Multidrug and Toxic Compound Extrusion Transporters: Ubiquitous Multifaceted Proteins in Microbes, Plants, and Their Interactions
Abstract
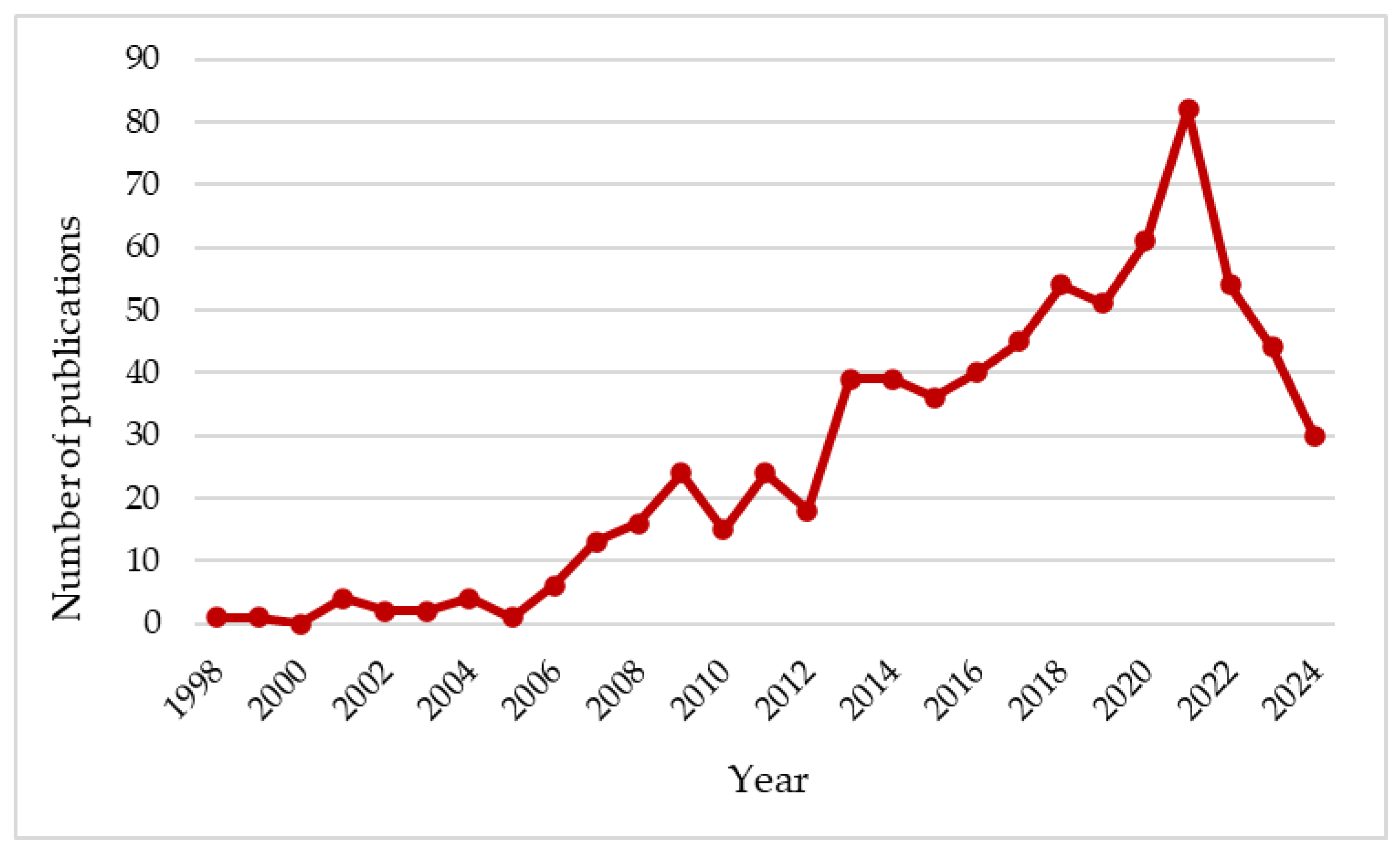
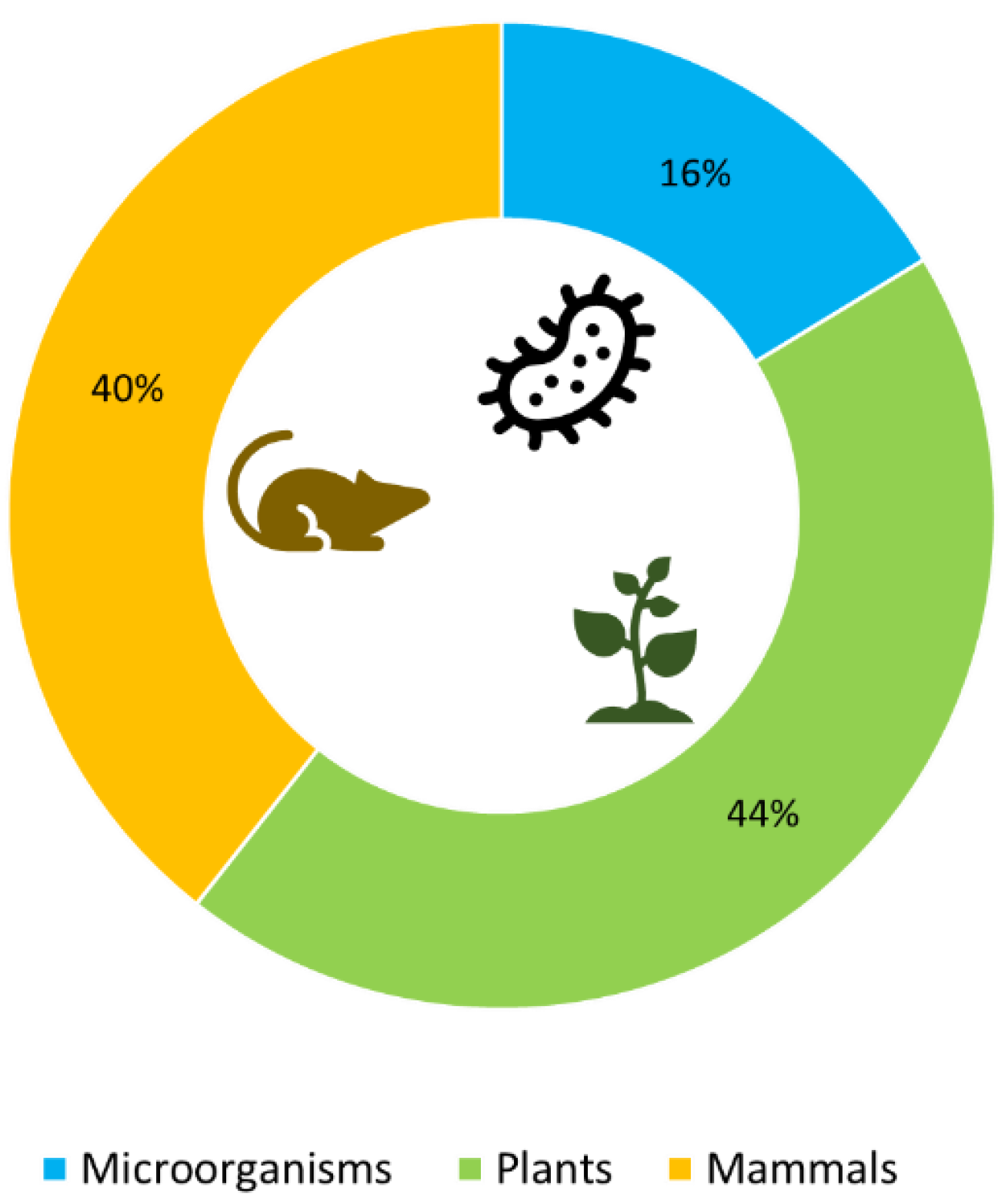
1. Introduction
2. MATE Transporters in Microorganisms
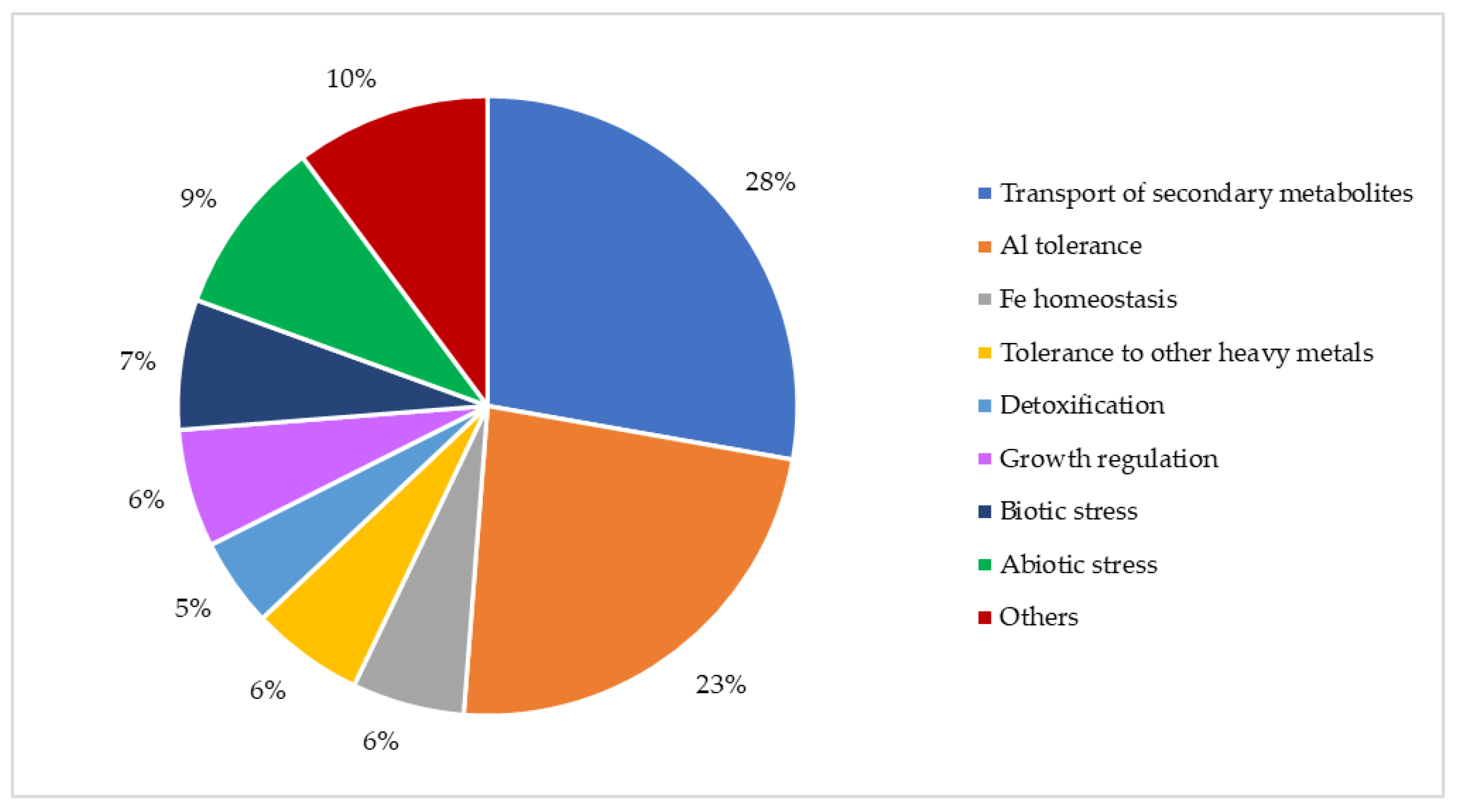
3. MATE Transporters in Plants
4. MATE Transporters in Plant–Pathogen Interactions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.-H.; Symersky, J.; Lu, M. Structure and Mechanism of a Redesigned Multidrug Transporter from the Major Facilitator Superfamily. Sci. Rep. 2020, 10, 3949. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xie, H.; Mehdipour, A.R.; Safarian, S.; Ermler, U.; Münke, C.; Thielmann, Y.; Hummer, G.; Ebersberger, I.; Wang, J.; et al. The Structure of the Aquifex aeolicus MATE Family Multidrug Resistance Transporter and Sequence Comparisons Suggest the Existence of a New Subfamily. Proc. Natl. Acad. Sci. USA 2021, 118, e2107335118. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Kodama, K.; Shiota, S.; Mine, T.; Kataoka, A.; Mizushima, T.; Tsuchiya, T. NorM, a Putative Multidrug Efflux Protein, of Vibrio parahaemolyticus and Its Homolog in Escherichia coli. Antimicrob. Agents Chemother. 1998, 42, 1778–1782. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.H.; Paulsen, I.T.; Skurray, R.A. The Multidrug Efflux Protein NorM Is a Prototype of a New Family of Transporters. Mol. Microbiol. 1999, 31, 394–395. [Google Scholar] [CrossRef]
- Omote, H.; Hiasa, M.; Matsumoto, T.; Otsuka, M.; Moriyama, Y. The MATE Proteins as Fundamental Transporters of Metabolic and Xenobiotic Organic Cations. Trends Pharmacol. Sci. 2006, 27, 587–593. [Google Scholar] [CrossRef]
- Diener, A.C.; Gaxiola, R.A.; Fink, G.R. Arabidopsis ALF5, a Multidrug Efflux Transporter Gene Family Member, Confers Resistance to Toxins. Plant Cell 2001, 13, 1625–1638. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsumoto, T.; Morimoto, R.; Arioka, S.; Omote, H.; Moriyama, Y. A Human Transporter Protein That Mediates the Final Excretion Step for Toxic Organic Cations. Proc. Natl. Acad. Sci. USA 2005, 102, 17923–17928. [Google Scholar] [CrossRef]
- Staud, F.; Cerveny, L.; Ahmadimoghaddam, D.; Ceckova, M. Multidrug and Toxin Extrusion Proteins (MATE/SLC47); Role in Pharmacokinetics. Int. J. Biochem. Cell Biol. 2013, 45, 2007–2011. [Google Scholar] [CrossRef]
- Goda, M.; Kanda, M.; Yoshioka, T.; Yoshida, A.; Murai, Y.; Zamami, Y.; Aizawa, F.; Niimura, T.; Hamano, H.; Okada, N.; et al. Effects of 5-HT₃ Receptor Antagonists on Cisplatin-Induced Kidney Injury. Clin. Transl. Sci. 2021, 14, 1906–1916. [Google Scholar] [CrossRef]
- Chung, Y.J.; Krueger, C.; Metzgar, D.; Saier, M.H. Size Comparisons among Integral Membrane Transport Protein Homologues in Bacteria, Archaea, and Eucarya. J. Bacteriol. 2001, 183, 1012–1021. [Google Scholar] [CrossRef]
- Hvorup, R.N.; Winnen, B.; Chang, A.B.; Jiang, Y.; Zhou, X.-F.; Saier, M.H., Jr. The Multidrug/Oligosaccharidyl-Lipid/Polysaccharide (MOP) Exporter Superfamily. Eur. J. Biochem. 2003, 270, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Jagessar, K.L.; Claxton, D.P.; Stein, R.A.; Mchaourab, H.S. Sequence and Structural Determinants of Ligand-Dependent Alternating Access of a MATE Transporter. Proc. Natl. Acad. Sci. USA 2020, 117, 4732–4740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wright, S.H. MATE1 Has an External COOH Terminus, Consistent with a 13-Helix Topology. Am. J. Physiol.-Ren. Physiol. 2009, 297, F263–F271. [Google Scholar] [CrossRef] [PubMed]
