Enhancing Morchella Mushroom Yield and Quality Through the Amendment of Soil Physicochemical Properties and Microbial Community with Wood Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of Experimental Site and Materials
2.2. Experimental Design and Pretreatment of Soil Microbial Samples
2.3. Determination of Fruiting Body Indices and Soil Physicochemical Properties
2.4. Extraction and Sequencing of Soil Microbial DNA
2.5. Data Analysis and Statistics
3. Results
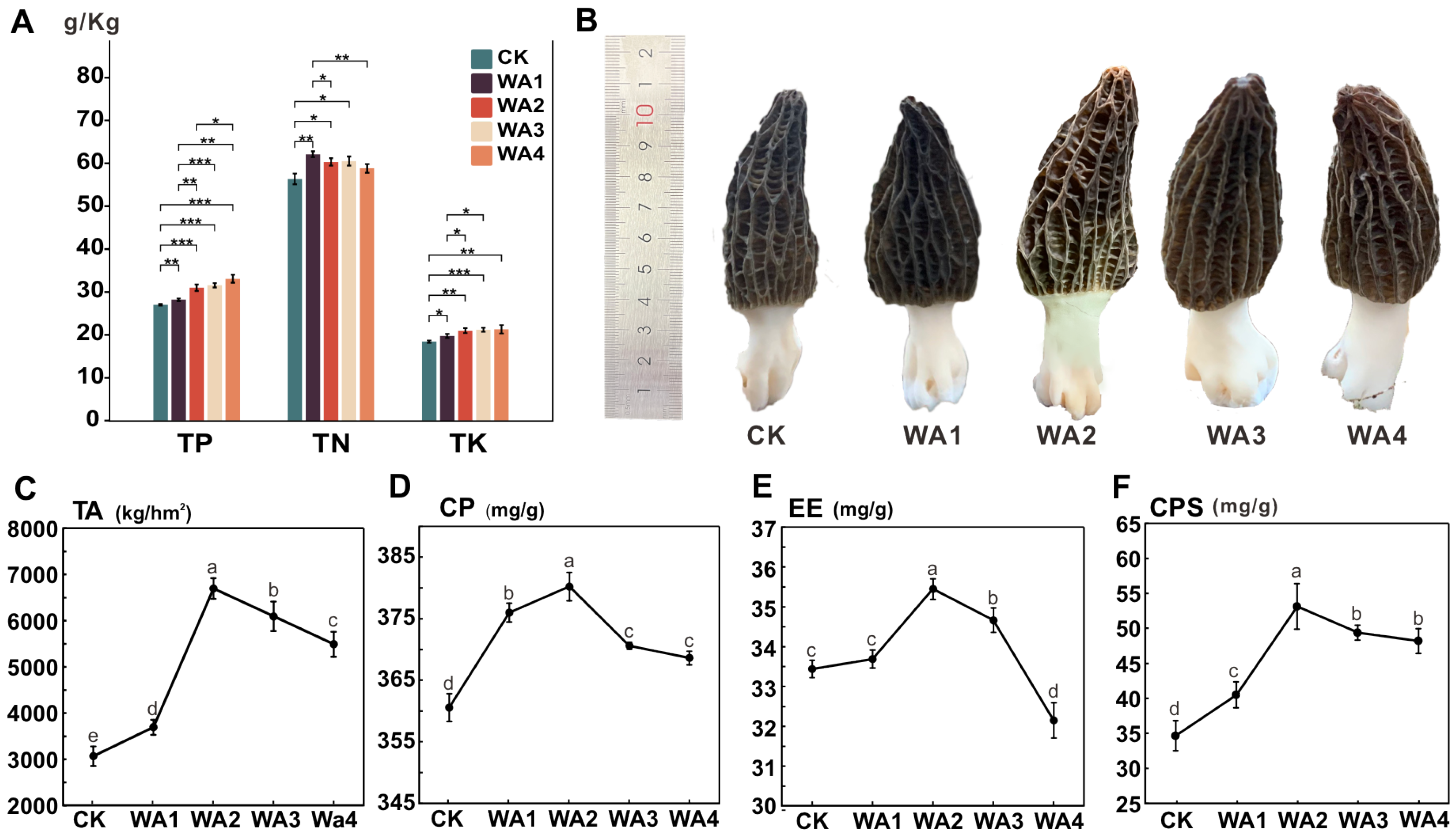
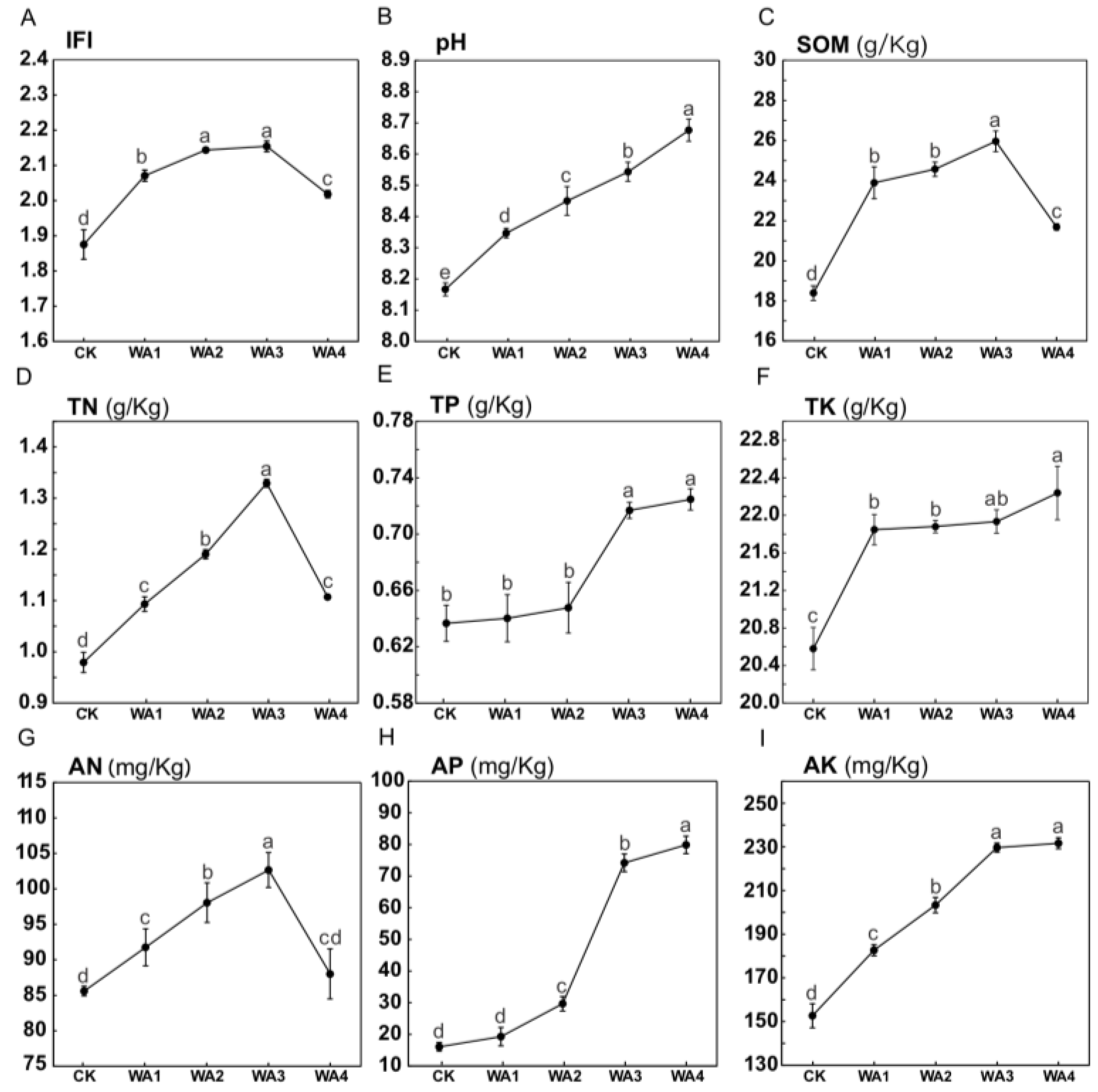
3.1. The Effect of Different Amounts of WA on the Yield and Quality of Morchella
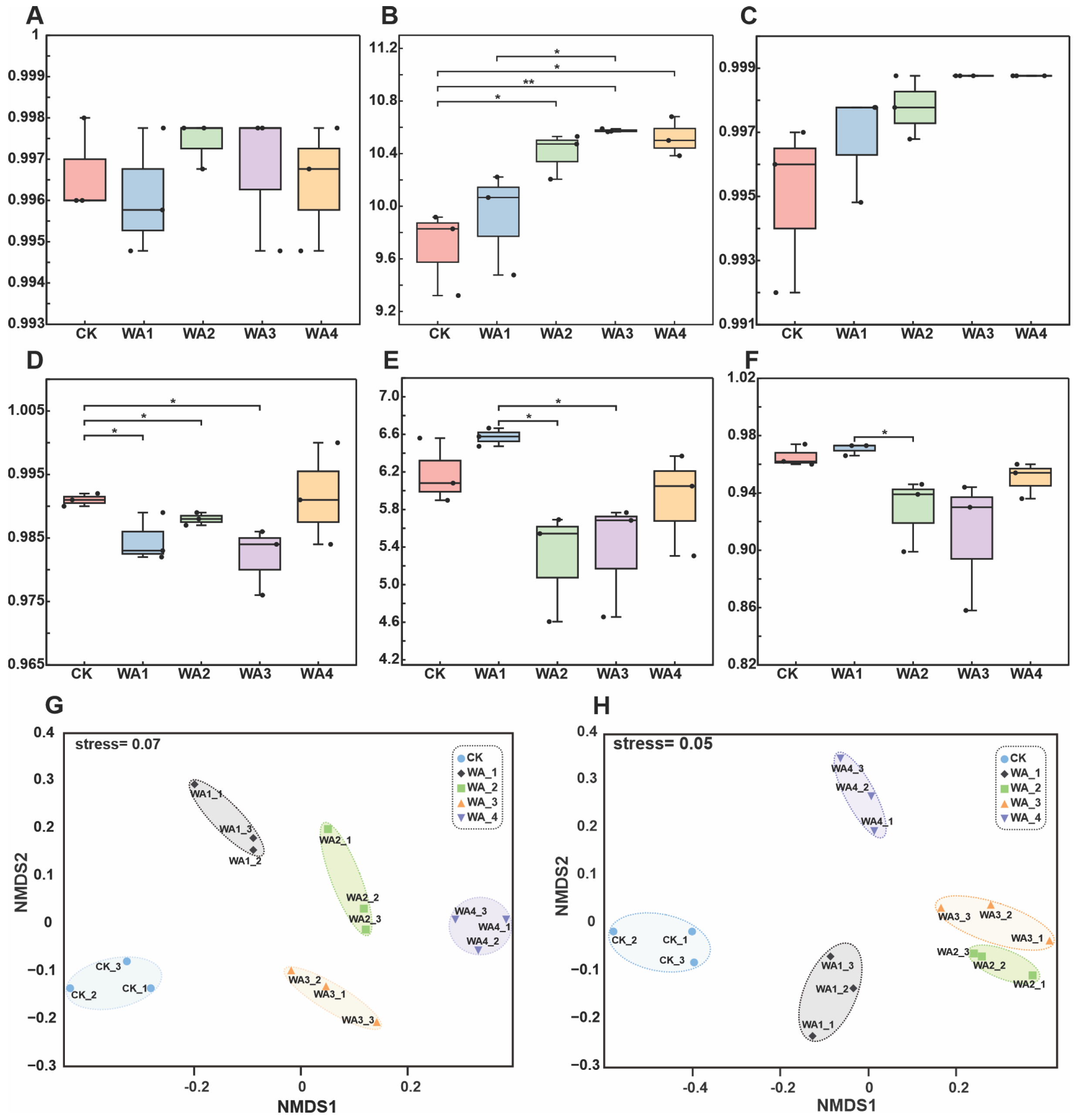
3.2. Microbial Community Diversity Analysis
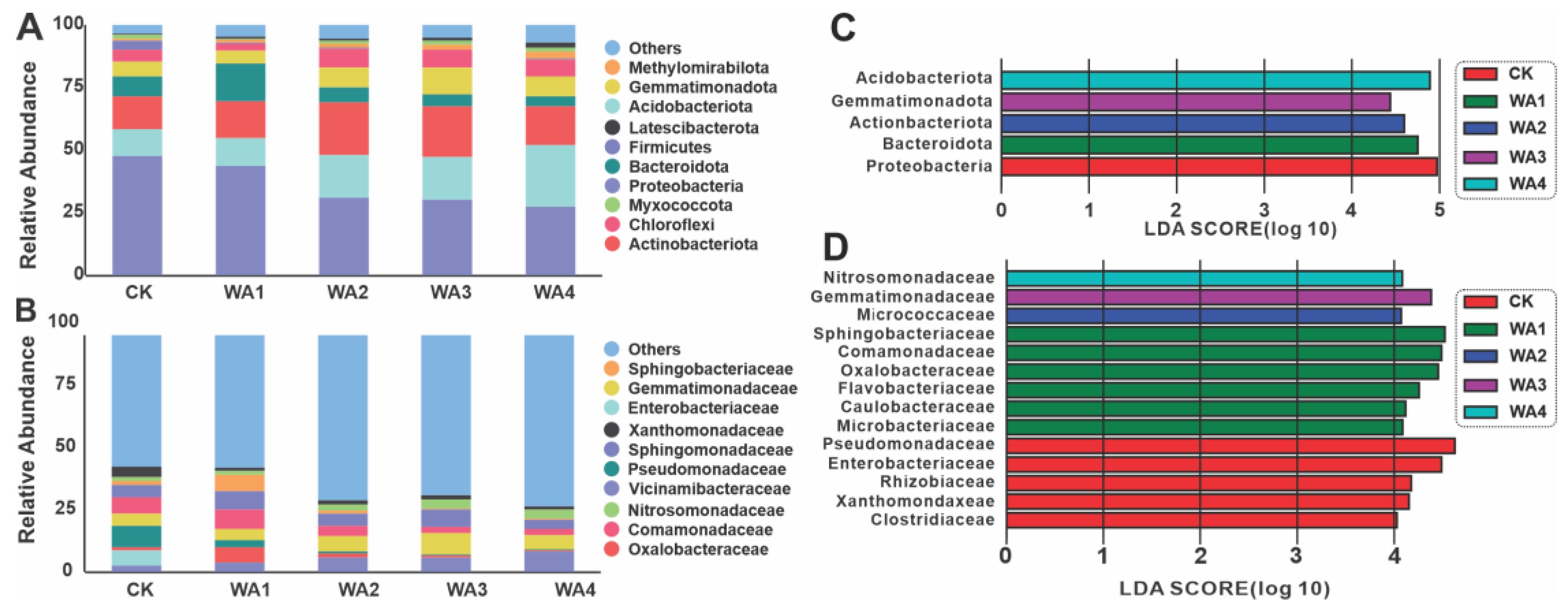
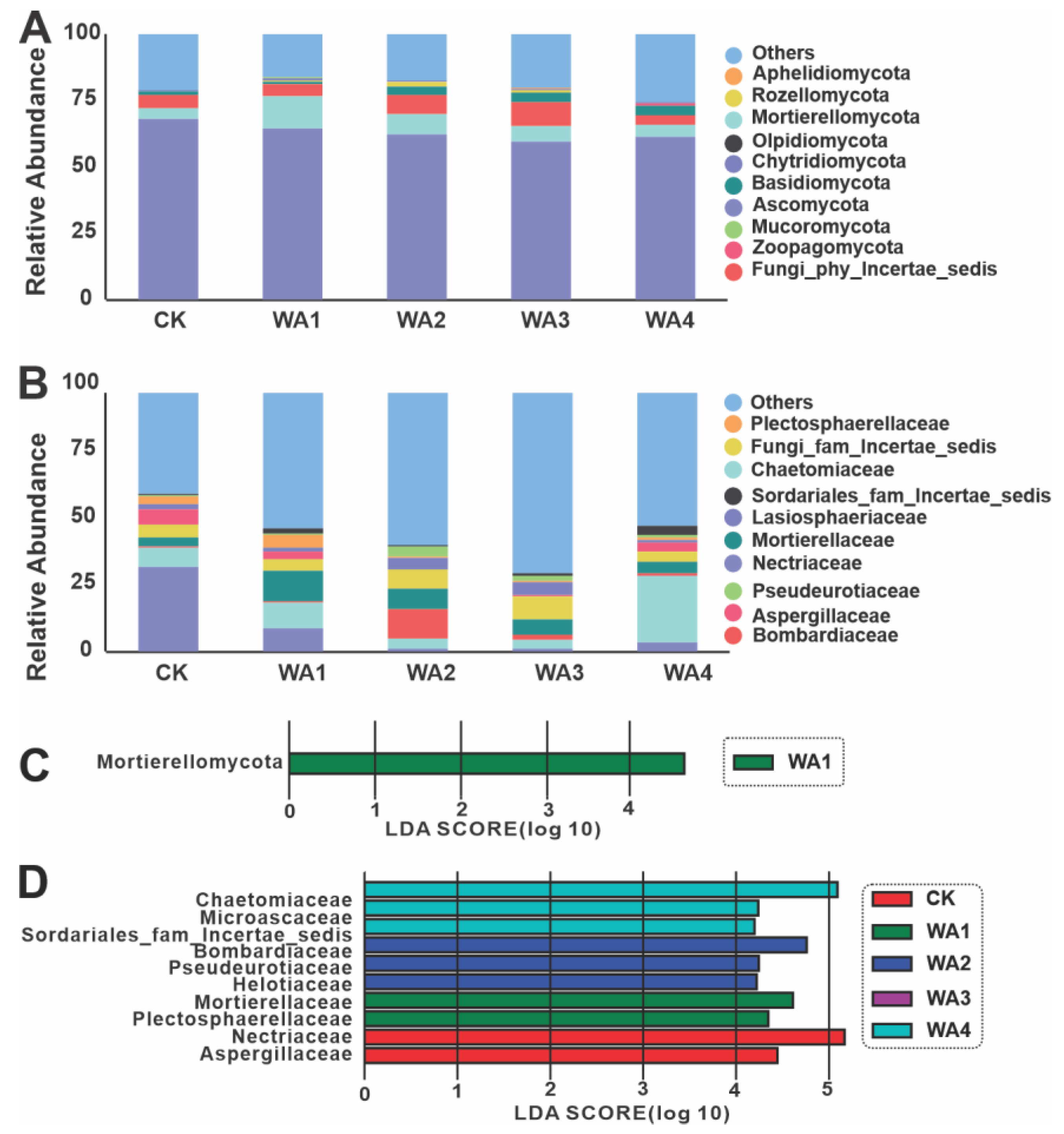
3.3. Microbial Community Compositional Variation Analysis
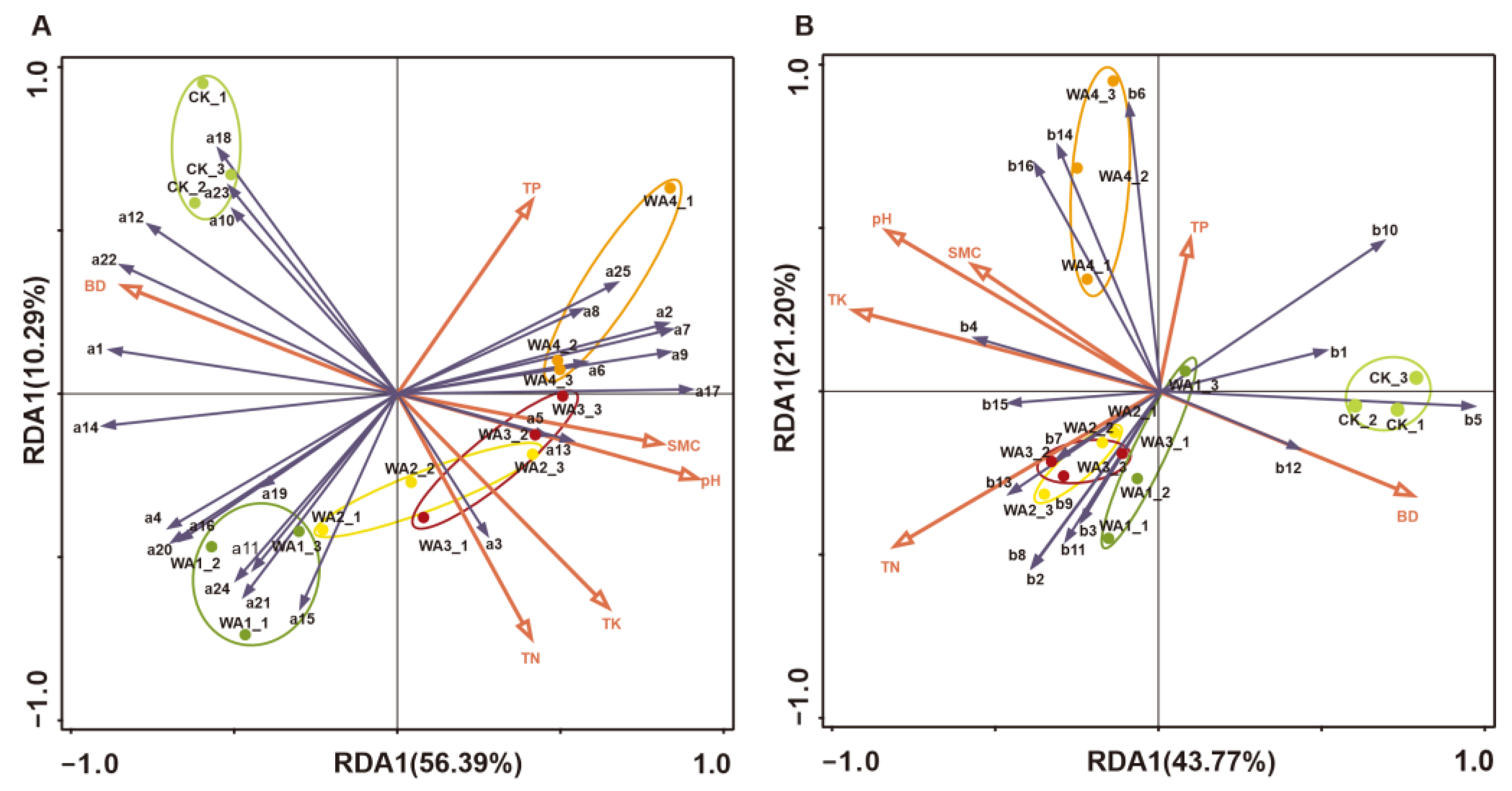
3.4. Redundancy Analysis (RDA) of the Correlation Between Soil Physicochemical Properties and Microbial Community
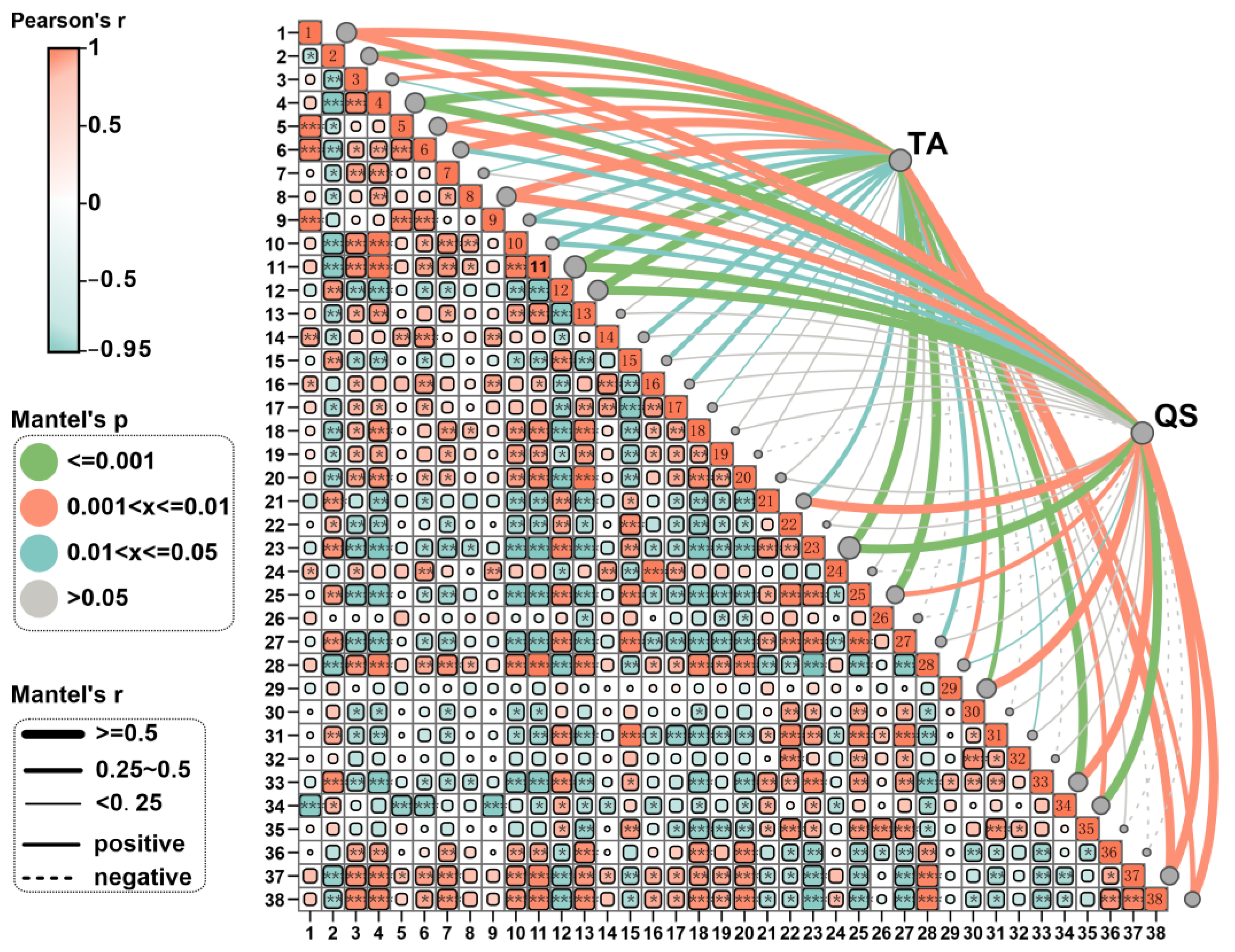
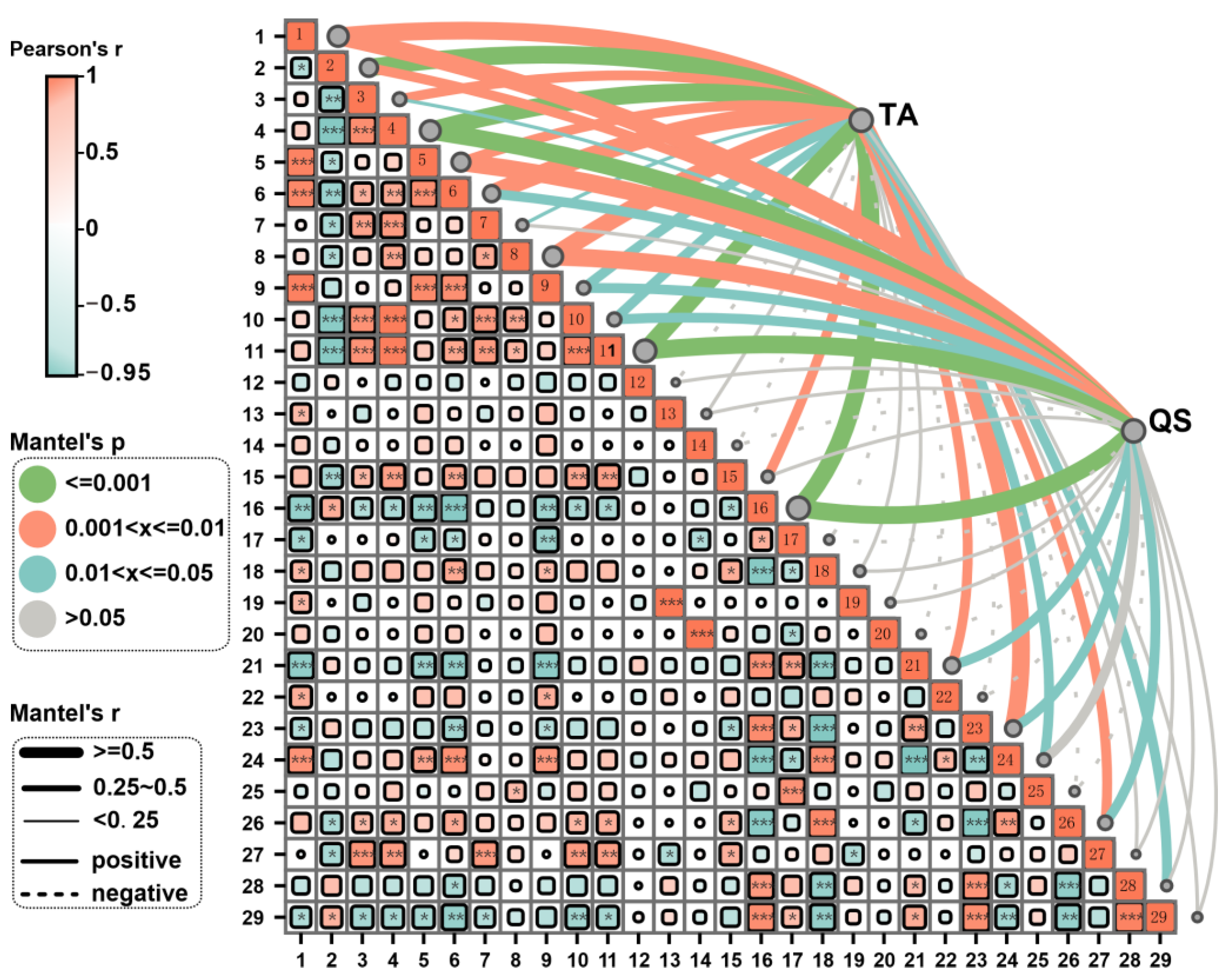
3.5. Correlation Analysis Between the Yield and Quality of Fruiting Body and the Integration of Key Microorganisms and Soil Fertility
4. Discussion
4.1. Effects of Different Levels of WA Additions on the Yield and Quality of Morchella
4.2. The Effect of Different Amounts of WA on Soil Physicochemical Properties
4.3. Effects of Different Levels of WA Additions on Soil Microbial Community Structure
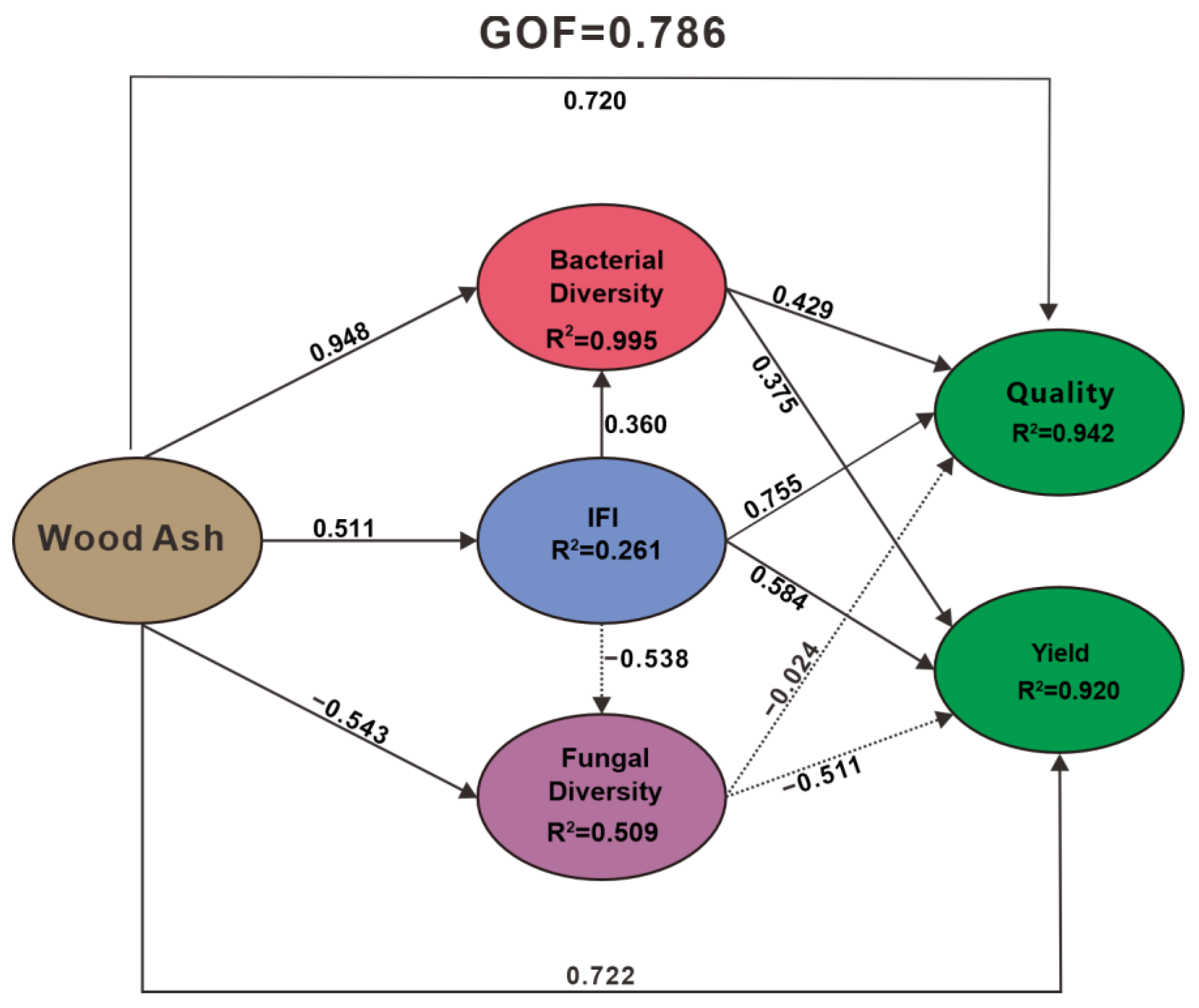
4.4. Effect of Soil Physicochemical Properties and Key Microbial Species on the Yield and Quality of Morchella
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunil, C.; Xu, B. Mycochemical profile and health-promoting effects of morel mushroom Morchella esculenta (L.)—A review. Food Res. 2022, 159, 111571. [Google Scholar] [CrossRef] [PubMed]
- Du, X.H.; Yang, Z.L. Mating Systems in True Morels (Morchella). Microbiol. Mol. Biol. 2021, 85, e0022020. [Google Scholar] [CrossRef] [PubMed]
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional value and biological properties of Chilean wild and commercial edible mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, L.W.; Yang, Z.L.; Bau, T.; Li, T.H.; Dai, Y.C. Resource diversity of Chinese macrofungi: Edible, medicinal and poisonous species. Fungal Divers. 2019, 98, 1–76. [Google Scholar] [CrossRef]
