Separating Infectious Proctitis from Inflammatory Bowel Disease—A Common Clinical Conundrum
Abstract
1. Introduction
2. Inflammatory Bowel Disease
2.1. Ulcerative Proctitis
2.2. Crohn’s Disease
3. Infective Proctitis
3.1. Neisseria gonorrhoeae

3.2. Chlamydia trachomatis
3.2.1. Non-LGV Chlamydia trachomatis
3.2.2. Lymphogranuloma Venereum Chlamydia trachomatis
3.3. Treponema pallidum
3.4. Herpes Simplex Virus
3.5. Mycoplasma genitalium
3.6. Mpox
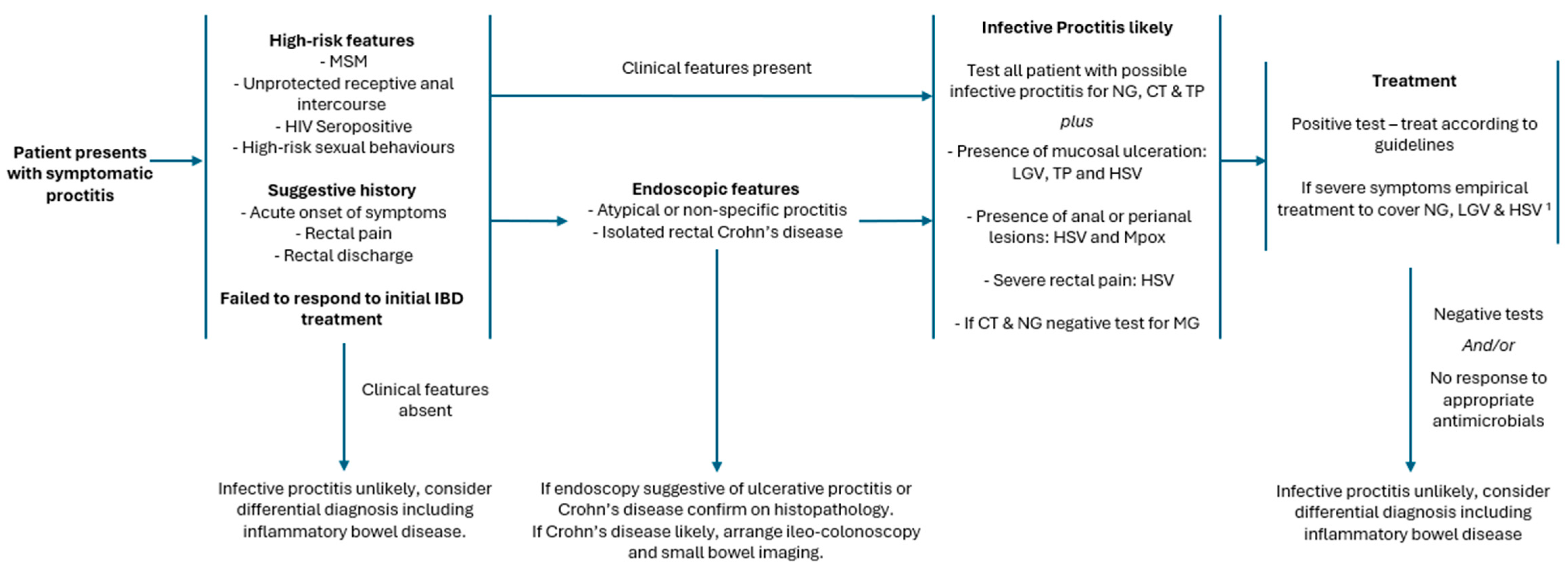
4. Distinguishing Inflammatory Bowel Disease and Infective Proctitis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Vries, H.J.C.; Nori, A.V.; Larsen, H.K.; Kreuter, A.; Padovese, V.; Pallawela, S.; Vall-Mayans, M.; Ross, J. 2021 European Guideline on the Management of Proctitis, Proctocolitis and Enteritis Caused by Sexually Transmissible Pathogens. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Tuddenham, S.; Hamill, M.M.; Ghanem, K.G. Diagnosis and Treatment of Sexually Transmitted Infections: A Review. JAMA 2022, 327, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Traeger, M.W.; Guy, R.; Asselin, J.; Patel, P.; Carter, A.; Wright, E.J.; Grulich, A.; McManus, H.; Fairley, C.K.; Chow, E.P.F.; et al. Real-World Trends in Incidence of Bacterial Sexually Transmissible Infections among Gay and Bisexual Men Using HIV Pre-Exposure Prophylaxis (PrEP) in Australia Following Nationwide PrEP Implementation: An Analysis of Sentinel Surveillance Data. Lancet Infect. Dis. 2022, 22, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical Presentation and Virological Assessment of Confirmed Human Monkeypox Virus Cases in Spain: A Prospective Observational Cohort Study. Lancet 2022, 400, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The Global Burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Fumery, M.; Singh, S.; Dulai, P.S.; Gower-Rousseau, C.; Peyrin-Biroulet, L.; Sandborn, W.J. Natural History of Adult Ulcerative Colitis in Population-Based Cohorts: A Systematic Review. Clin. Gastroenterol. Hepatol. 2018, 16, 343–356.e3. [Google Scholar] [CrossRef]
- Levy, I.; Gefen-Halevi, S.; Nissan, I.; Keller, N.; Pilo, S.; Wieder-Finesod, A.; Litchevski, V.; Shasha, D.; Kedem, E.; Rahav, G. Delayed Diagnosis of Colorectal Sexually Transmitted Diseases Due to Their Resemblance to Inflammatory Bowel Diseases. Int. J. Infect. Dis. 2018, 75, 34–38. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative Colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Dolinger, M.; Torres, J.; Vermeire, S. Crohn’s Disease. Lancet 2024, 403, 1177–1191. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Kontola, K.; Oksanen, P.; Huhtala, H.; Jussila, A. Increasing Incidence of Inflammatory Bowel Disease, with Greatest Change Among the Elderly: A Nationwide Study in Finland, 2000–2020. J. Crohn’s Colitis 2023, 17, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Holdsworth, C.D.; Read, N.W. Symptoms and Stool Patterns in Patients with Ulcerative Colitis. Gut 1988, 29, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal Classification of Inflammatory Bowel Disease: Controversies, Consensus, and Implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, Y.J.; Rhee, K.H.; Kim, Y.H.; Hong, S.N.; Kim, K.H.; Seo, S.I.; Cha, J.M.; Park, S.Y.; Jeong, S.K.; et al. A 30-Year Trend Analysis in the Epidemiology of Inflammatory Bowel Disease in the Songpa-Kangdong District of Seoul, Korea in 1986–2015. J. Crohn’s Colitis 2019, 13, 1410–1417. [Google Scholar] [CrossRef]
- Ng, S.C.; Zeng, Z.; Niewiadomski, O.; Tang, W.; Bell, S.; Kamm, M.A.; Hu, P.; De Silva, H.J.; Niriella, M.A.; Udara, W.S.A.A.Y.; et al. Early Course of Inflammatory Bowel Disease in a Population-Based Inception Cohort Study from 8 Countries in Asia and Australia. Gastroenterology 2016, 150, 86–95.e3. [Google Scholar] [CrossRef]
- Kyriacou, M.; Radford, S.; Moran, G.W. Delphi Consensus Survey: The Opinions of Patients Living with Refractory Ulcerative Proctitis and the Health Care Professionals Who Care for Them. BMJ Open Gastroenterol. 2023, 10, e001139. [Google Scholar] [CrossRef]
- Jayasooriya, N.; Baillie, S.; Blackwell, J.; Bottle, A.; Petersen, I.; Creese, H.; Saxena, S.; Pollok, R.C. Systematic Review with Meta-Analysis: Time to Diagnosis and the Impact of Delayed Diagnosis on Clinical Outcomes in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2023, 57, 635–652. [Google Scholar] [CrossRef]
