Development of Green Fluorescent Protein-Tagged Strains of Fusarium acuminatum via PEG-Mediated Genetic Transformation
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Culture Media and Plasmids
2.2. Antibiotic Resistance Screening
2.3. Preparation of Protoplast and Transformation
2.4. DNA Extraction and Detection of Transformants
2.5. Biological Determination of GFP Labeled Strain GFP-1
3. Results
3.1. Screening of Resistance Concentration of FDCY-5 to Hyg
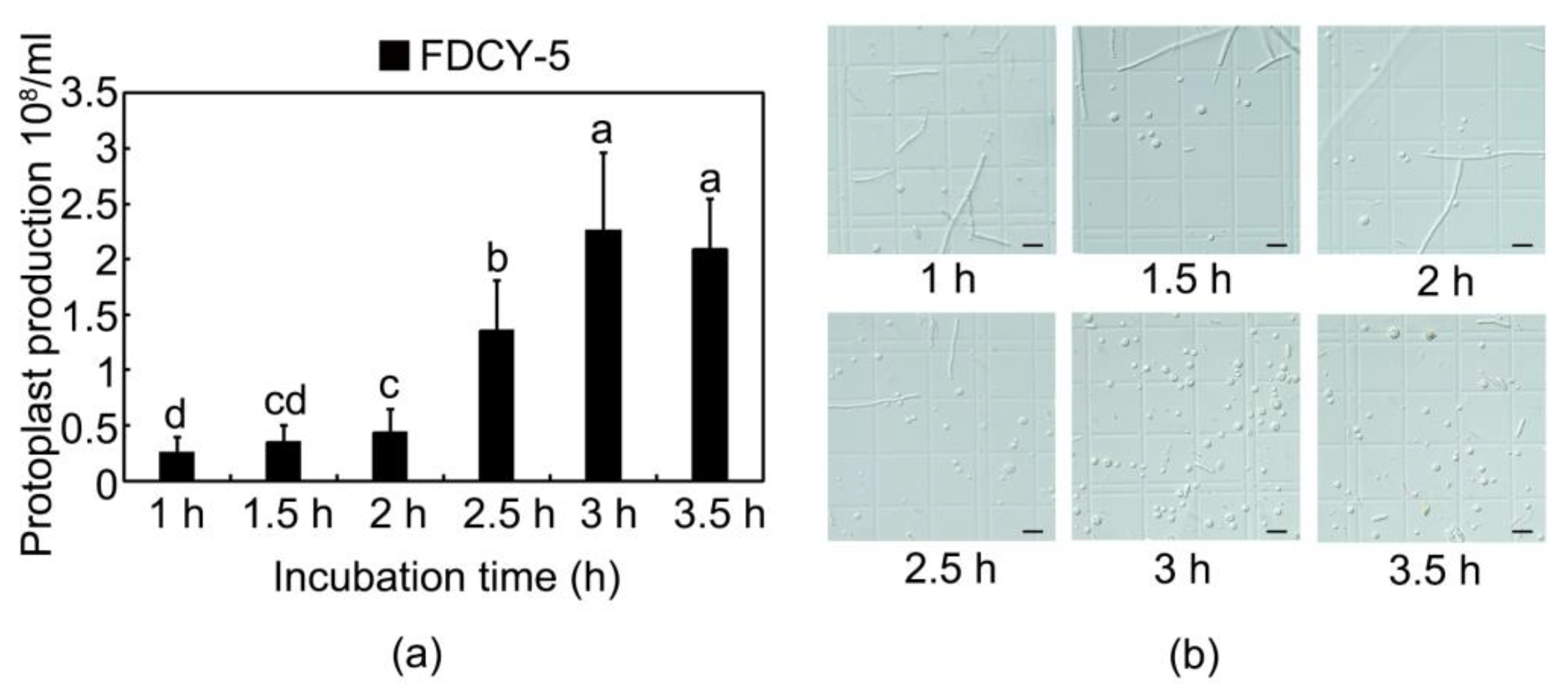
3.2. Effective Enzymatic Hydrolysis Time of Preparation of Protoplasts
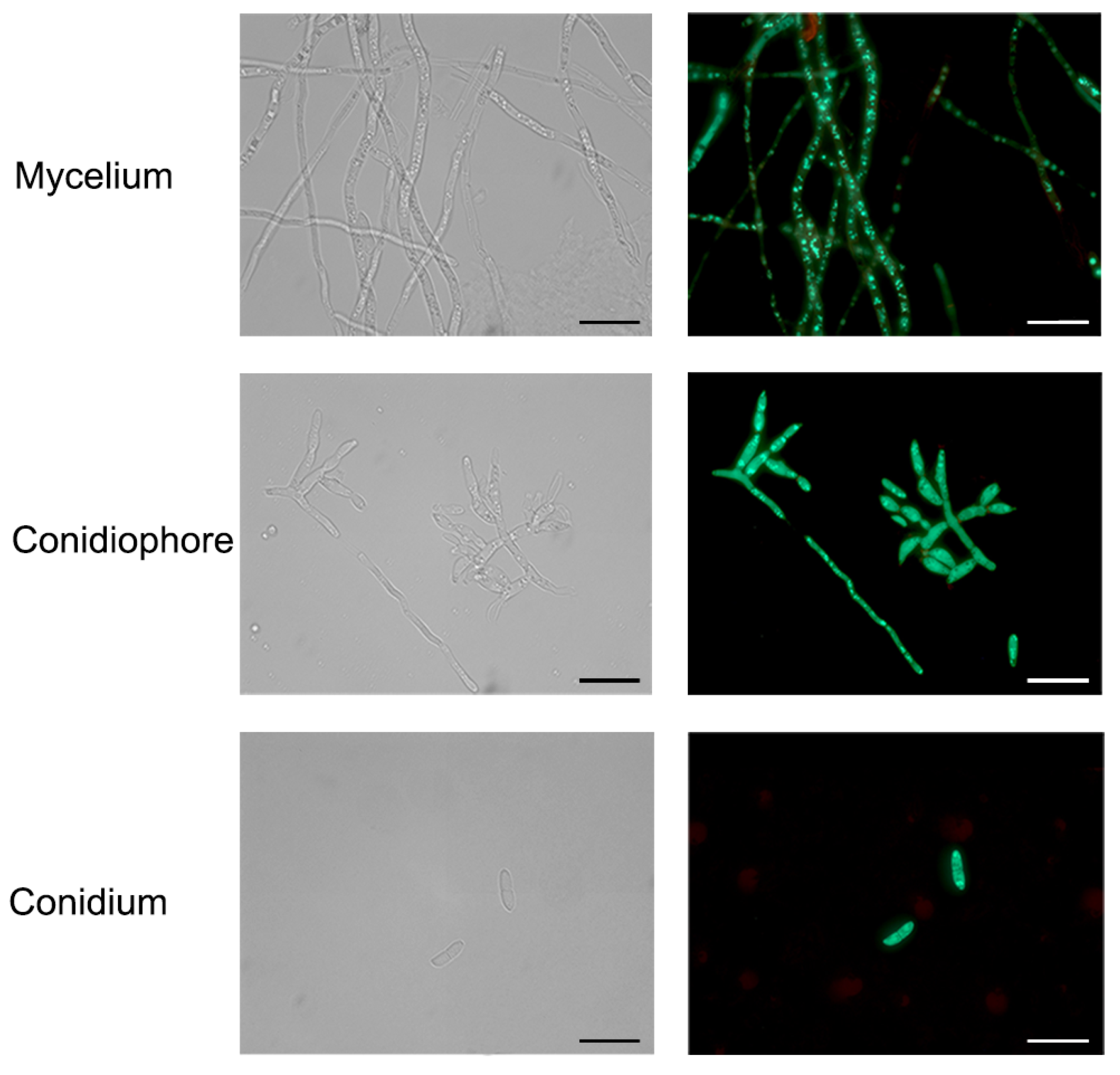
3.3. Transformation of F. acuminatum with GFP Gene
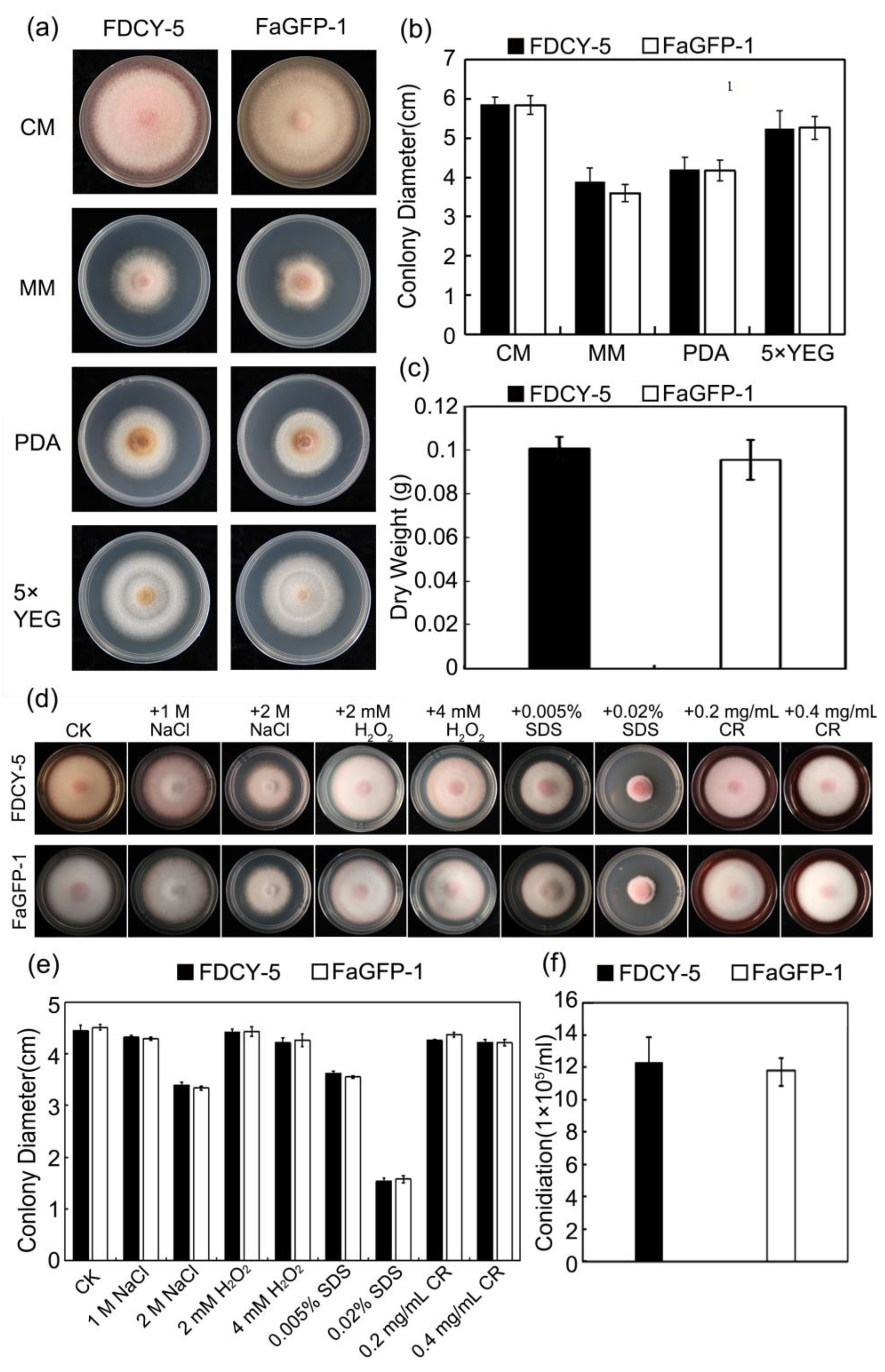
3.4. Phenotypic Results of the Wild FDCY-5 Strain and FaGFP-1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Engalycheva, I.; Kozar, E.; Frolova, S.; Vetrova, S.; Tikhonova, T.; Dzhos, E.; Engalychev, M.; Chizhik, V.; Martynov, V.; Shingaliev, A.; et al. Fusarium Species Causing Pepper Wilt in Russia: Molecular Identification and Pathogenicity. Microorganisms 2024, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, J.P.F.; Placinta, C.M.; Macdonald, A.M.C. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Glenn, A.E. Mycotoxigenic Fusarium species in animal feed. Anim. Feed Sci. Technol. 2007, 137, 213–240. [Google Scholar] [CrossRef]
- Osawa, H.; Sakamoto, Y.; Akino, S.; Kondo, N. Autumn potato seedling failure due to potato dry rot in Nagasaki Prefecture, Japan, caused by Fusarium acuminatum and Fusarium commune. J. Gen. Plant Pathol. 2021, 87, 46–50. [Google Scholar] [CrossRef]
- Leplat, J.; Friberg, H.; Abid, M.; Steinberg, C. Survival of Fusarium graminearum, the causal agent of Fusarium head blight. A review. Agron. Sustain. Dev. 2013, 33, 97–111. [Google Scholar] [CrossRef]
- Fravel, D.; Olivain, C.; Alabouvette, C. Fusarium oxysporum and its biocontrol. New Phytol. 2003, 157, 493–502. [Google Scholar] [CrossRef]