- Kusakizako, T.; Miyauchi, H.; Ishitani, R.; Nureki, O. Structural Biology of the Multidrug and Toxic Compound Extrusion Superfamily Transporters. Biochim. Biophys. Acta (BBA)—Biomembr. 2020, 1862, 183154. [Google Scholar] [CrossRef]
- Kuroda, T.; Tsuchiya, T. Multidrug Efflux Transporters in the MATE Family. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2009, 1794, 763–768. [Google Scholar] [CrossRef]
- Zakrzewska, S.; Mehdipour, A.R.; Malviya, V.N.; Nonaka, T.; Koepke, J.; Muenke, C.; Hausner, W.; Hummer, G.; Safarian, S.; Michel, H. Inward-Facing Conformation of a Multidrug Resistance MATE Family Transporter. Proc. Natl. Acad. Sci. USA 2019, 116, 12275–12284. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, H.; Moriyama, S.; Kusakizako, T.; Kumazaki, K.; Nakane, T.; Yamashita, K.; Hirata, K.; Dohmae, N.; Nishizawa, T.; Ito, K.; et al. Structural Basis for Xenobiotic Extrusion by Eukaryotic MATE Transporter. Nat. Commun. 2017, 8, 1633. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Liu, H.; Kang, L.; Geng, J.; Gai, Y.; Ding, Y.; Sun, H.; Li, Y. Identification and Expression Analysis of MATE Genes Involved in Flavonoid Transport in Blueberry Plants. PLoS ONE 2015, 10, e0118578. [Google Scholar] [CrossRef]
- Nawrath, C.; Heck, S.; Parinthawong, N.; Métraux, J.-P. EDS5, an Essential Component of Salicylic Acid–Dependent Signaling for Disease Resistance in Arabidopsis, Is a Member of the MATE Transporter Family. Plant Cell 2002, 14, 275–286. [Google Scholar] [CrossRef]
- Yokosho, K.; Yamaji, N.; Ma, J.F. Isolation and Characterisation of Two MATE Genes in Rye. Funct. Plant Biol. 2010, 37, 296–303. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, C.; Guo, X.; Liu, E.; Mao, K.; Mu, C.; Chen, N.; Zhang, W.; Liu, H. ELS1, a Novel MATE Transporter Related to Leaf Senescence and Iron Homeostasis in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2016, 476, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, H.; Harrington, K.C. Perspective: Root Exudation of Herbicides as a Novel Mode of Herbicide Resistance in Weeds. Pest Manag. Sci. 2020, 76, 2543–2547. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ryan, P.R.; Liu, C.; Li, H.; Hu, W.; Yan, W.; Huang, Y.; He, W.; Luo, B.; Zhang, X.; et al. ZmMATE6 from Maize Encodes a Citrate Transporter That Enhances Aluminum Tolerance in Transgenic Arabidopsis thaliana. Plant Sci. 2021, 311, 111016. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Iwaki, S.; Sasaki, A.; Tsukazaki, T. Crystal Structures of a Nicotine MATE Transporter Provide Insight into Its Mechanism of Substrate Transport. FEBS Lett. 2021, 595, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Slipski, C.J.; Zhanel, G.G.; Bay, D.C. Biocide Selective TolC-Independent Efflux Pumps in Enterobacteriaceae. J. Membr. Biol. 2018, 251, 15–33. [Google Scholar] [CrossRef]
- Burse, A.; Weingart, H.; Ullrich, M.S. NorM, an Erwinia amylovora Multidrug Efflux Pump Involved in In Vitro Competition with Other Epiphytic Bacteria. Appl. Environ. Microbiol. 2004, 70, 693–703. [Google Scholar] [CrossRef]