- Liu, W.; He, P.X.; Shi, X.F.; Zhang, Y.; Perez-Moreno, J.; Yu, F.Q. Large-Scale Field Cultivation of Morchella and Relevance of Basic Knowledge for Its Steady Production. J. Fungi 2023, 9, 855. [Google Scholar] [CrossRef]
- Tan, H.; Liu, T.H.; Yu, Y.; Tang, J.; Jiang, L.; Martin, F.M.; Peng, W.H. Morel production related to soil microbial diversity and evenness. Microbiol. Spectr. 2021, 9, e00229-21. [Google Scholar] [CrossRef]
- Yu, F.M.; Jayawardena, R.S.; Thongklang, N.; Lv, M.L.; Zhu, X.T.; Zhao, Q. Morel production associated with soil nitrogen-fixing and nitrifying microorganisms. J. Fungi 2022, 8, 299. [Google Scholar] [CrossRef]
- Larson, A.J.; Cansler, C.A.; Cowdery, S.G.; Hiebert, S.; Furniss, T.J.; Swanson, M.E.; Lutz, J.A. Post-fire morel (Morchella) mushroom abundance, spatial structure, and harvest sustainability. For. Ecol. Manag. 2016, 377, 16–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.F.; Luo, D.D.; Mao, P.; Rosazlina, R.; Martin, F.; Xu, L.L. Decline in Morel production upon continuous cropping is related to changes in soil mycobiome. J. Fungi 2023, 9, 492. [Google Scholar] [CrossRef]
- Laer, T.; Smedt, P.; Ronsse, F.; Ruysschaert, G.; Boeckx, P.; Verstraete, W.; Buysse, J.; Lavrysen, L.J. Legal constraints and opportunities for biochar: A case analysis of EU law. GCB Bioenergy 2013, 7, 14–24. [Google Scholar] [CrossRef]
- Piash, M.I.; Iwabuchi, K.; Itoh, T.; Uemura, K. Release of essential plant nutrients from manure- and wood-based biochars. Geoderma 2021, 397, 115100. [Google Scholar] [CrossRef]
- Paramisparam, P.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y.; Johan, P.D.; Hamidi, N.H. Co-Application of charcoal and wood ash to improve potassium availability in tropical mineral acid soils. Agronomy 2021, 11, 2083. [Google Scholar] [CrossRef]
- Moragues-Saitua, L.; Arias-González, A.; Gartzia-Bengoetxea, N. Effects of biochar and wood ash on soil hydraulic properties: A field experiment involving contrasting temperate soils. Geoderma 2017, 305, 144–152. [Google Scholar] [CrossRef]
- Han, G.J.; Huang, H.X.; Qu, Y.Y. The influence of potassium fertilizer types and application rates on soil water holding characteristics. Resour. Environ. Arid. Reg. 2013, 6, 26–29. [Google Scholar]
- Kang, M.W.; Yibeltal, M.; Kim, Y.H.; Oh, S.J.; Lee, J.C.; Kwon, E.E.; Lee, S.S. Enhancement of soil physical properties and soil water retention with biochar-based soil amendments. Sci. Total Environ. 2022, 836, 155746. [Google Scholar] [CrossRef]
