of Reference Genomes
3. Results
3.1. Morphological Characterization of b12.3 Strain
3.2. Strain Identification by the gyrB Gene Sequence
3.3. Screening of Strain b12.3’s Antagonistic Activity Against Bacterial Pathogens of Agricultural Crops
3.4. Testing the Fungicidal Activity of the Strain Against Fungal Plant Pathogens
3.5. The Genomic Properties of the Strain b12.3
3.5.1. The Basic Features of the Genome Assembly
3.5.2. Biosynthetic Gene Clusters and Insecticidal Loci Detected in the Strain b12.3
3.6. Genomic Comparisons with Closest Reference Assemblies
3.6.1. Reference Strain Selection and Description
3.6.2. The Distribution of BGCs and Toxin-Encoding Loci in the Genomic Dataset
3.7. Identifying Determinants of B. mycoides b12.3 Specificity Using Available Metadata
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANI | Average nucleotide identity |
| BIC | Bayesian information criterion |
| BPPRC | Bacterial Pesticidal Protein Resource Center |
| CDS | Coding sequence |
| HGT | Horizontal gene transfer |
| LB | Luria–Bertani |
| MDR | Multidrug-resistant |
| ML | Maximum likelihood |
| NRPS | Non-ribosomal peptide synthetases |
| PDB | Potato dextrose broth |
| PGPB | Plant growth-promoting bacteria |
| PKSs | Polyketide synthases |
| RCAM | Russian Collection of Agricultural Microorganisms |
| RiPPs | Ribosomally synthesized and post-translationally modified peptides |
| SNPs | Single-nucleotide polymorphisms |
References
- Hampton, S.E.; Izmest’eva, L.R.; Moore, M.V.; Katz, S.L.; Dennis, B.; Silow, E.A. Sixty Years of Environmental Change in the World’s Largest Freshwater Lake—Lake Baikal, Siberia. Glob. Chang. Biol. 2008, 14, 1947–1958. [Google Scholar] [CrossRef]
- Pavlova, O.N.; Chernitsyna, S.M.; Bukin, S.V.; Lomakina, A.V.; Shubenkova, O.V.; Smirnova, D.K.; Zemskaya, T.I. The Source of Thermophilic Bacteria in Lake Baikal Cold Sediments—Coastal Hydrotherms or Deep Fluids? Microbiology 2024, 93, 338–343. [Google Scholar] [CrossRef]
- Zemskaya, T.I.; Cabello-Yeves, P.J.; Pavlova, O.N.; Rodriguez-Valera, F. Microorganisms of Lake Baikal—The Deepest and Most Ancient Lake on Earth. Appl. Microbiol. Biotechnol. 2020, 104, 6079–6090. [Google Scholar] [CrossRef]
- Bashenkhaeva, M.V.; Galachyants, Y.P.; Khanaev, I.V.; Sakirko, M.V.; Petrova, D.P.; Likhoshway, Y.V.; Zakharova, Y.R. Comparative Analysis of Free-Living and Particle-Associated Bacterial Communities of Lake Baikal during the Ice-Covered Period. J. Great Lakes Res. 2020, 46, 508–518. [Google Scholar] [CrossRef]
- Axenov-Gribanov, D.V.; Kostka, D.V.; Vasilieva, U.A.; Shatilina, Z.M.; Krasnova, M.E.; Pereliaeva, E.V.; Zolotovskaya, E.D.; Morgunova, M.M.; Rusanovskaya, O.O.; Timofeyev, M.A. Cultivable Actinobacteria First Found in Baikal Endemic Algae Is a New Source of Natural Products with Antibiotic Activity. Int. J. Microbiol. 2020, 2020, 5359816. [Google Scholar] [CrossRef]
- Babich, O.; Shevchenko, M.; Ivanova, S.; Pavsky, V.; Zimina, M.; Noskova, S.; Anohova, V.; Chupakhin, E.; Sukhikh, S. Antimicrobial Potential of Microorganisms Isolated from the Bottom Sediments of Lake Baikal. Antibiotics 2021, 10, 927. [Google Scholar] [CrossRef]
- Sukhanova, E.; Zimens, E.; Kaluzhnaya, O.; Parfenova, V.; Belykh, O. Epilithic Biofilms in Lake Baikal: Screening and Diversity of PKs and NRPS Genes in the Genomes of Heterotrophic Bacteria. Pol. J. Microbiol. 2018, 67, 501–516. [Google Scholar] [CrossRef]
- Zakharenko, A.S.; Galachyants, Y.P.; Morozov, I.V.; Shubenkova, O.V.; Morozov, A.A.; Ivanov, V.G.; Pimenov, N.V.; Krasnopeev, A.Y.; Zemskaya, T.I. Bacterial Communities in Areas of Oil and Methane Seeps in Pelagic of Lake Baikal. Microb. Ecol. 2019, 78, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, F.H.; Cabello-Yeves, P.J.; Gonzalez-Serrano, R.; Rosselli, R.; López-Pérez, M.; Zemskaya, T.I.; Zakharenko, A.S.; Ivanov, V.G.; Rodriguez-Valera, F. New Viral Biogeochemical Roles Revealed through Metagenomic Analysis of Lake Baikal. Microbiome 2020, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Pereliaeva, E.V.; Dmitrieva, M.E.; Morgunova, M.M.; Belyshenko, A.Y.; Imidoeva, N.A.; Ostyak, A.S.; Axenov-Gribanov, D.V. The Use of Baikal Psychrophilic Actinobacteria for Synthesis of Biologically Active Natural Products from Sawdust Waste. Fermentation 2022, 8, 213. [Google Scholar] [CrossRef]
- Kurilkina, M.I.; Zakharova, Y.R.; Galachyants, Y.P.; Petrova, D.P.; Bukin, Y.S.; Domysheva, V.M.; Blinov, V.V.; Likhoshway, Y.V. Bacterial Community Composition in the Water Column of the Deepest Freshwater Lake Baikal as Determined by Next-Generation Sequencing. FEMS Microbiol. Ecol. 2016, 92, fiw094. [Google Scholar] [CrossRef]
- Zemskaya, T.I.; Bukin, S.V.; Lomakina, A.V.; Pavlova, O.N. Microorganisms in the Sediments of Lake Baikal, the Deepest and Oldest Lake in the World. Microbiology 2021, 90, 298–313. [Google Scholar] [CrossRef]
- Bedoshvili, Y.; Bayramova, E.; Sudakov, N.; Klimenkov, I.; Kurilkina, M.; Likhoshway, Y.; Zakharova, Y. Impact of Algicidal Bacillus mycoides on Diatom Ulnaria acus from Lake Baikal. Diversity 2021, 13, 469. [Google Scholar] [CrossRef]
- Wunderlin, T.; Junier, T.; Roussel-Delif, L.; Jeanneret, N.; Junier, P. Stage 0 Sporulation Gene A as a Molecular Marker to Study Diversity of Endospore-forming Firmicutes. Environ. Microbiol. Rep. 2013, 5, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Španová, A.; Rittich, B.; Štyriak, I.; Štyriaková, I.; Horák, D. Isolation of Polymerase Chain Reaction-Ready Bacterial DNA from Lake Baikal Sediments by Carboxyl-Functionalised Magnetic Polymer Microspheres. J. Chromatogr. A 2006, 1130, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.M.; Wiedmann, M.; Kovac, J. Proposal of a Taxonomic Nomenclature for the Bacillus cereus Group Which Reconciles Genomic Definitions of Bacterial Species with Clinical and Industrial Phenotypes. mBio 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Fiedoruk, K.; Drewnowska, J.M.; Mahillon, J.; Zambrzycka, M.; Swiecicka, I. Pan-Genome Portrait of Bacillus mycoides Provides Insights into the Species Ecology and Evolution. Microbiol. Spectr. 2021, 9, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Aslam, U.; Ferdous, S.; Qin, M.; Siddique, A.; Billah, M.; Naeem, M.; Mahmood, Z.; Kayani, S. Combined Effect of Endophytic Bacillus mycoides and Rock Phosphate on the Amelioration of Heavy Metal Stress in Wheat Plants. BMC Plant Biol. 2024, 24, 125. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, A.; Stefanski, T.; Lisboa, B.B.; Beneduzi, A.; Vargas, L.K.; Passaglia, L.M.P. Diazotrophic Bacilli Isolated from the Sunflower Rhizosphere and the Potential of Bacillus mycoides B38V as Biofertiliser. Ann. Appl. Biol. 2016, 168, 93–110. [Google Scholar] [CrossRef]
- Yi, Y.; de Jong, A.; Frenzel, E.; Kuipers, O.P. Comparative Transcriptomics of Bacillus mycoides Root Exudates Reveals Different Genetic Adaptation of Endophytic and Soil Isolates. Front. Microbiol. 2017, 8, 1487. [Google Scholar] [CrossRef] [PubMed]
- Neher, O.T.; Johnston, M.R.; Zidack, N.K.; Jacobsen, B.J. Evaluation of Bacillus mycoides Isolate BmJ and B. mojavensis Isolate 203-7 for the Control of Anthracnose of Cucurbits Caused by Glomerella cingulata var. orbiculare. Biol. Control 2009, 48, 140–146. [Google Scholar] [CrossRef]
- Paul, B.; Charles, R.; Bhatnagar, T. Biological Control of Pythium mamillatum Causing Damping-off of Cucumber Seedlings by a Soil Bacterium, Bacillus mycoides. Microbiol. Res. 1995, 150, 71–75. [Google Scholar] [CrossRef]
- Peng, Y.H.; Chou, Y.J.; Liu, Y.C.; Jen, J.F.; Chung, K.R.; Huang, J.W. Inhibition of Cucumber Pythium Damping-off Pathogen with Zoosporicidal Biosurfactants Produced by Bacillus mycoides. J. Plant Dis. Prot. 2017, 124, 481–491. [Google Scholar] [CrossRef]
- Sharma, N.; Gautam, N. Antibacterial Activity and Characterization of Bacteriocin of Bacillus mycoides Isolated from Whey. Proc. Indian J. Biotechnol. 2008, 7, 117–121. [Google Scholar]
- Wu, J.J.; Huang, J.W.; Deng, W.L. Phenylacetic Acid and Methylphenyl Acetate From the Biocontrol Bacterium Bacillus mycoides BM02 Suppress Spore Germination in Fusarium oxysporum f. sp. lycopersici. Front. Microbiol. 2020, 11, 569263. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, S.N.P.; Fernando, W.G.D.; Rashid, K.Y. Identification of Antifungal Antibiotics of Bacillus Species Isolated from Different Microhabitats Using Polymerase Chain Reaction and MALDI-TOF Mass Spectrometry. Can. J. Microbiol. 2009, 55, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Azizoglu, U. Bacillus thuringiensis as a Biofertilizer and Biostimulator: A Mini-Review of the Little-Known Plant Growth-Promoting Properties of Bt. Curr. Microbiol. 2019, 76, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Toppo, P.; Kagatay, L.L.; Gurung, A.; Singla, P.; Chakraborty, R.; Roy, S.; Mathur, P. Endophytic Fungi Mediates Production of Bioactive Secondary Metabolites via Modulation of Genes Involved in Key Metabolic Pathways and Their Contribution in Different Biotechnological Sector. 3 Biotech. 2023, 13, 191. [Google Scholar] [CrossRef]
- Poria, V.; Dębiec-Andrzejewska, K.; Fiodor, A.; Lyzohub, M.; Ajijah, N.; Singh, S.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) Integrated Phytotechnology: A Sustainable Approach for Remediation of Marginal Lands. Front. Plant Sci. 2022, 13, 999866. [Google Scholar] [CrossRef] [PubMed]
- Ngalimat, M.S.; Mohd Hata, E.; Zulperi, D.; Ismail, S.I.; Ismail, M.R.; Mohd Zainudin, N.A.I.; Saidi, N.B.; Yusof, M.T. Plant Growth-Promoting Bacteria as an Emerging Tool to Manage Bacterial Rice Pathogens. Microorganisms 2021, 9, 682. [Google Scholar] [CrossRef]
- Kulkova, I.; Dobrzyński, J.; Kowalczyk, P.; Bełżecki, G.; Kramkowski, K. Plant Growth Promotion Using Bacillus cereus. Int. J. Mol. Sci. 2023, 24, 9759. [Google Scholar] [CrossRef]
- Bi, Y.; An, H.; Chi, Z.; Xu, Z.; Deng, Y.; Ren, Y.; Wang, R.; Lu, X.; Guo, J.; Hu, R.; et al. The Acetyltransferase SCO0988 Controls Positively Specialized Metabolism and Morphological Differentiation in the Model Strains Streptomyces coelicolor and Streptomyces lividans. Front. Microbiol. 2024, 15, 1366336. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The Evolutionary Origins of Pesticide Resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Fenta, L.; Mekonnen, H. Microbial Biofungicides as a Substitute for Chemical Fungicides in the Control of Phytopathogens: Current Perspectives and Research Directions. Scientifica 2024, 2024, 5322696. [Google Scholar] [CrossRef]
- Khan, M.R.; Anwer, M.A. Fungal Bioinoculants for Plant Disease Management. In Microbes and Microbial Technology: Agricultural and Environmental Applications; Ahmad, I., Ahmad, F., Pichtel, J., Eds.; Springer: New York, NY, USA, 2011; pp. 447–488. ISBN 9781441979308. [Google Scholar]
- Ristaino, J.B.; Anderson, P.K.; Bebber, D.P.; Brauman, K.A.; Cunniffe, N.J.; Fedoroff, N.V.; Finegold, C.; Garrett, K.A.; Gilligan, C.A.; Jones, C.M.; et al. The Persistent Threat of Emerging Plant Disease Pandemics to Global Food Security. Proc. Natl. Acad. Sci. USA 2021, 118, e2022239118. [Google Scholar] [CrossRef]
- Landschoot, S.; Carrette, J.; Vandecasteele, M.; De Baets, B.; Höfte, M.; Audenaert, K.; Haesaert, G. Boscalid-Resistance in Alternaria alternata and Alternaria solani Populations: An Emerging Problem in Europe. Crop Prot. 2017, 92, 49–59. [Google Scholar] [CrossRef]
- Bashan, Y.; Azaizeh, M.; Diab, S.; Yunis, H.; Okon, Y. Crop Loss of Pepper Plants Artificially Infected with Xanthomonas campestris pv. vesicatoria in Relation to Symptom Expression. Crop Prot. 1985, 4, 77–84. [Google Scholar] [CrossRef]
- Hameed, A.; Zeeshan, M.; Binyamin, R.; Alam, M.W.; Ali, S.; Zaheer, M.S.; Ali, H.; Riaz, M.W.; Ali, H.H.; Elshikh, M.S.; et al. Molecular Characterization of Pectobacterium atrosepticum Infecting Potato and Its Management through Chemicals. PeerJ 2024, 12, e17518. [Google Scholar] [CrossRef]
- Tenssay, Z.W.; Ashenafi, M.; Eiler, A.; Bertilson, S. Isolation and Characterization of Bacillus thuringiensis from Soils in Contrasting Agroecological Zones of Ethiopia. SINET Ethiop. J. Sci. 2011, 32, 117–128. [Google Scholar] [CrossRef]
- Travers, R.S.; Martin, P.A.W.; Reichelderfer, C.F. Selective Process for Efficient Isolation of Soil Bacillus spp. Appl. Environ. Microbiol. 1987, 53, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.S.; Johnstone, K.; Hagelberg, E.; Ellar, D.J. Commitment of Bacterial Spores to Germinate. A Measure of the Trigger Reaction. Biochem. J. 1981, 198, 101–106. [Google Scholar] [CrossRef]
- Hendriksen, N.B.; Hansen, B.M. Diagnostic Properties of Three Conventional Selective Plating Media for Selection of Bacillus cereus, B. thuringiensis and B. weihenstephanensis. Folia Microbiol. 2011, 56, 535–539. [Google Scholar] [CrossRef]
- Schneider, S.; Hendriksen, N.B.; Melin, P.; Lundström, J.O.; Sundh, I. Chromosome-Directed PCR-Based Detection and Quantification of Bacillus cereus Group Members with Focus on B. thuringiensis Serovar Israelensis Active against Nematoceran Larvae. Appl. Environ. Microbiol. 2015, 81, 4894–4903. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA Sequencing with Chain-Terminating Inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Werle, E.; Schneider, C.; Renner, M.; Völker, M.; Fiehn, W. Convenient Single-Step, One Tube Purification of PCR Products for Direct Sequencing. Nucleic Acids Res. 1994, 22, 4354–4355. [Google Scholar] [CrossRef]
- Sci, W.J. A Comparison of Two Methods Used for Measuring the Antagonistic Activity of Bacillus Species. Culture 2008, 5, 161–171. [Google Scholar]