- Annese, V.; Daperno, M.; Rutter, M.D.; Amiot, A.; Bossuyt, P.; East, J.; Ferrante, M.; Götz, M.; Katsanos, K.H.; Kießlich, R.; et al. European Evidence Based Consensus for Endoscopy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2013, 7, 982–1018. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-Intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-Anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Lobatón, T.; Bessissow, T.; De Hertogh, G.; Lemmens, B.; Maedler, C.; Van Assche, G.; Vermeire, S.; Bisschops, R.; Rutgeerts, P.; Bitton, A.; et al. The Modified Mayo Endoscopic Score (MMES): A New Index for the Assessment of Extension and Severity of Endoscopic Activity in Ulcerative Colitis Patients. J. Crohn’s Colitis 2015, 9, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.P.L.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.F.; Feagan, B.G.; Hanauer, S.B.; Lémann, M.; Lichtenstein, G.R.; et al. Developing an Instrument to Assess the Endoscopic Severity of Ulcerative Colitis: The Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012, 61, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Doherty, G.; Peyrin-Biroulet, L.; Svrcek, M.; Borralho, P.; Walsh, A.; Carneiro, F.; Rosini, F.; De Hertogh, G.; Biedermann, L.; et al. ECCO Position Paper: Harmonization of the Approach to Ulcerative Colitis Histopathology. J. Crohn’s Colitis 2020, 14, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Feakins, R.M. Inflammatory Bowel Disease Biopsies: Updated British Society of Gastroenterology Reporting Guidelines. J. Clin. Pathol. 2013, 66, 1005–1026. [Google Scholar] [CrossRef]
- Feakins, R.M. Ulcerative Colitis or Crohn’s Disease? Pitfalls and Problems. Histopathology 2014, 64, 317–335. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Ayres, R.C.; Gillen, C.D.; Walmsley, R.S.; Allan, R.N. Progression of Ulcerative Proctosigmoiditis: Incidence and Factors Influencing Progression. Eur. J. Gastroenterol. Hepatol. 1996, 8, 555–558. [Google Scholar] [CrossRef]
- Roda, G.; Narula, N.; Pinotti, R.; Skamnelos, A.; Katsanos, K.H.; Ungaro, R.; Burisch, J.; Torres, J.; Colombel, J.F. Systematic Review with Meta-Analysis: Proximal Disease Extension in Limited Ulcerative Colitis. Aliment. Pharmacol. Ther. 2017, 45, 1481–1492. [Google Scholar] [CrossRef]
- Burisch, J.; Katsanos, K.H.; Christodoulou, D.K.; Barros, L.; Magro, F.; Pedersen, N.; Kjeldsen, J.; Vegh, Z.; Lakatos, P.L.; Eriksson, C.; et al. Natural Disease Course of Ulcerative Colitis during the First Five Years of Follow-up in a European Population-Based Inception Cohort-An Epi-IBD Study. J. Crohn’s Colitis 2019, 13, 198–208. [Google Scholar] [CrossRef]
- Dubois, E.; Moens, A.; Geelen, R.; Sabino, J.; Ferrante, M.; Vermeire, S. Long-Term Outcomes of Patients with Ulcerative Proctitis: Analysis from a Large Referral Centre Cohort. United Eur. Gastroenterol. J. 2020, 8, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.S.; Bryant, R.V.; Costello, S.P.; Barnett, M.; Schubert, J.; Rayner, C.K. Systematic Review of Therapies for Refractory Ulcerative Proctitis. J. Gastroenterol. Hepatol. 2023, 38, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Caron, B.; Sandborn, W.J.; Schreiber, S.; Panaccione, R.; Danese, S.; Peyrin-Biroulet, L. Drug Development for Ulcerative Proctitis: Current Concepts. Gut 2021, 70, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s Disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E. From Symptom to Diagnosis: Clinical Distinctions among Various Forms of Intestinal Inflammation. Gastroenterology 2004, 126, 1518–1532. [Google Scholar] [CrossRef]
- Mekhjian, H.S.; Switz, D.M.; Melnyk, C.S.; Rankin, G.B.; Brooks, R.K. Clinical Features and Natural History of Crohn’s Disease. Gastroenterology 1979, 77, 898–906. [Google Scholar] [CrossRef]
- Perler, B.; Ungaro, R.; Baird, G.; Mallette, M.; Bright, R.; Shah, S.; Shapiro, J.; Sands, B.E. Presenting Symptoms in Inflammatory Bowel Disease: Descriptive Analysis of a Community-Based Inception Cohort. BMC Gastroenterol. 2019, 19, 47. [Google Scholar] [CrossRef]
- Korelitz, B.I.; Aronoff, M.D.J. Crohn’s Proctitis: A Distinct Entity. Inflamm. Bowel Dis. 2010, 16, 721–722. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-Based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohn’s Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Tsai, L.; McCurdy, J.D.; Ma, C.; Jairath, V.; Singh, S. Epidemiology and Natural History of Perianal Crohn’s Disease: A Systematic Review and Meta-Analysis of Population-Based Cohorts. Inflamm. Bowel Dis. 2022, 28, 1477–1484. [Google Scholar] [CrossRef]
