Unveiling the Bioleaching Versatility of Acidithiobacillus ferrooxidans
Abstract
1. Introduction
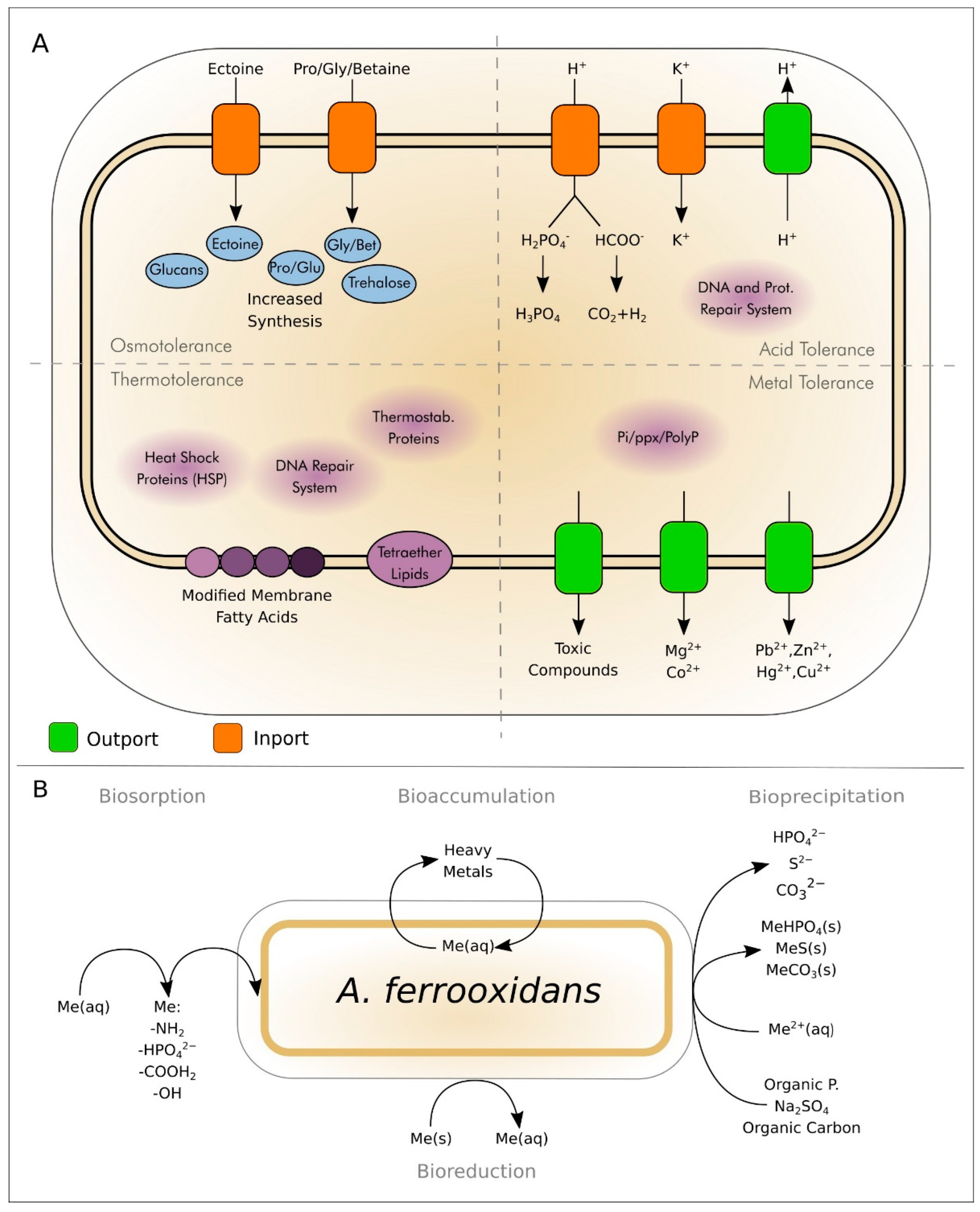
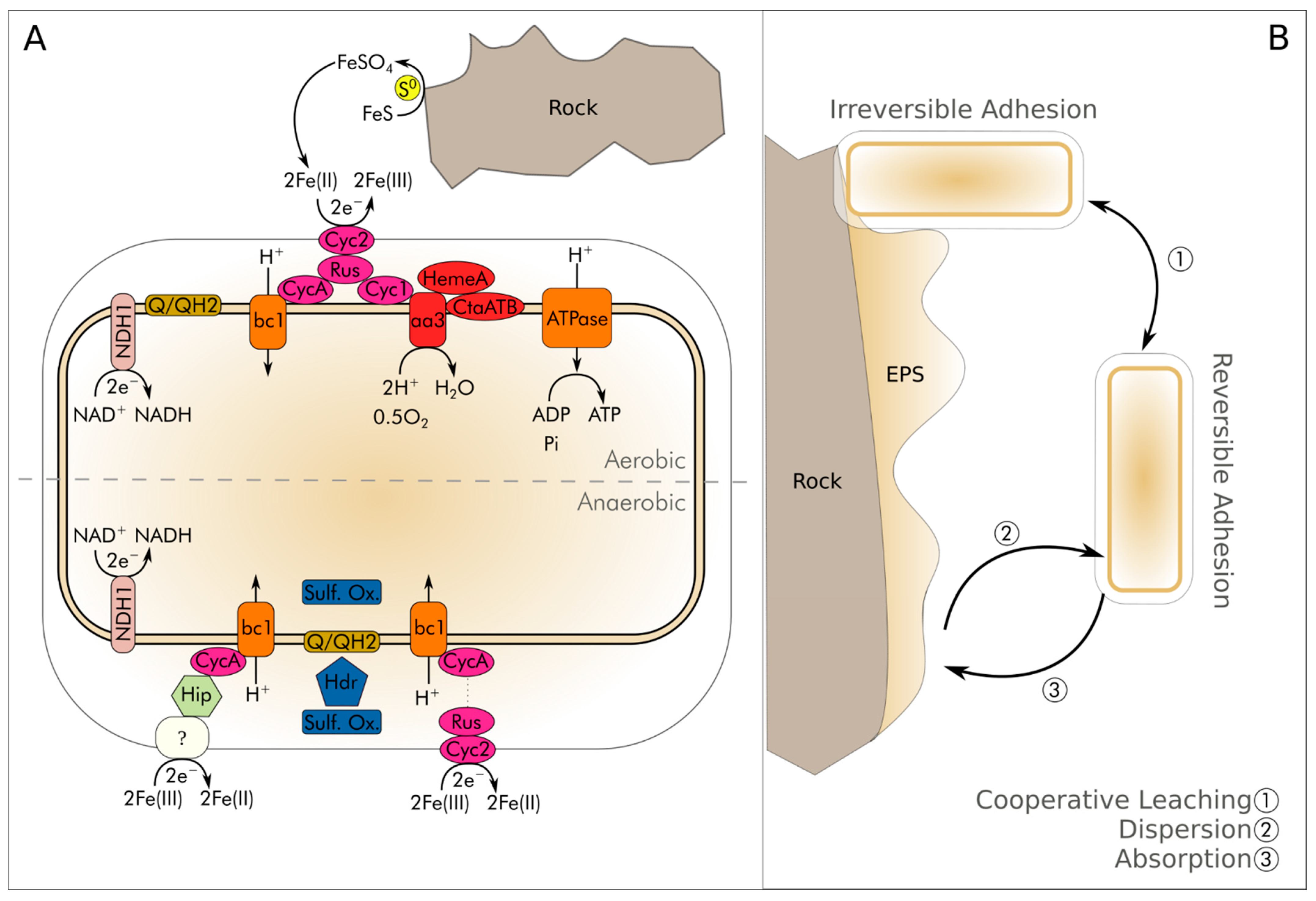
2. General Bioleaching Process
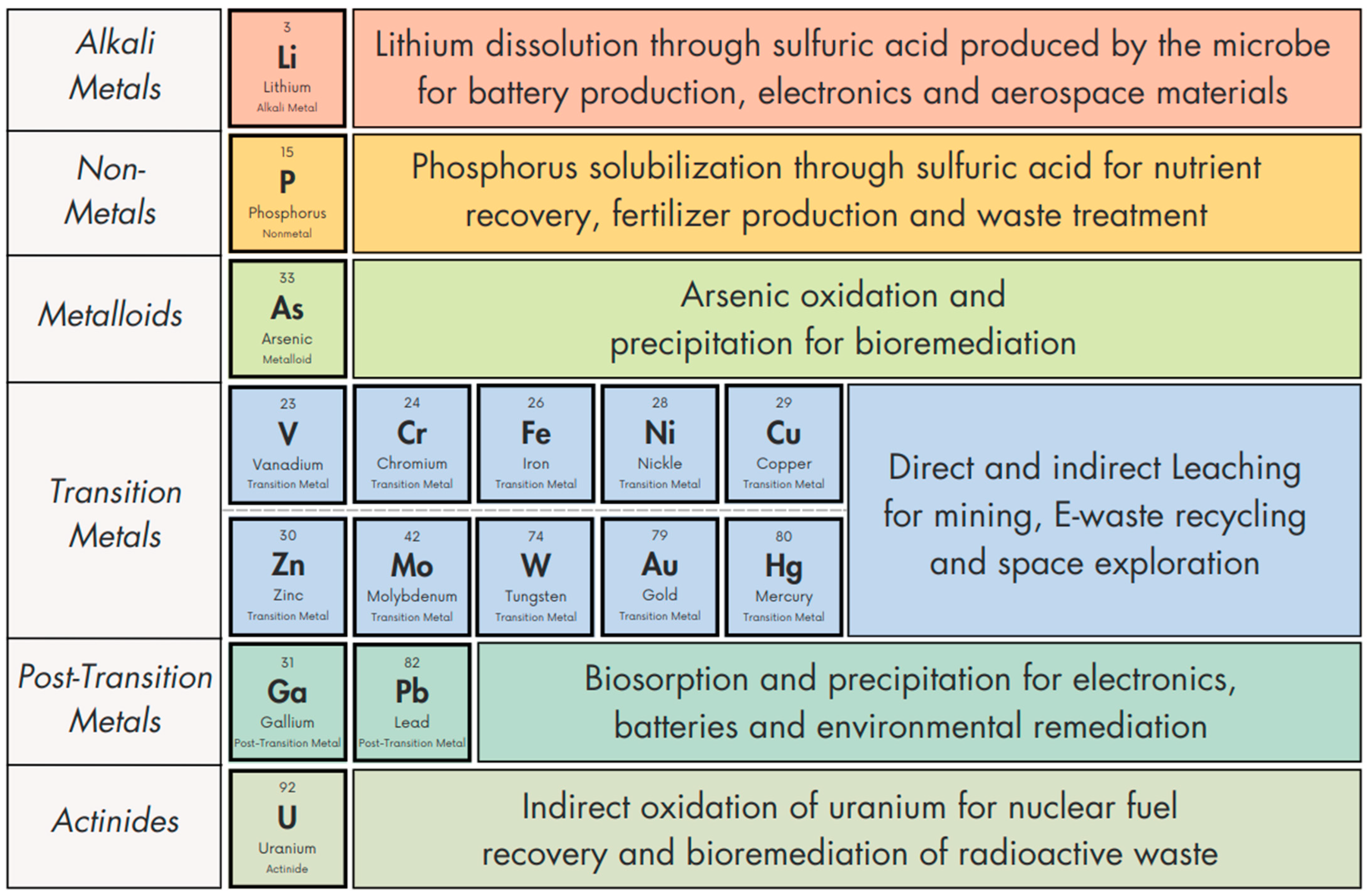
Chemical Elements Mobilized by A. ferrooxidans
3. Alkali Metals: Lithium
4. Non-Metals: Phosphorus
5. Transition Metals
5.1. Vanadium
5.2. Chromium
5.3. Iron
5.4. Nickel
5.5. Copper
5.6. Zinc
5.7. Molybdenum
5.8. Tungsten
5.9. Gold
5.10. Mercury
6. Post-Transition Metals
6.1. Gallium
6.2. Lead
7. Metalloids: Arsenic
8. Actinides: Uranium
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merino, N.; Aronson, H.S.; Bojanova, D.P.; Feyhl-Buska, J.; Wong, M.L.; Zhang, S.; Giovannelli, D. Living at the Extremes: Extremophiles and the Limits of Life in a Planetary Context. Front. Microbiol. 2019, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Ilyas, S.; Anwar, M.A.; Niazi, S.B.; Ghauri, M.A. Bioleaching of metals from electronic scrap by moderately thermophilic acidophilic bacteria. Hydrometallurgy 2007, 88, 180–188. [Google Scholar] [CrossRef]
- Navarro, C.A.; von Bernath, D.; Jerez, C.A. Heavy metal resistance strategies of acidophilic bacteria and their acquisition: Importance for biomining and bioremediation. Biol. Res. 2013, 46, 363–371. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, S. Chapter 7—Bioleaching of Electronic Waste Using Extreme Acidophiles. In Electronic Waste Management and Treatment Technology; Prasad, M.N.V., Vithanage, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 153–174. [Google Scholar]
- Niehaus, F.; Bertoldo, C.; Kähler, M.; Antranikian, G. Extremophiles as a source of novel enzymes for industrial application. Appl. Microbiol. Biotechnol. 1999, 51, 711–729. [Google Scholar] [CrossRef]
- Watling, H.R.; Shiers, D.W.; Collinson, D.M. Extremophiles in Mineral Sulphide Heaps: Some Bacterial Responses to Variable Temperature, Acidity and Solution Composition. Microorganisms 2015, 3, 364–390. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, P.; Vieira, G.A.; Otero, I.V.R.; Pellizzer, E.P.; Fontes, B.d.J.; Sette, L.D. Metal and organic pollutants bioremediation by extremophile microorganisms. J. Hazard. Mater. 2020, 382, 121024. [Google Scholar] [CrossRef]
- Cockell, C.S.; Santomartino, R.; Finster, K.; Waajen, A.C.; Eades, L.J.; Moeller, R.; Rettberg, P.; Fuchs, F.M.; Van Houdt, R.V.; Leys, N.; et al. Space station biomining experiment demonstrates rare earth element extraction in microgravity and Mars gravity. Nat. Commun. 2020, 11, 5523. [Google Scholar] [CrossRef] [PubMed]
- Santomartino, R.; Zea, L.; Cockell, C.S. The smallest space miners: Principles of space biomining. Extremophiles 2022, 26, 7. [Google Scholar] [CrossRef]
- Tonietti, L.; Barosa, B.; Pioltelli, E.; Giovannelli, D.; Covone, G.; Di Donato, P.; Cordone, A.; Inno, L.; Magliano, C.; Fiscale, S.; et al. Exploring the Development of Astrobiology Scientific Research through Bibliometric Network Analysis: A Focus on Biomining and Bioleaching. Minerals 2023, 13, 797. [Google Scholar] [CrossRef]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Dodson, R.J.; Tettelin, H.; BlakeII, R.; Eisen, J.A.; Holmes, D.S. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genom. 2008, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lin, J.-Q.; Liu, X.-M.; Pang, X.; Zhang, C.-J.; Yang, C.-L.; Gao, X.-Y.; Lin, C.-M.; Li, Y.-Q.; Li, Y.; et al. Sulfur Oxidation in the Acidophilic Autotrophic Acidithiobacillus spp. Front. Microbiol. 2019, 9, 3290. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, A.H.; Petersen, J. The Future of Biomining: Towards Sustainability in a Metal-Demanding World. In Biomining Technologies: Extracting and Recovering Metals from Ores and Wastes; Johnson, D.B., Bryan, C.G., Schlömann, M., Roberto, F.F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 295–314. [Google Scholar]
- Ibáñez, A.; Garrido-Chamorro, S.; Coque, J.J.R.; Barreiro, C. From Genes to Bioleaching: Unraveling Sulfur Metabolism in Acidithiobacillus Genus. Genes 2023, 14, 1772. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.J.; Banfield, J.F. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.F. Shouldn’t We Be Putting Our Sulphide-Rich Mine Tailings in the Ocean or in Lakes Rather than on Land? In Waters in Peril; Bendell-Young, L., Gallaugher, P., Eds.; Springer: Boston, MA, USA, 2001; pp. 151–162. [Google Scholar]
- Hafenbradl, D.; Keller, M.; Dirmeier, R.; Rachel, R.; Rossnagel, P.; Burggraf, S.; Huber, H.; Stetter, K.O. Ferroglobus placidus gen. nov., sp. nov., A novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch. Microbiol. 1996, 166, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Lee, J.-U. Effect of sulfur concentration on microbial removal of arsenic and heavy metals from mine tailings using mixed culture of Acidithiobacillus spp. J. Geochem. Explor. 2015, 148, 241–248. [Google Scholar] [CrossRef]
- Bruscella, P.; Appia-Ayme, C.; Levicán, G.; Ratouchniak, J.; Jedlicki, E.; Holmes, D.; & Bonnefoy, V. Differential expression of two bc1 complexes in the strict acidophilic chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans suggests a model for their respective roles in iron or sulfur oxidation. Microbiology 2007, 153 Pt 1, 102–110. [Google Scholar] [CrossRef]
- Quatrini, R.; Appia-Ayme, C.; Denis, Y.; Ratouchniak, J.; Veloso, F.; Valdes, J.; Lefimil, C.; Silver, S.; Roberto, F.; Orellana, O.; et al. Insights into the iron and sulfur energetic metabolism of Acidithiobacillus ferrooxidans by microarray transcriptome profiling. Hydrometallurgy 2006, 83, 263–272. [Google Scholar] [CrossRef]