- Reddy, G.K.; Leferink, N.G.H.; Umemura, M.; Ahmed, S.T.; Breitling, R.; Scrutton, N.S.; Takano, E. Exploring Novel Bacterial Terpene Synthases. PLoS ONE 2020, 15, e0232220. [Google Scholar] [CrossRef] [PubMed]
- López-González, R.C.; Juárez-Campusano, Y.S.; Rodríguez-Chávez, J.L.; Delgado-Lamas, G.; Medrano, S.M.A.; Martínez-Peniche, R.Á.; Pacheco-Aguilar, J.R. Antagonistic Activity of Bacteria Isolated from Apple in Different Fruit Development Stages against Blue Mold Caused by Penicillium expansum. Plant Pathol. J. 2021, 37, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, M.N.; Nesterenko, M.A.; Shikov, A.E.; Nizhnikov, A.A.; Antonets, K.S. Draft Genome Sequence Data of Lysinibacillus sphaericus Strain 1795 with Insecticidal Properties. Data 2023, 8, 167. [Google Scholar] [CrossRef]
- Saleem, F.; Shakoori, A.R. The First Cry2Ac-Type Protein Toxic to Helicoverpa armigera: Cloning and Overexpression of cry2ac7 Gene from SBS-BT1 Strain of Bacillus thuringiensis. Toxins 2017, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Spizizen, J. Transformation of Biochemically Deficient Strains of Bacillus subtilis by Deoxyribonucleate. Proc. Natl. Acad. Sci. USA 1958, 44, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Institute: Cambridge, UK, 2010. [Google Scholar]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference Sequence (RefSeq) Database at NCBI: Current Status, Taxonomic Expansion, and Functional Annotation. Nucleic Acids Res. 2016, 44, D733-45. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High Throughput ANI Analysis of 90K Prokaryotic Genomes Reveals Clear Species Boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.-L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic Gene Recognition and Translation Initiation Site Identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, J.; Bo, D.; Yu, Y.; Ye, W.; Peng, D.; Sun, M. BtToxin_Digger: A Comprehensive and High-Throughput Pipeline for Mining Toxin Protein Genes from Bacillus thuringiensis. Bioinformatics 2022, 38, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.E.; Malovichko, Y.V.; Skitchenko, R.K.; Nizhnikov, A.A.; Antonets, K.S. No More Tears: Mining Sequencing Data for Novel Bt Cry Toxins with CryProcessor. Toxins 2020, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A Structure-Based Nomenclature for Bacillus thuringiensis and Other Bacteria-Derived Pesticidal Proteins. J. Invertebr. Pathol. 2021, 186, 107438. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, G.D.; Prihoda, D.; Palicka, A.; Soukup, J.; Klempir, O.; Rampula, L.; Durcak, J.; Wurst, M.; Kotowski, J.; Chang, D.; et al. A Deep Learning Genome-Mining Strategy for Biosynthetic Gene Cluster Prediction. Nucleic Acids Res. 2019, 47, e110. [Google Scholar] [CrossRef]
- Barrett, T.; Clark, K.; Gevorgyan, R.; Gorelenkov, V.; Gribov, E.; Karsch-Mizrachi, I.; Kimelman, M.; Pruitt, K.D.; Resenchuk, S.; Tatusova, T.; et al. BioProject and BioSample Databases at NCBI: Facilitating Capture and Organization of Metadata. Nucleic Acids Res. 2012, 40, D57–D63. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Tonkin-Hill, G.; MacAlasdair, N.; Ruis, C.; Weimann, A.; Horesh, G.; Lees, J.A.; Gladstone, R.A.; Lo, S.; Beaudoin, C.; Floto, R.A.; et al. Producing Polished Prokaryotic Pangenomes with the Panaroo Pipeline. Genome Biol. 2020, 21, 180. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A New and Scalable Tool for the Selection of DNA and Protein Evolutionary Models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef]
- Page, A.J.; Taylor, B.; Delaney, A.J.; Soares, J.; Seemann, T.; Keane, J.A.; Harris, S.R. SNP-Sites: Rapid Efficient Extraction of SNPs from Multi-FASTA Alignments. Microb. Genom. 2016, 2, e000056. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A Fast, Scalable and User-Friendly Tool for Maximum Likelihood Phylogenetic Inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Tsen, H.Y. Discrimination of Bacillus cereus and Bacillus thuringiensis with 16S rRNA and gyrB Gene Based PCR Primers and Sequencing of Their Annealing Sites. J. Appl. Microbiol. 2002, 92, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, C.; Beccari, E.; Santini, T.; Pisaneschi, G.; Tecce, G. Colony Shape as a Genetic Trait in the Pattern-Forming Bacillus mycoides. BMC Microbiol. 2002, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Haldar, S.; Pandey, K.K.; Singh, R.P.; Singh, A.K.; Singh, P.C. Cultural, Morphological, Pathogenic and Molecular Variability amongst Tomato Isolates of Alternaria solani in India. World J. Microbiol. Biotechnol. 2008, 24, 1003–1009. [Google Scholar] [CrossRef]
- Chaerani, R.; Voorrips, R.E. Tomato Early Blight (Alternaria solani): The Pathogen, Genetics, and Breeding for Resistance. J. Gen. Plant Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Van Der Waals, J.E.; Korsten, L.; Slippers, B. Genetic Diversity among Alternaria solani Isolates from Potatoes in South Africa. Plant Dis. 2004, 88, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Lingwal, S.; Sinha, A.; Rai, J.P.; Prabhakar, C.S.; Srinivasaraghavan, A. Brown Spot of Potato Caused by Alternaria alternata: An Emerging Problem of Potato in Eastern India. Potato Res. 2022, 65, 693–705. [Google Scholar] [CrossRef]
- Parks, J.; Roberts, B.; Thayer, R.E. Pesticidal Genes and Methods of Use. Patent US11046973B2, 29 September 2021. [Google Scholar]
- Cao, B.; Shu, C.; Geng, L.; Song, F.; Zhang, J. Cry78Ba1, One Novel Crystal Protein from Bacillus thuringiensis with High Insecticidal Activity against Rice Planthopper. J. Agric. Food Chem. 2020, 68, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, H.; Nakashima, K.; Ishida, C.; Kawamura, T.; Matsuda, K. Cloning, Functional Characterization, and Mode of Action of a Novel Insecticidal Pore-Forming Toxin, Sphaericolysin, Produced by Bacillus sphaericus. Appl. Environ. Microbiol. 2007, 73, 3404–3411. [Google Scholar] [CrossRef] [PubMed]
- Stelder, S.K.; Mahmud, S.A.; Dekker, H.L.; de Koning, L.J.; Brul, S.; de Koster, C.G. Temperature Dependence of the Proteome Profile of the Psychrotolerant Pathogenic Food Spoiler Bacillus weihenstephanensis Type Strain WSBC J. Proteome Res. 2015, 14, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Jian, J.; Beno, S.M.; Wiedmann, M.; Kovac, J. Intraclade Variability in Toxin Production and Cytotoxicity of Bacillus cereus Group Type Strains and Dairy-Associated Isolates. Appl. Environ. Microbiol. 2018, 84, e02479-17. [Google Scholar] [CrossRef] [PubMed]
- Bucher, T.; Keren-Paz, A.; Hausser, J.; Olender, T.; Cytryn, E.; Kolodkin-Gal, I. An Active Β-lactamase Is a Part of an Orchestrated Cell Wall Stress Resistance Network of Bacillus subtilis and Related Rhizosphere Species. Environ. Microbiol. 2019, 21, 1068–1085. [Google Scholar] [CrossRef]
- Bağcıoğlu, M.; Fricker, M.; Johler, S.; Ehling-Schulz, M. Detection and Identification of Bacillus cereus, Bacillus cytotoxicus, Bacillus thuringiensis, Bacillus mycoides and Bacillus weihenstephanensis via Machine Learning Based FTIR Spectroscopy. Front. Microbiol. 2019, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Borah, M.; Mandal, M.; Konwar, B.K. Characterization of Probiotic Strains of Bacillus sp. from Fermented Palm Wine (Nypa fructicans sp.) and Exploration of Cellulolytic Potential for Use as an Addition in Animal Feed. Int. Microbiol. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.R.; Babusha, S.T.; George, J.; Ramana, K.V. Material Properties and Antimicrobial Activity of Polyhydroxybutyrate (PHB) Films Incorporated with Vanillin. Appl. Biochem. Biotechnol. 2015, 176, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Bashir, S.; Imran, M. Probiotic Characterization of Bacillus Species Strains Isolated from an Artisanal Fermented Milk Product Dahi. Folia Microbiol. 2023, 68, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Avendaño, E.; Carrillo, J.D.; Ndinga-Muniania, C.; Moreno, K.; Méndez-Bravo, A.; Guerrero-Analco, J.A.; Eskalen, A.; Reverchon, F. Antifungal Activity of Avocado Rhizobacteria against Fusarium euwallaceae and Graphium spp., Associated with Euwallacea spp. nr. fornicatus, and Phytophthora ciinnamomi. Antonie van Leeuwenhoek 2018, 111, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Hammad, A.M.M.; El-Mohandes, M.A.O. Controlling Fusarium Wilt Disease of Cucumber Plants via Antagonistic Microorganisms in Free and Immobilized States. Microbiol. Res. 1999, 154, 113–117. [Google Scholar] [CrossRef]
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Fischer, E.; Dinoor, A. Improving Biological Control by Combining Biocontrol Agents Each with Several Mechanisms of Disease Suppression. Phytopathology 2002, 92, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Guetsky, R.; Elad, Y.; Shtienberg, D.; Dinoor, A. Improved Biocontrol of Botrytis cinerea on Detached Strawberry Leaves by Adding Nutritional Supplements to a Mixture of Pichia guilermondii and Bacillus mycoides. Biocontrol Sci. Technol. 2002, 12, 625–630. [Google Scholar] [CrossRef]
- Mikhailov, I.S.; Bukin, Y.S.; Zakharova, Y.R.; Usoltseva, M.V.; Galachyants, Y.P.; Sakirko, M.V.; Blinov, V.V.; Likhoshway, Y.V. Co-Occurrence Patterns between Phytoplankton and Bacterioplankton across the Pelagic Zone of Lake Baikal during Spring. J. Microbiol. 2019, 57, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, O.N.; Adamovich, S.N.; Novikova, A.S.; Gorshkov, A.G.; Izosimova, O.N.; Ushakov, I.A.; Oborina, E.N.; Mirskova, A.N.; Zemskaya, T.I. Protatranes, Effective Growth Biostimulants of Hydrocarbon-Oxidizing Bacteria from Lake Baikal, Russia. Biotechnol. Rep. 2019, 24, e00371. [Google Scholar] [CrossRef]
- Parfenova, V.V.; Shimaraev, M.N.; Kostornova, T.Y.; Domysheva, V.M.; Levin, L.A.; Dryukker, V.V.; Zhdanov, A.A.; Gnatovskii, R.Y.; Tsekhanovskii, V.V.; Logacheva, N.F. On the Vertical Distribution of Microorganisms in Lake Baikal during Spring Deep-Water Renewal. Microbiology 2000, 69, 357–363. [Google Scholar] [CrossRef]
- Bannikova, G.E.; Lopatin, S.A.; Varlamov, V.P.; Kuznetsov, B.B.; Kozina, I.V.; Miroshnichenko, M.L.; Chernykh, N.A.; Turova, T.P.; Bonch-Osmolovskaya, E.A. The Thermophilic Bacteria Hydrolyzing Agar: Characterization of Thermostable Agarase. Appl. Biochem. Microbiol. 2008, 44, 366–371. [Google Scholar] [CrossRef]
- Lapteva, N.A.; Bel’kova, N.L.; Parfenova, V.V. Spatial Distribution and Species Composition of Prosthecate Bacteria in Lake Baikal. Microbiology 2007, 76, 480–486. [Google Scholar] [CrossRef]
- Galach’yants, A.D.; Bel’kova, N.L.; Sukhanova, E.V.; Galach’yants, Y.P.; Morozov, A.A.; Parfenova, V.V. Taxonomic Composition of Lake Baikal Bacterioneuston Communities. Microbiology 2017, 86, 241–249. [Google Scholar] [CrossRef]
- Chernitsina, S.M.; Zemskaya, T.I.; Vorob’eva, S.S.; Shubenkova, O.V.; Khlystov, O.M.; Kostornova, T.Y. Comparative Molecular Biological Analysis of the Microbial Community of the Holocene and Pleistocene Deposits of Posol’skaya Shoal, Lake Baikal. Microbiology 2007, 76, 102–111. [Google Scholar] [CrossRef]
- Voitsekhovskaia, I.; Paulus, C.; Dahlem, C.; Rebets, Y.; Nadmid, S.; Zapp, J.; Axenov-Gribanov, D.; Rückert, C.; Timofeyev, M.; Kalinowski, J.; et al. Baikalomycins A-C, New Aquayamycin-Type Angucyclines Isolated from Lake Baikal Derived Streptomyces sp. IB201691-2A. Microorganisms 2020, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Paulus, C.; Rebets, Y.; Zapp, J.; Rückert, C.; Kalinowski, J.; Luzhetskyy, A. New Alpiniamides From Streptomyces sp. IB2014/011-12 Assembled by an Unusual Hybrid Non-Ribosomal Peptide Synthetase Trans-AT Polyketide Synthase Enzyme. Front. Microbiol. 2018, 9, 1959. [Google Scholar] [CrossRef] [PubMed]
- Protasov, E.S.; Axenov-Gribanov, D.V.; Rebets, Y.V.; Voytsekhovskaya, I.V.; Tokovenko, B.T.; Shatilina, Z.M.; Luzhetskyy, A.N.; Timofeyev, M.A. The Diversity and Antibiotic Properties of Actinobacteria Associated with Endemic Deepwater Amphipods of Lake Baikal. Antonie van Leeuwenhoek 2017, 110, 1593–1611. [Google Scholar] [CrossRef] [PubMed]
- Axenov-Gribanov, D.V.; Voytsekhovskaya, I.V.; Rebets, Y.V.; Tokovenko, B.T.; Penzina, T.A.; Gornostay, T.G.; Adelshin, R.V.; Protasov, E.S.; Luzhetskyy, A.N.; Timofeyev, M.A. Actinobacteria Possessing Antimicrobial and Antioxidant Activities Isolated from the Pollen of Scots Pine (Pinus sylvestris) Grown on the Baikal Shore. Antonie van Leeuwenhoek 2016, 109, 1307–1322. [Google Scholar] [CrossRef]
- Axenov-Gribanov, D.; Rebets, Y.; Tokovenko, B.; Voytsekhovskaya, I.; Timofeyev, M.; Luzhetskyy, A. The Isolation and Characterization of Actinobacteria from Dominant Benthic Macroinvertebrates Endemic to Lake Baikal. Folia Microbiol. 2016, 61, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, I.S.; Oreshkova, S.F.; Ryabchikova, E.I.; Puchkova, L.I.; Blinova, N.N.; Repina, M.V.; Pechurkina, N.I.; Torok, T.; Repin, V.E. Genomic and Phenotypic Analyses of Microorganisms Isolated from the Sediments of Lake Baikal. Microbiology 2005, 74, 709–714. [Google Scholar] [CrossRef]
- Zakharova, Y.R.; Parfenova, V.V. A Method for Cultivation of Microorganisms Oxidizing Iron and Manganese in Bottom Sediments of Lake Baikal. Biol. Bull. 2007, 34, 236–241. [Google Scholar] [CrossRef]
- Lomakina, A.V.; Pavlova, O.N.; Shubenkova, O.V.; Zemskaya, T.I. Diversity of Cultured Aerobic Organisms in the Areas of Natural Oil Seepage on Lake Baikal. Biol. Bull. 2009, 36, 430–436. [Google Scholar] [CrossRef]
- Dambaev, V.B.; Gonchikov, G.G.; Buryukhaev, S.P.; Tsyrenov, B.S.; Zyakun, A.M.; Namsaraev, B.B. Microbiological and Isotopic Geochemical Research in the Arid Steppe Lakes and Sor Solonchaks of Western Transbaikalia. Microbiology 2011, 80, 857–866. [Google Scholar] [CrossRef]