- Schlunk, I.; Krause, K.; Wirth, S.; Kothe, E. A Transporter for Abiotic Stress and Plant Metabolite Resistance in the Ectomycorrhizal Fungus Tricholoma vaccinum. Environ. Sci. Pollut. Res. 2015, 22, 19384–19393. [Google Scholar] [CrossRef]
- Krause, K.; Henke, C.; Asiimwe, T.; Ulbricht, A.; Klemmer, S.; Schachtschabel, D.; Boland, W.; Kothe, E. Biosynthesis and Secretion of Indole-3-Acetic Acid and Its Morphological Effects on Tricholoma vaccinum-Spruce Ectomycorrhiza. Appl. Environ. Microbiol. 2015, 81, 7003–7011. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, D.; Wang, Z.; Tian, Z.; Yang, F.; Lu, X.; Long, C. Genome Sequencing and Transcriptome Analysis of Geotrichum citri-aurantii on Citrus Reveal the Potential Pathogenic- and Guazatine-Resistance Related Genes. Genomics 2020, 112, 4063–4071. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Li, P.; Luo, T.; Pu, X.; Zhou, Y.; Yu, J.; Liu, L. Plant Transporters: Roles in Stress Responses and Effects on Growth and Development. Plant Growth Regul. 2021, 93, 253–266. [Google Scholar] [CrossRef]
- Takanashi, K.; Shitan, N.; Yazaki, K. The Multidrug and Toxic Compound Extrusion (MATE) Family in Plants. Plant Biotechnol. 2014, 31, 417–430. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H.; Pan, Y.; Yu, Y.; Luan, S.; Li, L. A DTX/MATE-Type Transporter Facilitates Abscisic Acid Efflux and Modulates ABA Sensitivity and Drought Tolerance in Arabidopsis. Mol. Plant 2014, 7, 1522–1532. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Wang, W.; Gai, J.; Li, Y. Genome-Wide Analysis of MATE Transporters and Expression Patterns of a Subgroup of MATE Genes in Response to Aluminum Toxicity in Soybean. BMC Genom. 2016, 17, 223. [Google Scholar] [CrossRef]
- Gani, U.; Sharma, P.; Tiwari, H.; Nautiyal, A.K.; Kundan, M.; Wajid, M.A.; Kesari, R.; Nargotra, A.; Misra, P. Comprehensive Genome-Wide Identification, Characterization, and Expression Profiling of MATE Gene Family in Nicotiana tabacum. Gene 2021, 783, 145554. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Huang, Z.; Li, J.; Bao, J.; Tu, H.; Zeng, C.; Wu, Z.; Fu, H.; Xu, J.; Zhou, D.; et al. qTGW12a, a Naturally Varying QTL, Regulates Grain Weight in Rice. Theor. Appl. Genet. 2021, 134, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liao, L.; Xu, J.; Han, Y.; Li, L. Genome-Wide Identification, Characterization and Expression Analysis of MATE Family Genes in Apple (Malus × Domestica Borkh). BMC Genom. 2021, 22, 632. [Google Scholar] [CrossRef]
- Takanashi, K.; Yokosho, K.; Saeki, K.; Sugiyama, A.; Sato, S.; Tabata, S.; Ma, J.F.; Yazaki, K. LjMATE1: A Citrate Transporter Responsible for Iron Supply to the Nodule Infection Zone of Lotus japonicus. Plant Cell Physiol. 2013, 54, 585–594. [Google Scholar] [CrossRef]
- Li, N.; Meng, H.; Xing, H.; Liang, L.; Zhao, X.; Luo, K. Genome-Wide Analysis of MATE Transporters and Molecular Characterization of Aluminum Resistance in Populus. J. Exp. Bot. 2017, 68, 5669–5683. [Google Scholar] [CrossRef]
- Morales-Quintana, L.; Bustos, D.; González, J.; Urbina, D.C.; Herrera, R.; Ramos, P. PrMATE1 Is Differentially Expressed in Radiata Pine Exposed to Inclination and the Deduced Protein Displays High Affinity to Proanthocyanidin Substrates by a Computational Approach. J. Plant Growth Regul. 2019, 38, 14–29. [Google Scholar] [CrossRef]