- Zhan, Y.; Yang, M.; Zhang, S.; Zhao, D.; Duan, J.; Wang, W.; Yan, L. Iron and sulfur oxidation pathways of Acidithiobacillus ferrooxidans. World J. Microbiol. Biotechnol. 2019, 35, 60. [Google Scholar] [CrossRef]
- Liu, H.-L.; Lan, Y. Optimal production of sulphuric acid by Thiobacillus thiooxidans using response surface methodology. Process. Biochem. 2004, 39, 1953–1961. [Google Scholar] [CrossRef]
- Fortin, D.; Davis, B.; Southam, G.; Beveridge, T.J. Biogeochemical phenomena induced by bacteria within sulfidic mine tailings. J. Ind. Microbiol. 1995, 14, 178–185. [Google Scholar] [CrossRef]
- Pronk, J.T.; de Bruyn, J.C.; Bos, P.; Kuenen, J.G. Anaerobic Growth of Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 1992, 58, 2227–2230. [Google Scholar] [CrossRef] [PubMed]
- Plessis, C.A.D.; Slabbert, W.; Hallberg, K.B.; Johnson, D.B. Ferredox: A biohydrometallurgical processing concept for limonitic nickel laterites. Hydrometallurgy 2011, 109, 221–229. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- González-Paz, J.R.; del Carmen Monterrubio, M.; Ordaz, A.; García-Peña, E.I.; Guerrero-Barajas, C. Influence of Fe2+ and Fe3+ on the Performance and Microbial Community Composition of a MFC Inoculated with Sulfate-Reducing Sludge and Acetate as Electron Donor. J. Chem. 2022, 2022, e5685178. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Hedrich, S.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of microbial metal sulfide oxidation–part A. Appl. Microbiol. Biotechnol. 2022, 106, 6933–6952. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T.; Jozsa, P.-G.; Schippers, A. (Bio)chemistry of bacterial leaching—Direct vs. indirect bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Suzuki, I. Microbial leaching of metals from sulfide minerals. Biotechnol. Adv. 2001, 19, 119–132. [Google Scholar] [CrossRef]
- Van Gerven, T.; Moors, J.; Dutré, V.; Vandecasteele, C. Effect of CO2 on leaching from a cement-stabilized MSWI fly ash. Cem. Concr. Res. 2004, 34, 1103–1109. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, J.; Liu, S.; An, X.; Kang, Y.; Wang, L. Effect of Different CO2 Treatments on the Metal Leaching in Steel Slag Binders. Front. Energy Res. 2021, 9, 765519. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial Extracellular Polysaccharides Involved in Biofilm Formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.N.; Chen, M. Early stage adsorption behaviour of Acidithiobacillus ferrooxidans on minerals I: An experimental approach. Hydrometallurgy 2012, 119, 87–94. [Google Scholar] [CrossRef]
- Maluckov, B. The Catalytic Role of Acidithiobacillus Ferrooxidans for Metals Extraction from Mining-Metallurgical Resource. Biodivers. Int. J. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Nazari, B.; Jorjani, E.; Hani, H.; Manafi, Z.; Riahi, A. Formation of jarosite and its effect on important ions for Acidithiobacillus ferrooxidans bacteria. Trans. Nonferrous Met. Soc. China 2014, 24, 1152–1160. [Google Scholar] [CrossRef]
- Jerez, C.A. Biomining of metals: How to access and exploit natural resource sustainably. Microb. Biotechnol. 2017, 10, 1191–1193. [Google Scholar] [CrossRef]
- Metal Removal by Acidithiobacillus ferrooxidans Through Cells and Extra-Cellular Culture Supernatant in Biomachining-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1755581712000181 (accessed on 20 March 2024).
- Cai, G.; Ebrahimi, M.; Zheng, G.; Kaksonen, A.H.; Morris, C.; O’Hara, I.M.; Zhang, Z. Effect of ferrous iron loading on dewaterability, heavy metal removal and bacterial community of digested sludge by Acidithiobacillus ferrooxidans. J. Environ. Manag. 2021, 295, 113114. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, F.; Sugio, T. Volatilization and recovery of mercury from mercury-polluted soils and wastewaters using mercury-resistant Acidithiobacillus ferrooxidans strains SUG 2-2 and MON-1. Environ. Sci. Int. J. Environ. Physiol. Toxicol. 2006, 13, 305–316. [Google Scholar]
- Cabrera, G.; Viera, M.; Gómez, J.M.; Cantero, D.; Donati, E. Bacterial removal of chromium (VI) and (III) in a continuous system. Biodegradation 2007, 18, 505–513. [Google Scholar] [CrossRef]
- Camm, G.S.; Glass, H.J.; Bryce, D.W.; Butcher, A.R. Characterisation of a mining-related arsenic-contaminated site. Cornwall, UK. J. Geochem. Explor. 2004, 82, 1–15. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Oberholster, P.J.; Erasmus, M. Bioleaching of Metals from E-Waste Using Microorganisms: A Review. Minerals 2023, 13, 828. [Google Scholar] [CrossRef]
- Hubau, A.; Bryan, C.G. Metal Recovery from E-wastes. In Biomining Technologies: Extracting and Recovering Metals from Ores and Wastes; Johnson, D.B., Bryan, C.G., Schlömann, M., Roberto, F.F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 239–259. [Google Scholar]
- Hadler, K.; Martin, D.J.P.; Carpenter, J.; Cilliers, J.J.; Morse, A.; Starr, S.; Rasera, J.N.; Seweryn, K.; Reiss, P.; Meurisse, A. A universal framework for Space Resource Utilisation (SRU). Planet. Space Sci. 2020, 182, 104811. [Google Scholar] [CrossRef]
- Gumulya, Y.; Zea, L.; Kaksonen, A.H. In situ resource utilisation: The potential for space biomining. Miner. Eng. 2022, 176, 107288. [Google Scholar] [CrossRef]