- Wewer, M.D.; Zhao, M.; Nordholm-Carstensen, A.; Weimers, P.; Seidelin, J.B.; Burisch, J. The Incidence and Disease Course of Perianal Crohn’s Disease: A Danish Nationwide Cohort Study, 1997–2015. J. Crohn’s Colitis 2021, 15, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Eglinton, T.W.; Barclay, M.L.; Gearry, R.B.; Frizelle, F.A. The Spectrum of Perianal Crohn’s Disease in a Population-Based Cohort. Dis. Colon. Rectum 2012, 55, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350.e6. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Langner, C.; Driessen, A.; Ensari, A.; Geboes, K.; Mantzaris, G.J.; Villanacci, V.; Becheanu, G.; Nunes, P.B.; Cathomas, G.; et al. European Consensus on the Histopathology of Inflammatory Bowel Disease. J. Crohn’s Colitis 2013, 7, 827–851. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Coelho, R.; Ribeiro, T.; Abreu, N.; Gonçalves, R.; Macedo, G. Infectious Proctitis: What Every Gastroenterologist Needs to Know. Ann. Gastroenterol. 2023, 36, 275. [Google Scholar] [CrossRef]
- Dukers-Muijrers, N.H.T.M.; Schachter, J.; van Liere, G.A.F.S.; Wolffs, P.F.G.; Hoebe, C.J.P.A. What Is Needed to Guide Testing for Anorectal and Pharyngeal Chlamydia Trachomatis and Neisseria Gonorrhoeae in Women and Men? Evidence and Opinion. BMC Infect. Dis. 2015, 15, 533. [Google Scholar] [CrossRef]
- Chan, P.A.; Robinette, A.; Montgomery, M.; Almonte, A.; Cu-Uvin, S.; Lonks, J.R.; Chapin, K.C.; Kojic, E.M.; Hardy, E.J. Extragenital Infections Caused by Chlamydia Trachomatis and Neisseria Gonorrhoeae: A Review of the Literature. Infect. Dis. Obstet. Gynecol. 2016, 2016, 5758387. [Google Scholar] [CrossRef]
- Kent, C.K.; Chaw, J.K.; Wong, W.; Liska, S.; Gibson, S.; Hubbard, G.; Klausner, J.D. Prevalence of Rectal, Urethral, and Pharyngeal Chlamydia and Gonorrhea Detected in 2 Clinical Settings among Men Who Have Sex with Men: San Francisco, California, 2003. Clin. Infect. Dis. 2005, 41, 67–74. [Google Scholar] [CrossRef]
- Peters, R.P.H.; Nijsten, N.; Mutsaers, J.; Jansen, C.L.; Morré, S.A.; Van Leeuwen, A.P. Screening of Oropharynx and Anorectum Increases Prevalence of Chlamydia Trachomatis and Neisseria Gonorrhoeae Infection in Female STD Clinic Visitors. Sex. Transm. Dis. 2011, 38, 783–787. [Google Scholar] [CrossRef]
- Hascoet, J.L.; Dahoun, M.; Cohen, M.; Pommaret, E.; Pilmis, B.; Lemarchand, N.; Mizrahi, A.; Aubert, M.; de Parades, V.; Monnier, A. Le Clinical Diagnostic and Therapeutic Aspects of 221 Consecutive Anorectal Chlamydia Trachomatis and Neisseria Gonorrhoeae Sexually Transmitted Infections among Men Who Have Sex with Men. Int. J. Infect. Dis. 2018, 71, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Berti, V.; Blondel, J.; Spindler, L.; de Parades, V.; Aubert, M.; Le Monnier, A.; Lourtet-Hascoet, J. Infective Anoproctitis in Men Having Sex with Men: Don’t Forget Mycoplasma Genitalium. Infect. Dis. Now. 2023, 53, 104771. [Google Scholar] [CrossRef] [PubMed]
- Sigle, G.W.; Kim, R. Sexually Transmitted Proctitis. Clin. Colon. Rectal Surg. 2015, 28, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Hamlyn, E.; Taylor, C. Sexually Transmitted Proctitis. Postgrad. Med. J. 2006, 82, 733–736. [Google Scholar] [CrossRef]
- Cornelisse, V.J.; Chow, E.P.F.; Huffam, S.; Fairley, C.K.; Bissessor, M.; De Petra, V.; Howden, B.P.; Denham, I.; Bradshaw, C.S.; Williamson, D.; et al. Increased Detection of Pharyngeal and Rectal Gonorrhea in Men Who Have Sex with Men after Transition from Culture to Nucleic Acid Amplification Testing. Sex. Transm. Dis. 2017, 44, 114–117. [Google Scholar] [CrossRef]
- Merrick, R.; Cole, M.; Pitt, R.; Enayat, Q.; Ivanov, Z.; Day, M.; Sun, S.; Sinka, K.; Woodford, N.; Mohammed, H.; et al. Antimicrobial-Resistant Gonorrhoea: The National Public Health Response, England, 2013 to 2020. Eurosurveillance 2022, 27, 1. [Google Scholar] [CrossRef]
- Fifer, H.; Saunders, J.; Soni, S.; Sadiq, S.T.; Fitzgerald, M. 2018 UK National Guideline for the Management of Infection with Neisseria Gonorrhoeae. Int. J. STD AIDS 2019, 31, 4–15. [Google Scholar] [CrossRef]
- Unemo, M.; Ross, J.; Serwin, A.B.; Gomberg, M.; Cusini, M.; Jensen, J.S. 2020 European Guideline for the Diagnosis and Treatment of Gonorrhoea in Adults. Int. J. STD AIDS 2020, 32, 108–126. [Google Scholar] [CrossRef]
- Law, C.; Bhagat, V.; Patel, A.V. Lymphogranuloma Venereum Proctitis. Clin. Gastroenterol. Hepatol. 2020, 18, A20. [Google Scholar] [CrossRef]
- Di Altobrando, A.; Tartari, F.; Filippini, A.; D’antuono, A.; Patrizi, A.; Filippi, F.; Sechi, A.; Cuicchi, D.; Cosimo, N.; Salfi, M.; et al. Lymphogranuloma Venereum Proctitis Mimicking Inflammatory Bowel Diseases in 11 Patients: A 4-Year Single-Center Experience. Crohn’s Colitis 360 2019, 1, otz004. [Google Scholar] [CrossRef]
- You, J.H.; Cho, K.W.; Cha, Y.J.; Park, H.J. A Case of Rectal Syphilis Incidentally Found at Regular Medical Check-Up. Korean J. Gastroenterol. 2016, 68, 218. [Google Scholar] [CrossRef] [PubMed]