- Upadhyay, N.; Kar, D.; Deepak Mahajan, B.; Nanda, S.; Rahiman, R.; Panchakshari, N.; Bhagavatula, L.; Datta, S. The Multitasking Abilities of MATE Transporters in Plants. J. Exp. Bot. 2019, 70, 4643–4656. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Sugiyama, A.; Morita, M.; Shitan, N. Secondary Transport as an Efficient Membrane Transport Mechanism for Plant Secondary Metabolites. Phytochem. Rev. 2008, 7, 513–524. [Google Scholar] [CrossRef]
- Debeaujon, I.; Peeters, A.J.M.; Léon-Kloosterziel, K.M.; Koornneef, M. The TRANSPARENT TESTA12 Gene of Arabidopsis Encodes a Multidrug Secondary Transporter-like Protein Required for Flavonoid Sequestration in Vacuoles of the Seed Coat Endothelium. Plant Cell 2001, 13, 853–871. [Google Scholar] [CrossRef] [PubMed]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.-M.; Debeaujon, I.; Klein, M. The Arabidopsis MATE Transporter TT12 Acts as a Vacuolar Flavonoid/H+-Antiporter Active in Proanthocyanidin-Accumulating Cells of the Seed Coat. Plant Cell 2007, 19, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Oono, Y.; Narumi, I. Arabidopsis Pab1, a Mutant with Reduced Anthocyanins in Immature Seeds from Banyuls, Harbors a Mutation in the MATE Transporter FFT. Plant Mol. Biol. 2016, 90, 7–18. [Google Scholar] [CrossRef]
- Gomez, C.; Terrier, N.; Torregrosa, L.; Vialet, S.; Fournier-Level, A.; Verriès, C.; Souquet, J.-M.; Mazauric, J.-P.; Klein, M.; Cheynier, V.; et al. Grapevine MATE-Type Proteins Act as Vacuolar H+-Dependent Acylated Anthocyanin Transporters. Plant Physiol. 2009, 150, 402–415. [Google Scholar] [CrossRef]
- Watanabe, M.; Otagaki, S.; Matsumoto, S.; Shiratake, K. Genome-Wide Analysis of Multidrug and Toxic Compound Extruction Transporters in Grape. Front. Plant Sci. 2022, 13, 892638. [Google Scholar] [CrossRef]
- Sheng, J.; Chen, X.; Song, B.; Liu, H.; Li, J.; Wang, R.; Wu, J. Genome-Wide Identification of the MATE Gene Family and Functional Characterization of PbrMATE9 Related to Anthocyanin in Pear. Hortic. Plant J. 2023, 9, 1079–1094. [Google Scholar] [CrossRef]
- Morita, M.; Shitan, N.; Sawada, K.; Van Montagu, M.C.E.; Inzé, D.; Rischer, H.; Goossens, A.; Oksman-Caldentey, K.-M.; Moriyama, Y.; Yazaki, K. Vacuolar Transport of Nicotine Is Mediated by a Multidrug and Toxic Compound Extrusion (MATE) Transporter in Nicotiana tabacum. Proc. Natl. Acad. Sci. USA 2009, 106, 2447–2452. [Google Scholar] [CrossRef]
- Shitan, N.; Minami, S.; Morita, M.; Hayashida, M.; Ito, S.; Takanashi, K.; Omote, H.; Moriyama, Y.; Sugiyama, A.; Goossens, A.; et al. Involvement of the Leaf-Specific Multidrug and Toxic Compound Extrusion (MATE) Transporter Nt-JAT2 in Vacuolar Sequestration of Nicotine in Nicotiana tabacum. PLoS ONE 2014, 9, e108789. [Google Scholar] [CrossRef]
- Shoji, T.; Inai, K.; Yazaki, Y.; Sato, Y.; Takase, H.; Shitan, N.; Yazaki, K.; Goto, Y.; Toyooka, K.; Matsuoka, K.; et al. Multidrug and Toxic Compound Extrusion-Type Transporters Implicated in Vacuolar Sequestration of Nicotine in Tobacco Roots. Plant Physiol. 2009, 149, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Kar, D.; Pradhan, A.A.; Datta, S. The Role of Solute Transporters in Aluminum Toxicity and Tolerance. Physiol. Plant. 2021, 171, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Magalhaes, J.V.; Shaff, J.; Kochian, L.V. Aluminum-Activated Citrate and Malate Transporters from the MATE and ALMT Families Function Independently to Confer Arabidopsis Aluminum Tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Wang, N.; Dai, J.; Wang, T.; Kochian, L.V.; Liu, J.; Zuo, Y. AhFRDL1-Mediated Citrate Secretion Contributes to Adaptation to Iron Deficiency and Aluminum Stress in Peanuts. J. Exp. Bot. 2019, 70, 2873–2886. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Cui, W.; Gong, L.; He, Y.; Zhang, Q.; Meng, X.; Yang, Z.; You, J. Characterization of GmMATE13 in Its Contribution of Citrate Efflux and Aluminum Resistance in Soybeans. Front. Plant Sci. 2022, 13, 1027560. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; Vinecky, F.; Duarte, K.E.; Santiago, T.R.; das Chagas Noqueli Casari, R.A.; Hell, A.F.; da Cunha, B.A.D.B.; Martins, P.K.; da Cruz Centeno, D.; de Oliveira Molinari, P.A.; et al. Enhanced Aluminum Tolerance in Sugarcane: Evaluation of SbMATE Overexpression and Genome-Wide Identification of ALMTs in Saccharum spp. BMC Plant Biol. 2021, 21, 300. [Google Scholar] [CrossRef]
- Briat, J.-F.; Fobis-Loisy, I.; Grignon, N.; Lobréaux, S.; Pascal, N.; Savino, G.; Thoiron, S.; Von Wirén, N.; Van Wuytswinkel, O. Cellular and Molecular Aspects of Iron Metabolism in Plants. Biol. Cell 1995, 84, 69–81. [Google Scholar] [CrossRef]
- Durrett, T.P.; Gassmann, W.; Rogers, E.E. The FRD3-Mediated Efflux of Citrate into the Root Vasculature Is Necessary for Efficient Iron Translocation. Plant Physiol. 2007, 144, 197–205. [Google Scholar] [CrossRef]
- Rogers, E.E.; Wu, X.; Stacey, G.; Nguyen, H.T. Two MATE Proteins Play a Role in Iron Efficiency in Soybean. J. Plant Physiol. 2009, 166, 1453–1459. [Google Scholar] [CrossRef]
- Yokosho, K.; Yamaji, N.; Ueno, D.; Mitani, N.; Ma, J.F. OsFRDL1 Is a Citrate Transporter Required for Efficient Translocation of Iron in Rice. Plant Physiol. 2009, 149, 297–305. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Kakei, Y.; Shimo, H.; Bashir, K.; Sato, Y.; Sato, Y.; Uozumi, N.; Nakanishi, H.; Nishizawa, N.K. A Rice Phenolic Efflux Transporter Is Essential for Solubilizing Precipitated Apoplasmic Iron in the Plant Stele*. J. Biol. Chem. 2011, 286, 24649–24655. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Z.; Pandey, G.K.; Tsuchiya, T.; Luan, S. Functional Cloning and Characterization of a Plant Efflux Carrier for Multidrug and Heavy Metal Detoxification*. J. Biol. Chem. 2002, 277, 5360–5368. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, I.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Time Course Analysis of Gene Regulation under Cadmium Stress in Rice. Plant Soil 2009, 325, 97–108. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Y.; Mu, S.; Yan, D.; Xu, X.; Zhang, L.; Xu, B. Changes in Phenotype and Gene Expression under Lead Stress Revealed Key Genetic Responses to Lead Tolerance in Medicago sativa L. Gene 2021, 791, 145714. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, G.; Tian, W.; Li, D.; Meng, L.; Wu, D.; He, T. Genome-Wide Identification of MATE Gene Family in Potato (Solanum tuberosum L.) and Expression Analysis in Heavy Metal Stress. Front. Genet. 2021, 12, 650500. [Google Scholar] [CrossRef]
- Nimmy, M.S.; Kumar, V.; Singh, A.K.; Jain, P.K.; Srinivasan, R. Expression Analysis of a MATE-Type Transporter Gene of Arabidopsis and Its Orthologues in Rice and Chickpea under Salt Stress. Intern. J. Contemp. Microbiol. 2015, 75, 478. [Google Scholar] [CrossRef]