- Cinti, S.; Singh, S.; Covone, G.; Tonietti, L.; Ricciardelli, A.; Cordone, A.; Iacono, R.; Mazzoli, A.; Moracci, M.; Rotundi, A.; et al. Reviewing the state of biosensors and lab-on-a- chip technologies: Opportunities for extreme environments and space exploration. Front. Microbiol. 2023, 14, 1215529. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, V.; Ferretti, S.; Piccirillo, A.M.; Zakharov, V.; Di Paolo, F.; Rotundi, A.; Ammannito, E.; Amoroso, M.; Bertini, I.; Di Donato, P.; et al. DISC-the dust impact sensor and counter on-board Comet Interceptor: Characterization of the dust coma of a dynamically new comet. Adv. Space Res. 2023, 71, 3457–3467. [Google Scholar] [CrossRef]
- Magliano, C.; Covone, G.; Dobal, R.; Cacciapuoti, L.; Tonietti, L.; Giacalone, S.; Vines, J.I.; Inno, L.; Jenkins, J.S.; Lissauer, J.J.; et al. A systematic validation of hot Neptunes in TESS data. Mon. Not. R. Astron. Soc. 2023, 519, 1562–1577. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Z.; Han, Z.; Zhu, Y.; Li, Y.; Xie, K. Vanadium–Titanium Magnetite Concentrate, Calcium–Magnesium Composite Roasting and Sulfuric Acid Leaching for Vanadium Extraction from Pellets. Metals 2023, 13, 1135. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, B.; Feng, C.; Zhang, Z.; Lei, Z.; Shimizu, K.; Cao, X.; Liu, H.; Liu, H. Microbial vanadium (V) reduction in groundwater with different soils from vanadium ore mining areas. Chemosphere 2018, 202, 272–279. [Google Scholar] [CrossRef]
- Shi, C.; Cui, Y.; Lu, J.; Zhang, B. Sulfur-based autotrophic biosystem for efficient vanadium (V) and chromium (VI) reductions in groundwater. Chem. Eng. J. 2020, 395, 124972. [Google Scholar] [CrossRef]
- Tambat, V.S.; Patel, A.K.; Chen, C.-W.; Raj, T.; Chang, J.-S.; Singhania, R.R.; Dong, C.-D. A sustainable vanadium bioremediation strategy from aqueous media by two potential green microalgae. Environ. Pollut. 2023, 323, 121247. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; He, J.; Zhou, S.; Dong, H.; Rinklebe, J.; Ok, Y.S. Vanadium in the Environment: Biogeochemistry and Bioremediation. Environ. Sci. Technol. 2023, 57, 14770–14786. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, L.; Gao, Y. Hexavalent Chromium Leaching Influenced Factors in the Weathering Chrome Slag. Procedia Environ. Sci. 2013, 18, 783–787. [Google Scholar] [CrossRef]
- Garg, S.K.; Tripathi, M.; Srinath, T. Strategies for chromium bioremediation of tannery effluent. Rev. Environ. Contam. Toxicol. 2012, 217, 75–140. [Google Scholar] [PubMed]
- Arshadi, M.; Yaghmaei, S. Advances in bioleaching of copper and nickel from electronic waste using Acidithiobacillus ferrooxidans: Evaluating daily pH adjustment. Chem. Pap. 2020, 74, 2211–2227. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Dong, Z.; Lu, Y.; Liu, N.; Hao, X. The global copper material trade network and risk evaluation: A industry chain perspective. Resour. Policy 2021, 74, 102275. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Ning, Z.; Long, Q.; Xiao, L.; Huang, F.; Wang, W.; Xiao, Q.; Lan, X.; et al. Resources and extraction of gallium: A review. Hydrometallurgy 2017, 174, 105–115. [Google Scholar] [CrossRef]
- DenBaars, S.P.; Feezell, D.; Kelchner, K.; Pimputkar, S.; Pan, C.-C.; Yen, C.-C.; Tanaka, S.; Zhao, Y.; Pfaff, N.; Farrell, R.; et al. Development of gallium-nitride-based light-emitting diodes (LEDs) and laser diodes for energy-efficient lighting and displays. Acta Mater. 2013, 61, 945–951. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Boury, C.; Green, S.R.; Allanore, A. Production of Metallic Tungsten and Tungsten Carbide from Natural Wolframite and Scheelite via Sulfide Chemistry. Met. Mater. Trans. B 2023, 54, 3270–3287. [Google Scholar] [CrossRef]
- Han, Z.; Levett, A.; Edraki, M.; Jones, M.W.M.; Howard, D.; Southam, G. Accelerating bioleaching of tungsten mining wastes using indigenous acidophilic bacteria. J. Hazard. Mater. 2023, 454, 131490. [Google Scholar] [CrossRef] [PubMed]
- Hällström, L.; Alakangas, L.; Martinsson, O. Scheelite weathering and tungsten (W) mobility in historical oxidic-sulfidic skarn tailings at Yxsjöberg, Sweden. Environ. Sci. Pollut. Res. 2020, 27, 6180–6192. [Google Scholar] [CrossRef] [PubMed]
- El-Midany, A. Gold recovery from sulphide minerals: A bioprocessing approach. Afinidad-Barc. 2012, 68, 62–68. [Google Scholar]
- Medina, D.; Anderson, C.G. A Review of the Cyanidation Treatment of Copper-Gold Ores and Concentrates. Metals 2020, 10, 897. [Google Scholar] [CrossRef]
- Sen, C. Bioleaching of Gold: An alternative green mining technology for 21st century. Microbiol. World 2015, 3, 11–20. [Google Scholar]
- Işıldar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manag. 2016, 57, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Kamoldeen, A. Bioleaching of Lead from Nigerian Anglesite Ore by Acidithiobacillus ferrooxidans. Trends Appl. Sci. Res. 2012, 8, 5591–5598. [Google Scholar]
- Chen, L.; Liu, J.; Zhang, W.; Zhou, J.; Luo, D.; Li, Z. Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: A review. J. Hazard. Mater. 2021, 413, 125319. [Google Scholar] [CrossRef]
- Costine, A.; Nikoloski, A.N.; Costa, M.D.; Chong, K.F.; Hackl, R. Uranium extraction from a pure natural brannerite mineral by acidic ferric sulphate leaching. Miner. Eng. 2013, 53, 84–90. [Google Scholar] [CrossRef]
- Pal, S.; Pradhan, D.; Das, T.; Sukla, L.B.; Chaudhury, G.R. Bioleaching of low-grade uranium ore using Acidithiobacillus ferrooxidans. Indian J. Microbiol. 2010, 50, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Dye, J.L. The alkali metals: 200 years of surprises. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140174. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.V.; Ho, Y.-C.; Thant, M.M.M.; Han, D.S.; Lim, J.W. Lithium in a Sustainable Circular Economy: A Comprehensive Review. Processes 2023, 11, 418. [Google Scholar] [CrossRef]