- Husaini, A.M.; Sakina, A.; Cambay, S.R. Host-Pathogen Interaction in Fusarium oxysporum Infections: Where Do We Stand? Mol. Plant-Microbe Interact. 2018, 31, 889–898. [Google Scholar] [CrossRef]
- Ploetz, R.C. Fusarium Wilt of Banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Abbas, A.; Gavrilova, O.; Gagkaeva, T. Molecular Variation and Phylogeny within Fusarium avenaceum and Related Species. Diversity 2022, 14, 574. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Burgess, L.W.; Anelich, R.Y.; Lamprecht, S.C.; van Schalkwyk, D.J. Survey of Fusarium species associated with plant debris in South African soils. S. Afr. J. Bot. 1988, 54, 63–71. [Google Scholar] [CrossRef]
- Rabie, C.J.; Sydenham, E.W.; Thiel, P.G.; Lübben, A.; Marasas, W.F. T-2 toxin production by Fusarium acuminatum isolated from oats and barley. Appl. Environ. Microbiol. 1986, 52, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Burgess, L.W.; Forbes, G.A.; Windels, C.; Nelson, P.E.; Marasas, W.F.O.; Gott, K.P. Characterization and Distribution of Fusarium acuminatum Subsp. Armeniacum Subsp. Nov. Mycologia. 1993, 85, 119–124. [Google Scholar] [CrossRef]
- Merhej, J.; Richard-Forget, F.; Barreau, C. Regulation of trichothecene biosynthesis in Fusarium: Recent advances and new insights. Appl. Microbiol. Biotechnol. 2011, 91, 519–528. [Google Scholar] [CrossRef]
- Visconti, A.; Mirocha, C.J.; Logrieco, A.; Bottalico, A.; Solfrizzo, M. Mycotoxins produced by Fusarium acuminatum. Isolation and characterization of acuminatin: A new trichothecene. J. Agric. Food Chem. 1989, 37, 1348–1351. [Google Scholar] [CrossRef]
- Chakroun, Y.; Oueslati, S.; Pinson-Gadais, L.; Abderrabba, M.; Savoie, J.-M. Characterization of Fusarium acuminatum: A Potential Enniatins Producer in Tunisian Wheat. J. Fungi. 2022, 8, 458. [Google Scholar] [CrossRef]
- Bugingo, C.; Brelsford, M.; Burrows, M. Fungicide sensitivity of Fusarium oxysporum f. sp. lentis and Fusarium acuminatum affecting lentil in the Northern Great Plains. Plant Dis. 2023, 108, 286–290. [Google Scholar] [CrossRef]