- Lu, P.; Magwanga, R.O.; Kirungu, J.N.; Hu, Y.; Dong, Q.; Cai, X.; Zhou, Z.; Wang, X.; Zhang, Z.; Hou, Y.; et al. Overexpression of Cotton a DTX/MATE Gene Enhances Drought, Salt, and Cold Stress Tolerance in Transgenic Arabidopsis. Front. Plant Sci. 2019, 10, 299. [Google Scholar] [CrossRef]
- Gandia-Herrero, F.; Lorenz, A.; Larson, T.; Graham, I.A.; Bowles, D.J.; Rylott, E.L.; Bruce, N.C. Detoxification of the Explosive 2,4,6-Trinitrotoluene in Arabidopsis: Discovery of Bifunctional O- and C-Glucosyltransferases. Plant J. 2008, 56, 963–974. [Google Scholar] [CrossRef]
- Ramel, F.; Sulmon, C.; Cabello-Hurtado, F.; Taconnat, L.; Martin-Magniette, M.-L.; Renou, J.-P.; El Amrani, A.; Couée, I.; Gouesbet, G. Genome-Wide Interacting Effects of Sucrose and Herbicide-Mediated Stress in Arabidopsis thaliana: Novel Insights into Atrazine Toxicity and Sucrose-Induced Tolerance. BMC Genom. 2007, 8, 450. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Li, S.; Qin, G.; Novák, O.; Pěnčík, A.; Ljung, K.; Aoyama, T.; Liu, J.; Murphy, A.; et al. ADP1 Affects Plant Architecture by Regulating Local Auxin Biosynthesis. PLoS Genet. 2014, 10, e1003954. [Google Scholar] [CrossRef]
- Upadhyay, N.; Kar, D.; Datta, S. A Multidrug and Toxic Compound Extrusion (MATE) Transporter Modulates Auxin Levels in Root to Regulate Root Development and Promotes Aluminium Tolerance. Plant Cell Environ. 2020, 43, 745–759. [Google Scholar] [CrossRef]
- Wang, P.; Yu, W.; Zhang, J.; Rengel, Z.; Xu, J.; Han, Q.; Chen, L.; Li, K.; Yu, Y.; Chen, Q. Auxin Enhances Aluminium-Induced Citrate Exudation through Upregulation of GmMATE and Activation of the Plasma Membrane H+-ATPase in Soybean Roots. Ann. Bot. 2016, 118, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Won, S.-K.; Lee, Y.-J.; Lee, H.-Y.; Heo, Y.-K.; Cho, M.; Cho, H.-T. Cis-Element- and Transcriptome-Based Screening of Root Hair-Specific Genes and Their Functional Characterization in Arabidopsis. Plant Physiol. 2009, 150, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Bock, K.W.; Honys, D.; Ward, J.M.; Padmanaban, S.; Nawrocki, E.P.; Hirschi, K.D.; Twell, D.; Sze, H. Integrating Membrane Transport with Male Gametophyte Development and Function through Transcriptomics. Plant Physiol. 2006, 140, 1151–1168. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Rathjen, J.P. Plant Immunity: Towards an Integrated View of Plant–Pathogen Interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Feussner, I. Isochorismate-Derived Biosynthesis of the Plant Stress Hormone Salicylic Acid. Science 2019, 365, 498–502. [Google Scholar] [CrossRef]
- Serrano, M.; Wang, B.; Aryal, B.; Garcion, C.; Abou-Mansour, E.; Heck, S.; Geisler, M.; Mauch, F.; Nawrath, C.; Métraux, J.-P. Export of Salicylic Acid from the Chloroplast Requires the Multidrug and Toxin Extrusion-Like Transporter EDS5. Plant Physiol. 2013, 162, 1815–1821. [Google Scholar] [CrossRef]
- Ishihara, T.; Sekine, K.-T.; Hase, S.; Kanayama, Y.; Seo, S.; Ohashi, Y.; Kusano, T.; Shibata, D.; Shah, J.; Takahashi, H. Overexpression of the Arabidopsis thaliana EDS5 Gene Enhances Resistance to Viruses. Plant Biol. 2008, 10, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Dobritzsch, M.; Lübken, T.; Eschen-Lippold, L.; Gorzolka, K.; Blum, E.; Matern, A.; Marillonnet, S.; Böttcher, C.; Dräger, B.; Rosahl, S. MATE Transporter-Dependent Export of Hydroxycinnamic Acid Amides. Plant Cell 2016, 28, 583–596. [Google Scholar] [CrossRef]