- Wang, T.-W.; Liu, T.; Sun, H. Direct recycling for advancing sustainable battery solutions. Mater. Today Energy 2023, 38, 101434. [Google Scholar] [CrossRef]
- Ding, T.; Zheng, M.; Peng, S.; Lin, Y.; Zhang, X.; Li, M. Lithium extraction from salt lakes with different hydrochemical types in the Tibet Plateau. Geosci. Front. 2023, 14, 101485. [Google Scholar] [CrossRef]
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef]
- Marcinov, V.; Klimko, J.; Takáčová, Z.; Pirošková, J.; Miškufová, A.; Sommerfeld, M.; Dertmann, C.; Friedrich, B.; Orac, D. Lithium Production and Recovery Methods: Overview of Lithium Losses. Metals 2023, 13, 1213. [Google Scholar] [CrossRef]
- Szlugaj, J.; Radwanek-Bąk, B. Lithium sources and their current use. Gospod. Surowcami Miner.-Miner. Resour. Manag. 2022, 38, 61–87. [Google Scholar]
- Guo, H.; Kuang, G.; Wan, H.; Yang, Y.; Yu, H.; Wang, H. Enhanced acid treatment to extract lithium from lepidolite with a fluorine-based chemical method. Hydrometallurgy 2019, 183, 9–19. [Google Scholar] [CrossRef]
- Moazzam, P.; Boroumand, Y.; Rabiei, P.; Baghbaderani, S.S.; Mokarian, P.; Mohagheghian, F. Lithium bioleaching: An emerging approach for the recovery of Li from spent lithium ion batteries. Chemosphere 2021, 277, 130196. [Google Scholar] [CrossRef]
- Sedlakova-Kadukova, J.; Marcincakova, R.; Luptakova, A.; Vojtko, M.; Fujda, M.; Pristas, P. Comparison of three different bioleaching systems for Li recovery from lepidolite. Sci. Rep. 2020, 10, 14594. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.K.; Balasubramanian, R. Recovery of valuable metals from spent lithium-ion batteries using microbial agents for bioleaching: A review. Front. Microbiol. 2023, 14, 1197081. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Zhang, D.; Zhang, X.; Xia, Y.; Wu, F.; Chen, S.; Li, L. Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria. Bioresour. Technol. 2009, 100, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Kim, D.-J.; Ralph, D.E.; Ahn, J.-G.; Rhee, Y.-H. Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Manag. 2008, 28, 333–338. [Google Scholar] [CrossRef]
- Yao, B.; Kuznetsov, V.L.; Xiao, T.; Slocombe, D.R.; Rao, C.N.R.; Hensel, F.; Edwards, P.P. Metals and non-metals in the periodic table. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20200213. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Interactions 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- El Wali, M.; Golroudbary, S.R.; Kraslawski, A. Circular economy for phosphorus supply chain and its impact on social sustainable development goals. Sci. Total. Environ. 2021, 777, 146060. [Google Scholar] [CrossRef]
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Simulations and Laboratory Tests for Assessing Phosphorus Recovery Efficiency from Sewage Sludge. Resources 2018, 7, 54. [Google Scholar] [CrossRef]
- Mabrouk, O.; Hamdi, H.; Sayadi, S.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H.; Zouari, N. Reuse of Sludge as Organic Soil Amendment: Insights into the Current Situation and Potential Challenges. Sustainability 2023, 15, 6773. [Google Scholar] [CrossRef]
- Tytła, M. Assessment of Heavy Metal Pollution and Potential Ecological Risk in Sewage Sludge from Municipal Wastewater Treatment Plant Located in the Most Industrialized Region in Poland—Case Study. Int. J. Environ. Res. Public Health 2019, 16, 2430. [Google Scholar] [CrossRef]
- Jama-Rodzeńska, A.; Sowiński, J.; Koziel, J.A.; Białowiec, A. Phosphorus Recovery from Sewage Sludge Ash Based on Cradle-to-Cradle Approach—Mini-Review. Minerals 2021, 11, 985. [Google Scholar] [CrossRef]
- Priha, O.; Sarlin, T.; Blomberg, P.; Wendling, L.; Mäkinen, J.; Arnold, M.; Kinnunen, P. Bioleaching phosphorus from fluorapatites with acidophilic bacteria. Hydrometallurgy 2014, 150, 269–275. [Google Scholar] [CrossRef]
- Calle-Castañeda, S.M.; Márquez-Godoy, M.A.; Hernández-Ortiz, J.P. Phosphorus recovery from high concentrations of low-grade phosphate rocks using the biogenic acid produced by the acidophilic bacteria Acidithiobacillus thiooxidans. Miner. Eng. 2018, 115, 97–105. [Google Scholar] [CrossRef]
- Chi, R.; Xiao, C.; Gao, H. Bioleaching of phosphorus from rock phosphate containing pyrites by Acidithiobacillus ferrooxidans. Miner. Eng. 2006, 19, 979–981. [Google Scholar] [CrossRef]
- Cortés, M.P.; Acuña, V.; Travisany, D.; Siegel, A.; Maass, A.; Latorre, M. Integration of Biological Networks for Acidithiobacillus thiooxidans Describes a Modular Gene Regulatory Organization of Bioleaching Pathways. Front. Mol. Biosci. 2020, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.K.; Heinonen-Tanski, H.; Veijalainen, A.-M.; Peräniemi, S.; Torvinen, E. Phosphorus Recovery from Sewage Sludge Using Acidithiobacilli. Int. J. Environ. Res. Public. Health 2021, 18, 7135. [Google Scholar] [CrossRef] [PubMed]
- Durrant, M.C. Transition Metal Chemistry; past, present and future. Transit. Met. Chem. 2019, 44, 1–3. [Google Scholar] [CrossRef]
- Giovannelli, D. Trace metal availability and the evolution of biogeochemistry. Nat. Rev. Earth Environ. 2023, 4, 597–598. [Google Scholar] [CrossRef]
- Mele, B.H.; Monticelli, M.; Leone, S.; Bastoni, D.; Barosa, B.; Cascone, M.; Migliaccio, F.; Montemagno, F.; Ricciardelli, A.; Tonietti, L.; et al. Oxidoreductases and metal cofactors in the functioning of the earth. Essays Biochem. 2023, 67, 653–670. [Google Scholar]