- Pennycook, S.R. Fungal fruit rots of Actinidia deliciosa (kiwifruit). N. Z. J. Exp. Agric. 1985, 13, 289–299. [Google Scholar]
- Wang, C.W.; Ai, J.; Fan, S.T.; Lv, H.Y.; Qin, H.Y.; Yang, Y.M.; Liu, Y.X. Fusarium acuminatum: A New Pathogen Causing Postharvest Rot on Stored Kiwifruit in China. Plant Dis. 2015, 99, 1644. [Google Scholar] [CrossRef]
- Tang, T.; Wang, F.; Guo, J.; Guo, X.; Duan, Y.; You, J. Fusarium acuminatum Associated with Root Rot of Ophiopogon japonicus (Linn. f.) in China. Plant Dis. 2020, 15, 1860. [Google Scholar]
- Xu, J.; Jiao, B.; Xia, H.; Dai, T. First Report of Fusarium acuminatum Causing Dianthus chinensis Root Rot and Foliage Blight in China. Plant Dis. 2023, 107, 2254. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Ma, Y.; Zhang, X.; Yang, H.; Li, G.; Li, X.; Wang, M.; Zhao, X.; Wang, J.; et al. Rapid and specific detection of Fusarium acuminatum and Fusarium solani (Neocosmospora) associated with root rot on Astragalus membranaceus using loop-mediated isothermal amplification (LAMP). Eur. J. Plant Pathol. 2022, 163, 305–320. [Google Scholar] [CrossRef]
- Zheng, Y.; Cui, Q.; Li, Q.; Tian, P.; Peng, W.; Guo, X.; Xu, S.; Yang, X.; Zhang, X.; Xie, C.; et al. First Report of Atractylodes lancea Leaf Spot Caused by Fusarium acuminatum in China. Plant Dis. 2023, 107, 968. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sun, Q.; Zhang, S.; Shi, X.; Herrera-Balandrano, D.D.; Wang, S.-Y.; Laborda, P. First Report of Fusarium acuminatum Causing Leaf Blight on Garlic in China. Plant Dis. 2022, 107, 213. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Feng, J.; Lu, S.; Chen, Z.; Chen, C.; He, Y.; Yi, X.; Xi, L. Genetic transformation of fungi. Int. J. Dev. Biol. 2017, 61, 375–381. [Google Scholar] [CrossRef]