- Su, Q.; Rong, W.; Zhang, Z. The Pathogen-Induced MATE Gene TaPIMA1 Is Required for Defense Responses to Rhizoctonia cerealis in Wheat. Int. J. Mol. Sci. 2022, 23, 3377. [Google Scholar] [CrossRef]
- Sun, X.; Gilroy, E.M.; Chini, A.; Nurmberg, P.L.; Hein, I.; Lacomme, C.; Birch, P.R.J.; Hussain, A.; Yun, B.-W.; Loake, G.J. ADS1 Encodes a MATE-Transporter That Negatively Regulates Plant Disease Resistance. New Phytol. 2011, 192, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Sharma, D.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression of OsMATE1 and OsMATE2 Alters Development, Stress Responses and Pathogen Susceptibility in Arabidopsis. Sci. Rep. 2014, 4, 3964. [Google Scholar] [CrossRef]
- Brown, D.G.; Swanson, J.K.; Allen, C. Two Host-Induced Ralstonia Solanacearum Genes, acrA and dinF, Encode Multidrug Efflux Pumps and Contribute to Bacterial Wilt Virulence. Appl. Environ. Microbiol. 2007, 73, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Cerboneschi, M.; Decorosi, F.; Biancalani, C.; Ortenzi, M.V.; Macconi, S.; Giovannetti, L.; Viti, C.; Campanella, B.; Onor, M.; Bramanti, E.; et al. Indole-3-Acetic Acid in Plant–Pathogen Interactions: A Key Molecule for in Planta Bacterial Virulence and Fitness. Res. Microbiol. 2016, 167, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Tegli, S.; Bini, L.; Calamai, S.; Cerboneschi, M.; Biancalani, C. A MATE Transporter Is Involved in Pathogenicity and IAA Homeostasis in the Hyperplastic Plant Pathogen Pseudomonas savastanoi pv. nerii. Microorganisms 2020, 8, 156. [Google Scholar] [CrossRef]
- Castillo-Lizardo, M.G.; Aragón, I.M.; Carvajal, V.; Matas, I.M.; Pérez-Bueno, M.L.; Gallegos, M.-T.; Barón, M.; Ramos, C. Contribution of the Non-Effector Members of the HrpL Regulon, iaaL and matE, to the Virulence of Pseudomonas syringae pv. tomato DC3000 in Tomato Plants. BMC Microbiol. 2015, 15, 165. [Google Scholar] [CrossRef]
- Biała-Leonhard, W.; Zanin, L.; Gottardi, S.; de Brito Francisco, R.; Venuti, S.; Valentinuzzi, F.; Mimmo, T.; Cesco, S.; Bassin, B.; Martinoia, E.; et al. Identification of an Isoflavonoid Transporter Required for the Nodule Establishment of the Rhizobium-Fabaceae Symbiotic Interaction. Front. Plant Sci. 2021, 12, 758213. [Google Scholar] [CrossRef]
- Zhong, Y.; Xun, W.; Wang, X.; Tian, S.; Zhang, Y.; Li, D.; Zhou, Y.; Qin, Y.; Zhang, B.; Zhao, G.; et al. Root-Secreted Bitter Triterpene Modulates the Rhizosphere Microbiota to Improve Plant Fitness. Nat. Plants 2022, 8, 887–896. [Google Scholar] [CrossRef]
- Julião, M.H.M.; Silva, S.R.; Ferro, J.A.; Varani, A.M. A Genomic and Transcriptomic Overview of MATE, ABC, and MFS Transporters in Citrus sinensis Interaction with Xanthomonas citri Subsp. citri. Plants 2020, 9, 794. [Google Scholar] [CrossRef]
| Organism | MATE Transporter | Substrates | Physiological and Biochemical Processes | References | |
|---|---|---|---|---|---|
| Microorganisms | Erwinia amylovora | NorM | Norfloxacin, ethidium bromide, berberine | Resistance to biocides and antibiotics | [26] |
| Vibrio parahaemolyticus | NorM | Norfloxacin, ciprofloxacin, ethidium, kanamycin, streptomycin | Resistance to biocides and antibiotics | [3] | |
| Escherichia coli | YdhE | Norfloxacin, ciprofloxacin, acriflavine, tetraphenylphosphonium ion | Resistance to antibiotics | [3] | |