- Cabello-Yeves, P.J.; Zemskaya, T.I.; Rosselli, R.; Coutinho, F.H.; Zakharenko, A.S.; Blinov, V.V.; Rodriguez-Valera, F. Genomes of Novel Microbial Lineages Assembled from the Sub-Ice Waters of Lake Baikal. Appl. Environ. Microbiol. 2018, 84, e02132-17. [Google Scholar] [CrossRef] [PubMed]
- Bel’kova, N.L.; Parfenova, V.V.; Suslova, M.Y.; Ahn, T.S.; Tazaki, K. Biodiversity and Activity of the Microbial Community in the Kotelnikovsky Hot Springs (Lake Baikal). Biol. Bull. 2005, 32, 549–555. [Google Scholar] [CrossRef]
- Pavlova, O.N.; Zemskaya, T.I.; Gorshkov, A.G.; Parfenova, V.V.; Suslova, M.Y.; Khlystov, O.M. Study on the Lake Baikal Microbial Community in the Areas of the Natural Oil Seeps. Appl. Biochem. Microbiol. 2008, 44, 287–291. [Google Scholar] [CrossRef]
- Parfenova, V.V.; Terkina, I.A.; Kostornova, T.Y.; Nikulina, I.G.; Chernykh, V.I.; Maksimova, E.A. Microbial Community of Freshwater Sponges in Lake Baikal. Biol. Bull. 2008, 35, 374–379. [Google Scholar] [CrossRef]
- Pavlova, O.N.; Tupikin, A.E.; Chernitsyna, S.M.; Bukin, Y.S.; Lomakina, A.V.; Pogodaeva, T.V.; Nikonova, A.A.; Bukin, S.V.; Zemskaya, T.I.; Kabilov, M.R. Description and Genomic Analysis of the First Facultatively Lithoautotrophic, Thermophilic Bacteria of the Genus Thermaerobacter Isolated from Low-Temperature Sediments of Lake Baikal. Microb. Ecol. 2023, 86, 1604–1619. [Google Scholar] [CrossRef]
- Butina, T.V.; Bukin, Y.S.; Krasnopeev, A.S.; Belykh, O.I.; Tupikin, A.E.; Kabilov, M.R.; Sakirko, M.V.; Belikov, S.I. Estimate of the Diversity of Viral and Bacterial Assemblage in the Coastal Water of Lake Baikal. FEMS Microbiol. Lett. 2019, 366, fnz094. [Google Scholar] [CrossRef] [PubMed]
- Haro-Moreno, J.M.; Cabello-Yeves, P.J.; Garcillán-Barcia, M.P.; Zakharenko, A.; Zemskaya, T.I.; Rodriguez-Valera, F. A Novel and Diverse Group of Candidatus Patescibacteria from Bathypelagic Lake Baikal Revealed through Long-Read Metagenomics. Environ. Microbiome 2023, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Podosokorskaya, O.A.; Elcheninov, A.G.; Novikov, A.A.; Merkel, A.Y.; Kublanov, I.V. Fontisphaera Persica Gen. Nov., Sp. Nov., a Thermophilic Hydrolytic Bacterium from a Hot Spring of Baikal Lake Region, and Proposal of Fontisphaeraceae fam. nov., and Limisphaeraceae fam. nov. within the Limisphaerales ord. nov. (Verrucomicrobiota). Syst. Appl. Microbiol. 2023, 46, 126438. [Google Scholar] [CrossRef]
- Soutourina, O.A.; Semenova, E.A.; Parfenova, V.V.; Danchin, A.; Bertin, P. Control of Bacterial Motility by Environmental Factors in Polarly Flagellated and Peritrichous Bacteria Isolated from Lake Baikal. Appl. Environ. Microbiol. 2001, 67, 3852–3859. [Google Scholar] [CrossRef] [PubMed]
- Evseev, P.; Tikhonova, I.; Krasnopeev, A.; Sorokovikova, E.; Gladkikh, A.; Timoshkin, O.; Miroshnikov, K.; Belykh, O. Tychonema sp. BBK16 Characterisation: Lifestyle, Phylogeny and Related Phages. Viruses 2023, 15, 442. [Google Scholar] [CrossRef] [PubMed]
- Chernogor, L.; Bakhvalova, K.; Belikova, A.; Belikov, S. Isolation and Properties of the Bacterial Strain Janthinobacterium sp. SLB01. Microorganisms 2022, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, Y.R.; Galachyants, Y.P.; Kurilkina, M.I.; Likhoshvay, A.V.; Petrova, D.P.; Shishlyannikov, S.M.; Ravin, N.V.; Mardanov, A.V.; Beletsky, A.V.; Likhoshway, Y.V. The Structure of Microbial Community and Degradation of Diatoms in the Deep Near-Bottom Layer of Lake Baikal. PLoS ONE 2013, 8, e59977. [Google Scholar] [CrossRef] [PubMed]
- Chernogor, L.; Eliseikina, M.; Petrushin, I.; Chernogor, E.; Khanaev, I.; Belikov, S.I. Janthinobacterium sp. Strain SLB01 as Pathogenic Bacteria for Sponge Lubomirskia baikalensis. Pathogens 2022, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Shchapova, E.; Nazarova, A.; Vasilyeva, U.; Gurkov, A.; Ostyak, A.; Mutin, A.; Adelshin, R.; Belkova, N.; Timofeyev, M. Cellular Immune Response of an Endemic Lake Baikal Amphipod to Indigenous Pseudomonas sp. Mar. Biotechnol. 2021, 23, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Belikov, S.; Belkova, N.; Butina, T.; Chernogor, L.; Martynova-Van Kley, A.; Nalian, A.; Rorex, C.; Khanaev, I.; Maikova, O.; Feranchuk, S. Diversity and Shifts of the Bacterial Community Associated with Baikal Sponge Mass Mortalities. PLoS ONE 2019, 14, e0213926. [Google Scholar] [CrossRef] [PubMed]
- Kadnikov, V.V.; Mardanov, A.V.; Beletsky, A.V.; Shubenkova, O.V.; Pogodaeva, T.V.; Zemskaya, T.I.; Ravin, N.V.; Skryabin, K.G. Microbial Community Structure in Methane Hydrate-Bearing Sediments of Freshwater Lake Baikal. FEMS Microbiol. Ecol. 2012, 79, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, I.S.; Zakharova, Y.R.; Galachyants, Y.P.; Usoltseva, M.V.; Petrova, D.P.; Sakirko, M.V.; Likhoshway, Y.V.; Grachev, M.A. Similarity of Structure of Taxonomic Bacterial Communities in the Photic Layer of Lake Baikal’s Three Basins Differing in Spring Phytoplankton Composition and Abundance. Dokl. Biochem. Biophys. 2015, 465, 413–419. [Google Scholar] [CrossRef]
- Podosokorskaya, O.A.; Elcheninov, A.G.; Novikov, A.A.; Kublanov, I.V. Fontivita pretiosa gen. nov., sp. nov., a Thermophilic Planctomycete of the Order Tepidisphaerales from a Hot Spring of Baikal Lake Region. Syst. Appl. Microbiol. 2022, 45, 126375. [Google Scholar] [CrossRef] [PubMed]
- Chernitsyna, S.M.; Elovskaya, I.S.; Bukin, S.V.; Bukin, Y.S.; Pogodaeva, T.V.; Kwon, D.A.; Zemskaya, T.I. Genomic and Morphological Characterization of a New Thiothrix Species from a Sulfide Hot Spring of the Zmeinaya Bay (Northern Baikal, Russia). Antonie van Leeuwenhoek 2024, 117, 23. [Google Scholar] [CrossRef]
- Pavlova, O.N.; Zemskaya, T.I.; Gorshkov, A.G.; Kostornova, T.Y.; Khlystov, O.M.; Parfenova, V.V. Comparative Characterization of Microbial Communities in Two Regions of Natural Oil Seepage in Lake Baikal. Biol. Bull. 2008, 35, 287–293. [Google Scholar] [CrossRef]
- Dagurova, O.P.; Namsaraev, B.B.; Kozyreva, L.P.; Zemskaya, T.I.; Dulov, L.E. Bacterial Processes of the Methane Cycle in Bottom Sediments of Lake Baikal. Microbiology 2004, 73, 202–210. [Google Scholar] [CrossRef]
- Kaluzhnaya, O.V.; Itskovich, V.B. Phylogenetic Diversity of Microorganisms Associated with the Deep-Water Sponge Baikalospongia intermedia. Russ. J. Genet. 2014, 50, 667–676. [Google Scholar] [CrossRef]
- Voytsekhovskaya, I.V.; Axenov-Gribanov, D.V.; Murzina, S.A.; Pekkoeva, S.N.; Protasov, E.S.; Gamaiunov, S.V.; Timofeyev, M.A. Estimation of Antimicrobial Activities and Fatty Acid Composition of Actinobacteria Isolated from Water Surface of Underground Lakes from Badzheyskaya and Okhotnichya Caves in Siberia. PeerJ 2018, 6, e5832. [Google Scholar] [CrossRef]
- Terkina, I.A.; Parfenova, V.V.; Ahn, T.S. Antagonistic Activity of Actinomycetes of Lake Baikal. Appl. Biochem. Microbiol. 2006, 42, 173–176. [Google Scholar] [CrossRef]
- Lomakina, A.V.; Pogodaeva, T.V.; Morozov, I.V.; Zemskaya, T.I. Microbial Communities of the Discharge Zone of Oil- and Gas-Bearing Fluids in Low-Mineral Lake Baikal. Microbiology 2014, 83, 278–287. [Google Scholar] [CrossRef]
- Petrushin, I.; Belikov, S.; Chernogor, L. Cooperative Interaction of Janthinobacterium sp. SLB01 and Flavobacterium sp. SLB02 in the Diseased Sponge Lubomirskia baicalensis. Int. J. Mol. Sci. 2020, 21, 8128. [Google Scholar] [CrossRef]
- Shishlyannikova, T.A.; Kuzmin, A.V.; Fedorova, G.A.; Shishlyannikov, S.M.; Lipko, I.A.; Sukhanova, E.V.; Belkova, N.L. Ionofore Antibiotic Polynactin Produced by Streptomyces sp. 156A Isolated from Lake Baikal. Nat. Prod. Res. 2017, 31, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Sobolevskaya, M.P.; Terkina, I.A.; Buzoleva, L.S.; Li, I.A.; Kusaikin, M.I.; Verigina, N.S.; Mazeika, A.N.; Shevchenko, L.S.; Burtseva, Y.V.; Zvyagintseva, T.N.; et al. Biologically Active Compounds from Lake Baikal Streptomycetes. Chem. Nat. Compd. 2006, 42, 82–87. [Google Scholar] [CrossRef]
- Sokolova, T.G.; Kostrikina, N.A.; Chernyh, N.A.; Kolganova, T.V.; Tourova, T.P.; Bonch-Osmolovskaya, E.A. Thermincola carboxydiphila gen. nov., sp. nov., a Novel Anaerobic, Carboxydotrophic, Hydrogenogenic Bacterium from a Hot Spring of the Lake Baikal Area. Int. J. Syst. Evol. Microbiol. 2005, 55, 2069–2073. [Google Scholar] [CrossRef]
- Zhilina, T.N.; Kevbrin, V.V.; Tourova, T.P.; Lysenko, A.M.; Kostrikina, N.A.; Zavarzin, G.A. Clostridium alkalicellum sp. nov., an Obligately Alkaliphilic Cellulolytic Bacterium from a Soda Lake in the Baikal Region. Microbiology 2005, 74, 557–566. [Google Scholar] [CrossRef]
- Kaluzhnaya, O.V.; Krivich, A.A.; Itskovich, V.B. Diversity of 16S RRNA Genes in Metagenomic Community of the Freshwater Sponge Lubomirskia baicalensis. Russ. J. Genet. 2012, 48, 855–858. [Google Scholar] [CrossRef]
- Safronova, V.I.; Sazanova, A.L.; Kuznetsova, I.G.; Belimov, A.A.; Andronov, E.E.; Chirak, E.R.; Popova, J.P.; Verkhozina, A.V.; Willems, A.; Tikhonovich, I.A. Phyllobacterium zundukense sp. nov., a Novel Species of Rhizobia Isolated from Root Nodules of the Legume Species Oxytropis triphylla (Pall.) Pers. Int. J. Syst. Evol. Microbiol. 2018, 68, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, V.V.; Gladkikh, A.S.; Belykh, O.I. Comparative Analysis of Biodiversity in the Planktonic and Biofilm Bacterial Communities in Lake Baikal. Microbiology 2013, 82, 91–101. [Google Scholar] [CrossRef]
- Safronova, V.; Belimov, A.; Sazanova, A.; Chirak, E.; Kuznetsova, I.; Andronov, E.; Pinaev, A.; Tsyganova, A.; Seliverstova, E.; Kitaeva, A.; et al. Two Broad Host Range Rhizobial Strains Isolated From Relict Legumes Have Various Complementary Effects on Symbiotic Parameters of Co-Inoculated Plants. Front. Microbiol. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Likhoshvay, A.; Lomakina, A.; Grachev, M. The Complete Alk Sequences of Rhodococcus erythropolis from Lake Baikal. Springerplus 2014, 3, 621. [Google Scholar] [CrossRef] [PubMed]
- Kaluzhnaya, O.V.; Itskovich, V.B. Distinctive Features of the Microbial Diversity and the Polyketide Synthase Genes Spectrum in the Community of the Endemic Baikal Sponge Swartschewskia papyracea. Russ. J. Genet. 2016, 52, 38–48. [Google Scholar] [CrossRef]
- Gladkikh, A.S.; Belykh, O.I.; Klimenkov, I.V.; Tikhonova, I.V. Nitrogen-Fixing Cyanobacterium Trichormus variabilis of the Lake Baikal Phytoplankton. Microbiology 2008, 77, 726–733. [Google Scholar] [CrossRef]
- Mikhailov, I.S.; Zakharova, Y.R.; Bukin, Y.S.; Galachyants, Y.P.; Petrova, D.P.; Sakirko, M.V.; Likhoshway, Y.V. Co-Occurrence Networks Among Bacteria and Microbial Eukaryotes of Lake Baikal During a Spring Phytoplankton Bloom. Microb. Ecol. 2019, 77, 96–109. [Google Scholar] [CrossRef]
- Galachyants, A.D.; Krasnopeev, A.Y.; Podlesnaya, G.V.; Potapov, S.A.; Sukhanova, E.V.; Tikhonova, I.V.; Zimens, E.A.; Kabilov, M.R.; Zhuchenko, N.A.; Gorshkova, A.S.; et al. Diversity of Aerobic Anoxygenic Phototrophs and Rhodopsin-Containing Bacteria in the Surface Microlayer, Water Column and Epilithic Biofilms of Lake Baikal. Microorganisms 2021, 9, 842. [Google Scholar] [CrossRef] [PubMed]
- Galachyants, A.D.; Tomberg, I.V.; Sukhanova, E.V.; Shtykova, Y.R.; Suslova, M.Y.; Zimens, E.A.; Blinov, V.V.; Sakirko, M.V.; Domysheva, V.M.; Belykh, O.I. Bacterioneuston in Lake Baikal: Abundance, Spatial and Temporal Distribution. Int. J. Environ. Res. Public Health 2018, 15, 2587. [Google Scholar] [CrossRef] [PubMed]
- Lomakina, A.V.; Bukin, S.V.; Pogodaeva, T.V.; Turchyn, A.V.; Khlystov, O.M.; Khabuev, A.V.; Ivanov, V.G.; Krylov, A.A.; Zemskaya, T.I. Microbial Diversity and Authigenic Siderite Mediation in Sediments Surrounding the Kedr-1 Mud Volcano, Lake Baikal. Geobiology 2023, 21, 770–790. [Google Scholar] [CrossRef] [PubMed]
- Bashenkhaeva, M.V.; Zakharova, Y.R.; Petrova, D.P.; Khanaev, I.V.; Galachyants, Y.P.; Likhoshway, Y.V. Sub-Ice Microalgal and Bacterial Communities in Freshwater Lake Baikal, Russia. Microb. Ecol. 2015, 70, 751–765. [Google Scholar] [CrossRef]
- Dmitrieva, M.E.; Malygina, E.V.; Belyshenko, A.Y.; Shelkovnikova, V.N.; Imidoeva, N.A.; Morgunova, M.M.; Telnova, T.Y.; Vlasova, A.A.; Axenov-Gribanov, D.V. The Effects of a High Concentration of Dissolved Oxygen on Actinobacteria from Lake Baikal. Metabolites 2023, 13, 830. [Google Scholar] [CrossRef]
- Chernitsyna, S.M.; Khalzov, I.A.; Sitnikova, T.Y.; Naumova, T.V.; Khabuev, A.V.; Zemskaya, T.I. Microbial Communities Associated with Bentic Invertebrates of Lake Baikal. Curr. Microbiol. 2021, 78, 3020–3031. [Google Scholar] [CrossRef] [PubMed]
- Belykh, O.I.; Gladkikh, A.S.; Sorokovikova, E.G.; Tikhonova, I.V.; Butina, T.V. Identification of Toxic Cyanobacteria in Lake Baikal. Dokl. Biochem. Biophys. 2015, 463, 220–224. [Google Scholar] [CrossRef]
- Galach’yants, A.D.; Bel’kova, N.L.; Sukhanova, E.V.; Romanovskaya, V.A.; Gladka, G.V.; Bedoshvili, E.D.; Parfenova, V.V. Diversity and Physiological and Biochemical Properties of Heterotrophic Bacteria Isolated from Lake Baikal Neuston. Microbiology 2016, 85, 604–613. [Google Scholar] [CrossRef]
- Butina, T.V.; Petrushin, I.S.; Khanaev, I.V.; Bukin, Y.S. Metagenomic Assessment of DNA Viral Diversity in Freshwater Sponges, Baikalospongia bacillifera. Microorganisms 2022, 10, 480. [Google Scholar] [CrossRef]
- Belykh, O.I.; Tikhonova, I.V.; Kuzmin, A.V.; Sorokovikova, E.G.; Fedorova, G.A.; Khanaev, I.V.; Sherbakova, T.A.; Timoshkin, O.A. First Detection of Benthic Cyanobacteria in Lake Baikal Producing Paralytic Shellfish Toxins. Toxicon 2016, 121, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Bukin, S.V.; Pavlova, O.N.; Manakov, A.Y.; Kostyreva, E.A.; Chernitsyna, S.M.; Mamaeva, E.V.; Pogodaeva, T.V.; Zemskaya, T.I. The Ability of Microbial Community of Lake Baikal Bottom Sediments Associated with Gas Discharge to Carry Out the Transformation of Organic Matter under Thermobaric Conditions. Front. Microbiol. 2016, 7, 690. [Google Scholar] [CrossRef]