- Niazian, M.; Noori, S.A.S.; Galuszka, P.; Mortazavian, S.M.M. Tissue culture-based Agrobacterium-mediated and in planta transformation methods. Czech J. Genet. Plant Breed. 2017, 53, 133–143. [Google Scholar] [CrossRef]
- Li, D.; Tang, Y.; Lin, J.; Cai, W. Methods for genetic transformation of filamentous fungi. Microb. Cell Factories. 2017, 16, 168. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, C.; Zhou, E.; Shu, C. The latest research progress on genetic transformation system of filamentous fungi. Genom. Appl. Biol. 2019, 38, 1138–1143. [Google Scholar]
- Yan, S.Y.; Du, J.; Yang, F.L.; Ren, M.M.; Li, J.H.; Gu, P.W. Establishment of genetic transformation system of Lycium barbarum endophytic fungus Fusarium nematophilum NQ8G 4 using PEG II-mediated method and transformants evaluation. Acta Hortic. Sin. 2020, 47, 2385–2396. [Google Scholar]
- Kawai, S.; Hashimoto, W.; Murata, K. Transformation of Saccharomyces cerevisiae and other fungi. Bioeng. Bugs. 2010, 1, 395–403. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- Liu, Z.; Friesen, T.L. Polyethylene Glycol (PEG)-Mediated Transformation in Filamentous Fungal Pathogens. In Plant Fungal Pathogens; Bolton, M.D., Thomma, B.P.J., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 835, pp. 365–375. [Google Scholar]
- Court, D.L.; Sawitzke, J.A.; Thomason, L.C. Genetic Engineering Using Homologous Recombination. Annu. Rev. Genet. 2002, 36, 361–388. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wang, Q.; Dou, X.; Wang, W.; Zhao, Q.; Lv, R.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. MoSwi6, an APSES family transcription factor, interacts with MoMps1 and is required for hyphal and conidial morphogenesis, appressorial function and pathogenicity of Magnaporthe oryzae. Mol. Plant Pathol. 2012, 13, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.; Von Wettstein, D.; Schäfer, W.; Kogel, K.-H.; Felk, A.; Maier, F.J. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 2005, 102, 16892–16897. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-R.; Tian, X.-L.; Shen, B.-M.; Mao, Z.-C.; Chen, G.; Xie, B.-Y. Transformation of the endophytic fungus Acremonium implicatum with GFP and evaluation of its biocontrol effect against Meloidogyne incognita. World J. Microbiol. Biotechnol. 2015, 31, 549–556. [Google Scholar] [CrossRef]