- Costales-Cantrell, J.K.; Dong, E.Y.; Wu, B.U.; Nomura, J.H. Syphilitic Proctitis Presenting as a Rectal Mass: A Case Report and Review of the Literature. J. Gen. Intern. Med. 2021, 36, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Sandgren, K.E.; Price, N.B.; Bishop, W.P.; McCarthy, P.J. Herpes Simplex Proctitis Mimicking Inflammatory Bowel Disease in a Teenaged Male. Case Rep. Pediatr. 2017, 2017, 3547230. [Google Scholar] [CrossRef] [PubMed]
- Mavilia, M.G.; Putra, I.; Song, X.; Cappa, J. Endoscopic and Histologic Assessment of Monkey Pox-Associated Proctitis. Clin. Gastroenterol. Hepatol. 2023, 21, A19. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Lamb, E.I.M.; Mansfield, J.C.; Sankar, K.N. Sexually Transmitted Infections Manifesting as Proctitis. Frontline Gastroenterol. 2013, 4, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Prestage, G.P.; Mao, L.; Kippax, S.C.; Pell, C.M.; Donovan, B.; Cunningham, P.H.; Templeton, D.J.; Kaldor, J.M.; Grulich, A.E. Incidence and Risk Factors for Urethral and Anal Gonorrhoea and Chlamydia in a Cohort of HIV-Negative Homosexual Men: The Health in Men Study. Sex. Transm. Infect. 2007, 83, 113–119. [Google Scholar] [CrossRef]
- Chandra, N.L.; Broad, C.; Folkard, K.; Town, K.; Harding-Esch, E.M.; Woodhall, S.C.; Saunders, J.M.; Sadiq, S.T.; Dunbar, J.K. Detection of Chlamydia Trachomatis in Rectal Specimens in Women and Its Association with Anal Intercourse: A Systematic Review and Meta-Analysis. Sex. Transm. Infect. 2018, 94, 320–326. [Google Scholar] [CrossRef]
- Visser, M.; Hoebe, C.J.P.A.; Wolffs, P.F.G.; Heijne, J.C.M. Anorectal Neisseria Gonorrhoeae Infections in Women with and without Reported Anal Sex and Sex Workers in Sexual Health Centres in the Netherlands: A Retrospective Cohort Study. Lancet Microbe 2024, 5, e326–e334. [Google Scholar] [CrossRef]
- Quinn, T.C.; Goodell, S.E.; Mkrtichian, E.; Schuffler, M.D.; Wang, S.-P.; Stamm, W.E.; Holmes, K.K. Chlamydia Trachomatis Proctitis. N. Engl. J. Med. 1981, 305, 195–200. [Google Scholar] [CrossRef]
- Ootani, A.; Mizuguchi, M.; Tsunada, S.; Sakata, H.; Iwakiri, R.; Toda, S.; Fujimoto, K. Chlamydia Trachomatis Proctitis. Gastrointest. Endosc. 2004, 60, 161–162. [Google Scholar] [CrossRef]
- Van Der Helm, J.J.; Hoebe, C.J.P.A.; van Rooijen, M.S.; Brouwers, E.E.H.G.; Fennema, H.S.A.; Thiesbrummel, H.F.J.; Dukers-Muijrers, N.H.T.M. High Performance and Acceptability of Self-Collected Rectal Swabs for Diagnosis of Chlamydia Trachomatis and Neisseria Gonorrhoeae in Men Who Have Sex with Men and Women. Sex. Transm. Dis. 2009, 36, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Nwokolo, N.C.; Dragovic, B.; Patel, S.; Tong, C.Y.W.; Barker, G.; Radcliffe, K. 2015 UK National Guideline for the Management of Infection with Chlamydia Trachomatis. Int. J. STD AIDS 2016, 27, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, A.; Foschi, C.; Tartari, F.; Gaspari, V.; Re, M.C. Lymphogranuloma Venereum Genovariants in Men Having Sex with Men in Italy. Sex. Transm. Infect. 2021, 97, 441–445. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, L.; Svidler López, L.; Entrocassi, A.C.; López Aquino, D.; Caffarena, D.; Büttner, K.A.; Gallo Vaulet, M.L.; Rodríguez Fermepin, M. Chlamydia Trachomatis Anorectal Infections by LGV (L1, L2 and L2b) and Non-LGV Serotypes in Symptomatic Patients in Buenos Aires, Argentina. Int. J. STD AIDS 2021, 32, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, R.F.; Ossewaarde, J.M.; Götz, H.M.; Dees, J.; Thio, H.B.; Thomeer, M.G.J.; Den Hollander, J.C.; Neumann, M.H.A.; Meijden, W.I. Van Der Resurgence of Lymphogranuloma Venereum in Western Europe: An Outbreak of Chlamydia Trachomatis Serovar L2 Proctitis in The Netherlands among Men Who Have Sex with Men. Clin. Infect. Dis. 2004, 39, 996–1003. [Google Scholar] [CrossRef]
- Chi, K.H.; De Voux, A.; Morris, M.; Katz, S.S.; Pillay, A.; Danavall, D.; Bowden, K.E.; Gaynor, A.M.; Kersh, E.N. Detection of Lymphogranuloma Venereum-Associated Chlamydia Trachomatis L2 Serovars in Remnant Rectal Specimens Collected from 7 US Public Health Laboratories. Sex. Transm. Dis. 2022, 49, E26–E28. [Google Scholar] [CrossRef]
- Cole, M.J.; Field, N.; Pitt, R.; Amato-Gauci, A.J.; Begovac, J.; French, P.D.; Keše, D.; Klavs, I.; Lepej, S.Z.; Pöcher, K.; et al. Substantial Underdiagnosis of Lymphogranuloma Venereum in Men Who Have Sex with Men in Europe: Preliminary Findings from a Multicentre Surveillance Pilot. Sex. Transm. Infect. 2020, 96, 137–142. [Google Scholar] [CrossRef]
- Peuchant, O.; Laurier-Nadalié, C.; Albucher, L.; Balcon, C.; Dolzy, A.; Hénin, N.; Touati, A.; Bébéar, C.; Agsous, M.; Alauzet, C.; et al. Anorectal Lymphogranuloma Venereum among Men Who Have Sex with Men: A 3-Year Nationwide Survey, France, 2020 to 2022. Eurosurveillance 2024, 29, 2300520. [Google Scholar] [CrossRef]