- Bondarenko, N.A.; Belykh, O.I.; Golobokova, L.P.; Artemyeva, O.V.; Logacheva, N.F.; Tikhonova, I.V.; Lipko, I.A.; Kostornova, T.Y.; Parfenova, V.V.; Khodzher, T.V.; et al. Stratified Distribution of Nutrients and Extremophile Biota within Freshwater Ice Covering the Surface of Lake Baikal. J. Microbiol. 2012, 50, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Zemskaya, T.I.; Chernitsyna, S.M.; Dul’tseva, N.M.; Sergeeva, V.N.; Pogodaeva, T.V.; Namsaraev, B.B. Colorless Sulfur Bacteria Thioploca from Different Sites in Lake Baikal. Microbiology 2009, 78, 117–124. [Google Scholar] [CrossRef]
- Sorokovikova, E.G.; Belykh, O.I.; Gladkikh, A.S.; Kotsar, O.V.; Tikhonova, I.V.; Timoshkin, O.A.; Parfenova, V.V. Diversity of Cyanobacterial Species and Phylotypes in Biofilms from the Littoral Zone of Lake Baikal. J. Microbiol. 2013, 51, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Chernogor, L.; Klimenko, E.; Khanaev, I.; Belikov, S. Microbiome Analysis of Healthy and Diseased Sponges Lubomirskia Baicalensis by Using Cell Cultures of Primmorphs. PeerJ 2020, 8, e9080. [Google Scholar] [CrossRef] [PubMed]
- Kulakova, N.V.; Sakirko, M.V.; Adelshin, R.V.; Khanaev, I.V.; Nebesnykh, I.A.; Pérez, T. Brown Rot Syndrome and Changes in the Bacterial Community of the Baikal Sponge Lubomirskia baicalensis. Microb. Ecol. 2018, 75, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, V.V.; Shchetinina, E.V.; Kraĭkivskaia, O.V.; Maksimov, V.N.; Maksimova, E.A. The Classification and the Monitoring of the State of Mouth Riverine and Lacustrine Ecosystems in Lake Baikal Based on the Composition of Local Microbiocenoses and Their Activity. Microbiology 2002, 71, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.S.; Pimenov, N.V.; Ivanova, V.G.; Zemskaya, T.I. Detection of Methane in the Water Column at Gas and Oil Seep Sites in Central and Southern Lake Baikal. Microbiology 2015, 84, 90–97. [Google Scholar] [CrossRef]
- Kozyreva, L.; Egorova, D.; Anan’ina, L.; Plotnikova, E.; Ariskina, E.; Prisyazhnaya, N.; Radnaeva, L.; Namsaraev, B. Belliella buryatensis sp. nov., Isolated from Alkaline Lake Water. Int. J. Syst. Evol. Microbiol. 2016, 66, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Bel’kova, N.L.; Driukker, V.V.; Xong, S.K.; An, T.S. Study of the Aquatic Bacterial Community Composition of Baikal Lake by in situ Hybridization Assay. Mikrobiologiia 2003, 72, 282–283. [Google Scholar] [CrossRef]
- Pimenov, N.V.; Zakharova, E.E.; Bryukhanov, A.L.; Korneeva, V.A.; Kuznetsov, B.B.; Tourova, T.P.; Pogodaeva, T.V.; Kalmychkov, G.V.; Zemskaya, T.I. Activity and Structure of the Sulfate-Reducing Bacterial Community in the Sediments of the Southern Part of Lake Baikal. Microbiology 2014, 83, 47–55. [Google Scholar] [CrossRef]
- Kalashnikov, A.M.; Gaisin, V.A.; Sukhacheva, M.V.; Namsaraev, B.B.; Panteleeva, A.N.; Nuyanzina-Boldareva, E.N.; Kuznetsov, B.B.; Gorlenko, V.M. Anoxygenic Phototrophic Bacteria from Microbial Communities of Goryachinsk Thermal Spring (Baikal Area, Russia). Microbiology 2014, 83, 407–421. [Google Scholar] [CrossRef]
- Spiglazov, L.P.; Drucker, V.V.; Ahn, T.S. Bacterial Aggregates Formation after Addition of Glucose in Lake Baikal Water. J. Microbiol. 2004, 42, 357–360. [Google Scholar] [PubMed]
- Zelenkina, T.S.; Eshinimayev, B.T.; Dagurova, O.P.; Suzina, N.E.; Namsarayev, B.B.; Trotsenko, Y.A. Aerobic Methanotrophs from the Coastal Thermal Springs of Lake Baikal. Microbiology 2009, 78, 492–497. [Google Scholar] [CrossRef]
- Zakharyuk, A.G.; Kozyreva, L.P.; Khijniak, T.V.; Namsaraev, B.B.; Shcherbakova, V.A. Desulfonatronum zhilinae sp. nov., a Novel Haloalkaliphilic Sulfate-Reducing Bacterium from Soda Lake Alginskoe, Trans-Baikal Region, Russia. Extremophiles 2015, 19, 673–680. [Google Scholar] [CrossRef]
- Garnova, E.S.; Zhilina, T.N.; Tourova, T.P.; Lysenko, A.M. Anoxynatronum sibiricum gen. nov., sp nov. Alkaliphilic Saccharolytic Anaerobe from Cellulolytic Community of Nizhnee Beloe (Transbaikal Region). Extremophiles 2003, 7, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Grachev, M.; Zubkov, I.; Tikhonova, I.; Ivacheva, M.; Kuzmin, A.; Sukhanova, E.; Sorokovikova, E.; Fedorova, G.; Galkin, A.; Suslova, M.; et al. Extensive Contamination of Water with Saxitoxin Near the Dam of the Irkutsk Hydropower Station Reservoir (East Siberia, Russia). Toxins 2018, 10, 402. [Google Scholar] [CrossRef]
- Jung, D.; Seo, E.-Y.; Epstein, S.S.; Joung, Y.; Han, J.; Parfenova, V.V.; Belykh, O.I.; Gladkikh, A.S.; Ahn, T.S. Application of a New Cultivation Technology, I-Tip, for Studying Microbial Diversity in Freshwater Sponges of Lake Baikal, Russia. FEMS Microbiol. Ecol. 2014, 90, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Dul’tseva, N.M.; Chernitsina, S.M.; Zemskaya, T.I. Isolation of Bacteria of the Genus Variovorax from the Thioploca Mats of Lake Baikal. Microbiology 2012, 81, 67–78. [Google Scholar] [CrossRef]
- Denisova, L.I.; Bel’kova, N.L.; Tulokhonov, I.I.; Zaĭchikov, E.F. Diversity of Bacteria at Various Depths in the Southern Part of Lake Baikal as Detected by 16S RRNA Sequencing. Mikrobiologiia 1999, 68, 547–556. [Google Scholar] [PubMed]
- Pavlova, O.N.; Lomakina, A.V.; Gorshkov, A.G.; Suslova, M.Y.; Likhoshvai, A.V.; Zemskaya, T.I. Microbial Communities and Their Ability to Oxidize N-Alkanes in the Area of Release of Gas- and Oil-Containing Fluids in Mid-Baikal (Cape Gorevoi Utes). Biol. Bull. 2012, 39, 458–463. [Google Scholar] [CrossRef]
- Gladkikh, A.S.; Kalyuzhnaya, O.V.; Belykh, O.I.; Ahn, T.S.; Parfenova, V.V. Analysis of Bacterial Communities of Two Lake Baikal Endemic Sponge Species. Microbiology 2014, 83, 787–797. [Google Scholar] [CrossRef]
- Kovaleva, O.L.; Merkel, A.Y.; Novikov, A.A.; Baslerov, R.V.; Toshchakov, S.V.; Bonch-Osmolovskaya, E.A. Tepidisphaera mucosa gen. nov., sp. nov., a Moderately Thermophilic Member of the Class Phycisphaerae in the Phylum Planctomycetes, and Proposal of a New Family, Tepidisphaeraceae fam. nov., and a New Order, Tepidisphaerales ord. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Chernitsyna, S.M.; Khal’zov, I.A.; Khanaeva, T.A.; Morozov, I.V.; Klimenkov, I.V.; Pimenov, N.V.; Zemskaya, T.I. Microbial Community Associated with Thioploca sp. Sheaths in the Area of the Posolsk Bank Methane Seep, Southern Baikal. Microbiology 2016, 85, 562–569. [Google Scholar] [CrossRef]
- Pavlova, O.N.; Izosimova, O.N.; Chernitsyna, S.M.; Ivanov, V.G.; Pogodaeva, T.V.; Khabuev, A.V.; Gorshkov, A.G.; Zemskaya, T.I. Anaerobic Oxidation of Petroleum Hydrocarbons in Enrichment Cultures from Sediments of the Gorevoy Utes Natural Oil Seep under Methanogenic and Sulfate-Reducing Conditions. Microb. Ecol. 2022, 83, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.I.; Galperin, M.Y.; Glagolev, A.N.; Skulachev, V.P. Utilization of Energy Stored in the Form of Na + and K + Ion Gradients by Bacterial Cells. Eur. J. Biochem. 1983, 134, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Tulupova, Y.R.; Parfenova, V.V.; Sitnikova, T.Y.; Sorokovnikova, E.G.; Khanaev, I.B. First Report on Bacteria of the Family Spirochaetaceae from Digestive Tract of Endemic Gastropods from Lake Baikal. Microbiology 2012, 81, 460–467. [Google Scholar] [CrossRef]
- Maksimenko, S.Y.; Zemskaya, T.I.; Pavlova, O.N.; Ivanov, V.G.; Buryukhaev, S.P. Microbial Community of the Water Column of the Selenga River-Lake Baikal Biogeochemical Barrier. Microbiology 2008, 77, 587–594. [Google Scholar] [CrossRef]
- Shubenkova, O.V.; Zemskaya, T.I.; Chernitsyna, S.M.; Khlystov, O.M.; Triboi, T.I. The First Results of an Investigation into the Phylogenetic Diversity of Microorganisms in Southern Baikal Sediments in the Region of Subsurface Discharge of Methane Hydrates. Microbiology 2005, 74, 314–320. [Google Scholar] [CrossRef]
- Maksimov, V.V.; Shchetinina, E.V.; Kraykivskaya, O.V.; Maksimova, E.A. Response of Microbial Communities of Lake Baikal to Extreme Temperatures. Microbiology 2006, 75, 653–657. [Google Scholar] [CrossRef]
- Kaluzhnaya, O.V.; Itskovich, V.B. Phototrophic Microorganisms in the Symbiotic Communities of Baikal Sponges: Diversity of psbA Gene (Encoding D1 Protein of Photosystem II) Sequences. Mol. Biol. 2017, 51, 372–378. [Google Scholar] [CrossRef]
- Bel’kova, N.L.; Parfenova, V.V.; Kostopnova, T.I.; Denisova, L.I.; Zaĭchikov, E.F. Microbial Biodiversity in the Lake Baikal Water. Mikrobiologiia 2003, 72, 239–249. [Google Scholar] [CrossRef]
- Gainutdinova, E.A.; Eshinimaev, B.T.; Tsyrenzhapova, I.S.; Dagurova, O.P.; Suzina, N.E.; Khmelenina, V.N.; Namsaraev, B.B.; Trotsenko, Y.A. Aerobic Methanotrophic Communities in the Bottom Sediments of Lake Baikal. Microbiology 2005, 74, 486–494. [Google Scholar] [CrossRef]
- Belykh, O.I.; Glagkikh, A.S.; Tikhonova, I.V.; Kuz’min, A.V.; Mogil’nikova, T.A.; Fedorova, G.A.; Sorokovikova, E.G. Identification of Cyanobacterial Producers of Shellfish Paralytic Toxins in Lake Baikal and Reservoirs of the Angara River. Microbiology 2015, 84, 98–99. [Google Scholar] [CrossRef]
- Parfenova, V.V.; Kravchenko, O.S.; Pavlova, O.N.; Suslova, M.I.; Bedoshvili, E.D. Effect of Different Calcium Hypochlorite Concentrations on the Survival of Potentially Pathogenic Microorganisms Isolated from Baikal Lake. Gigiena i Sanitariia 2012, 8–12. [Google Scholar]
- Semenova, E.A.; Kuznedelov, K.D.; Grachev, M.A. Nucleotide Sequences of Fragments of 16S RRNA of the Baikal Natural Populations and Laboratory Cultures of Cyanobacteria. Mol. Biol. 2001, 35, 405–410. [Google Scholar] [CrossRef]
- Terkina, I.A.; Drukker, V.V.; Parfenova, V.V.; Kostornova, T.Y. The Biodiversity of Actinomycetes in Lake Baikal. Microbiology 2002, 71, 346–349. [Google Scholar] [CrossRef]
- Lavrenteva, E.V.; Shagzhina, A.P.; Babasanova, O.B.; Dunaevsky, Y.E.; Namsaraev, Z.B.; Barkhutova, D.D. The Study of Two Alkaliphilic Thermophile Bacteria of the Anoxybacillus Genus as Producers of Extracellular Proteinase. Appl. Biochem. Microbiol. 2009, 45, 484–488. [Google Scholar] [CrossRef]
- Andreeva, I.S.; Pechurkina, N.I.; Morozova, O.V.; Ryabchikova, E.I.; Belikov, S.I.; Puchkova, L.I.; Emel’yanova, E.K.; Torok, T.; Repin, V.E. The New Eubacterium Roseomonas baikalica sp. nov. Isolated from Core Samples Collected by Deep-Hole Drilling of the Bottom of Lake Baikal. Microbiology 2007, 76, 487–493. [Google Scholar] [CrossRef]
- Sorokovikova, E.G.; Tikhonova, I.V.; Belykh, O.I.; Klimenkov, I.V.; Likhoshwai, E.V. Identification of Two Cyanobacterial Strains Isolated from the Kotel’nikovskii Hot Spring of the Baikal Rift. Microbiology 2008, 77, 365–372. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Responses of Beneficial Bacillus amyloliquefaciens SQR9 to Different Soilborne Fungal Pathogens through the Alteration of Antifungal Compounds Production. Front. Microbiol. 2014, 5, 636. [Google Scholar] [CrossRef] [PubMed]
- Bargabus, R.L.; Zidack, N.K.; Sherwood, J.E.; Jacobsen, B.J. Characterisation of Systemic Resistance in Sugar Beet Elicited by a Non-Pathogenic, Phyllosphere-Colonizing Bacillus mycoides, Biological Control Agent. Physiol. Mol. Plant Pathol. 2002, 61, 289–298. [Google Scholar] [CrossRef]
- Bargabus, R.L.; Zidack, N.K.; Sherwood, J.E.; Jacobsen, B.J. Oxidative Burst Elicited by Bacillus mycoides Isolate Bac J, a Biological Control Agent, Occurs Independently of Hypersensitive Cell Death in Sugar Beet. Mol. Plant-Microbe Interact. 2003, 16, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, S.A.; Surgenor, R.R.; Khey, K.M.W.; Vederas, J.C. Total Synthesis and Stereochemical Assignment of the Antimicrobial Lipopeptide Cerexin A1. Org. Lett. 2015, 17, 5428–5431. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Barajas, C.; Constantino-Salinas, E.A.; Amora-Lazcano, E.; Tlalapango-Ángeles, D.; Mendoza-Figueroa, J.S.; Cruz-Maya, J.A.; Jan-Roblero, J. Bacillus mycoides A1 and Bacillus tequilensis A3 Inhibit the Growth of a Member of the Phytopathogen Colletotrichum gloeosporioides Species Complex in Avocado. J. Sci. Food Agric. 2020, 100, 4049–4056. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernando, W.G.D.; De Kievit, T.R.; Berry, C.; Daayf, F.; Paulitz, T.C. Detection of Antibiotic-Related Genes from Bacterial Biocontrol Agents with Polymerase Chain Reaction. Can. J. Microbiol. 2006, 52, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Koppisch, A.T.; Browder, C.C.; Moe, A.L.; Shelley, J.T.; Kinkel, B.A.; Hersman, L.E.; Iyer, S.; Ruggiero, C.E. Petrobactin Is the Primary Siderophore Synthesized by Bacillus anthracis str. Sterne under Conditions of Iron Starvation. BioMetals 2005, 18, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Koppisch, A.T.; Dhungana, S.; Hill, K.K.; Boukhalfa, H.; Heine, H.S.; Colip, L.A.; Romero, R.B.; Shou, Y.; Ticknor, L.O.; Marrone, B.L.; et al. Petrobactin Is Produced by Both Pathogenic and Non-Pathogenic Isolates of the Bacillus cereus Group of Bacteria. BioMetals 2008, 21, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for Plant Growth Promotion and Stress Resilience: What Have We Learned? Plants 2022, 11, 2482. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.P.; Hartmann, A.; Gao, X.W.; Borriss, R. Biocontrol Mechanism by Root-Associated Bacillus amyloliquefaciens FZB42—A Review. Front. Microbiol. 2015, 6, 780. [Google Scholar] [CrossRef]