- Cai, N.; Wang, F.; Nong, X.; Wang, G.; McNeill, M.; Cao, G.; Hao, K.; Liu, S.; Zhang, Z. Visualising confirmation of the endophytic relationship of Metarhizium anisopliae with maize roots using molecular tools and fluorescent labelling. Biocontrol Sci. Technol. 2019, 29, 1023–1036. [Google Scholar] [CrossRef]
- Moradi, S.; Sanjarian, F.; Safaie, N. Fusarium graminearum transformation via protoplast preparation. New Biotechnol. 2009, 25, S316. [Google Scholar] [CrossRef]
- Roth, M.G.; Chilvers, M.I. A protoplast generation and transformation method for soybean sudden death syndrome causal agents Fusarium virguliforme and F. brasiliense. Fungal Biol. Biotechnol. 2019, 6, 7. [Google Scholar] [CrossRef]
- Visser, M.; Gordon, T.R.; Wingfield, B.D.; Wingfield, M.J.; Viljoen, A. Transformation of Fusarium oxysporum f. sp. cubense, causal agent of Fusarium wilt of banana, with the green fluorescent protein (GFP) gene. Australas. Plant Pathol. 2004, 33, 69. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Q.; Wang, G.; Zhang, X.; Liu, H.; Jiang, C. Combatting Fusarium head blight: Advances in molecular interactions between Fusarium graminearum and wheat. Phytopathol. Res. 2022, 4, 37. [Google Scholar] [CrossRef]
- Zang, W.; Zhao, W.; Huang, J. PEG-mediated genetic transformation of Fusarium oxysporum (Neocosmospora) f. sp. conglutinans to study pathogenesis in cabbage. Chiang Mai J. Sci. 2014, 41, 945–956. [Google Scholar]
- CHANGYu-mei, H.Z. Research on Gene Knockout and Function of FgPDE1 in Fusarium graminearum. China Biotechnol. 2015, 35, 59–67. [Google Scholar]