- Ndiate, N.I.; Zaman, Q.U.; Francis, I.N.; Dada, O.A.; Rehman, A.; Asif, M.; Goffner, D.; Kane, A.; Liqun, C.; Haider, F.U. Soil amendment with arbuscular mycorrhizal fungi and biochar Improves salinity tolerance, growth, and lipid metabolism of common wheat (Triticum aestivum L.). Sustainability 2022, 14, 3210. [Google Scholar] [CrossRef]
- Mikajlo, I.; Lerch, T.Z.; Louvel, B.; Hynšt, J.; Záhora, J.; Pourrut, B. Composted biochar versus compost with biochar: Effects on soil properties and plant growth. Biochar 2024, 6, 85. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Yang, T.; Lv, G.; Li, W.; Chen, Y.; Wu, D. Mechanisms underlying the succession of plant rhizosphere microbial community structure and function in an alpine open-pit coal mining disturbance zone. J. Environ. Manag. 2023, 325, 116571. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Luo, X.; Fu, X.; Yang, Y.; Cai, P.; Peng, S.; Chen, W.; Huang, Q. Microbial communities play important roles in modulating paddy soil fertility. Sci. Rep. 2016, 6, 20326. [Google Scholar] [CrossRef]
- Peltoniemi, K.; Pyrhönen, M.; Laiho, R.; Moilanen, M.; Fritze, H. Microbial communities after wood ash fertilization in a boreal drained peatland forest. Eur. J. Soil Biol. 2016, 76, 95–102. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Bang-Andreasen, T.; Nielsen, J.T.; Voriskova, J.; Heise, J.; Rønn, R.; Kjøller, R.; Hansen, H.C.B.; Jacobsen, C.S. Wood Ash Induced pH Changes Strongly Affect Soil Bacterial Numbers and Community Composition. Front. Microbiol. 2017, 8, 1400. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Paredes, C.; Wallander, H.; Kjøller, R.; Rousk, J. Using community trait-distributions to assign microbial responses to pH changes and Cd in forest soils treated with wood ash. Soil. Biol. Biochem. 2017, 112, 153–164. [Google Scholar] [CrossRef]
- Vestergård, M.; Bang-Andreasen, T.; Buss, S.M.; Cruz-Paredes, C.; Bentzon-Tiliva, S.; Ekelund, F.; Kjøller, R.; Hindborg Mortensen, L.; Rønn, R. The relative importance of the bacterial pathway and soil inorganic nitrogen increase across an extreme wood-ash application gradient. GCB Bioenergy 2018, 10, 320–334. [Google Scholar] [CrossRef]
- Wen, Y.; Wu, R.; Qi, D. The effect of AMF combined with biochar on plant growth and soil quality under saline-alkali stress: Insights from microbial community analysis. Ecotoxicol. Environ. Saf. 2024, 281, 116592. [Google Scholar] [CrossRef]
- Wan, Y.; Sheng, H.F.; He, Y.; Wu, J.Y.; Jiang, Y.X.; Tam, N.; Zhou, H.W. Comparson of the levels of bacteral diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar]