- White, D.; Levy, L. Vanadium: Environmental Hazard or Environmental Opportunity? A Perspective on Some Key Research Needs. Environ. Sci. Process. Impacts 2021, 23, 527–534. [Google Scholar] [CrossRef]
- Yang, B.; He, J.; Zhang, G.; Guo, J. (Eds.) Chapter 15—Vanadium catalysts. In Vanadium; Elsevier: Amsterdam, The Netherlands, 2021; pp. 415–443. [Google Scholar]
- Zhang, C.; Zhang, L.; Ding, Y.; Peng, S.; Guo, X.; Zhao, Y.; He, G.; Yu, G. Progress and prospects of next-generation redox flow batteries. Energy Storage Mater. 2018, 15, 324–350. [Google Scholar] [CrossRef]
- Odebiyi, O.S.; Gao, F.; Du, H.; Liu, B.; Wang, S. Theoretical Modeling and Experimental Evaluation of Vanadium Recovery from Mechanical Activation-Assisted Vanadium Titano-Magnetite Ore (NH4)2C2O4 Leaching. Min. Met. Explor. 2023, 40, 2505–2517. [Google Scholar] [CrossRef]
- He, D.; Feng, Q.; Zhang, G.; Ou, L.; Lu, Y. An environmentally-friendly technology of vanadium extraction from stone coal. Miner. Eng. 2007, 20, 1184–1186. [Google Scholar] [CrossRef]
- Rastegar, O.; Mousavi, S.M.; Shojaosadati, S.; Sarraf-Mamoory, R. Bioleaching of V, Ni, and Cu from residual produced in oil fired furnaces using Acidithiobacillus ferrooxidans. Hydrometallurgy 2015, 157, 50–59. [Google Scholar] [CrossRef]
- Cockell, C.S.; Santomartino, R.; Finster, K.; Waajen, A.C.; Nicholson, N.; Loudon, C.-M.; Eades, L.J.; Moeller, R.; Rettberg, P.; Fuchs, F.M.; et al. Microbially-Enhanced Vanadium Mining and Bioremediation Under Micro- and Mars Gravity on the International Space Station. Front. Microbiol. 2021, 12, 641387. [Google Scholar] [CrossRef] [PubMed]
- Bredberg, K.; Karlsson, H.; Holst, O. Reduction of vanadium(V) with Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Bioresour. Technol. 2004, 92, 93–96. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Yang, M.; Lin, H. Bioleaching of vanadium by Acidithiobacillus ferrooxidans from vanadium-bearing resources: Performance and mechanisms. J. Hazard. Mater. 2021, 416, 125843. [Google Scholar] [CrossRef] [PubMed]
- Shearer, C.K.; McKay, G.; Papike, J.; Karner, J.M. Valence state partitioning of vanadium between olivine-liquid: Estimates of the oxygen fugacity of Y980459 and application to other olivine-phyric Martian basalts. Am. Miner. 2006, 91, 1657–1663. [Google Scholar] [CrossRef]
- Peng, H. A Literature Review on Leaching and Recovery of Vanadium. J. Environ. Chem. Eng. 2019, 7, 103313. [Google Scholar] [CrossRef]
- Zeiner, M.; Rezić, I.; Ujević, D.; Steffan, I. Determination of total chromium in tanned leather samples used in car industry. Coll. Antropol. 2011, 35, 89–92. [Google Scholar]
- Anekwe, I.M.S.; Isa, Y.M. Bioremediation of acid mine drainage—Review. Alex. Eng. J. 2023, 65, 1047–1075. [Google Scholar] [CrossRef]
- Santos, A.L.; Dybowska, A.; Schofield, P.F.; Herrington, R.J.; Cibin, G.; Johnson, D.B. Chromium (VI) Inhibition of Low pH Bioleaching of Limonitic Nickel-Cobalt Ore. Front. Microbiol. 2022, 12, 802991. [Google Scholar] [CrossRef] [PubMed]
- Meruane, G.; Vargas, T. Bacterial oxidation of ferrous iron by Acidithiobacillus ferrooxidans in the pH range 2.5–7.0. Hydrometallurgy 2003, 71, 149–158. [Google Scholar] [CrossRef]
- Bolaños-Benítez, V.; Van Hullebusch, E.D.; Lens, P.N.L.; Quantin, C.; Van de Vossenberg, J.; Subramanian, S.; Sivry, Y. (Bio)leaching Behavior of Chromite Tailings. Minerals 2018, 8, 261. [Google Scholar] [CrossRef]
- Teixeira, A.; Tristão, J.; Araujo, M.; Oliveira, L.C.; Moura, F.; Ardisson, J.; Amorim, C.C.; Lago, R.M. Iron: A versatile element to produce materials for environmental applications. J. Braz. Chem. Soc. 2012, 23, 1579–1593. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, G.; Hu, Y.; Sun, X.; Li, J.; Gu, G. Bioleaching of pyrite by A. ferrooxidans and L. ferriphilum. Trans. Nonferrous Met. Soc. China 2008, 18, 1415–1420. [Google Scholar] [CrossRef]
- Watling, H. Microbiological Advances in Biohydrometallurgy. Minerals 2016, 6, 49. [Google Scholar] [CrossRef]
- Manikandan, S.; Inbakandan, D.; Nachiyar, C.V.; Namasivayam, S.K.R. Towards sustainable metal recovery from e-waste: A mini review. Sustain. Chem. Environ. 2023, 2, 100001. [Google Scholar] [CrossRef]
- Qiu, R.; Lin, M.; Ruan, J.; Fu, Y.; Hu, J.; Deng, M.; Tang, Y.; Qui, R. Recovering full metallic resources from waste printed circuit boards: A refined review. J. Clean. Prod. 2020, 244, 118690. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Extraction of metals from high grade waste printed circuit board by conventional and hybrid bioleaching using Acidithiobacillus ferrooxidans. Hydrometallurgy 2018, 177, 132–139. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Rossini, G.; Bernardes, A.M. Galvanic sludge metals recovery by pyrometallurgical and hydrometallurgical treatment. J. Hazard. Mater. 2006, 131, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C.; Gómez-Arroyave, L. Bioleaching Techniques for Sustainable Recovery of Metals from Solid Matrices. Sustainability 2023, 15, 10222. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public. Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Mousavi, S.M. Multi-objective optimization of heavy metals bioleaching from discarded mobile phone PCBs: Simultaneous Cu and Ni recovery using Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2015, 147, 210–219. [Google Scholar] [CrossRef]
- Seck, G.S.; Hache, E.; Bonnet, C.; Simoën, M.; Carcanague, S. Copper at the crossroads: Assessment of the interactions between low-carbon energy transition and supply limitations. Resour. Conserv. Recycl. 2020, 163, 105072. [Google Scholar] [CrossRef]