- Charles, H.; Prochazka, M.; Sinka, K.; contributors. Trends of Lymphogranuloma venereum (LGV) in England: 2019; Public Health England: London, UK, 2020. [Google Scholar]
- Ward, H.; Martin, I.; Macdonald, N.; Alexander, S.; Simms, I.; Fenton, K.; French, P.; Dean, G.; Ison, C. Lymphogranuloma Venereum in the United Kingdom. Clin. Infect. Dis. 2007, 44, 26–32. [Google Scholar] [CrossRef]
- van Aar, F.; Kroone, M.M.; de Vries, H.J.C.; Götz, H.M.; van Benthem, B.H.B. Increasing Trends of Lymphogranuloma Venereum among HIV-Negative and Asymptomatic Men Who Have Sex with Men, the Netherlands, 2011 to 2017. Eurosurveillance 2020, 25, 1900377. [Google Scholar] [CrossRef]
- Prochazka, M.; Charles, H.; Allen, H.; Cole, M.; Hughes, G.; Sinka, K. Rapid Increase in Lymphogranuloma Venereum among Hiv-Negative Men Who Have Sex with Men, England, 2019. Emerg. Infect. Dis. 2021, 27, 2695–2699. [Google Scholar] [CrossRef] [PubMed]
- De Baetselier, I.; Tsoumanis, A.; Florence, E.; Van Den Berghe, W.; Crucitti, T.; Van Den Bossche, D.; Kenyon, C. Did Pre-Exposure Prophylaxis Roll-Out Influence the Epidemic of Rectal Lymphogranuloma Venereum in Belgium? Results From the National Surveillance System. J. Acquir. Immune Defic. Syndr. (1988) 2021, 86, E1–E5. [Google Scholar] [CrossRef] [PubMed]
- White, J.; O’Farrell, N.; Daniels, D. 2013 UK National Guideline for the Management of Lymphogranuloma Venereum: Clinical Effectiveness Group of the British Association for Sexual Health and HIV (CEG/BASHH) Guideline Development Group. Int. J. STD AIDS 2013, 24, 593–601. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, N.H.N.; Versteeg, B.; Bruisten, S.M.; Van Rooijen, M.S.; Van Der Helm, J.J.; De Vries, H.J.C. Low Prevalence of Urethral Lymphogranuloma Venereum Infections Among Men Who Have Sex with Men: A Prospective Observational Study, Sexually Transmitted Infection Clinic in Amsterdam, the Netherlands. Sex. Transm. Dis. 2017, 44, 547–550. [Google Scholar] [CrossRef]
- Ward, H.; Alexander, S.; Carder, C.; Dean, G.; French, P.; Ivens, D.; Ling, C.; Paul, J.; Tong, W.; White, J.; et al. The Prevalence of Lymphogranuloma Venereum Infection in Men Who Have Sex with Men: Results of a Multicentre Case Finding Study. Sex. Transm. Infect. 2009, 85, 173–175. [Google Scholar] [CrossRef]
- Soni, S.; Srirajaskanthan, R.; Lucas, S.B.; Alexander, S.; Wong, T.; White, J.A. Lymphogranuloma Venereum Proctitis Masquerading as Inflammatory Bowel Disease in 12 Homosexual Men. Aliment. Pharmacol. Ther. 2010, 32, 59–65. [Google Scholar] [CrossRef]
- Pallawela, S.N.S.; Sullivan, A.K.; Macdonald, N.; French, P.; White, J.; Dean, G.; Smith, A.; Winter, A.J.; Mandalia, S.; Alexander, S.; et al. Clinical Predictors of Rectal Lymphogranuloma Venereum Infection: Results from a Multicentre Case-Control Study in the U.K. Sex. Transm. Infect. 2014, 90, 269–274. [Google Scholar] [CrossRef]
- Saxon, C.; Hughes, G.; Ison, C. Asymptomatic Lymphogranuloma Venereum in Men Who Have Sex with Men, United Kingdom. Emerg. Infect. Dis. 2016, 22, 112. [Google Scholar] [CrossRef]
- Chromy, D.; Sadoghi, B.; Gasslitter, I.; Skocic, M.; Okoro, A.; Grabmeier-Pfistershammer, K.; Willinger, B.; Weninger, W.; Öllinger, A.; Sarcletti, M.; et al. Asymptomatic Lymphogranuloma Venereum Is Commonly Found among Men Who Have Sex with Men in Austria. J. Dtsch. Dermatol. Ges. 2024, 22, 389–397. [Google Scholar] [CrossRef]
- Craxford, L.; Fox, A. Lymphogranuloma Venereum: A Rare and Forgotten Cause of Rectal Stricture Formation. Int. J. STD AIDS 2018, 29, 1133–1135. [Google Scholar] [CrossRef]
- Bancil, A.S.; Alexakis, C.; Pollok, R. Delayed Diagnosis of Lymphogranuloma Venereum-Associated Colitis in a Man First Suspected to Have Rectal Cancer. JRSM Open 2017, 8, 205427041666093. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, R.; Correia, C.; Estorninho, J.; Gravito-Soares, E.; Gravito-Soares, M.; Figueiredo, P. Lymphogranuloma Venereum-Associated Proctitis Mimicking a Malignant Rectal Neoplasia: Searching for Diagnosis. GE Port. J. Gastroenterol. 2022, 29, 267. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.J.C.; de Barbeyrac, B.; de Vrieze, N.H.N.; Viset, J.D.; White, J.A.; Vall-Mayans, M.; Unemo, M. 2019 European Guideline on the Management of Lymphogranuloma Venereum. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Hook, E.W. Syphilis. Lancet 2017, 389, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Janier, M.; Unemo, M.; Dupin, N.; Tiplica, G.S.; Potočnik, M.; Patel, R. 2020 European Guideline on the Management of Syphilis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 574–588. [Google Scholar] [CrossRef]