- Lysøe, E.; Klemsdal, S.S.; Bone, K.R.; Frandsen, R.J.N.; Johansen, T.; Thrane, U.; Giese, H. The PKS4 Gene of Fusarium graminearum Is Essential for Zearalenone Production. Appl. Environ. Microbiol. 2006, 72, 3924–3932. [Google Scholar] [CrossRef] [PubMed]
- Lysøe, E.; Klemsdal, S.S.; Bone, K.R.; Frandsen, R.J.N.; Johansen, T.; Thrane, U.; Giese, H. Host-induced gene silencing: An effective control strategy against Fusarium species. J. Plant Dis. Prot. 2022, 129, 1025–1030. [Google Scholar]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef]
- Ma, L.-J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium Pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Okungbowa, F.; Shittu, H. Fusarium Wilts: An Overview. Environ. Res. J. 2014, 6, 83–104. [Google Scholar]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight from a Microbiome Perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef]
- Ajmal, M.; Hussain, A.; Ali, A.; Chen, H.; Lin, H. Strategies for Controlling the Sporulation in Fusarium spp. J. Fungi. 2023, 9, 10. [Google Scholar] [CrossRef]
- Cormack, M.W. Fusarium spp.as root parasites of alfalfa and swect clover in Alberta. Can. J. Res. 1937, 15, 493–510. [Google Scholar] [CrossRef]
- Seif El-Nasr, H.I. Crown and Root Fungal Diseases of Alfalfa in Egypt. Plant Dis. 1983, 67, 509. [Google Scholar] [CrossRef]
- Turner, V. Crown Rot of Alfalfa in Utah. Phytopathology 1983, 73, 1333. [Google Scholar] [CrossRef]
- Han, G.; Shao, Q.; Li, C.; Zhao, K.; Jiang, L.; Fan, J.; Jiang, H.; Tao, F. An efficient Agrobacterium-mediated transformation method for aflatoxin generation fungus Aspergillus flavus. J. Microbiol. 2018, 56, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Michielse, C.B.; Hooykaas, P.J.J.; van den Hondel, C.A.M.J.J.; Ram, A.F.J. Agrobacterium-mediated transformation of the filamentous fungus Aspergillus awamori. Nat. Protoc. 2008, 3, 1671–1678. [Google Scholar] [CrossRef]