- Li, J.J.; Xu, Y.B. Effects of continuous cropping years of lily on soil microbial diversities under greenhouse cultivation. Chin. J. Soil Sci. 2020, 51, 343–351. [Google Scholar]
- Wan, W.J.; Grossart, H.; He, D.L.; Liu, W.Z.; Wang, S.; Yang, Y.Y. Differentiation strategies for planktonic bacteria and eukaryotes in response to aggravated algal blooms in urban lakes. iMeta 2023, 2, e84. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, A.; Xin, X.; Yang, W.L.; Zhang, J.B.; Ding, S.J. Tillage and residue management for long-term wheat-maize cropping in the North China Plain: I. Crop yield and integrated soil fertility index. Field Crops Res. 2018, 221, 157–165. [Google Scholar] [CrossRef]
- Gao, J.; Fang, D.; Muinde Kimatu, B.; Chen, X.; Wu, X.; Du, J.; Yang, Q.; Chen, H.; Zheng, H.; An, X. Analysis of umami taste substances of morel mushroom (Morchella sextelata) hydrolysates derived from different enzymatic systems. Food Chem. 2021, 362, 130192. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.W.; Xu, Z.S.; Huo, Y.Z.; Sun, Q.; Wang, Y.; Che, Y.H.; Wang, J.C.; Li, W.; Zhang, H.H. Effects of salt concentration, pH, and their interaction on plant growth, nutrient uptake, and photochemistry of alfalfa (Medicago sativa) leaves. Plant Signal. Behav. 2020, 15, 1832373. [Google Scholar]
- Ogunyemi, A.M.; Otegbayo, B.O.; Fagbenro, J.A. Effects of NPK and biochar fertilized soil on the proximate composition and mineral evaluation of maize flour. Food Sci. Nutr. 2018, 6, 2308–2313. [Google Scholar] [CrossRef] [PubMed]
- Muiesan, M.L.; Buso, G.; Agabiti Rosei, C. Less sodium and more potassium to reduce cardiovascular risk. Eur. Heart J. Suppl. 2023, 25, B108–B110. [Google Scholar] [CrossRef]
- Yang, X.; Lin, R.; Xu, K.; Guo, L.; Yu, H. Comparative Proteomic Analysis within the Developmental Stages of the Mushroom White Hypsizygus marmoreus. J. Fungi 2021, 7, 1064. [Google Scholar] [CrossRef]
- Agbede, T.M.; Adekiya, A.O. Effect of wood ash, poultry manure and NPK fertilizer on soil and leaf nutrient composition, growth and yield of okra (Abelmoschus esculentus). Food Agric. 2012, 24, 314–321. [Google Scholar]
- Liu, M.; Linna, C.; Ma, S.M.; Ma, Q.; Guo, J.E.; Wang, F.F.; Wang, L.C. Effects of biochar with inorganic and organic fertilizers on agronomic traits and nutrient absorption of soybean and fertility and microbes in purple soil. Front. Plant Sci. 2022, 13, 871021. [Google Scholar] [CrossRef]