- Rawlings, D.E. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Factories 2005, 4, 13. [Google Scholar] [CrossRef]
- Lennox, J.; Blaha, T. “Leaching of Copper Ore” by Thiobacillus Ferrooxidans. Am. Biol. Teach. 1991, 53, 361–368. [Google Scholar] [CrossRef]
- Schippers, A.; Hedrich, S.; Vasters, J.; Drobe, M.; Sand, W.; Willscher, S. Biomining: Metal Recovery from Ores with Microorganisms. In Geobiotechnology I: Metal-Related Issues; Schippers, A., Glombitza, F., Sand, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–47. [Google Scholar]
- Villares, M.; Işıldar, A.; Beltran, A.M.; Guinee, J. Applying an ex-ante life cycle perspective to metal recovery from e-waste using bioleaching. J. Clean. Prod. 2016, 129, 315–328. [Google Scholar] [CrossRef]
- Yi, Q.; Wu, S.; Southam, G.; Robertson, L.; You, F.; Liu, Y.; Wang, S.; Saha, N.; Webb, R.; Wykes, J.; et al. Acidophilic Iron- and Sulfur-Oxidizing Bacteria, Acidithiobacillus ferrooxidans, Drives Alkaline pH Neutralization and Mineral Weathering in Fe Ore Tailings. Environ. Sci. Technol. 2021, 55, 8020–8034. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, M.; Sheikh, T.M.M.; Mumtaz, M.Z.; Chohan, T.A.; Shamim, S.; Liu, Y. Zinc Essentiality, Toxicity, and Its Bacterial Bioremediation: A Comprehensive Insight. Front. Microbiol. 2022, 13, 900740. [Google Scholar] [CrossRef] [PubMed]
- Picazo-Rodríguez, N.G.; de J, M.; Martínez-Luévanos, A.; Almaguer-Guzmán, I.; Chaidez-Félix, J.; Carrillo-Pedroza, F.R. Direct Acid Leaching of Sphalerite: An Approach Comparative and Kinetics Analysis. Minerals 2020, 10, 359. [Google Scholar] [CrossRef]
- Xu, H.; Wei, C.; Li, C.; Fan, G.; Deng, Z.; Li, M.; Li, X. Sulfuric acid leaching of zinc silicate ore under pressure. Hydrometallurgy 2010, 105, 186–190. [Google Scholar] [CrossRef]
- Fowler, T.A.; Crundwell, F.K. Leaching of Zinc Sulfide by Thiobacillus ferrooxidans: Experiments with a Controlled Redox Potential Indicate No Direct Bacterial Mechanism. Appl. Environ. Microbiol. 1998, 64, 3570–3575. [Google Scholar] [CrossRef]
- Oehlerking, F.; Stawovy, M.T.; Ohm, S.; Imandoust, A. Microstructural characterization and mechanical properties of additively manufactured molybdenum and molybdenum alloys. Int. J. Refract. Met. Hard Mater. 2022, 109, 105971. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Hu, D.-W.; Zhao, F.-J. Molybdenum: More than an essential element. J. Exp. Bot. 2022, 73, 1766–1774. [Google Scholar] [CrossRef]
- Xu, G.; Wang, J.; Liu, L.; Wang, Y.; Xiao, H. Negligible emissions and highly efficient recovery of Mo, V, and Al from spent hydroprocessing catalysts through alkaline leaching and solvent extraction. J. Environ. Chem. Eng. 2023, 11, 111052. [Google Scholar] [CrossRef]
- Pistaccio, L.; Curutchet, G.; Donati, E.; Tedesco, P. Analysis of molybdenite bioleaching by Thiobacillus ferrooxidans in the absence of iron (II). Biotechnol. Lett. 1994, 16, 189–194. [Google Scholar] [CrossRef]
- Aracena, A.; Azocar, A.; Ibáñez, J.P.; Jerez, O. Mechanism and leaching kinetics of molybdenite concentrate in a hydrogen peroxide-acid system. Physicochem. Probl. Miner. Process. 2018, 55, 140–152. [Google Scholar]
- Wan, H.; Yang, W.; Cao, W.; He, T.; Liu, Y.; Yang, J.; Guo, L.; Peng, Y. The Interaction between Ca2+ and Molybdenite Edges and Its Effect on Molybdenum Flotation. Minerals 2017, 7, 141. [Google Scholar] [CrossRef]
- Permyakov, E.A. Metal Binding Proteins. Encyclopedia 2021, 1, 261–292. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Won, S.; Ha, M.-G.; Nguyen, D.D.; Kang, H.Y. Bioleaching for environmental remediation of toxic metals and metalloids: A review on soils, sediments, and mine tailings. Chemosphere 2021, 282, 131108. [Google Scholar] [CrossRef] [PubMed]
- Zelin, X.; Hu, M.; Zhang, H.; Peacock, C.; Liu, X.; Nie, N.; Xue, Q.; Lei, M.; Tie, B. Natural organic matter decreases uptake of W(VI), and reduces W(VI) to W(V), during adsorption to ferrihydrite. Chem. Geol. 2020, 540, 119567. [Google Scholar]
- Han, Z.; Levett, A.; Edraki, M.; Jones, M.; Howard, D.; Southam, G. Microbially Influenced Tungsten Mobilisation and Formation of Secondary Minerals in Wolframite Tailings. J. Hazard. Mater. 2023, 445, 130508. [Google Scholar] [CrossRef]
- Martins, J.I. Leaching Systems of Wolframite and Scheelite: A Thermodynamic Approach. Miner. Process. Extr. Met. Rev. 2014, 35, 23–43. [Google Scholar] [CrossRef]
- Ciriminna, R.; Falletta, E.; Della Pina, C.; Teles, J.H.; Pagliaro, M. Industrial Applications of Gold Catalysis. Angew. Chem. Int. Ed. 2016, 55, 14210–14217. [Google Scholar] [CrossRef] [PubMed]
- Alvillo-Rivera, A.; Garrido-Hoyos, S.; Buitrón, G.; Thangarasu-Sarasvathi, P.; Rosano-Ortega, G. Biological treatment for the degradation of cyanide: A review. J. Mater. Res. Technol. 2021, 12, 1418–1433. [Google Scholar] [CrossRef]
- Wang, J.; Faraji, F.; Ramsay, J.; Ghahreman, A. A review of biocyanidation as a sustainable route for gold recovery from primary and secondary low-grade resources. J. Clean. Prod. 2021, 296, 126457. [Google Scholar] [CrossRef]
- Hylander, L.D.; Meili, M. 500 years of mercury production: Global annual inventory by region until 2000 and associated emissions. Sci. Total. Environ. 2003, 304, 13–27. [Google Scholar] [CrossRef]
- Sznopek, J.L.; Goonan, T.G. The Materials Flow of Mercury in the Economies of the United States and the World; Circular/1197; U.S. Geological Survey: Reston, VA, USA, 2000.