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Chakraborty, R.D. Bacillibactin Class of Siderophore Antibiotics from a Marine Symbiotic Bacillus as Promising Antibacterial Agents. Appl. Microbiol. Biotechnol. 2022, 106, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, A.; Theologidis, I.; Benaki, D.; Koukounia, M.; Zervakou, A.; Tzima, A.; Diallinas, G.; Hatzinikolaou, D.G.; Skandalis, N. Direct Antibiotic Activity of Bacillibactin Broadens the Biocontrol Range of Bacillus amyloliquefaciens MBI600. mSphere 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Andrić, S.; Rigolet, A.; Argüelles Arias, A.; Steels, S.; Hoff, G.; Balleux, G.; Ongena, L.; Höfte, M.; Meyer, T.; Ongena, M. Plant-Associated Bacillus Mobilizes Its Secondary Metabolites upon Perception of the Siderophore Pyochelin Produced by a Pseudomonas competitor. ISME J. 2023, 17, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Lagzian, A.; Riseh, R.S.; Sarikhan, S.; Ghorbani, A.; Khodaygan, P.; Borriss, R.; Guzzi, P.H.; Veltri, P. Genome Mining Conformance to Metabolite Profile of Bacillus Strains to Control Potato Pathogens. Sci. Rep. 2023, 13, 19095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Qiang, R.; Zhou, Z.; Pan, Y.; Yu, S.; Yuan, W.; Cheng, J.; Wang, J.; Zhao, D.; Zhu, J.; et al. Biocontrol and Action Mechanism of Bacillus subtilis Lipopeptides’ Fengycins against Alternaria Solani in Potato as Assessed by a Transcriptome Analysis. Front. Microbiol. 2022, 13, 861113. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Garcia-Gonzalez, E.; Mainz, A.; Hertlein, G.; Heid, N.C.; Mösker, E.; Van Den Elst, H.; Overkleeft, H.S.; Genersch, E.; Süssmuth, R.D. Paenilamicin: Structure and Biosynthesis of a Hybrid Nonribosomal Peptide/Polyketide Antibiotic from the Bee Pathogen Paenibacillus larvae. Angew. Chem. Int. Ed. 2014, 53, 10821–10825. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, E.; Müller, S.; Hertlein, G.; Heid, N.; Süssmuth, R.D.; Genersch, E. Biological Effects of Paenilamicin, a Secondary Metabolite Antibiotic Produced by the Honey Bee Pathogenic Bacterium Paenibacillus larvae. Microbiologyopen 2014, 3, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Liu, H.; Zheng, J.; Xie, C.; Gao, Y.; Dai, D.; Peng, D.; Ruan, L.; Chen, H.; Sun, M. In silico Analysis Highlights the Diversity and Novelty of Circular Bacteriocins in Sequenced Microbial Genomes. mSystems 2020, 5, 10-1128. [Google Scholar] [CrossRef]
- Zhu, S.; Hegemann, J.D.; Fage, C.D.; Zimmermann, M.; Xie, X.; Linne, U.; Marahiel, M.A. Insights into the Unique Phosphorylation of the Lasso Peptide Paeninodin. J. Biol. Chem. 2016, 291, 13662–13678. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Jiang, H.; Jiang, X.; Li, T.; Lu, P.; Yin, X.; Wei, Y. Comparative Genomic and Functional Analyses of Paenibacillus peoriae ZBSF16 with Biocontrol Potential against Grapevine Diseases, Provide Insights into Its Genes Related to Plant Growth-Promoting and Biocontrol Mechanisms. Front. Microbiol. 2022, 13, 975344. [Google Scholar] [CrossRef] [PubMed]
- Kotake, C.; Yamasaki, T.; Moriyama, T.; Shinoda, M.; Komiyama, N.; Furumai, T.; Konishi, M.; Oki, T. Butyrolactols A and B, New Antifungal Antibiotics Taxonomy, Isolation, Physico-Chemical Properties, Structure and Biological Activity. J. Antibiot. 1992, 45, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Harunari, E.; Komaki, H.; Igarashi, Y. Biosynthetic Origin of Butyrolactol A, an Antifungal Polyketide Produced by a Marine-Derived Streptomyces. Beilstein J. Org. Chem. 2017, 13, 441–450. [Google Scholar] [CrossRef]
- Dementiev, A.; Board, J.; Sitaram, A.; Hey, T.; Kelker, M.S.; Xu, X.; Hu, Y.; Vidal-Quist, C.; Chikwana, V.; Griffin, S.; et al. The Pesticidal Cry6Aa Toxin from Bacillus thuringiensis Is Structurally Similar to HlyE-Family Alpha Pore-Forming Toxins. BMC Biol. 2016, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Sampson, K.S.; Tomso, D.J.; Agarwal, S.; McNulty, B.; Campbell, C. Toxin Genes and Methods for Their Use. U.S. Patent 8,461,421 B2, 19 June 2013. [Google Scholar]
- Lai, L.; Villanueva, M.; Muruzabal-Galarza, A.; Fernández, A.B.; Unzue, A.; Toledo-Arana, A.; Caballero, P.; Caballero, C.J. Bacillus thuringiensis Cyt Proteins as Enablers of Activity of Cry and Tpp Toxins against Aedes albopictus. Toxins 2023, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Best, H.L.; Williamson, L.J.; Lipka-Lloyd, M.; Waller-Evans, H.; Lloyd-Evans, E.; Rizkallah, P.J.; Berry, C. The Crystal Structure of Bacillus thuringiensis Tpp80Aa1 and Its Interaction with Galactose-Containing Glycolipids. Toxins 2022, 14, 863. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zavala, S.A.; Barboza-Pérez, U.E.; Hernández-Guzmán, G.; Bideshi, D.K.; Barboza-Corona, J.E. Chitinases of Bacillus thuringiensis: Phylogeny, Modular Structure, and Applied Potentials. Front. Microbiol. 2020, 10, 3032. [Google Scholar] [CrossRef] [PubMed]
- Dalhammar, G.; Steiner, H. Characterization of Inhibitor A, a Protease from Bacillus thuringiensis Which Degrades Attacins and Cecropins, Two Classes of Antibacterial Proteins in Insects. Eur. J. Biochem. 1984, 139, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, L.; Huang, Q.; Zheng, J.; Zhou, W.; Peng, D.; Ruan, L.; Sun, M. Bacillus thuringiensis Metalloproteinase Bmp1 Functions as a Nematicidal Virulence Factor. Appl. Environ. Microbiol. 2013, 79, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Malovichko, Y.V.; Nizhnikov, A.A.; Antonets, K.S. Repertoire of the Bacillus thuringiensis Virulence Factors Unrelated to Major Classes of Protein Toxins and Its Role in Specificity of Host-Pathogen Interactions. Toxins 2019, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Méric, G.; Mageiros, L.; Pascoe, B.; Woodcock, D.J.; Mourkas, E.; Lamble, S.; Bowden, R.; Jolley, K.A.; Raymond, B.; Sheppard, S.K. Lineage-Specific Plasmid Acquisition and the Evolution of Specialized Pathogens in Bacillus thuringiensis and the Bacillus cereus Group. Mol. Ecol. 2018, 27, 1524–1540. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.K.; Maheshwari, D.K.; Kim, K.; Bajpai, V.K. Termitarium-Inhabiting Bacillus endophyticus TSH42 and Bacillus cereus TSH77 Colonizing Curcuma longa L.: Isolation, Characterization, and Evaluation of Their Biocontrol and Plant-Growth-Promoting Activities. Can. J. Microbiol. 2016, 62, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lou, H.; He, H.; He, X.; Wang, Z.; Gao, X.; Liu, J. Genomic and Biological Control of Sclerotinia sclerotiorum Using an Extracellular Extract from Bacillus velezensis 20507. Front. Microbiol. 2024, 15, 1385067. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Brader, G.; Corretto, E.; Aleti, G.; Abaidullah, M.; Sessitsch, A.; Hafeez, F.Y. Qualitative Analysis of Biosurfactants from Bacillus Species Exhibiting Antifungal Activity. PLoS ONE 2018, 13, e0198107. [Google Scholar] [CrossRef]
- Lalanne-Tisné, G.; Barral, B.; Taibi, A.; Coulibaly, Z.K.; Burguet, P.; Rasoarahona, F.; Quinton, L.; Meile, J.-C.; Boubakri, H.; Kodja, H. Exploring the Phytobeneficial and Biocontrol Capacities of Endophytic Bacteria Isolated from Hybrid Vanilla Pods. Microorganisms 2023, 11, 1754. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Velandia, C.A.; Ongena, M.; Cotes, A.M. Effects of Fengycins and Iturins on Fusarium oxysporum f. sp. physali and Root Colonization by Bacillus velezensis Bs006 Protect Golden Berry Against Vascular Wilt. Phytopathology 2021, 111, 2227–2237. [Google Scholar] [CrossRef]
- Kiesewalter, H.T.; Lozano-Andrade, C.N.; Wibowo, M.; Strube, M.L.; Maróti, G.; Snyder, D.; Jørgensen, T.S.; Larsen, T.O.; Cooper, V.S.; Weber, T.; et al. Genomic and Chemical Diversity of Bacillus subtilis Secondary Metabolites against Plant Pathogenic Fungi. mSystems 2021, 6. [Google Scholar] [CrossRef]
- Boiu-Sicuia, O.-A.; Toma, R.C.; Diguță, C.F.; Matei, F.; Cornea, C.P. In vitro Evaluation of Some Endophytic Bacillus to Potentially Inhibit Grape and Grapevine Fungal Pathogens. Plants 2023, 12, 2553. [Google Scholar] [CrossRef] [PubMed]
- Vega-Portalatino, E.J.; Rosales-Cuentas, M.M.; Tamariz-Angeles, C.; Olivera-Gonzales, P.; Espinoza-Espinoza, L.A.; Moreno-Quispe, L.A.; Portalatino-Zevallos, J.C. Diversity of Endophytic Bacteria with Antimicrobial Potential Isolated from Marine Macroalgae from Yacila and Cangrejos Beaches, Piura-Peru. Arch. Microbiol. 2024, 206, 372. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Song, X.; Li, C.; Zhou, W.; Qin, L.; Wei, L.; Di, W.; Huang, S.; Li, B.; Huang, Q.; et al. Antifungal Mechanism of Bacillus amyloliquefaciens Strain GKT04 against Fusarium Wilt Revealed Using Genomic and Transcriptomic Analyses. Microbiologyopen 2021, 10, e1192. [Google Scholar] [CrossRef]
- Phazna, T.A.; Ngashangva, N.G.; Yentrembam, R.-B.S.; Maurya, R.; Mukherjee, P.; Sharma, C.; Verma, P.K.; Sarangthem, I. Draft Genome Sequence and Functional Analysis of Lysinibacillus xylanilyticus T26, a Plant Growth-Promoting Bacterium Isolated from Capsicum Chinense Rhizosphere. J. Biosci. 2022, 47, 36. [Google Scholar] [CrossRef]
- Balthazar, C.; Novinscak, A.; Cantin, G.; Joly, D.L.; Filion, M. Biocontrol Activity of Bacillus spp. and Pseudomonas spp. Against Botrytis cinerea and Other Cannabis Fungal Pathogens. Phytopathology 2022, 112, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Ezrari, S.; Mhidra, O.; Radouane, N.; Tahiri, A.; Polizzi, G.; Lazraq, A.; Lahlali, R. Potential Role of Rhizobacteria Isolated from Citrus Rhizosphere for Biological Control of Citrus Dry Root Rot. Plants 2021, 10, 872. [Google Scholar] [CrossRef]
- Caulier, S.; Gillis, A.; Colau, G.; Licciardi, F.; Liépin, M.; Desoignies, N.; Modrie, P.; Legrève, A.; Mahillon, J.; Bragard, C. Versatile Antagonistic Activities of Soil-Borne Bacillus spp. and Pseudomonas spp. against Phytophthora infestans and Other Potato Pathogens. Front. Microbiol. 2018, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, X.; Ye, J.; Wu, T.; Liu, H. Forest Rhizosphere Bacterium Bacillus mycoides JYZ-SD5 and Application Thereof. Patent CN109355228B, 24 January 2020. [Google Scholar]
- Niu, F.; Zhao, B.; Li, P.; Li, Q.; Zhang, S. Bacillus mycoides Strain and Application Thereof. Patent CN105002120A, 30 January 2018. [Google Scholar]
- Dorozhkin, N.A.; Novikova, L.M.; Viktorchik, I.V.; Belskaya, S.I.; Kolesnikov, V.S.; Kedrova, I.I. Strain of Bacteria Bacillus mycoides for Production of Preparation against Potato Disease Agent. Patent RU1771639C, 30 November 1992. [Google Scholar]
- Yang, C.-H.; Liu, X. Pseudomonas Strains and Their Metabolites to Control Plant Diseases. Patent US20230165260A1 (pending Application Approbation), 30 January 2023. [Google Scholar]
- Yang, C.-H.; Liu, X. Pseudomonas chlororaphis Species and Its Use in the Control of Diseases Caused by Bacteria and Fungi. Patent US20220232834A1 (pending Application Approbation), 28 January 2022. [Google Scholar]
- Méndez Acevedo, M.; Carroll, L.M.; Mukherjee, M.; Mills, E.; Xiaoli, L.; Dudley, E.G.; Kovac, J. Novel Effective Bacillus cereus Group Species “Bacillus clarus” Is Represented by Antibiotic-Producing Strain ATCC 21929 Isolated from Soil. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Jun’ichi Shoji, H.; Mikao Mayama, I.; Shinzo Matsuura, I.; Kouichi Matsumoto, T.; Yoshiharu Wakisaka, T. Antibiotic 60-6 and Production Thereof. Patent US3923979A, 2 December 1974. [Google Scholar]
- Hung, J.-C.; Huang, T.-P.; Huang, J.; Chang, C.J.; Jan, F.-J. The Efficacy of Orange Terpene and Bacillus mycoides Strain BM103 on the Control of Periwinkle Leaf Yellowing Phytoplasma. Plant Dis. 2024. [Google Scholar] [CrossRef]
- Elamary, R.; Salem, W.M. Optimizing and Purifying Extracellular Amylase from Soil Bacteria to Inhibit Clinical Biofilm-Forming Bacteria. PeerJ 2020, 8, e10288. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, N.; Paul, A.; Qiu, T.; Combs, D.B.; Hosseinzadeh, S.; Underhill, A.; Jiang, Y.; Cadle-Davidson, L.E.; Gold, K.M. Non-Destructive Monitoring of Foliar Fungicide Efficacy with Hyperspectral Sensing in Grapevine. Phytopathology 2024, 114, 464–473. [Google Scholar] [CrossRef]
- Yuan, X.; Gdanetz, K.; Outwater, C.A.; Slack, S.M.; Sundin, G.W. Evaluation of Plant Defense Inducers and Plant Growth Regulators for Fire Blight Management Using Transcriptome Studies and Field Assessments. Phytopathology 2023, 113, 2152–2164. [Google Scholar] [CrossRef] [PubMed]
- Ambas, I.; Buller, N.; Fotedar, R. Isolation and Screening of Probiotic Candidates from Marron, Cherax cainii (Austin, 2002) Gastrointestinal Tract (GIT) and Commercial Probiotic Products for the Use in Marron Culture. J. Fish. Dis. 2015, 38, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Martinez, C.; Simao-Beaunoir, A.-M.; Bélanger, R.R.; Tweddell, R.J. Selection of Antagonist Microorganisms Against Helminthosporium solani, Causal Agent of Potato Silver Scurf. Plant Dis. 2002, 86, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.A.; Kochman, J.K.; Brown, J.F. Phylloplane Bacteria Antagonistic to the Sunflower Pathogen Alternaria helianthi. Australas. Plant Pathol. 1997, 26, 85. [Google Scholar] [CrossRef]
- Luo, T.; Hou, S.; Yang, L.; Qi, G.; Zhao, X. Nematodes Avoid and Are Killed by Bacillus mycoides-Produced Styrene. J. Invertebr. Pathol. 2018, 159, 129–136. [Google Scholar] [CrossRef]
- Fravel, D.R. Biocontrol of Tobacco Brown-Spot Disease by Bacillus cereus subsp. mycoides in a Controlled Environment. Phytopathology 1977, 77, 930. [Google Scholar] [CrossRef]
- de la Huerta-Bengoechea, P.; Gil-Serna, J.; Melguizo, C.; Ramos, A.J.; Prim, M.; Vázquez, C.; Patiño, B. Biocontrol of Mycotoxigenic Fungi Using Bacteria Isolated from Ecological Vineyard Soils. J. Fungi 2022, 8, 1136. [Google Scholar] [CrossRef]