- Crowhurst, R.N.; Rees-George, J.; Rikkerink, E.H.A.; Templeton, M.D. High efficiency transformation of Fusarium solani f. sp. cucurbitae race 2 (mating population V). Curr. Genet. 1992, 21, 463–469. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Ke, X.; Kang, Z.; Huang, L. Development of genetic transformation system of Valsa mali of apple mediated by PEG. Wei Sheng Wu Xue Bao 2011, 51, 1194–1199. [Google Scholar] [PubMed]
- Liu, L.L.; Wang, Y.L.; Xiong, D.G.; Xu, X.; Tian, C.M.; Liang, Y.M. Genetic transformation system of Cytospora chrysosperma, the causal agent of poplar canker. Microbiol. China 2017, 44, 2487–2497. [Google Scholar]
- Moradi, S.; Sanjarian, F.; Safaie, N.; Mousavi, A.; Bakhshi Khaniki, G.R. A modified method for transformation of Fusarium graminearum. J. Crop Prot. 2013, 2, 297–304. [Google Scholar]
- Fitzgerald, A.M.; Mudge, A.M.; Gleave, A.P.; Plummer, K.M. Agrobacterium and PEG-mediated transformation of the phytopathogen Venturia inaequalis. Mycol. Res. 2003, 107, 803–810. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, F.; Qi, Z.; Tan, J.; Dai, T. Development of Green Fluorescent Protein-Tagged Strains of Fusarium acuminatum via PEG-Mediated Genetic Transformation. Microorganisms 2024, 12, 2427. https://doi.org/10.3390/microorganisms12122427
Ju F, Qi Z, Tan J, Dai T. Development of Green Fluorescent Protein-Tagged Strains of Fusarium acuminatum via PEG-Mediated Genetic Transformation. Microorganisms. 2024; 12(12):2427. https://doi.org/10.3390/microorganisms12122427
Chicago/Turabian StyleJu, Fangyi, Zhongqiang Qi, Jiajin Tan, and Tingting Dai. 2024. "Development of Green Fluorescent Protein-Tagged Strains of Fusarium acuminatum via PEG-Mediated Genetic Transformation" Microorganisms 12, no. 12: 2427. https://doi.org/10.3390/microorganisms12122427
APA StyleJu, F., Qi, Z., Tan, J., & Dai, T. (2024). Development of Green Fluorescent Protein-Tagged Strains of Fusarium acuminatum via PEG-Mediated Genetic Transformation. Microorganisms, 12(12), 2427. https://doi.org/10.3390/microorganisms12122427