- Pitman, R.M. Wood ash use in forestry—A review of the environmental impacts. Forestry 2006, 79, 563–588. [Google Scholar] [CrossRef]
- Moyin-Jesu, E.I. Use of plant residues for improving soil fertility, pod nutrients, root growth and pod weight of okra (Abelmoschus esculentum L.). Bioresour. Technol. 2007, 98, 2057–2064. [Google Scholar] [CrossRef]
- Khanna, P.K.; Raison, R.J.; Falkiner, R.A. Chemical-Properties of Ash Derived from Eucalyptus Litter and Its Effects on Forest Soils. Forest Ecol. Manag. 1994, 66, 107–125. [Google Scholar] [CrossRef]
- Park, B.B.; Yanai, R.D.; Sahm, J.M.; Lee, D.K.; Abrahamson, L.P. Wood ash effects on plant and soil in a willow bioenergy plantation. Biomass Bioenergy 2005, 28, 355–365. [Google Scholar] [CrossRef]
- Raison, R.J.; McGarity, J.W. Some effects of plant ash on the chemical properties of soils and aqueous suspensions. Plant Soil 1980, 55, 339–352. [Google Scholar] [CrossRef]
- Oliveira, W.C.M.; Bonfim-Silva, E.M.; Ferraz, A.P.F.; Guimaraes, S.L.; Silva, T.J.A.; Duarte, T.F. Soil quality indicators for fertilized with wood ash. Rev. Bras. Eng. Agric. Ambient. 2023, 27, 241–249. [Google Scholar] [CrossRef]
- Winder, R.S. Cultural studies of Morchella elata. Mycol. Res. 2006, 110, 612–623. [Google Scholar] [CrossRef]
- Reeves, D.W. The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil Till. Res. 1997, 43, 131–167. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wang, N.; Zhang, J.Y.; Hu, Y.B.; Cai, D.J.; Guo, J.H.; Wu, D.; Sun, G.Y. Soil Physicochemical Properties and the Rhizosphere Soil Fungal Community in a Mulberry (Morus alba L.)/Alfalfa (Medicago sativa L.) Intercropping System. Forests 2019, 10, 167. [Google Scholar] [CrossRef]
- Singh, B. Are Nitrogen Fertilizers Deleterious to Soil Health? Agronomy 2018, 8, 48. [Google Scholar] [CrossRef]
- Su, J.Q.; Ma, Y.; Xu, Z.H.; Liu, Y.Z.; Zhao, Y.; Li, X.R.; Hu, Y.H. Cumulative effects of experimental nitrogen deposition on soil chemistry in a desert steppe: A 12-year field study. Sci. Total Environ. 2024, 950, 175388. [Google Scholar] [CrossRef]
- Constancias, F.; Prévost-Bouré, N.C.; Terrat, S.; Aussems, S.; Nowak, V.; Guillemin, J.P.; Bonnotte, A.; Biju-Duval, L.; Navel, A.; Martins, J.M.F. Microscale evidence for a high decrease of soil bacterial density and diversity by cropping. Agron. Sustain. Dev. 2014, 34, 831–840. [Google Scholar] [CrossRef]