- Sandilya, S.P.; Jeevan, B.; Subrahmanyam, G.; Dutta, K.; Vijay, N.; Bhattacharyya, N.; Chutia, M. Co-Inoculation of Native Multi-Trait Plant Growth Promoting Rhizobacteria Promotes Plant Growth and Suppresses Alternaria Blight Disease in Castor (Ricinus communis L.). Heliyon 2022, 8, e11886. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, P.; Li, H.-B.; Song, Q.-Q.; Guo, D.-J.; Solanki, M.K.; Verma, K.K.; Malviya, M.K.; Song, X.-P.; Lakshmanan, P.; et al. Diversity of Nitrogen-Fixing Rhizobacteria Associated with Sugarcane: A Comprehensive Study of Plant-Microbe Interactions for Growth Enhancement in Saccharum spp. BMC Plant Biol. 2020, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Irvin, A.D. The Inhibition of Listeria monocytogenes by an Organism, Resembling Bacillus mycoides, Present in Normal Silage. Res. Vet. Sci. 1969, 10, 106–108. [Google Scholar] [CrossRef]
- Czaban, J.; Ksiezniak, A.; Wróblewska, B.; Paszkowski, W.L. An Attempt to Protect Winter Wheat against Gaeumannomyces graminis var. tritici by the Use of Rhizobacteria Pseudomonas fluorescent and Bacillus mycoides. Pol. J. Microbiol. 2004, 53, 101–110. [Google Scholar] [PubMed]
- Piacenza, E.; Presentato, A.; Zonaro, E.; Lemire, J.A.; Demeter, M.; Vallini, G.; Turner, R.J.; Lampis, S. Antimicrobial Activity of Biogenically Produced Spherical Se-nanomaterials Embedded in Organic Material against Pseudomonas aeruginosa and Staphylococcus aureus Strains on Hydroxyapatite-coated Surfaces. Microb. Biotechnol. 2017, 10, 804–818. [Google Scholar] [CrossRef]
- Ambas, I.; Suriawan, A.; Fotedar, R. Immunological Responses of Customised Probiotics-Fed Marron, Cherax tenuimanus, (Smith 1912) When Challenged with Vibrio mimicus. Fish. Shellfish Immunol. 2013, 35, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Dinoor, A. Combining Biocontrol Agents to Reduce the Variability of Biological Control. Phytopathology 2001, 91, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Azarova, I.N.; Parfenova, V.V.; Baram, G.I.; Terkina, I.A.; Pavlova, O.N.; Suslova, M.I. Degradation of Bis(2-Ethylhexyl)Phthalate by Microorganisms of Water and Sediments of the Selenga River and Baikal Lake under Experimental Conditions. Prikl. Biokhim. Mikrobiol. 2003, 39, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Mácha, H.; Marešová, H.; Juříková, T.; Švecová, M.; Benada, O.; Škríba, A.; Baránek, M.; Novotný, Č.; Palyzová, A. Killing Effect of Bacillus velezensis Fzb42 on a (Xcc) Strain Newly Isolated from Cabbage Brassica oleracea convar. capitata (L.): A Metabolomic Study. Microorganisms 2021, 9, 1410. [Google Scholar] [CrossRef]
- Pengproh, R.; Thanyasiriwat, T.; Sangdee, K.; Saengprajak, J.; Kawicha, P.; Sangdee, A. Evaluation and Genome Mining of Bacillus stercoris Isolate B.PNR1 as Potential Agent for Fusarium Wilt Control and Growth Promotion of Tomato. Plant Pathol. J. 2023, 39, 430–448. [Google Scholar] [CrossRef]
- Nifakos, K.; Tsalgatidou, P.C.; Thomloudi, E.-E.; Skagia, A.; Kotopoulis, D.; Baira, E.; Delis, C.; Papadimitriou, K.; Markellou, E.; Venieraki, A.; et al. Genomic Analysis and Secondary Metabolites Production of the Endophytic Bacillus velezensis Bvel1: A Biocontrol Agent against Botrytis cinerea Causing Bunch Rot in Post-Harvest Table Grapes. Plants 2021, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Fatima, R.; Mahmood, T.; Moosa, A.; Aslam, M.N.; Shakeel, M.T.; Maqsood, A.; Shafiq, M.U.; Ahmad, T.; Moustafa, M.; Al-Shehri, M. Bacillus thuringiensis CHGP12 Uses a Multifaceted Approach for the Suppression of Fusarium oxysporum f. sp. siceris and to Enhance the Biomass of Chickpea Plants. Pest. Manag. Sci. 2023, 79, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Doty, S.L.; Joubert, P.M.; Firrincieli, A.; Sher, A.W.; Tournay, R.; Kill, C.; Parikh, S.S.; Okubara, P. Potential Biocontrol Activities of Populus Endophytes against Several Plant Pathogens Using Different Inhibitory Mechanisms. Pathogens 2022, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-X.; Xu, W.-H.; Chen, Z.-N.; Hu, J.-L.; Luo, S.-Q.; Wang, Z.-G. Complete Genome Sequence of Bacillus velezensis WB, an Isolate from the Watermelon Rhizosphere: Genomic Insights into Its Antifungal Effects. J. Glob. Antimicrob. Resist. 2022, 30, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Nakkeeran, S.; Saranya, N.; Senthilraja, C.; Renukadevi, P.; Krishnamoorthy, A.S.; El Enshasy, H.A.; El-Adawi, H.; Malathi, V.G.; Salmen, S.H.; Ansari, M.J.; et al. Mining the Genome of Bacillus velezensis VB7 (CP047587) for MAMP Genes and Non-Ribosomal Peptide Synthetase Gene Clusters Conferring Antiviral and Antifungal Activity. Microorganisms 2021, 9, 2511. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.; Lee, Y.; Balaraju, K.; Jeon, Y. Characterization and Evaluation of Bacillus subtilis GYUN-2311 as a Biocontrol Agent against Colletotrichum spp. on Apple and Hot Pepper in Korea. Front. Microbiol. 2024, 14, 1322641. [Google Scholar] [CrossRef] [PubMed]
- Tsalgatidou, P.C.; Thomloudi, E.-E.; Baira, E.; Papadimitriou, K.; Skagia, A.; Venieraki, A.; Katinakis, P. Integrated Genomic and Metabolomic Analysis Illuminates Key Secreted Metabolites Produced by the Novel Endophyte Bacillus halotolerans Cal.l.30 Involved in Diverse Biological Control Activities. Microorganisms 2022, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Song, C.; Li, Z.; Kuipers, O.P. Antimicrobial Activity Screening of Rhizosphere Soil Bacteria from Tomato and Genome-Based Analysis of Their Antimicrobial Biosynthetic Potential. BMC Genomics 2021, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.; Guo, D.; Naeimi, S.; Ahmadi, J. Perception of Biocontrol Potential of Bacillus inaquosorum KR2-7 against Tomato Fusarium Wilt through Merging Genome Mining with Chemical Analysis. Biology 2022, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rodriguez, E.; Moreno-Ulloa, A.; Castro-Longoria, E.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Integrated Omics Approaches for Deciphering Antifungal Metabolites Produced by a Novel Bacillus Species, B. cabrialesii TE3T, against the Spot Blotch Disease of Wheat (Triticum turgidum L. subsp. durum). Microbiol. Res. 2021, 251, 126826. [Google Scholar] [CrossRef] [PubMed]
- Nimbeshaho, F.; Nihorimbere, G.; Arias, A.A.; Liénard, C.; Steels, S.; Nibasumba, A.; Nihorimbere, V.; Legrève, A.; Ongena, M. Unravelling the Secondary Metabolome and Biocontrol Potential of the Recently Described Species Bacillus nakamurai. Microbiol. Res. 2024, 288, 127841. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yin, S.; An, L.; Zhang, G.; Cheng, H.; Xi, Y.; Cui, G.; Zhang, F.; Zhang, L. Complete Genome Sequence of Bacillus subtilis BSD-2, a Microbial Germicide Isolated from Cultivated Cotton. J. Biotechnol. 2016, 230, 26–27. [Google Scholar] [CrossRef]
- Li, S.; He, P.; Fan, H.; Liu, L.; Yin, K.; Yang, B.; Li, Y.; Huang, S.-M.; Li, X.; Zheng, S.-J. A Real-Time Fluorescent Reverse Transcription Quantitative PCR Assay for Rapid Detection of Genetic Markers’ Expression Associated with Fusarium Wilt of Banana Biocontrol Activities in Bacillus. J. Fungi 2021, 7, 353. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Zhao, Q.; Yang, X.; Li, Y.; Zhou, H.; Zhao, M.; Zheng, H. Whole-Genome Analysis Revealed the Growth-Promoting and Biological Control Mechanism of the Endophytic Bacterial Strain Bacillus halotolerans Q2H2, with Strong Antagonistic Activity in Potato Plants. Front. Microbiol. 2024, 14, 1287921. [Google Scholar] [CrossRef]
- Ferreira, W.T.; Hong, H.A.; Hess, M.; Adams, J.R.G.; Wood, H.; Bakun, K.; Tan, S.; Baccigalupi, L.; Ferrari, E.; Brisson, A.; et al. Micellar Antibiotics of Bacillus. Pharmaceutics 2021, 13, 1296. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Sun, L.; Xu, H.; Gu, Y.; Lei, P. Bacillus atrophaeus NX-12 Utilizes Exosmotic Glycerol from Fusarium oxysporum f. sp. cucumerinum for Fengycin Production. J. Agric. Food Chem. 2023, 71, 10565–10574. [Google Scholar] [CrossRef]
- dos Santos, J.B.; Cruz, J.d.O.; Geraldo, L.C.; Dias, E.G.; Queiroz, P.R.M.; Monnerat, R.G.; Borges, M.; Blassioli-Moraes, M.C.; Blum, L.E.B. Detection and Evaluation of Volatile and Non-Volatile Antifungal Compounds Produced by Bacillus spp. Strains. Microbiol. Res. 2023, 275, 127465. [Google Scholar] [CrossRef]
- Adeniji, A.A.; Aremu, O.S.; Babalola, O.O. Selecting Lipopeptide-producing, Fusarium-Suppressing Bacillus spp.: Metabolomic and Genomic Probing of Bacillus velezensis NWUMFkBS10.5. Microbiologyopen 2019, 8, e00742. [Google Scholar] [CrossRef]
- Jiao, R.; Cai, Y.; He, P.; Munir, S.; Li, X.; Wu, Y.; Wang, J.; Xia, M.; He, P.; Wang, G.; et al. Bacillus amyloliquefaciens YN201732 Produces Lipopeptides With Promising Biocontrol Activity Against Fungal Pathogen Erysiphe cichoracearum. Front. Cell. Infect. Microbiol. 2021, 11, 598999. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, J.; Fu, L.; Xu, G.; Lin, X.; Qiao, J.; Xia, Y. Biocontrol of Wheat Crown Rot Using Bacillus halotolerans QTH8. Pathogens 2022, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Ali, H.; Hassan, N.; Tawab, A.; Salman, M.; Jawad, I.; de Jong, A.; Moreno, C.M.; Kuipers, O.P.; Feroz, Y.; et al. Food Safety and Biological Control; Genomic Insights and Antimicrobial Potential of Bacillus velezensis FB2 against Agricultural Fungal Pathogens. PLoS ONE 2023, 18, e0291975. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yang, P.; Zhong, M.; Bilal, M.; Xu, H.; Zhang, Q.; Xu, J.; Liang, N.; Liu, S.; Zhao, L.; et al. Isolation of a Potential Probiotic Strain Bacillus amyloliquefaciens LPB-18 and Identification of Antimicrobial Compounds Responsible for Inhibition of Food-borne Pathogens. Food Sci. Nutr. 2023, 11, 2186–2196. [Google Scholar] [CrossRef]
- Santos-Lima, D.; de Castro Spadari, C.; de Morais Barroso, V.; Carvalho, J.C.S.; de Almeida, L.C.; Alcalde, F.S.C.; Ferreira, M.J.P.; Sannomiya, M.; Ishida, K. Lipopeptides from an Isolate of Bacillus Subtilis Complex Have Inhibitory and Antibiofilm Effects on Fusarium Solani. Appl. Microbiol. Biotechnol. 2023, 107, 6103–6120. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Kim, S.E.; Lee, W.J.; Fumei, Z.; Cho, M.S.; Moon, J.S.; Oh, H.-W.; Park, H.-Y.; Kim, S.U. Isolation and Characterization of a High Iturin Yielding Bacillus velezensis UV Mutant with Improved Antifungal Activity. PLoS ONE 2020, 15, e0234177. [Google Scholar] [CrossRef] [PubMed]
- Afordoanyi, D.M.; Diabankana, R.G.C.; Komissarov, E.N.; Kuchaev, E.S.; Validov, S.Z. Characterization of a Novel Bacillus glycinifermentans Strain MGMM1 Based on Full Genome Analysis and Phenotypic Properties for Biotechnological Applications. Microorganisms 2023, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Quan, C.; Wang, Y.; Wang, J.; Fan, S. Bacillus amyloliquefaciens Q-426 as a Potential Biocontrol Agent against Fusarium oxysporum f. sp. spinaciae. J. Basic Microbiol. 2014, 54, 448–456. [Google Scholar] [CrossRef]
- Vignesh, M.; Shankar, S.R.M.; MubarakAli, D.; Hari, B.N.V. A Novel Rhizospheric Bacterium: Bacillus velezensis NKMV-3 as a Biocontrol Agent Against Alternaria Leaf Blight in Tomato. Appl. Biochem. Biotechnol. 2022, 194, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Song, C.; Muñoz, C.Y.; Kuipers, O.P. Bacillus cabrialesii BH5 Protects Tomato Plants Against Botrytis cinerea by Production of Specific Antifungal Compounds. Front. Microbiol. 2021, 12, 707609. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of Two Plant-Growth Promoting Bacillus selezensis Isolates Against Ralstonia solanacearum and Fusarium sxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, Z.; Chen, Y.; Wang, J.; Xiao, R.; Wang, X.; Liu, B.; Chen, M.; He, J. Lipopeptide C 17 Fengycin B Exhibits a Novel Antifungal Mechanism by Triggering Metacaspase-Dependent Apoptosis in Fusarium oxysporum. J. Agric. Food. Chem. 2024, 72, 7943–7953. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Z.; Xu, W. Induced Oxidative Equilibrium Damage and Reduced Toxin Synthesis in Fusarium oxysporum f. sp. niveum by Secondary Metabolites from Bacillus velezensis WB. FEMS Microbiol. Ecol. 2022, 98, fiac080. [Google Scholar] [CrossRef]
- Ali, S.; Hameed, S.; Shahid, M.; Iqbal, M.; Lazarovits, G.; Imran, A. Functional Characterization of Potential PGPR Exhibiting Broad-Spectrum Antifungal Activity. Microbiol. Res. 2020, 232, 126389. [Google Scholar] [CrossRef] [PubMed]
- Jan, F.; Arshad, H.; Ahad, M.; Jamal, A.; Smith, D.L. In vitro Assessment of Bacillus subtilis FJ3 Affirms Its Biocontrol and Plant Growth Promoting Potential. Front. Plant Sci. 2023, 14, 1205894. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutar, D.M.K.; Alzawar, N.S.A.; Noman, M.; Azizullah; Li, D.; Song, F. Suppression of Fusarium Wilt in Watermelon by Bacillus amyloliquefaciens DHA55 through Extracellular Production of Antifungal Lipopeptides. J. Fungi 2023, 9, 336. [Google Scholar] [CrossRef]
- Li, Q.; Liao, S.; Zhi, H.; Xing, D.; Xiao, Y.; Yang, Q. Characterization and Sequence Analysis of Potential Biofertilizer and Biocontrol Agent Bacillus subtilis Strain SEM-9 from Silkworm Excrement. Can. J. Microbiol. 2019, 65, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, J.; Yang, X.; Yang, J.; Zhao, S.; Zhou, Q.; Chen, L. Identification of Lipopeptide Iturin A Produced by Bacillus amyloliquefaciens NCPSJ7 and Its Antifungal Activities against Fusarium oxysporum f. sp. siveum. Foods 2022, 11, 2996. [Google Scholar] [CrossRef]
- Berlanga-Clavero, M.V.; Molina-Santiago, C.; Caraballo-Rodríguez, A.M.; Petras, D.; Díaz-Martínez, L.; Pérez-García, A.; de Vicente, A.; Carrión, V.J.; Dorrestein, P.C.; Romero, D. Bacillus subtilis Biofilm Matrix Components Target Seed Oil Bodies to Promote Growth and Anti-Fungal Resistance in Melon. Nat. Microbiol. 2022, 7, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Ghazala, I.; Charfeddine, S.; Charfeddine, M.; Gargouri-Bouzid, R.; Ellouz-Chaabouni, S.; Haddar, A. Antimicrobial and Antioxidant Activities of Bacillus mojavensis I4 Lipopeptides and Their Potential Application against the Potato Dry Rot Causative Fusarium solani. Arch. Microbiol. 2022, 204, 484. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Nikolaidis, M.; Antequera-Gómez, M.L.; Cámara-Almirón, J.; Romero, D.; Moschakis, T.; Amoutzias, G.D.; Karaoglanidis, G.S. Whole Genome Sequencing and Root Colonization Studies Reveal Novel Insights in the Biocontrol Potential and Growth Promotion by Bacillus subtilis MBI 600 on Cucumber. Front. Microbiol. 2021, 11, 600393. [Google Scholar] [CrossRef]