- Klausner, J.D.; Kohn, R.; Kent, C. Etiology of Clinical Proctitis among Men Who Have Sex with Men. Clin. Infect. Dis. 2004, 38, 300–302. [Google Scholar] [CrossRef]
- Bissessor, M.; Fairley, C.K.; Read, T.; Denham, I.; Bradshaw, C.; Chen, M. The Etiology of Infectious Proctitis in Men Who Have Sex with Men Differs According to HIV Status. Sex. Transm. Dis. 2013, 40, 768–770. [Google Scholar] [CrossRef]
- Arnold, C.A.; Limketkai, B.N.; Illei, P.B.; Montgomery, E.; Voltaggio, L. Syphilitic and Lymphogranuloma Venereum (LGV) Proctocolitis: Clues to a Frequently Missed Diagnosis. Am. J. Surg. Pathol. 2013, 37, 38–46. [Google Scholar] [CrossRef]
- Patil, R.V.; Stephenson, I.; Richards, C.J.; Griffin, Y. Rectal Cancer Mimic: A Rare Case of Syphilitic Proctitis. BMJ Case Rep. CP 2020, 13, e235522. [Google Scholar] [CrossRef]
- Lopez, C.C.; Herrera-Gonzalez, S.; Shamoon, D.; Dacosta, T.J.; Bains, Y. The Great Mimicker Gets Caught: A Rare Case of Syphilis in the Gastrointestinal Tract. Cureus 2024, 16, e59222. [Google Scholar] [CrossRef]
- Chiang, Y.; Hsieh, K.L.C.; Kung, C.H. Syphilitic Proctitis Imitating Rectal Malignancy. Eur. J. Gastroenterol. Hepatol. 2020, 32, 285–286. [Google Scholar] [CrossRef]
- Bronson, T.; Behdad, A.; Pezhouh, M.K. Syphilitic Proctitis with Clinical Features of Lymphoma. Clin. Gastroenterol. Hepatol. 2021, 19, A31. [Google Scholar] [CrossRef] [PubMed]
- Ferzacca, E.S.; Dunne, D.; Barbieri, A.; Aoun-Barakat, L.; Carvalhaes, C.G.; Sader, H.S.; Rhomberg, P.R.; Castanheira, M.; Mendes, R.E. 1546. Update in Syphilis Proctitis: Insights from a Case Series and Literature Review. Open Forum Infect. Dis. 2020, 7, S772–S773. [Google Scholar] [CrossRef]
- Struyve, M.; Meersseman, W.; Van Moerkercke, W. Primary Syphilitic Proctitis: Case Report and Literature Review. Acta Gastroenterol. Belg. 2018, 81, 430–432. [Google Scholar] [PubMed]
- Kingston, M.; Apea, V.; Evans, C.; Fifer, H.; Foster, K.; Patrick, P.; Grant, A.; Manns, V.; Ramsden, S.; Sinka, K.; et al. BASHH UK Guidelines for the Management of Syphilis 2024. Int. J. STD AIDS 2024, 35, 1142–1160. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.P.F.; Lee, D.; Bond, S.; Fairley, C.K.; Maddaford, K.; Wigan, R.; Fehler, G.; Lange, S.A.; De Petra, V.; Bissessor, M.; et al. Nonclassical Pathogens as Causative Agents of Proctitis in Men Who Have Sex With Men. Open Forum Infect. Dis. 2021, 8, ofab137. [Google Scholar] [CrossRef]
- Pinto-Sander, N.; Parkes, L.; Fitzpatrick, C.; Richardson, D. Symptomatic Sexually Transmitted Proctitis in Men Who Have Sex with Men. Sex. Transm. Infect. 2019, 95, 471. [Google Scholar] [CrossRef]
- Hoentjen, F.; Rubin, D.T. Infectious Proctitis: When to Suspect It Is Not Inflammatory Bowel Disease. Dig. Dis. Sci. 2012, 57, 269–273. [Google Scholar] [CrossRef]
- Goodell, S.E.; Quinn, T.C.; Mkrtichian, E.; Schuffler, M.D.; Holmes, K.K.; Corey, L. Herpes Simplex Virus Proctitis in Homosexual Men. N. Engl. J. Med. 1983, 308, 868–871. [Google Scholar] [CrossRef]
- Lavery, E.A.; Coyle, W.J. Herpes Simplex Virus and the Alimentary Tract. Curr. Gastroenterol. Rep. 2008, 10, 417–423. [Google Scholar] [CrossRef]
- Patel, R.; Green, J.; Moran, B.; Clarke, E.; Seneviratne, K.; Evans, C.; Young, F.; Nicholson, M.; Pelosi, E.; Kingston, M.; et al. British Association of Sexual Health and HIV UK National Guideline for the Management of Anogenital Herpes, 2024. Int. J. STD AIDS 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Alexander, S.; Verlander, N.; Saunders, P.; Richardson, D.; Fisher, M.; Ison, C. The Prevalence of Urethral and Rectal Mycoplasma Genitalium and Its Associations in Men Who Have Sex with Men Attending a Genitourinary Medicine Clinic. Sex. Transm. Infect. 2010, 86, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Read, T.R.H.; Murray, G.L.; Danielewski, J.A.; Fairley, C.K.; Doyle, M.; Worthington, K.; Su, J.; Mokany, E.; Tan, L.T.; Lee, D.; et al. Symptoms, Sites, and Significance of Mycoplasma Genitalium in Men Who Have Sex with Men. Emerg. Infect. Dis. 2019, 25, 719. [Google Scholar] [CrossRef] [PubMed]
- Lê, A.-S.; Labbé, A.-C.; Fourmigue, A.; Dvorakova, M.; Cox, J.; Fortin, C.; Martin, I.; Grace, D.; Hart, T.; Moore, D.; et al. Mycoplasma Genitalium Infection among Gay, Bisexual and Other Men Who Have Sex with Men in Montréal, Canada. Can. Commun. Dis. Rep. 2023, 49, 477–486. [Google Scholar] [CrossRef]
- Khosropour, C.M.; Jensen, J.S.; Soge, O.O.; Leipertz, G.; Unutzer, A.; Pascual, R.; Barbee, L.A.; Dombrowski, J.C.; Golden, M.R.; Manhart, L.E. High Prevalence of Vaginal and Rectal Mycoplasma Genitalium Macrolide Resistance Among Female Sexually Transmitted Disease Clinic Patients in Seattle, Washington. Sex. Transm. Dis. 2020, 47, 321–325. [Google Scholar] [CrossRef]