- Ning, Q.; Chen, L.; Jia, Z.J.; Zhang, C.Z.; Ma, D.H.; Li, F.; Zhang, J.B.; Li, D.M.; Han, X.R.; Cai, Z.J. Multiple long-term observations reveal a strategy for soil pH-dependent fertilization and fungal communities in support of agricultural production. Agric. Ecosyst. Environ. 2020, 293, 106837. [Google Scholar] [CrossRef]
- Hsiao, C.J.; Sassenrath, G.F.; Zeglin, L.H.; Hettiarachchi, G.M.; Rice, C.W. Temporal Variation of Soil Microbial Properties in a Corn–Wheat–Soybean System. Soil Sci. Soc. Am. J. 2019, 83, 1696–1711. [Google Scholar] [CrossRef]
- Hu, X.J.; Liu, J.J.; Wei, D.; Zhu, P.; Cui, X.; Zhou, B.K.; Chen, X.L.; Jin, J.; Liu, X.B.; Wang, G.H. Effects of over 30-year of different fertilization regimes on fungal community compositions in the black soils of northeast China. Agric. Ecosyst. Environ. 2017, 248, 113–122. [Google Scholar] [CrossRef]
- Sugiyama, A.; Ueda, Y.; Zushi, T.; Takase, H.; Yazaki, K. Changes in the bacterial community of soybean rhizospheres during growth in the field. PLoS ONE 2014, 9, e100709. [Google Scholar] [CrossRef] [PubMed]
- Abdi, D.; Ziadi, N.; Shi, Y.C.; Gagnon, B.; Lalande, R.; Hamel, C. Residual effects of paper mill biosolids and liming materials on soil microbial biomass and community structure. Can. J. Soil Sci. 2017, 97, 188–199. [Google Scholar] [CrossRef]
- He, X.J.; Xie, H.; Gao, D.M.; Rahman, M.K.U.; Zhou, X.A.; Wu, F.Z. Biochar and intercropping with potato-onion enhanced the growth and yield advantages of tomato by regulating the soil properties, nutrient uptake, and soil microbial community. Front. Microbiol. 2021, 12, 695447. [Google Scholar] [CrossRef]
- Zhao, H.L.; Tian, X.H.; Jiang, Y.H.; Zhao, Y.; Si, B.C. Effect of combining straw-derived materials and wood ash on alkaline soil carbon content and the microbial community. Eur. J. Soil Sci. 2021, 72, 1863–1878. [Google Scholar] [CrossRef]
- She, S.Y.; Niu, J.J.; Zhang, C.; Xiao, Y.H.; Chen, W.; Dai, L.J.; Liu, X.D.; Yin, H.Q. Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch. Microbiol. 2017, 199, 267–275. [Google Scholar] [CrossRef]
- Wang, R.; Xiao, Y.; Lv, F.; Hu, L.; Wei, L.; Yuan, Z.; Lin, H. Bacterial community structure and functional potential of rhizosphere soils as influenced by nitrogen addition and bacterial wilt disease under continuous sesame cropping. Appl. Soil Ecol. 2018, 125, 117–127. [Google Scholar] [CrossRef]
- Ye, G.P.; Lin, Y.X.; Luo, J.F.; Di, H.J.; Lindsey, S.; Liu, D.Y.; Fan, J.B.; Ding, W.X. Responses of soil fungal diversity and community composition to long-term fertilization: Field experiment in an acidic ultisol and literature synthesis. Appl. Soil Ecol. 2020, 145, 103305. [Google Scholar] [CrossRef]