- Wang, C.; Ye, X.; Ng, T.B.; Zhang, W. Study on the Biocontrol Potential of Antifungal Peptides Produced by Bacillus velezensis against Fusarium solani That Infects the Passion Fruit Passiflora edulis. J. Agric. Food Chem. 2021, 69, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Avendaño, E.; Lizette Pérez-Molina, M.; Luis Monribot-Villanueva, J.; Marian Cortazar-Murillo, E.; Ramírez-Vázquez, M.; Reverchon, F.; Antonio Guerrero-Analco, J. Identification of Antifungal Compounds from Avocado Rhizobacteria (Bacillus spp.) against Fusarium spp., by a Bioassay-Guided Fractionation Approach. Chem. Biodivers 2022, 19, e202200687. [Google Scholar] [CrossRef]
- Al-Mutar, D.M.K.; Noman, M.; Alzawar, N.S.A.; Qasim, H.H.; Li, D.; Song, F. The Extracellular Lipopeptides and Volatile Organic Compounds of Bacillus subtilis DHA41 Display Broad-Spectrum Antifungal Activity against Soil-Borne Phytopathogenic Fungi. J. Fungi 2023, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, X.; Li, Z.; Chu, R.; Shah, S.; Wang, X.; Zhang, X. Antagonistic Effect of Bacillus and Pseudomonas Combinations against Fusarium oxysporum and Their Effect on Disease Resistance and Growth Promotion in Watermelon. J. Appl. Microbiol. 2024, 135, lxae074. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Stanković, S.; Nišavić, M.; Petković, M.; Ristivojević, P.; Fira, D.; Berić, T. The Profile and Antimicrobial Activity of Bacillus Lipopeptide Extracts of Five Potential Biocontrol Strains. Front. Microbiol. 2017, 8, 925. [Google Scholar] [CrossRef] [PubMed]
- Báez-Vallejo, N.; Camarena-Pozos, D.A.; Monribot-Villanueva, J.L.; Ramírez-Vázquez, M.; Carrión-Villarnovo, G.L.; Guerrero-Analco, J.A.; Partida-Martínez, L.P.; Reverchon, F. Forest Tree Associated Bacteria for Potential Biological Control of Fusarium solani and of Fusarium kuroshium, Causal Agent of Fusarium Dieback. Microbiol. Res. 2020, 235, 126440. [Google Scholar] [CrossRef]
- Moreno-Velandia, C.A.; Ongena, M.; Kloepper, J.W.; Cotes, A.M. Biosynthesis of Cyclic Lipopeptides by Bacillus velezensis Bs006 and Its Antagonistic Activity Are Modulated by the Temperature and Culture Media Conditions. Curr. Microbiol. 2021, 78, 3505–3515. [Google Scholar] [CrossRef]
- Izquierdo-García, L.F.; González-Almario, A.; Cotes, A.M.; Moreno-Velandia, C.A. Trichoderma virens Gl006 and Bacillus velezensis Bs006: A Compatible Interaction Controlling Fusarium Wilt of Cape Gooseberry. Sci. Rep. 2020, 10, 6857. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Xu, W. Production-optimized Fermentation of Antifungal Compounds by Bacillus velezensis LZN01 and Transcriptome Analysis. Microb. Biotechnol. 2024, 17, e70026. [Google Scholar] [CrossRef] [PubMed]
- Bóka, B.; Manczinger, L.; Kocsubé, S.; Shine, K.; Alharbi, N.S.; Khaled, J.M.; Münsterkötter, M.; Vágvölgyi, C.; Kredics, L. Genome Analysis of a Bacillus subtilis Strain Reveals Genetic Mutations Determining Biocontrol Properties. World J. Microbiol. Biotechnol. 2019, 35, 52. [Google Scholar] [CrossRef] [PubMed]
- Velho, R.V.; Medina, L.F.C.; Segalin, J.; Brandelli, A. Production of Lipopeptides among Bacillus Strains Showing Growth Inhibition of Phytopathogenic Fungi. Folia Microbiol. 2011, 56, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Wei, Z.; Guan, Z.; Cai, Y.; Liao, X. Antifungal Activity of Isolated Bacillus amyloliquefaciens SYBC H47 for the Biocontrol of Peach Gummosis. PLoS ONE 2016, 11, e0162125. [Google Scholar] [CrossRef] [PubMed]
- Assena, M.W.; Pfannstiel, J.; Rasche, F. Inhibitory Activity of Bacterial Lipopeptides against Fusarium oxysporum f. sp. strigae. BMC Microbiol. 2024, 24, 227. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Liu, B.; Zhu, Y.; Xiao, R.; Yang, W.; Ge, C.; Chen, Z. Biocontrol of Tomato Bacterial Wilt by the New Strain Bacillus velezensis FJAT-46737 and Its Lipopeptides. BMC Microbiol. 2020, 20, 160. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yang, Q.; Zhao, W.; Cui, L.; Wang, B.; Zhang, L.; Cheng, H.; Song, S.; Zhang, L. Genomics and LC-MS Reveal Diverse Active Secondary Metabolites in Bacillus amyloliquefaciens WS-8. J. Microbiol. Biotechnol. 2020, 30, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.S.; Hwang, S.H.; Maung, C.E.H.; Cho, J.-Y.; Kim, K.Y. Enhanced Control Efficacy of Bacillus subtilis NM4 via Integration of Chlorothalonil on Potato Early Blight Caused by Alternaria solani. Microb. Pathog. 2024, 190, 106604. [Google Scholar] [CrossRef] [PubMed]
- Nanjundan, J.; Ramasamy, R.; Uthandi, S.; Ponnusamy, M. Antimicrobial Activity and Spectroscopic Characterization of Surfactin Class of Lipopeptides from Bacillus amyloliquefaciens SR1. Microb. Pathog. 2019, 128, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zeng, Q.; Jiang, W.; Wang, L.; Zhang, J.; Wang, Z.; Wang, Q.; Li, Y. Genome Sequencing and Characterization of Bacillus velezensis N23 as Biocontrol Agent against Plant Pathogens. Microorganisms 2024, 12, 294. [Google Scholar] [CrossRef]
- Ananev, A.A.; Ogneva, Z.V.; Nityagovsky, N.N.; Suprun, A.R.; Kiselev, K.V.; Aleynova, O.A. Whole Genome Sequencing of Bacillus velezensis AMR25, an Effective Antagonist Strain against Plant Pathogens. Microorganisms 2024, 12, 1533. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Yang, J.-J.; Mao, Z.-C.; Ho, H.-H.; Wu, Y.-X.; He, Y.-Q. Enhancement of Biocontrol Activities and Cyclic Lipopeptides Production by Chemical Mutagenesis of Bacillus subtilis XF-1, a Biocontrol Agent of Plasmodiophora brassicae and Fusarium solani. Indian J. Microbiol. 2014, 54, 476–479. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. Antifungal Activity of Lipopeptides from Bacillus XT1 CECT 8661 Against Botrytis cinerea. Front. Microbiol 2018, 9, 1315. [Google Scholar] [CrossRef]
- Jemil, N.; Besbes, I.; Gharbi, Y.; Triki, M.A.; Cheffi, M.; Manresa, A.; Nasri, M.; Hmidet, N. Bacillus methylotrophicus DCS1: Production of Different Lipopeptide Families, in vitro Antifungal Activity and Suppression of Fusarium Wilt in Tomato Plants. Curr. Microbiol 2024, 81, 142. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, P.; Ye, W.; Chen, Y.; Wei, D.; Qiao, C.; Zhou, B.; Xiao, J. Biological Control of Root Rot of Strawberry by Bacillus amyloliquefaciens Strains CMS5 and CMR12. J. Fungi 2024, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Raza, W.; Huang, Q.; Shen, Q. The Ultrasound-assisted Extraction and Identification of Antifungal Substances from B. amyloliquefaciens Strain NJN-6 Suppressing Fusarium oxysporum. J. Basic Microbiol. 2012, 52, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, Y.; Diop, A.; Gancel, F.; Béchet, M.; Jacques, P.; Drider, D. Polynucleotide Phosphorylase Is Involved in the Control of Lipopeptide Fengycin Production in Bacillus subtilis. Arch. Microbiol. 2018, 200, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, M.; Huang, R.; Qu, Q.; Li, G.; Zhou, J.; Zhang, K.; Lu, K.; Niu, X.; Luo, J. Induction of Chlamydospore Formation in Fusarium by Cyclic Lipopeptide Antibiotics from Bacillus subtilis C2. J. Chem. Ecol. 2012, 38, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K. Plant Methyl Salicylate Induces Defense Responses in the Rhizobacterium Bacillus subtilis. Environ. Microbiol. 2015, 17, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Cazorla, F.M.; Romero, D.; Pérez-García, A.; Lugtenberg, B.J.J.; de Vicente, A.; Bloemberg, G. Isolation and Characterization of Antagonistic Bacillus subtilis Strains from the Avocado Rhizoplane Displaying Biocontrol Activity. J. Appl. Microbiol. 2007, 103, 1950–1959. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, Y.; Wang, J.; Dhanasekaran, S.; Yue, Q.; Feng, F.; Gu, X.; Li, B.; Zhao, L.; Zhang, H. Characterization of a Bacillus velezensis Strain as a Potential Biocontrol Agent against Soft Rot of Eggplant Fruits. Int. J. Food Microbiol. 2024, 410, 110480. [Google Scholar] [CrossRef]
- Rocha, G.T.; Queiroz, P.R.M.; Grynberg, P.; Togawa, R.C.; de Lima Ferreira, A.D.C.; do Nascimento, I.N.; Gomes, A.C.M.M.; Monnerat, R. Biocontrol Potential of Bacteria Belonging to the Bacillus subtilis Group against Pests and Diseases of Agricultural Interest through Genome Exploration. Antonie van Leeuwenhoek 2023, 116, 599–614. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Zhang, Y.; He, L.L.; Ji, Z.L.; Tong, Y.H. Identification of a Small Antimycotic Peptide Produced by Bacillus amyloliquefaciens 6256. Pestic Biochem. Physiol. 2018, 150, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Benitez, L.B.; Velho, R.V.; Lisboa, M.P.; da Costa Medina, L.F.; Brandelli, A. Isolation and Characterization of Antifungal Peptides Produced by Bacillus amyloliquefaciens LBM5006. J. Microbiol. 2010, 48, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Kefi, A.; Ben Slimene, I.; Karkouch, I.; Rihouey, C.; Azaeiz, S.; Bejaoui, M.; Belaid, R.; Cosette, P.; Jouenne, T.; Limam, F. Characterization of Endophytic Bacillus Strains from Tomato Plants (Lycopersicon esculentum) Displaying Antifungal Activity against Botrytis cinerea Pers. World J. Microbiol. Biotechnol 2015, 31, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Zeriouh, H.; Romero, D.; García-Gutiérrez, L.; Cazorla, F.M.; de Vicente, A.; Pérez-García, A. The Iturin-like Lipopeptides Are Essential Components in the Biological Control Arsenal of Bacillus Subtilis Against Bacterial Diseases of Cucurbits. Mol. Plant-Microbe Interact. 2011, 24, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Guo, Q.; Ma, Y.; Li, S.; Lu, X.; Zhang, X.; Ma, P. DegQ Regulates the Production of Fengycins and Biofilm Formation of the Biocontrol Agent Bacillus subtilis NCD-2. Microbiol. Res. 2015, 178, 42–50. [Google Scholar] [CrossRef]
- Xu, Z.; Shao, J.; Li, B.; Yan, X.; Shen, Q.; Zhang, R. Contribution of Bacillomycin D in Bacillus amyloliquefaciens SQR9 to Antifungal Activity and Biofilm Formation. Appl. Environ. Microbiol. 2013, 79, 808–815. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Xu, Y.; Zhang, G.; Shen, Q.; Zhang, R. Exploring Elicitors of the Beneficial Rhizobacterium Bacillus amyloliquefaciens SQR9 to Induce Plant Systemic Resistance and Their Interactions With Plant Signaling Pathways. Mol. Plant-Microbe Interact. 2018, 31, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; Wang, Y.; Guo, Q.; Lu, X.; Li, S.; Ma, P. Lipopeptides, a Novel Protein, and Volatile Compounds Contribute to the Antifungal Activity of the Biocontrol Agent Bacillus atrophaeus CAB-1. Appl. Microbiol. Biotechnol. 2013, 97, 9525–9534. [Google Scholar] [CrossRef] [PubMed]
- Toure, Y.; Ongena, M.; Jacques, P.; Guiro, A.; Thonart, P. Role of Lipopeptides Produced by Bacillus subtilis GA1 in the Reduction of Grey Mould Disease Caused by Botrytis Cinerea on Apple. J. Appl. Microbiol. 2004, 96, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.R.; Park, J.S.; Jung, W.-J. Antifungal Evaluation of Fengycin Isoforms Isolated from Bacillus amyloliquefaciens PPL against Fusarium oxysporum f. sp. lycopersici. Microb. Pathog. 2020, 149, 104509. [Google Scholar] [CrossRef]
- Lin, X.; Wang, J.; Hou, Z.; Ren, S.; Wang, W.; Yang, Y.; Yi, Y.; Zhang, Y.; Li, R. Antifungal Potential and Mechanism of Bacillus velezensis HeN-7 Isolated from Tobacco Leaves on Bipolaris sorokiniana. Curr. Microbiol. 2024, 81, 340. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Ruiz, V.; Robles-Montoya, R.I.; Parra-Cota, F.I.; Santoyo, G.; del Carmen Orozco-Mosqueda, M.; Rodríguez-Ramírez, R.; de los Santos-Villalobos, S. Draft Genome Sequence of Bacillus paralicheniformis TRQ65, a Biological Control Agent and Plant Growth-Promoting Bacterium Isolated from Wheat (Triticum turgidum subsp. durum) Rhizosphere in the Yaqui Valley, Mexico. 3 Biotech 2019, 9, 436. [Google Scholar] [CrossRef]
- Morales Sandoval, P.H.; Ortega Urquieta, M.E.; Valenzuela Ruíz, V.; Montañez Acosta, K.; Campos Castro, K.A.; Parra Cota, F.I.; Santoyo, G.; de los Santos Villalobos, S. Improving Beneficial Traits in Bacillus cabrialesii subsp. cabrialesii TE3T through UV-Induced Genomic Changes. Plants 2024, 13, 2578. [Google Scholar] [CrossRef]
- Walaszczyk, A.; Jasińska, A.; Bernat, P.; Płaza, G.; Paraszkiewicz, K. Microplastics Influence on Herbicides Removal and Biosurfactants Production by a Bacillus sp. Strain Active against Fusarium culmorum. Sci. Rep. 2023, 13, 14618. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, Y.; Wang, P.; Zhu, L.; Zheng, J.; Li, R.; Ruan, L.; Peng, D.; Sun, M. Complete Genome Sequence of Bacillus subtilis BSn5, an Endophytic Bacterium of Amorphophallus konjac with Antimicrobial Activity for the Plant Pathogen Erwinia carotovora subsp. carotovora. J. Bacteriol. 2011, 193, 2070–2071. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Zhang, Q.; Zhu, X.; Yao, X.; Zhang, L. The Complete Genome Sequence Resource of Rhizospheric Soil-Derived Bacillus velezensis Yao, with Biocontrol Potential Against Fusarium solani -Induced Pepper Root Rot. Phytopathology 2023, 113, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Grady, E.N.; MacDonald, J.; Ho, M.T.; Weselowski, B.; McDowell, T.; Solomon, O.; Renaud, J.; Yuan, Z.-C. Characterization and Complete Genome Analysis of the Surfactin-Producing, Plant-Protecting Bacterium Bacillus zelezensis 9D-6. BMC Microbiol. 2019, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Mu, F.; Chen, X.; Fu, Z.; Wang, X.; Guo, J.; Zhao, X.; Zhang, B. Genome and Transcriptome Analysis to Elucidate the Biocontrol Mechanism of Bacillus amyloliquefaciens XJ5 against Alternaria solani. Microorganisms 2023, 11, 2055. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, L.; Wu, H.; Gu, W.; Lu, Y.; Liu, C.; Zhang, J.; Li, W.; Zhou, C.; Geng, H.; et al. Bacillus velezensis YXDHD1-7 Prevents Early Blight Disease by Promoting Growth and Enhancing Defense Enzyme Activities in Tomato Plants. Microorganisms 2024, 12, 921. [Google Scholar] [CrossRef]