- Hylander, L.D.; Meili, M. The Rise and Fall of Mercury: Converting a Resource to Refuse After 500 Years of Mining and Pollution. Crit. Rev. Environ. Sci. Technol. 2005, 35, 1–36. [Google Scholar] [CrossRef]
- Feng, X. Mercury Pollution in China—An Overview. In Dynamics of Mercury Pollution on Regional and Global Scales; Pirrone, N., Mahaffey, K.R., Eds.; Springer: Boston, MA, USA, 2005; pp. 657–678. [Google Scholar]
- Schippers, A.; Sand, W. Bacterial Leaching of Metal Sulfides Proceeds by Two Indirect Mechanisms via Thiosulfate or via Polysulfides and Sulfur. Appl. Environ. Microbiol. 1999, 65, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zeng, K.; Wang, Q.; Yang, X.; Wang, K. In vitro studies on dissolved substance of cinnabar: Chemical species and biological properties. J. Ethnopharmacol. 2010, 131, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Yang, Y.J.; Li, D.P.; Hu, H.F.; Li, H.Y.; He, X.H. Bioxidative dissolution of cinnabar by iron-oxidizing bacteria. Biochem. Eng. J. 2013, 74, 102–106. [Google Scholar] [CrossRef]
- Devasia, P.; Natarajan, K.A. Adhesion of Acidithiobacillus ferrooxidans to mineral surfaces. Int. J. Miner. Process. 2010, 94, 135–139. [Google Scholar] [CrossRef]
- Kinzler, K.; Gehrke, T.; Telegdi, J.; Sand, W. Bioleaching—A result of interfacial processes caused by extracellular polymeric substances (EPS). Hydrometallurgy 2003, 71, 83–88. [Google Scholar] [CrossRef]
- Yujian, W.; Xiaojuan, Y.; Wei, T.; Hongyu, L. High-rate ferrous iron oxidation by immobilized Acidithiobacillus ferrooxidans with complex of PVA and sodium alginate. J. Microbiol. Methods 2007, 68, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Breed, A.W.; Hansford, G.S. Effect of pH on ferrous-iron oxidation kinetics of Leptospirillum ferrooxidans in continuous culture. Biochem. Eng. J. 1999, 3, 193–201. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Ding, X.; Qiao, L. Recent advances in the electrochemistry of layered post-transition metal chalcogenide nanomaterials for hydrogen evolution reaction. J. Energy Chem. 2021, 60, 451–479. [Google Scholar] [CrossRef]
- Tang, S.; He, T.; Yu, H.; Ou, Z.; Ren, Z.; Li, M.; Sheng, W. A novel coating to avoid corrosion effect between eutectic gallium–indium alloy and heat sink metal for X-ray optics cooling. Rev. Sci. Instrum. 2022, 93, 123102. [Google Scholar] [CrossRef]
- Ueberschaar, M.; Otto, S.J.; Rotter, V.S. Challenges for critical raw material recovery from WEEE—The case study of gallium. Waste Manag. 2017, 60, 534–545. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M. Enhancement of copper, nickel, and gallium recovery from LED waste by adaptation of Acidithiobacillus ferrooxidans. Waste Manag. 2018, 79, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Maneesuwannarat, S.; Vangnai, A.S.; Yamashita, M.; Thiravetyan, P. Bioleaching of gallium from gallium arsenide by Cellulosimicrobium funkei and its application to semiconductor/electronic wastes. Process Saf. Environ. Prot. 2016, 99, 80–87. [Google Scholar] [CrossRef]
- Ghazi, A.M.; Millette, J.R. 4-Lead. In Environmental Forensics; Morrison, R.D., Murphy, B.L., Eds.; Academic Press: Burlington, ON, Canada, 1964; pp. 55–79. [Google Scholar]
- Cheriton, L.W.; Gupta, J.P. Building Materials. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 304–314. [Google Scholar]
- Grant, R.M. Lead Production. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssiere, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 4439–4442. [Google Scholar]
- Jiang, L.; Zhou, H.; Peng, X.; Ding, Z. Bio-oxidation of galena particles by Acidithiobacillus ferrooxidans. Particuology 2008, 6, 99–105. [Google Scholar] [CrossRef]
- Páez-Espino, D.; Tamames, J.; de Lorenzo, V.; Cánovas, D. Microbial responses to environmental arsenic. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2009, 22, 117–130. [Google Scholar] [CrossRef]
- Liu, J.; Waalkes, M.P. Liver is a Target of Arsenic Carcinogenesis. Toxicol. Sci. 2008, 105, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Zargar, K.; Hoeft, S.; Oremland, R.; Saltikov, C.W. Identification of a Novel Arsenite Oxidase Gene, arxA, in the Haloalkaliphilic, Arsenite-Oxidizing Bacterium Alkalilimnicola ehrlichii Strain MLHE-1. J. Bacteriol. 2010, 192, 3755–3762. [Google Scholar] [CrossRef]
- Chen, P.; Xu, R.; Yan, L.; Wu, Z.; Wei, Y.; Zhao, W.; Wang, X.; Xie, Q.; Li, H. Properties of realgar bioleaching using an extremely acidophilic bacterium and its antitumor mechanism as an anticancer agent. Biol. Res. 2017, 50, 17. [Google Scholar] [CrossRef]
- Chen, P.; Yan, L.; Li, H. Optimal parameters for bioleaching of realgar using Acidithiobacillus ferrooxidans under different growth conditions and mathematical analysis. Biocatal. Biotransformation 2013, 31, 33–41. [Google Scholar] [CrossRef]
- Allard, B.; Olofsson, U.; Torstenfelt, B. Environmental actinide chemistry. Inorganica Chim. Acta 1984, 94, 205–221. [Google Scholar] [CrossRef]
- Vázquez-Campos, X.; Kinsela, A.S.; Collins, R.N.; Neilan, B.A.; Waite, T.D. Uranium extraction from a low-grade, stockpiled, non-sulfidic ore: Impact of added iron and the native microbial consortia. Hydrometallurgy 2017, 167, 81–91. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Lakaniemi, A.-M.; Tuovinen, O.H. Acid and ferric sulfate bioleaching of uranium ores: A review. J. Clean. Prod. 2020, 264, 121586. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Castro, M.; Barriga, A.; Jerez, C.A.; Guiliani, N. The extremophile Acidithiobacillus ferrooxidans possesses a c-di-GMP signalling pathway that could play a significant role during bioleaching of minerals. Lett. Appl. Microbiol. 2012, 54, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Romero-González, M.; Nwaobi, B.C.; Hufton, J.M.; Gilmour, D.J. Ex-situ Bioremediation of U(VI) from Contaminated Mine Water Using Acidithiobacillus ferrooxidans Strains. Front. Environ. Sci. 2016, 4, 39. [Google Scholar] [CrossRef]