- Chen, B.; Shao, G.G.; Zhou, T.; Fan, Q.H.; Yang, N.L.; Cui, M.; Zhang, J.W.; Wu, X.L.; Zhang, B.X.; Zhang, R.Y. Dazomet changes microbial communities and improves morel mushroom yield under continuous cropping. Front. Microbiol. 2023, 14, 1200226. [Google Scholar] [CrossRef]
- Liu, B.S.; Hu, Y.H.; Wang, Y.; Xue, H.H.; Li, Z.H.; Li, M. Effects of saline-alkali stress on bacterial and fungal community diversity in rhizosphere soil. Environ. Sci. Pollut. R. 2022, 29, 70000–70013. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.N.; He, X.H.; Gao, N.; Li, Q.; Qiu, Z.J.; Hou, Y.X.; Shen, W.S. Soil pH amendment alters the abundance, diversity, and composition of microbial communities in two contrasting agricultural soils. Microbiol. Spectr. 2024, 12, e0416523. [Google Scholar] [CrossRef] [PubMed]
- Füzesi, I.; Heil, B.; Kovács, G. Effects of wood ash on the chemical properties of soil and crop vitality in small plot experiments. Acta Silv. Lignaria Hung. 2015, 11, 55–64. [Google Scholar] [CrossRef]
- Zhang, W.; Zwiazek, J.J. Effects of root medium pH on root water transport and apoplastic pH in red-osier dogwood (Cornus sericea) and paper birch (Betula papyrifera) seedlings. Plant Biol. 2016, 18, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Xie, J.H.; Li, L.L.; Effah, Z.; Xie, L.H.; Luo, Z.Z.; Zhou, Y.J.; Jiang, Y.J. Fertilization treatments affect soil CO emission through regulating soil bacterial community composition in the semiarid Loess Plateau. Sci. Rep. 2022, 12, 20123. [Google Scholar]
- Chen, W.Q.; Wang, J.Y.; Chen, X.; Meng, Z.X.; Xu, R.; Duoji, D.; Zhang, J.H.; He, J.; Wang, Z.A.; Chen, J. Soil microbial network complexity predicts ecosystem function along elevation gradients on the Tibetan Plateau. Soil Biol. Biochem. 2022, 172, 108766. [Google Scholar] [CrossRef]
- Filho, A.S.S.; Silva, E.M.B.; Ferraz, A.P.F.; Simeon, B.G.; Silva, T.J.A.; Duarte, T.F. Productive characteristics and structure of Paiaguás grass pasture fertilized with wood ash in the Brazilian Cerrado. Aust. J. Crop Sci. 2023, 377–384. [Google Scholar] [CrossRef]
- Lan, J.; Wang, S.; Long, Q.; Wang, J.; Qi, X.; Huang, M.; Liu, L.; Yue, K. The Influence of Afforestation on Soil Bacterial Community Composition in Karst Rocky Desertification Areas of Guizhou Province, China. Available online: https://www.researchsquare.com/article/rs-2920989/v1 (accessed on 1 July 2024).