| Saccharomyces cerevisiae | ERC1 | Ethionine | Resistance to antimetabolite | [4,5] | |
| Tricholoma vaccinum | Mte1 | Hygromycin B, Opus, indole-3-acetic acid | Resistance to antibiotics and fungicides, ectomycorrhiza formation | [27,28] | |
| Ralstonia solanacearum | DinF | Toxic compounds | Plant–pathogen interaction | [83] | |
| Pseudomonas savastanoi pv. nerii | MATE | Indole-3-acetic acid | Plant–pathogen interaction | [84,85] | |
| Geotrichum citri-aurantii | MATE | Guazatine | Resistance to fungicide | [29] | |
| Plants | Arabidopsis thaliana | TT12 | Glycosylated flavan-3-ol monomers | Transport of secondary metabolites | [44] |
| FFT/DTX35 | Anthocyanin | Transport of secondary metabolites | [45] | ||
| AtMATE | Citrate | Al tolerance | [53] | ||
| AtFRD3 | Citrate | Fe homeostasis | [58] | ||
| AtDTX1 | Antibiotics, cadmium | Resistance to antibiotics, heavy metal tolerance | [62] | ||
| AtDTX3 | TNT | Bioremediation | [68] | ||
| ADP1 | Auxin | Auxin homeostasis Growth and development | [70] | ||
| AtDTX21 | Unknown | Atrazine detoxification | [69] | ||
| AtDTX30 | Auxin | Auxin homeostasis Root development Al tolerance | [71] | ||
| EDS5 | Isochorismate | Plant–pathogen interaction | [76] | ||
| DTX18 | Coumaroylagmatine | Plant–pathogen interaction | [79] | ||
| DTX50 | Abscisic acid | Growth regulation | [33] | ||
| Vitis vinifera | AM1, AM3 | Anthocyanin | Transport of secondary metabolites | [46] | |
| VvMATE38 | Anthocyanin | Transport of secondary metabolites | [47] | ||
| Nicotiana tabacum | NtMATE1, NtMATE2 | Nicotine | Transport of secondary metabolites | [24] | |
| NtJAT1, NtJAT2 | Nicotine | Transport of secondary metabolites | [49,50] | ||
| Populus trichocarpa | PtrMATE1, PtrMATE2 | Citrate | Al tolerance | [39] | |
| Arachis hypogea | AhFRDL1 | Citrate | Al tolerance | [54] | |
| Glycine max | GmMATE13 | Citrate | Al tolerance | [55] | |
| GmFRD3a, GmFRD3b | Citrate | Fe homeostasis | [59] | ||
| GmMATE | Citrate | Al tolerance | [72] | ||
| Oryza sativa | OsFRDL1 | Citrate | Fe homeostasis | [60] | |
| Lotus japonicus | LjMATE | Citrate | Fe homeostasis | [38] | |
| Lupinus albus | LaMATE | Genistein | Symbiotic interaction | [87] | |
| Cucumis melo | CmMATE | Cucurbitacin B | Plant–microbiome interaction | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastacaldi, C.; Gaudioso, D.; Tegli, S. Multidrug and Toxic Compound Extrusion Transporters: Ubiquitous Multifaceted Proteins in Microbes, Plants, and Their Interactions. Microorganisms 2024, 12, 2433. https://doi.org/10.3390/microorganisms12122433
Pastacaldi C, Gaudioso D, Tegli S. Multidrug and Toxic Compound Extrusion Transporters: Ubiquitous Multifaceted Proteins in Microbes, Plants, and Their Interactions. Microorganisms. 2024; 12(12):2433. https://doi.org/10.3390/microorganisms12122433
Chicago/Turabian StylePastacaldi, Chiara, Dario Gaudioso, and Stefania Tegli. 2024. "Multidrug and Toxic Compound Extrusion Transporters: Ubiquitous Multifaceted Proteins in Microbes, Plants, and Their Interactions" Microorganisms 12, no. 12: 2433. https://doi.org/10.3390/microorganisms12122433
APA StylePastacaldi, C., Gaudioso, D., & Tegli, S. (2024). Multidrug and Toxic Compound Extrusion Transporters: Ubiquitous Multifaceted Proteins in Microbes, Plants, and Their Interactions. Microorganisms, 12(12), 2433. https://doi.org/10.3390/microorganisms12122433