- Bissessor, M.; Tabrizi, S.N.; Bradshaw, C.S.; Fairley, C.K.; Hocking, J.S.; Garland, S.M.; Twin, J.; Poljak, M.; Peel, J.; Chen, M.Y. The Contribution of Mycoplasma Genitalium to the Aetiology of Sexually Acquired Infectious Proctitis in Men Who Have Sex with Men. Clin. Microbiol. Infect. 2016, 22, 260–265. [Google Scholar] [CrossRef]
- Ong, J.J.; Aung, E.; Read, T.R.H.; Fairley, C.K.; Garland, S.M.; Murray, G.; Chen, M.Y.; Chow, E.P.F.; Bradshaw, C.S. Clinical Characteristics of Anorectal Mycoplasma Genitalium Infection and Microbial Cure in Men Who Have Sex With Men. Sex. Transm. Dis. 2018, 45, 522–526. [Google Scholar] [CrossRef]
- Francis, S.C.; Kent, C.K.; Klausner, J.D.; Rauch, L.; Kohn, R.; Hardick, A.; Gaydos, C.A. Prevalence of Rectal Trichomonas Vaginalis and Mycoplasma Genitalium in Male Patients at the San Francisco STD Clinic, 2005–2006. Sex. Transm. Dis. 2008, 35, 797. [Google Scholar] [CrossRef]
- Latimer, R.L.; Vodstrcil, L.; De Petra, V.; Fairley, C.K.; Read, T.R.H.; Williamson, D.; Doyle, M.; Chow, E.P.F.; Bradshaw, C. Extragenital Mycoplasma Genitalium Infections among Men Who Have Sex with Men. Sex. Transm. Infect. 2020, 96, 10–18. [Google Scholar] [CrossRef]
- Jensen, J.S.; Cusini, M.; Gomberg, M.; Moi, H.; Wilson, J.; Unemo, M. 2021 European Guideline on the Management of Mycoplasma Genitalium Infections. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 641–650. [Google Scholar] [CrossRef]
- Soni, S.; Horner, P.; Rayment, M.; Pinto-Sander, N.; Naous, N.; Parkhouse, A.; Bancroft, D.; Patterson, C.; Fifer, H. British Association for Sexual Health and HIV National Guideline for the Management of Infection with Mycoplasma Genitalium (2018). Int. J. STD AIDS 2019, 30, 938–950. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. WHO Director-General Declares Mpox Outbreak a Public Health Emergency of International Concern. Available online: https://www.who.int/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-public-health-emergency-of-international-concern (accessed on 10 October 2024).
- Hoffmann, C.; Jessen, H.; Wyen, C.; Grunwald, S.; Noe, S.; Teichmann, J.; Krauss, A.S.; Kolarikal, H.; Scholten, S.; Schuler, C.; et al. Clinical Characteristics of Monkeypox Virus Infections among Men with and without HIV: A Large Outbreak Cohort in Germany. HIV Med. 2023, 24, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Fontoura, D.D.S.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical Features and Novel Presentations of Human Monkeypox in a Central London Centre during the 2022 Outbreak: Descriptive Case Series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- de Nicolas-Ruanes, B.; Vivancos, M.J.; Azcarraga-Llobet, C.; Moreno, A.M.; Rodriguez-Dominguez, M.; Berna-Rico, E.D.; Garcia-Mouronte, E.; Carron-Herrero, A.; McGee, A.; Galan, J.C.; et al. Monkeypox Virus Case with Maculopapular Exanthem and Proctitis during the Spanish Outbreak in 2022. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e658–e660. [Google Scholar] [CrossRef]
- Candela, C.; Raccagni, A.R.; Bruzzesi, E.; Bertoni, C.; Rizzo, A.; Gagliardi, G.; Canetti, D.; Gianotti, N.; Mileto, D.; Gismondo, M.R.; et al. Human Monkeypox Experience in a Tertiary Level Hospital in Milan, Italy, between May and October 2022: Epidemiological Features and Clinical Characteristics. Viruses 2023, 15, 667. [Google Scholar] [CrossRef]
- Yakubovsky, M.; Shasha, D.; Reich, S.; Tau, L.; Friedel, N.; Halutz, O.; Dekel, M.; Meijer, S.; Ben-Ami, R.; Paran, Y. Mpox Presenting as Proctitis in Men Who Have Sex With Men. Clin. Infect. Dis. 2023, 76, 528–530. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Palich, R.; Ghosn, J.; Walmsley, S.; Moschese, D.; Cortes, C.P.; Galliez, R.M.; Garlin, A.B.; Nozza, S.; Mitja, O.; et al. Human Monkeypox Virus Infection in Women and Non-Binary Individuals during the 2022 Outbreaks: A Global Case Series. Lancet 2022, 400, 1953–1965. [Google Scholar] [CrossRef]
- Mazzotta, V.; Scorzolini, L.; Falasca, L.; Lionetti, R.; Aguglia, C.; Kontogiannis, D.; Colombo, D.; Colavita, F.; De Palo, M.G.; Carletti, F.; et al. Lymphofollicular Lesions Associated with Monkeypox (Mpox) Virus Proctitis. Int. J. Infect. Dis. 2023, 130, 48–51. [Google Scholar] [CrossRef]
- NHS England. Tecovirimat as Treatment for Patients Hospitalised Due to Monkeypox Virus Infection. Available online: https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2022/09/B2008-Tecovirimat-treatment-for-patients-hospitalised-due-to-monkeypox.pdf (accessed on 8 October 2024).