- Kpalari, D.F.; Hamani, A.K.M.; Hui, C.; Sogbedji, J.M.; Liu, J.M.; Le, Y.; Kama, R.; Gao, Y.; Morcia, C. Soil Bacterial Community and Greenhouse Gas Emissions as Responded to the Coupled Application of Nitrogen Fertilizer and Microbial Decomposing Inoculants in Wheat (Triticum aestivum L.) Seedling Stage under Different Water Regimes. Agronomy 2023, 13, 2950. [Google Scholar] [CrossRef]
- Loganathachetti, D.S.; Venkatachalam, S.; Jabir, T.; Vipindas, P.V.; Krishnan, K.P. Total nitrogen influence bacterial community structure of active layer permafrost across summer and winter seasons in Ny-Ålesund, Svalbard. World J. Microb. Biot. 2022, 38, 28. [Google Scholar] [CrossRef]
- Chun, S.J.; Kim, Y.J.; Cui, Y.; Nam, K.H. Ecological network analysis reveals distinctive microbial modules associated with heavy metal contamination of abandoned mine soils in Korea. Environ. Pollut. 2021, 289, 117851. [Google Scholar] [CrossRef]
- Vicente, J.G.; Holub, E.B. Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol. Plant Pathol. 2013, 14, 2–18. [Google Scholar]
- Goto, L.S.; Alexandrino, A.V.; Pereira, C.M.; Martins, C.S.; Pereira, H.D.; Brandao-Neto, J.; Novo-Mansur, M.T.M. Structural and functional characterization of the phosphoglucomutase from Xanthomonas citri subsp. citri. BBA-Proteins Proteom. 2016, 1864, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, I.; Pesic, A.; Petras, D.; Royer, M.; Süssmuth, R.D.; Cociancich, S. What makes Xanthomonas albilineans unique amongst xanthomonads? Front. Plant Sci. 2015, 6, 289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, B.; Wang, S.; Wu, Q.; Shi, L.; Shi, C. Effect of Solarization to Kill Bradysia Cellarum on Chinese Chive Growth and Soil Microbial Diversity. Front. Agric. Sci. Eng. 2022, 9, 52–62. [Google Scholar]
- Bai, X.L.; Zhang, E.; Wu, J.M.; Ma, D.H.; Zhang, C.H.; Zhang, B.Y.; Liu, Y.P.; Zhang, Z.; Tian, F.; Zhao, H.; et al. Soil fungal community is more sensitive than bacterial community to modified materials application in saline-alkali land of Hetao Plain. Front. Microbiol. 2024, 15, 1255536. [Google Scholar] [CrossRef]
- Guo, H.N.; Huang, Z.J.; Li, M.Q.; Min, W. Response of Soil Fungal Community Structure and Diversity to Saline Water Irrigation in Alluvial Grey Desert Soils. Appl. Ecol. Environ. Res. 2020, 18, 4969–4985. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, C.; Yao, R.J.; Wang, X.P.; Liu, G.M. Response of Soil Fungal Community in Coastal Saline Soil to Short-Term Water Management Combined with Bio-Organic Fertilizer. Agronomy 2024, 14, 1441. [Google Scholar] [CrossRef]
- Farooq, T.H.; Kumar, U.; Shakoor, A.; Albasher, G.; Alkahtani, S.; Rizwana, H.; Tayyab, M.; Dobaria, J.; Hussain, M.I.; Wu, P.F. Influence of Intraspecific Competition Stress on Soil Fungal Diversity and Composition in Relation to Tree Growth and Soil Fertility in Sub-Tropical Soils under Chinese Fir Monoculture. Sustainability 2021, 13, 10688. [Google Scholar] [CrossRef]
- Chandrakasan, G.; Gastauer, M.; Marcus, G. Mapping, Distribution, Function, and High-Throughput Methodological Strategies for Soil Microbial Communities in the Agroecosystem in the Last Decades. Span. J. Soil Sci. 2024, 14, 12080. [Google Scholar] [CrossRef]
- Sahu, P.K.; Singh, D.P.; Prabha, R.; Meena, K.K.; Abhilash, P.C. Connecting microbial capabilities with the soil and plant health: Options for agricultural sustainability. Ecol. Indic. 2019, 105, 601–612. [Google Scholar] [CrossRef]
- Duan, Y.N.; Jiang, W.T.; Zhang, R.; Chen, R.; Chen, X.S.; Yin, C.M.; Mao, Z.Q. Discovery of f. sp. Causing Apple Replant Disease in China. Plant Dis. 2022, 106, 2958–2966. [Google Scholar] [CrossRef] [PubMed]
- Popp, C.; Wamhoff, D.; Winkelmann, T.; Maiss, E.; Grunewaldt-Stöcker, G. Molecular identification of Nectriaceae in infections of apple replant disease affected roots collected by Harris Uni-Core punching or laser microdissection. J. Plant Dis. Protect. 2020, 127, 571–582. [Google Scholar] [CrossRef]
- Sweany, R.R.; Mack, B.M.; Gebru, S.T.; Mammel, M.K.; Cary, J.W.; Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H.; Gilbert, M.K. Divergent Aspergillus flavus corn population is composed of prolific conidium producers: Implications for saprophytic disease cycle. Mycologia 2024, 116, 536–557. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Li, L.; Wu, W.; Pu, K.; Qi, W.; Qi, J.; Li, M. Enhancing Morchella Mushroom Yield and Quality Through the Amendment of Soil Physicochemical Properties and Microbial Community with Wood Ash. Microorganisms 2024, 12, 2406. https://doi.org/10.3390/microorganisms12122406
Huang K, Li L, Wu W, Pu K, Qi W, Qi J, Li M. Enhancing Morchella Mushroom Yield and Quality Through the Amendment of Soil Physicochemical Properties and Microbial Community with Wood Ash. Microorganisms. 2024; 12(12):2406. https://doi.org/10.3390/microorganisms12122406
Chicago/Turabian StyleHuang, Kai, Ling Li, Weijun Wu, Kunlun Pu, Wei Qi, Jianzhao Qi, and Minglei Li. 2024. "Enhancing Morchella Mushroom Yield and Quality Through the Amendment of Soil Physicochemical Properties and Microbial Community with Wood Ash" Microorganisms 12, no. 12: 2406. https://doi.org/10.3390/microorganisms12122406
APA StyleHuang, K., Li, L., Wu, W., Pu, K., Qi, W., Qi, J., & Li, M. (2024). Enhancing Morchella Mushroom Yield and Quality Through the Amendment of Soil Physicochemical Properties and Microbial Community with Wood Ash. Microorganisms, 12(12), 2406. https://doi.org/10.3390/microorganisms12122406