- Sharma, A.; Kaushik, N.; Sharma, A.; Bajaj, A.; Rasane, M.; Shouche, Y.S.; Marzouk, T.; Djébali, N. Screening of Tomato Seed Bacterial Endophytes for Antifungal Activity Reveals Lipopeptide Producing Bacillus siamensis Strain NKIT9 as a Potential Bio-Control Agent. Front. Microbiol. 2021, 12, 609482. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, I.; Berić, T.; Dimkić, I.; Popović, T.; Lozo, J.; Fira, D.; Stanković, S. Biological Control of Pseudomonas syringae pv. aptata on Sugar Beet with Bacillus pumilus SS-10.7 and Bacillus amyloliquefaciens (SS-12.6 and SS-38.4) Strains. J. Appl. Microbiol 2019, 126, 165–176. [Google Scholar] [CrossRef]
- Tang, Y.; Lei, J.; Ma, X.; Li, J.; Li, H.; Liu, Z. Identification and Characterization of a Novel Bacteriocin Gene Cluster in Lysinibacillus boronitolerans. Biotechnol. Appl. Biochem. 2023, 70, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Jiang, H.; Ding, T.; Xu, Q.; Chai, W.; Cheng, B. Bacillus amyloliquefaciens FZB42 Represses Plant miR846 to Induce Systemic Resistance via a Jasmonic Acid-dependent Signalling Pathway. Mol. Plant Pathol. 2018, 19, 1612–1623. [Google Scholar] [CrossRef]
- Dong, H.; Gao, R.; Dong, Y.; Yao, Q.; Zhu, H. Bacillus velezensis RC116 Inhibits the Pathogens of Bacterial Wilt and Fusarium Wilt in Tomato with Multiple Biocontrol Traits. Int. J. Mol. Sci 2023, 24, 8527. [Google Scholar] [CrossRef]
- Mosela, M.; Andrade, G.; Massucato, L.R.; de Araújo Almeida, S.R.; Nogueira, A.F.; de Lima Filho, R.B.; Zeffa, D.M.; Mian, S.; Higashi, A.Y.; Shimizu, G.D.; et al. Bacillus velezensis Strain Ag75 as a New Multifunctional Agent for Biocontrol, Phosphate Solubilization and Growth Promotion in Maize and Soybean Crops. Sci. Rep. 2022, 12, 15284. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.K.; Orellana, L.A.G.; Bryan, D.W.; Moore, A.; Munafo, J.P.; den Bakker, H.C.; Denes, T.G. Phylogeny of the Bacillus altitudinis Complex and Characterization of a Newly Isolated Strain with Antilisterial Activity. J. Food Prot. 2021, 84, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhang, R.; Liu, Y.; Liu, Y. Evaluation of the Biocontrol Efficiency of Bacillus subtilis Wettable Powder on Pepper Root Rot Caused by Fusarium solani. Pathogens 2023, 12, 225. [Google Scholar] [CrossRef]
- Ashajyothi, M.; Mahadevakumar, S.; Venkatesh, Y.N.; Sarma, P.V.S.R.N.; Danteswari, C.; Balamurugan, A.; Prakash, G.; Khandelwal, V.; Tarasatyavathi, C.; Podile, A.R.; et al. Comprehensive Genomic Analysis of Bacillus subtilis and Bacillus paralicheniformis Associated with the Pearl Millet Panicle Reveals Their Antimicrobial Potential against Important Plant Pathogens. BMC Plant Biol. 2024, 24, 197. [Google Scholar] [CrossRef]
- Li, B.; Yang, P.; Feng, Y.; Du, C.; Qi, G.; Zhao, X. Rhizospheric Microbiota of Suppressive Soil Protect Plants against Fusarium solani Infection. Pest Manag. Sci. 2024, 80, 4186–4198. [Google Scholar] [CrossRef] [PubMed]
- Cheffi, M.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Belka, M.; Vallat, A.; Rateb, M.E.; Tounsi, S.; Triki, M.A.; Belbahri, L. Olea europaea L. Root Endophyte Bacillus velezensis OEE1 Counteracts Oomycete and Fungal Harmful Pathogens and Harbours a Large Repertoire of Secreted and Volatile Metabolites and Beneficial Functional Genes. Microorganisms 2019, 7, 314. [Google Scholar] [CrossRef]
- Mnif, I.; Hammami, I.; Triki, M.A.; Azabou, M.C.; Ellouze-Chaabouni, S.; Ghribi, D. Antifungal Efficiency of a Lipopeptide Biosurfactant Derived from Bacillus subtilis SPB1 versus the Phytopathogenic Fungus, Fusarium solani. Environ. Sci. Pollut. Res. 2015, 22, 18137–18147. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhao, L.; Jiang, W.; Chen, R.; Zhang, R.; Chen, X.; Yin, C.; Mao, Z. The Phlorizin-Degrading Bacillus licheniformis XNRB-3 Mediates Soil Microorganisms to Alleviate Apple Replant Disease. Front. Microbiol. 2022, 13, 839484. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Gao, Y.; Gao, T.; Zhang, D. Inhibitory Abilities of Bacillus Isolates and Their Culture Filtrates against the Gray Mold Caused by Botrytis cinerea on Postharvest Fruit. Plant Pathol. J. 2019, 35, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; Salvatierra-Martínez, R.; González, M.; Araya, M. The Role of Surfactin Production by Bacillus velezensis on Colonization, Biofilm Formation on Tomato Root and Leaf Surfaces and Subsequent Protection (ISR) against Botrytis cinerea. Microorganisms 2021, 9, 2251. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Karaoglanidis, G.S.; Tzelepis, G. Insights into the Multitrophic Interactions between the Biocontrol Agent Bacillus Subtilis MBI 600, the Pathogen Botrytis Cinerea and Their Plant Host. Microbiol. Res. 2021, 248, 126752. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.P.; Teixeira, G.M.; de Jesus, M.L.A.; Bertê, R.; Higashi, A.; Mosela, M.; da Silva, D.V.; de Oliveira, J.P.; Sanches, D.S.; Brancher, J.D.; et al. Antifungal Activity and Genomic Characterization of the Biocontrol Agent Bacillus velezensis CMRP 4489. Sci. Rep. 2022, 12, 17401. [Google Scholar] [CrossRef] [PubMed]
- Balderas-Ruíz, K.A.; Gómez-Guerrero, C.I.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Aranda-Ocampo, S.; Juárez, A.M.; Leyva, E.; Galindo, E.; Serrano-Carreón, L. Bacillus velezensis 83 Increases Productivity and Quality of Tomato (Solanum lycopersicum L.): Pre and Postharvest Assessment. Curr. Res. Microb. Sci. 2021, 2, 100076. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wan, J.; Sha, J.; Tian, M.; Wang, M.; Zhang, X.; Sun, W.; Mao, Y.; Min, J.; Qin, Y.; et al. Genomics Assisted Functional Characterization of Bacillus velezensis E as a Biocontrol and Growth Promoting Bacterium for Lily. Front. Microbiol. 2022, 13, 976918. [Google Scholar] [CrossRef] [PubMed]
- Altimira, F.; Godoy, S.; Arias-Aravena, M.; Araya, B.; Montes, C.; Castro, J.F.; Dardón, E.; Montenegro, E.; Pineda, W.; Viteri, I.; et al. Genomic and Experimental Analysis of the Biostimulant and Antagonistic Properties of Phytopathogens of Bacillus safensis and Bacillus siamensis. Microorganisms 2022, 10, 670. [Google Scholar] [CrossRef]
- Thomloudi, E.-E.; Tsalgatidou, P.C.; Baira, E.; Papadimitriou, K.; Venieraki, A.; Katinakis, P. Genomic and Metabolomic Insights into Secondary Metabolites of the Novel Bacillus halotolerans Hil4, an Endophyte with Promising Antagonistic Activity against Gray Mold and Plant Growth Promoting Potential. Microorganisms 2021, 9, 2508. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Wu, J.; Chen, L.; Dong, W. Isolated Bacillus subtilis Strain 330-2 and Its Antagonistic Genes Identified by the Removing PCR. Sci. Rep. 2017, 7, 1777. [Google Scholar] [CrossRef]
- Ji, S.; Tian, Y.; Li, J.; Xu, G.; Zhang, Y.; Chen, S.; Chen, Y.; Tang, X. Complete Genome Sequence of Bacillus cereus Z4, a Biocontrol Agent against Tobacco Black Shank, Isolated from the Western Pacific Ocen. Mar. Genom. 2023, 72, 101071. [Google Scholar] [CrossRef]
- Eltokhy, M.A.; Saad, B.T.; Eltayeb, W.N.; Yahia, I.S.; Aboshanab, K.M.; Ashour, M.S.E. Exploring the Nature of the Antimicrobial Metabolites Produced by Paenibacillus ehimensis Soil Isolate MZ921932 Using a Metagenomic Nanopore Sequencing Coupled with LC-Mass Analysis. Antibiotics 2021, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.; Loll, B.; Müller, S.; Skobalj, R.; Ebeling, J.; Bulatov, T.; Gensel, S.; Göbel, J.; Wahl, M.C.; Genersch, E.; et al. Molecular Basis of Antibiotic Self-Resistance in a Bee Larvae Pathogen. Nat. Commun. 2022, 13, 2349. [Google Scholar] [CrossRef] [PubMed]
- Priyanto, J.A.; Prastya, M.E.; Astuti, R.I.; Kristiana, R. The Antibacterial and Antibiofilm Activities of the Endophytic Bacteria Associated with Archidendron pauciflorum against Multidrug-Resistant Strains. Appl. Biochem. Biotechnol. 2023, 195, 6653–6674. [Google Scholar] [CrossRef]
- Kukreti, A.; Kotasthane, A.S.; Tandon, A.L.; Nekkanti, A.; Prasannakumar, M.K.; Devanna, P.; Aravindaram, K.; Sreedevi, K.; Sushil, S.N.; Manjunatha, C. Hybrid de Novo Whole Genome Assembly of Lipopeptide Producing Novel Bacillus thuringiensis Strain NBAIR BtAr Exhibiting Antagonistic Activity against Sclerotium rolfsii. Microb. Pathog. 2024, 195, 106867. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, N.F.K.; Hashim, A.M.; Hanish, I.; Zulkarnain, A.; Raja Nhari, R.M.H.; Abdul Sani, A.A.; Abbasiliasi, S.; Ariff, A.; Mustafa, S.; Rahim, R.A. The Discovery of New Antilisterial Proteins From Paenibacillus polymyxa Kp10 via Genome Mining and Mass Spectrometry. Front. Microbiol. 2020, 11, 960. [Google Scholar] [CrossRef] [PubMed]

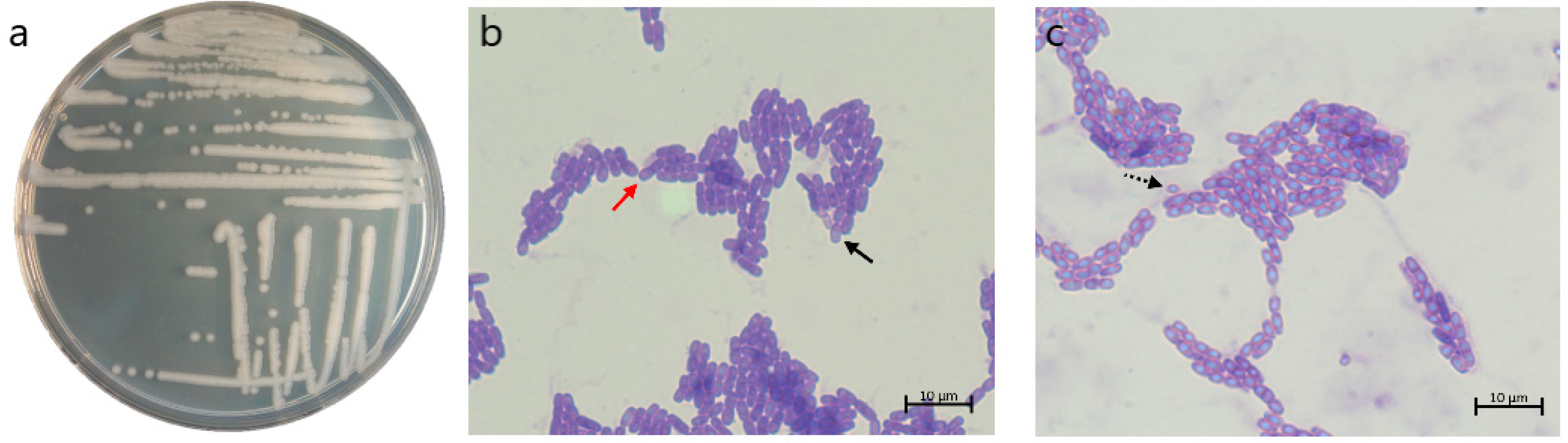
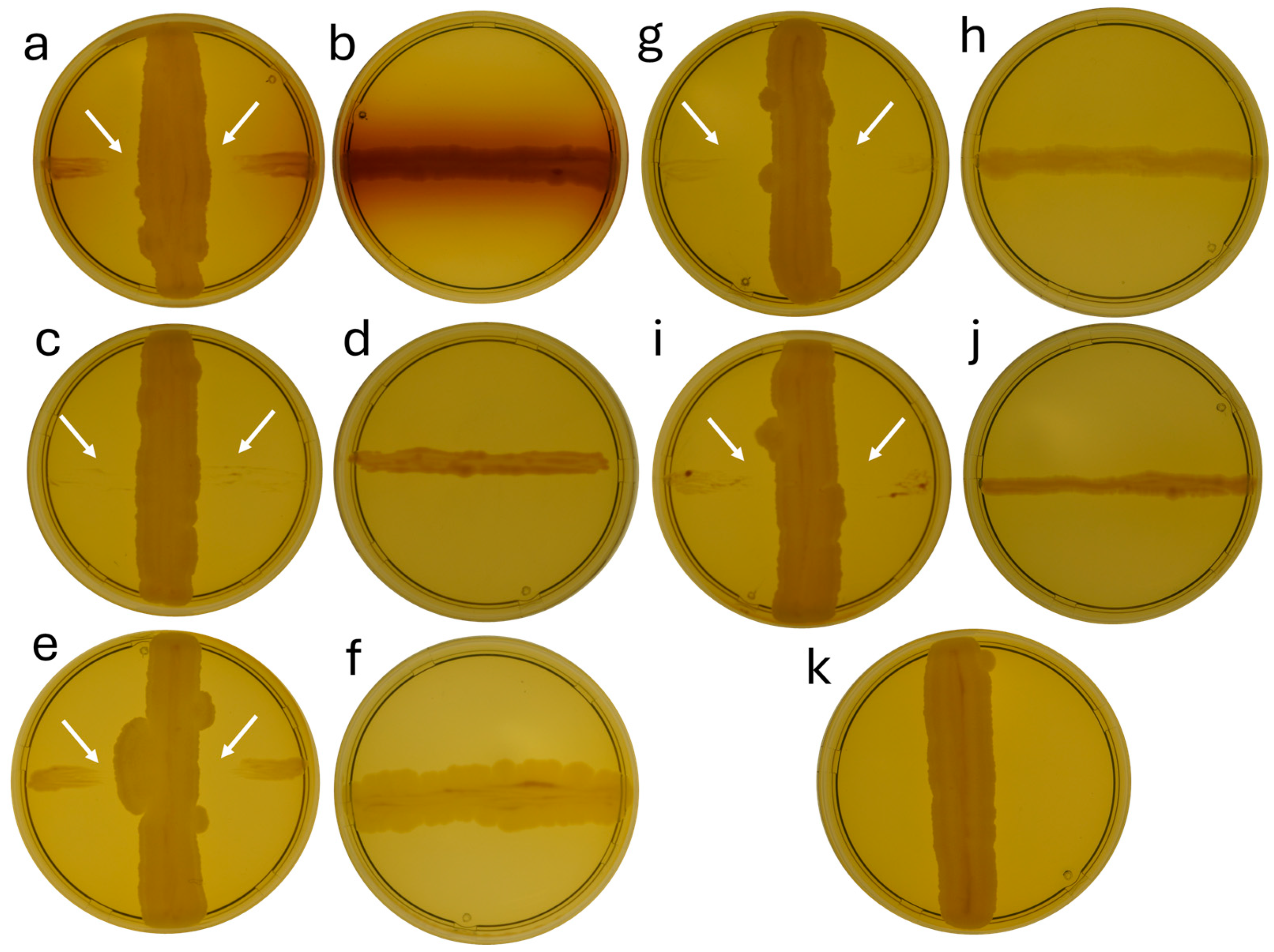
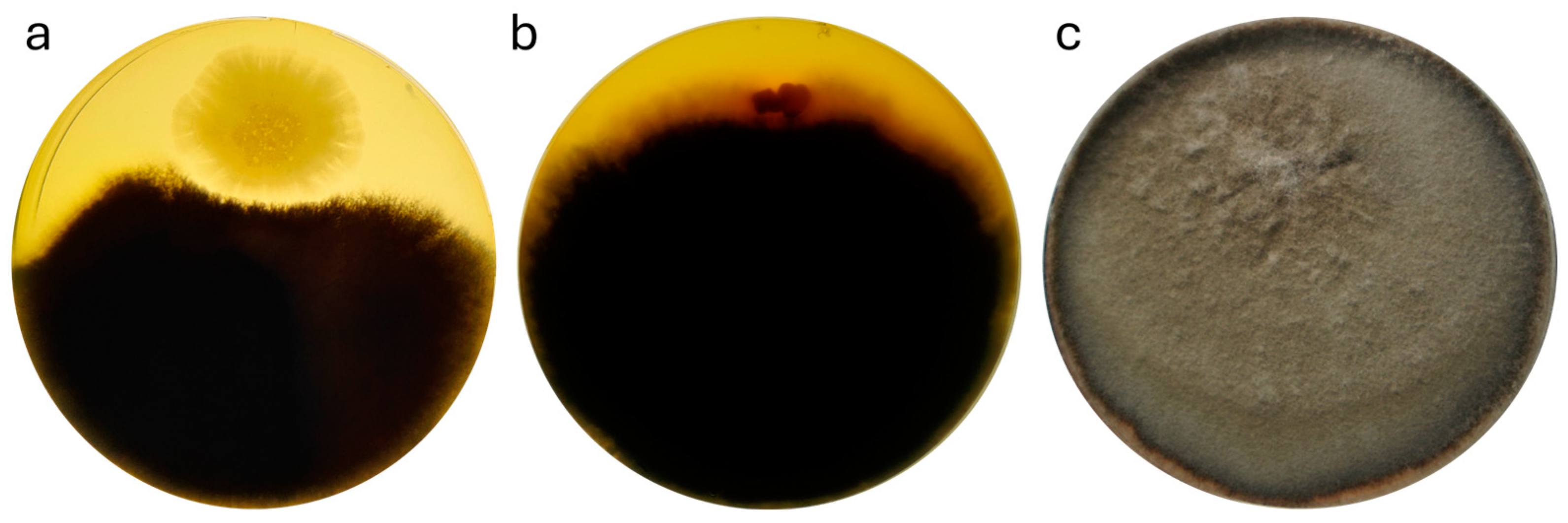
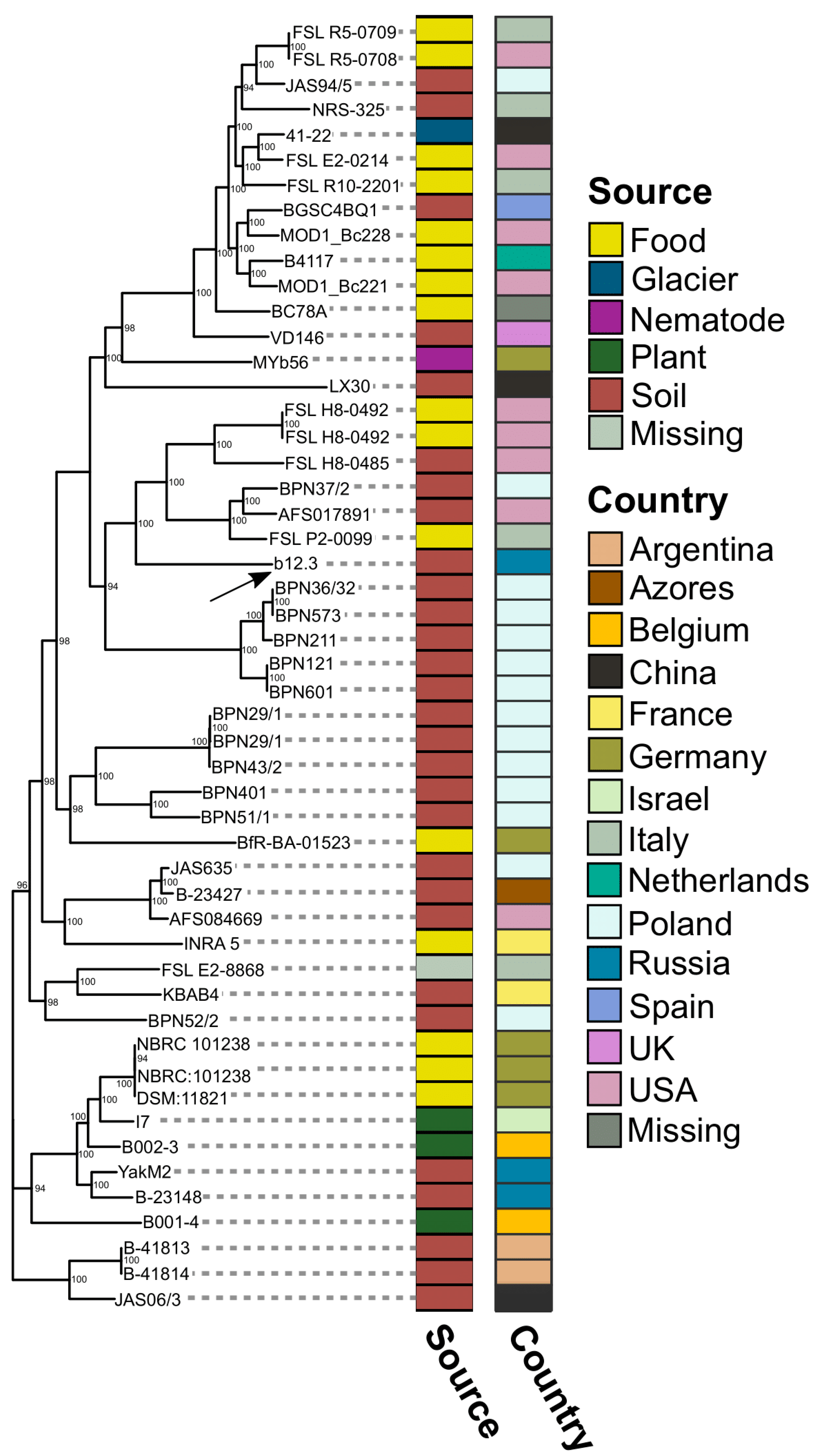
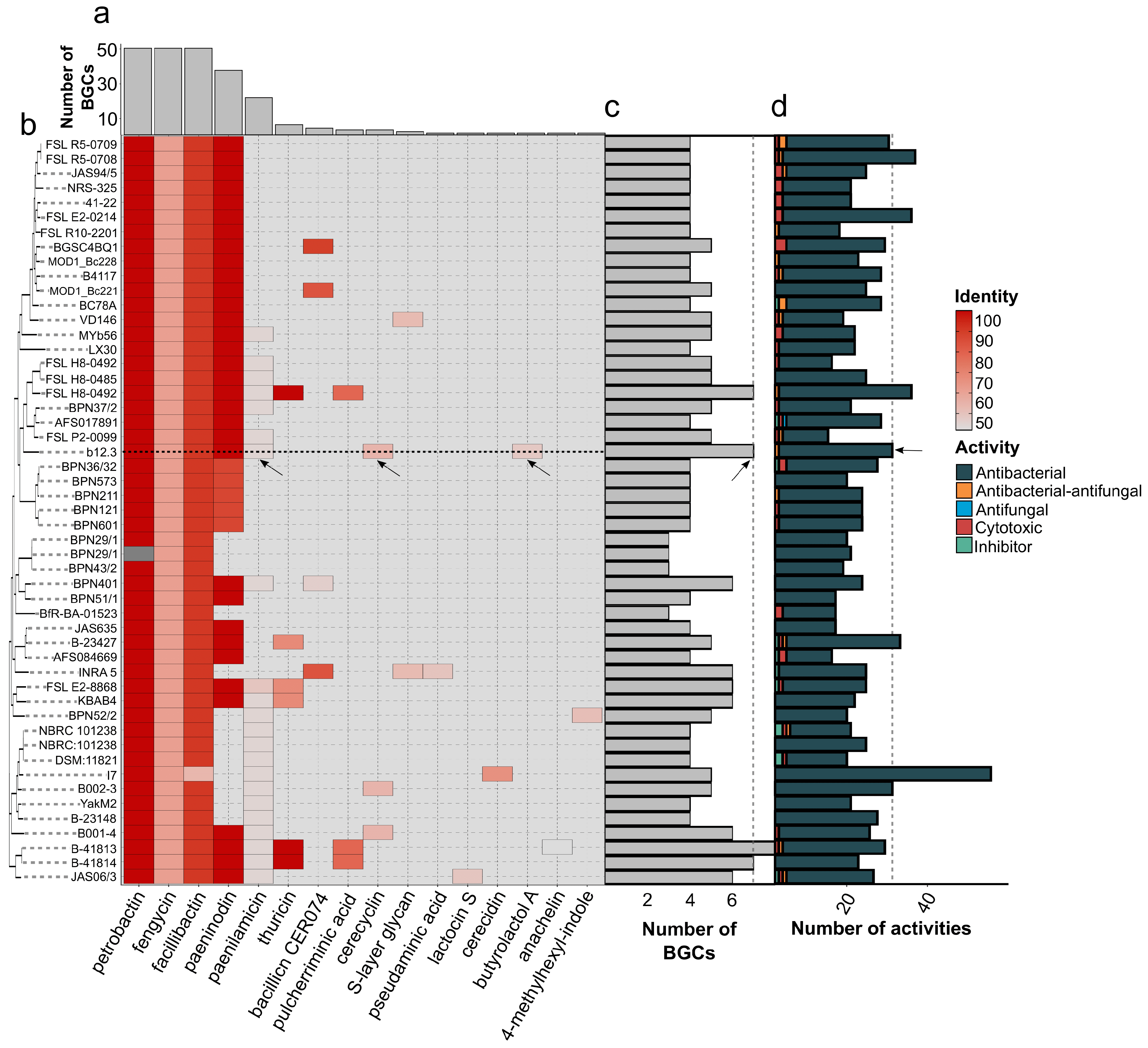
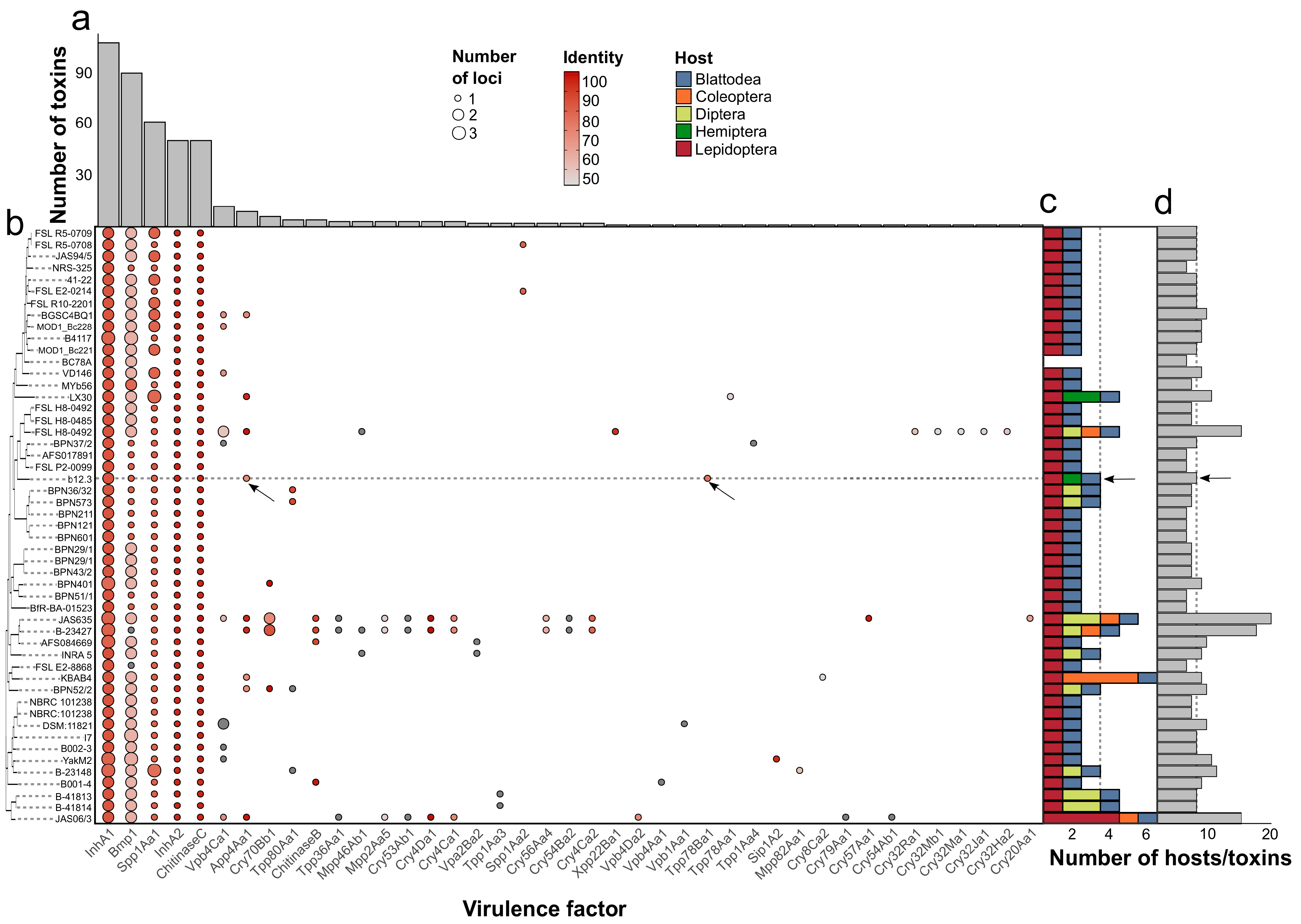
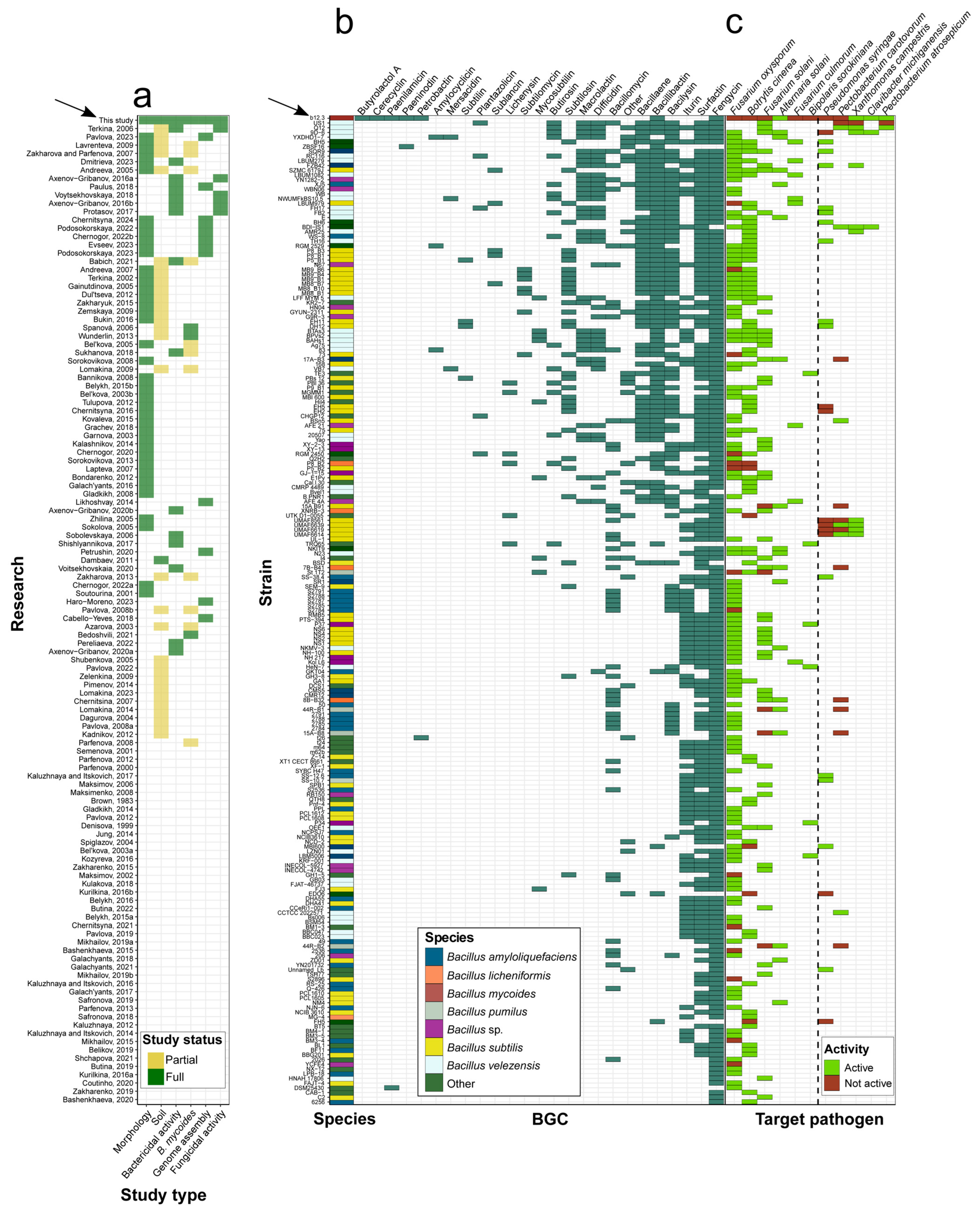
| Pathogen Type | Species | Strain(s)/Pathovar(s) | Provided by * |
|---|---|---|---|
| Pseudomonas sp. | 8949 | Dr. A.M.Lazarev, FSBSI VIZR | |
| Bacteria | Pectobacterium atrosepticum | 5128 | |
| Clavibacter michiganensis | 5351 | ||
| Xanthomonas campestris | 23 | ||
| Pseudomonas syringae | 03278 and 00904 | RCAM, ARRIAM | |
| P. atrosepticum | 01726 and 01000 | ||
| Pectobacterium carotovorum | 01001 | ||
| X. campestris | 01002 | ||
| C. michiganensis | VIZR | Dr. Antonova I.A., FSBSI VIZR, deposited in State Collection of Microorganisms, FSBSI VIZR | |
| P. syringae | pv. maculicola and tomato | ||
| Fungi | Fusarium oxysporum | 01187 | RCAM, ARRIAM |
| Alternaria solani | 03318 | ||
| Fusarium culmorum | 58800 | Dr. T. Yu. Gagkaeva, FSBSI VIZR | |
| F. oxysporum | 60521 | ||
| Fusarium solani | 46011 | ||
| A. solani | 46011 | ||
| Bipolaris sorokiniana | |||
| Botrytis sp. | Mrs. A.V.Erofeeva, ARRIAM |
| Species | Strain/Pathovar | Activity Intensity * |
|---|---|---|
| P. syringae | 03278 | - |
| 00904 | - | |
| pv. maculicola | - | |
| pv. tomato | - | |
| P. atrosepticum | 01726 | Lack of growth |
| 5128 | 9 ± 1.1 mm | |
| 01000 | - | |
| C. michiganensis | VIZR | - |
| 5351 | 6 ± 0.75 mm | |
| X. campestris | 01002 | Lack of growth |
| 23 | 5 ± 0.67 mm | |
| P. carotovorum | 01001 | - |
| Pseudomonas sp. | 8949 | - |
| Species | Strain | Activity Intensity * |
|---|---|---|
| F. oxysporum | 01187 | - |
| 60521 | - | |
| F. solani | 46011 | - |
| F. culmorum | 58800 | - |
| A. solani | 46011 | + |
| 03318 | - | |
| B. sorokiniana | - | |
| Botrytis sp. | - |
| Contig | Genomic Coordinate (b.p.) * | Known Substance | Identity | Chemical Class | Predicted Activity |
|---|---|---|---|---|---|
| 1 | 12,670–64,422 (51,752) | Bacillibactin | 85.71% | NRPS | - |
| 1 | 230,027–255,265 (25,238) | Fengycin | 40% | Betalactone | - |
| 1 | 275,519–322,529 (47,010) | - | - | NRPS | - |
| 1 | 345,867–356,142 (10,275) | - | - | RiPP-like | - |
| 2 | 187,808–211,342 (23,534) | - | - | LAP | Bactericidal |
| 9 | 1741–87,617 (85,876) | Butyrolactol A | 20% | Polyketide | - |
| 15 | 4606–48,187 (43,581) | - | - | NRPS-like | - |
| 16 | 6269–19,986 (13,717) | Petrobactin | 100% | Siderophore | - |
| 16 | 57,070–112,887 (55,817) | Paenilamicin | 14.29% | NRP + Polyketide | Bactericidal |
| 18 | 53,670–63,993 (10,323) | Cerecyclin | 30% | RiPP | - |
| 19 | 70,740–92,593 (21,853) | - | - | Terpene | - |
| 26 | 22,545–46,459 (23,914) | Paeninodin | 30% | RiPP | - |
| 29 | 42–65,910 (65,868) | - | - | NRPS | - |
| Encoded Toxin | Similarity Percent | Affected Host Order | Affected Host Species | References |
|---|---|---|---|---|
| App4Aa1 | 68% | Lepidoptera | Plutella xylostella | [81] |
| Tpp78Ba1 | 75.2% | Hemiptera | Laodelphax striatellus | [82] |
| Spp1Aa1 | 80.1% | Lepidoptera | Spodoptera litura | [83] |
| Blattodea | Blattella germanica |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanenko, M.N.; Shikov, A.E.; Savina, I.A.; Shmatov, F.M.; Nizhnikov, A.A.; Antonets, K.S. Genomic Insights into the Bactericidal and Fungicidal Potential of Bacillus mycoides b12.3 Isolated in the Soil of Olkhon Island in Lake Baikal, Russia. Microorganisms 2024, 12, 2450. https://doi.org/10.3390/microorganisms12122450
Romanenko MN, Shikov AE, Savina IA, Shmatov FM, Nizhnikov AA, Antonets KS. Genomic Insights into the Bactericidal and Fungicidal Potential of Bacillus mycoides b12.3 Isolated in the Soil of Olkhon Island in Lake Baikal, Russia. Microorganisms. 2024; 12(12):2450. https://doi.org/10.3390/microorganisms12122450
Chicago/Turabian StyleRomanenko, Maria N., Anton E. Shikov, Iuliia A. Savina, Fedor M. Shmatov, Anton A. Nizhnikov, and Kirill S. Antonets. 2024. "Genomic Insights into the Bactericidal and Fungicidal Potential of Bacillus mycoides b12.3 Isolated in the Soil of Olkhon Island in Lake Baikal, Russia" Microorganisms 12, no. 12: 2450. https://doi.org/10.3390/microorganisms12122450
APA StyleRomanenko, M. N., Shikov, A. E., Savina, I. A., Shmatov, F. M., Nizhnikov, A. A., & Antonets, K. S. (2024). Genomic Insights into the Bactericidal and Fungicidal Potential of Bacillus mycoides b12.3 Isolated in the Soil of Olkhon Island in Lake Baikal, Russia. Microorganisms, 12(12), 2450. https://doi.org/10.3390/microorganisms12122450