- Jerez, C.A. 3.60-Bioleaching and Biomining for the Industrial Recovery of Metals. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, ON, Canada, 2011; pp. 717–729. [Google Scholar]
- Johnson, D. The Biogeochemistry of Biomining. In Geomicrobiology: Molecular and Environmental Perspective; Springer: Dordrecht, Germany, 2010; pp. 401–426. [Google Scholar]
- Brune, K.D.; Bayer, T.S. Engineering microbial consortia to enhance biomining and bioremediation. Front. Microbiol. 2012, 3, 203. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Gomes, H.I.; Funari, V.; Ferrari, R. Bioleaching for resource recovery from low-grade wastes like fly and bottom ashes from municipal incinerators: A SWOT analysis. Sci. Total. Environ. 2020, 715, 136945. [Google Scholar] [CrossRef]
| Element | Mineral | Chemical Formula | Reactions |
|---|---|---|---|
| Li | Lepidolite | KLi2Al(Si4O10)(F,OH)2 | 3Li2O*2K2O*5Al2O3*10SiO2*2SiF4 + 20H2SO4 → 3Li2SO4 + 2K2SO4 + 5Al2(SO4)3 + 11SiO2 + H2SiF6 + 18H2O + 2HF |
| P | P-Sludge | Ca3(PO)4 | 3H2SO4 + Ca3(PO4)2 → 2H3PO4 + 3CaSO4 |
| V | V-Ti-Magnetite and Vanadates | (V,Ti),Fe2+Fe3+2O4 and V2O3 | V2O3 + 2Fe3+ + 2H+ →2VO2+ + 2Fe2+ + H2O VO2 + Fe3+ → VO2+ + Fe2+ 10VO2 + 10Fe3+ + 8H2O → HV10O255− + 10Fe2+ + 15H+ |
| Cr | Cr-Slug | Ca3Al2(H4O4, CrO4)3 | Cr6+ + 3Fe2+ → Cr3+ + 3Fe3+ |
| Ni | Ni-Sludge | Ni0 | Fe2(SO4)3 + Ni0 → Ni2+ + SO42− + 2FeSO4 |
| Cu | Chalcopyrite | CuFeS2 | CuFeS2 + 2Fe2(SO4)3 → CuSO4 + 5FeSO4 + 2S0 CuFeS2 + 2H2O + 3O2 → Cu2+ + Fe2+ + 2H2SO4 CuFeS2 + H2SO4 → Cu2+ + FeSO4 + 2H+ + 2S0 CuFeS2 + 4H+ + O2 → Cu2+ + Fe2+ + 2H2O + 2S0 3FeSO4 + H2SO4 + 0.5O2 → Fe2(SO4)3 + FeSO4 + H2O |
| As | Realgar | As2S2 or alpha As4S4 | 4Fe2+ + O2 + 4H+ → 4Fe3+ + 2H2O As2S2 + 6Fe3+ → 2As3+ + 2S0 + 6Fe2+ H3AsO3 + H2O → H3AsO4 + 2H+ + 2e− H3AsO4 + Fe3+ → FeAsO4 + 3H+ As2S2 + 14H2O → 2H3AsO3 + 2HSO4− + 20H+ +18e− |
| Mo | Mo Sulfide | MoS2 | 4MoS2 + 4Fe3+ + 16H2O → 4MoO42− + 4Fe2+ + 32H+ + 8S0 |
| W | Tungstate Salts | WO42− | H2SO4 + FeWO4 → FeSO4 + H2WO4 |
| Au | Au Ores | Au0 | 4Au0 + 8NaCN + 2H2O + O2 → 4NaAu(CN)2 + 4NaOH |
| Hg | Cinnabar | HgS | HgS + 2Fe3+ → Hg2+ + 2Fe2+ + S0 2HgS + 4Fe3+ +1.5O2 +2H2O → 2Hg2+ + 4Fe2+ + 2H2SO4 |
| U | U Oxide | UO2 | U(IV)O2 + 2Fe3+ → U(VI)O22+ + 2Fe2+ |
| Element | Main Alloys | Application Fields | Metal Printing | Electronics | Extraction Yield (ktons/year) d | Biomining on Earth | Biomining in Space * | Biorecovery | References |
|---|---|---|---|---|---|---|---|---|---|
| Ti | Alpha/BetaTi alloys | Aerospace, Construction, Automobile | Yes | Major (hardware) | 6300 | Yes (A) | No | Yes (A,B,C) | [52] |
| V | Ferrovanadium | Structural, Fusion Reactor | No | Minor (semiconductors) | 81 | Yes (A) | Yes (B) | Yes (A,B,C) | [9,10,53,54,55,56] |
| Cr | FeCr, Stainless steel | Automobile, High Temperature | Yes | Minor (anticorrosive coating) | 38,600 | Yes (A) | No | Yes (A,B,C) | [43,57,58,59] |
| Fe | Steel, Inox | Construction, Automobile, Aerospace, | Yes | Major (solder) | 3,040,000 | Yes (A,B,C) | Yes (B) | Yes (A,B,C) | [9,10,22] |
| Ni | NiCrFe | Medical, Aerospace, Energy, Isolators, Automotive, Cables | Yes | Minor (plating agent) | 2700 | Yes (A) | Yes (B) | Yes (A,B,C) | [9,10,60] |
| Cu | Brass, Bronze | Construction, Marine, Automotive, Medical, Aerospace | Yes | Major (cables) | 20,700 | Yes (A,B,C) 1 | Yes (B) | Yes (A,B,C) | [9,10,61] |
| Ga | GaAlZn alloys | Semiconductors, Diodes, Circuits | No | Major (diodes) | 0.380 | Yes (A) | No | No | [62,63] |
| As | As Bronze | Automobile, Ammunition | No | Minor (batteries) | NA | Yes (A,B,C) | No | Yes (A,B,C) | [44,64] |
| W | W,Ni,Cu and W,Ni,Fe alloys | Aerospace, Medical, Automobile | Yes | Minor (light bulbs) | 91.5 | Yes (A) | No | Yes (A,B,C) | [65,66,67] |
| Au | AuPt alloys | Increasing Corrosion Resistance | No | Major (connector) | 3.3 | Yes (A) | No | No | [68,69,70,71] |
| Pb | PbCu, alloys | Automobile, Ammunition, Batteries | No | Major (solder) | 4700 | Yes (A,C) | No | Yes (A,B,C) | [72,73] |
| U | Mulberry | Increasing Corrosion Resistance | No | None | 53 | Yes (A) | No | Yes (A,B,C) | [74,75,76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonietti, L.; Esposito, M.; Cascone, M.; Barosa, B.; Fiscale, S.; Muscari Tomajoli, M.T.; Sbaffi, T.; Santomartino, R.; Covone, G.; Cordone, A.; et al. Unveiling the Bioleaching Versatility of Acidithiobacillus ferrooxidans. Microorganisms 2024, 12, 2407. https://doi.org/10.3390/microorganisms12122407
Tonietti L, Esposito M, Cascone M, Barosa B, Fiscale S, Muscari Tomajoli MT, Sbaffi T, Santomartino R, Covone G, Cordone A, et al. Unveiling the Bioleaching Versatility of Acidithiobacillus ferrooxidans. Microorganisms. 2024; 12(12):2407. https://doi.org/10.3390/microorganisms12122407
Chicago/Turabian StyleTonietti, Luca, Mattia Esposito, Martina Cascone, Bernardo Barosa, Stefano Fiscale, Maria Teresa Muscari Tomajoli, Tomasa Sbaffi, Rosa Santomartino, Giovanni Covone, Angelina Cordone, and et al. 2024. "Unveiling the Bioleaching Versatility of Acidithiobacillus ferrooxidans" Microorganisms 12, no. 12: 2407. https://doi.org/10.3390/microorganisms12122407
APA StyleTonietti, L., Esposito, M., Cascone, M., Barosa, B., Fiscale, S., Muscari Tomajoli, M. T., Sbaffi, T., Santomartino, R., Covone, G., Cordone, A., Rotundi, A., & Giovannelli, D. (2024). Unveiling the Bioleaching Versatility of Acidithiobacillus ferrooxidans. Microorganisms, 12(12), 2407. https://doi.org/10.3390/microorganisms12122407