- Arnold, C.A.; Roth, R.; Arsenescu, R.; Harzman, A.; Lam-Himlin, D.M.; Limketkai, B.N.; Montgomery, E.A.; Voltaggio, L. Sexually Transmitted Infectious Colitis vs Inflammatory Bowel Disease: Distinguishing Features from a Case-Controlled Study. Am. J. Clin. Pathol. 2015, 144, 771–781. [Google Scholar] [CrossRef]
- Siwak, E.; Suchacz, M.M.; Cielniak, I.; Kubicka, J.; Firląg-Burkacka, E.; Wiercińska-Drapało, A. Inflammatory Bowel Disease in Adult HIV-Infected Patients-Is Sexually Transmitted Infections Misdiagnosis Possible? J. Clin. Med. 2022, 11, 5324. [Google Scholar] [CrossRef] [PubMed]
- Høie, S.; Knudsen, L.S.; Gerstoft, J. Lymphogranuloma Venereum Proctitis: A Differential Diagnose to Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2011, 46, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, M.; Bradly, D.; Jakate, S.; Keshavarzian, A. Lymphogranuloma Venereum Proctosigmoiditis Is a Mimicker of Inflammatory Bowel Disease. World J. Gastroenterol. WJG 2012, 18, 3317. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Hay, P. Reminder of Important Clinical Lesson: Lymphogranuloma Venereum and HIV Infection: Misdiagnosed as Crohn’s Disease. BMJ Case Rep. 2010, 2010, bcr0220102771. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Churchill, D. Lesson of the Week: Ulcerative Proctitis in Men Who Have Sex with Men: An Emerging Outbreak. BMJ Br. Med. J. 2006, 332, 99. [Google Scholar] [CrossRef]
- Tinmouth, J.; Rachlis, A.; Wesson, T.; Hsieh, E. Lymphogranuloma Venereum in North America: Case Reports and an Update for Gastroenterologists. Clin. Gastroenterol. Hepatol. 2006, 4, 469–473. [Google Scholar] [CrossRef]
- Richardson, D.; Pakianathan, M.; Ewens, M.; Mitchell, H.; Mohammed, H.; Wiseman, E.; Tweed, M.; Nichols, K.; Rawdah, W.; Cooper, R.; et al. British Association of Sexual Health and HIV (BASHH) United Kingdom National Guideline for the Management of Sexually Transmitted Enteric Infections 2023. Int. J. STD AIDS 2023, 34, 588–602. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J. Crohn’s Colitis 2019, 13, 144–164K. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Romeo, M.; Palladino, G.; Cipullo, M.; Iadanza, G.; Olivieri, S.; Zagaria, G.; De Gennaro, N.; Santonastaso, A.; et al. Quality of Bowel Preparation in Patients with Inflammatory Bowel Disease Undergoing Colonoscopy: What Factors to Consider? World J. Gastrointest. Endosc. 2023, 15, 133–145. [Google Scholar] [CrossRef]
- Surawicz, C.M.; Haggitt, R.C.; Husseman, M.; McFarland, L. V Mucosal Biopsy Diagnosis of Colitis: Acute Self-Limited Colitis and Idiopathic Inflammatory Bowel Disease. Gastroenterology 1994, 107, 755–763. [Google Scholar] [CrossRef]
- Surawicz, C.M.; Belic, L. Rectal Biopsy Helps to Distinguish Acute Self-Limited Colitis from Idiopathic Inflammatory Bowel Disease. Gastroenterology 1984, 86, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, G.; Sandstedt, B.; Kollberg, B. A Prospective Study of First Attacks of Inflammatory Bowel Disease and Infectious Colitis. Clinical Findings and Early Diagnosis. Scand. J. Gastroenterol. 1994, 29, 265–274. [Google Scholar] [CrossRef] [PubMed]
| Risk Factors for Infective Proctitis |
|---|
| MSM or transgender women |
| HIV seropositive status |
| Unprotected receptive anal intercourse |
| Other sexually transmitted infection in previous six months |
| High-risk sexual behaviours |
| Traumatic sex |
| Multiple sexual partners |
| Group sex |
| Chemsex 1 |
| Common Clinical Features | Endoscopic Features | Diagnostic Test | First-Line Treatment | |
|---|---|---|---|---|
| Neisseria gonorrhoeae | Rectal pain, rectal bleeding, purulent discharge, tenesmus. | Purulent discharge, erythema, and loss of vascular pattern. Ulceration is not common. | NAAT via rectal swab or tissue sampling. Culture to assess antibiotic resistance. | Ceftriaxone, 1 g IM once if sensitivities are unknown. Ciprofloxacin if known to be sensitive [57]. |
| Chlamydia trachomatis serovars D-K | Usually asymptomatic. Rectal pain, tenesmus, mucopurulent or bloody discharge. | Mild inflammation with erythema, friability, and erosions. Ulceration is rare. | NAAT via rectal swab or tissue sampling. | Doxycycline, 100 mg PO BD for 7 days or azithromycin, 1 g PO as a single dose [72]. |
| Chlamydia trachomatis serovars L1-3 (LGV) | Rectal pain, mucopurulent discharge, anorectal bleeding, tenesmus, and constipation. | Mucopurulent exudate and ulceration. Erythematous and friable mucosa. Fistulas, strictures, abscesses, and masses can be seen. | NAAT for CT via rectal swab followed by LGV-specific NAAT. | Doxycycline, 100 mg BD for 21 days [84]. |
| Treponema pallidum | Rectal bleeding, rectal pain, abdominal pain, tenesmus, diarrhoea, mucous discharge. | Anorectal ulceration, rectal masses. Fissures, fistulas, and abscesses can be present. | Non-treponemal and treponemal serology. Tissue biopsy with staining. | Penicillin G benzathine, 2.4 million units IM, single dose [106]. |
| Herpes simplex virus | Severe rectal pain, tenesmus, constipation, rectal discharge, perianal ulceration, sacral paraesthesia. | Vesicular lesions, mucosal oedema, and ulceration. Confined to distal rectum. | NAAT via rectal swab or biopsy. | Acyclovir, 400 mg TDS PO for 5 days, or valaciclovir 500 mg BD for 5 days [112]. |
| Mycoplasma genitalium | Rectal pain and rectal discharge. | Non-specific erythema, erosions. | NAAT via rectal swab, only if NG and CT are excluded. | Doxycycline, 100 mg BD PO for 7 days followed by azithromycin, 1 g PO once, followed by 500 mg PO OD for 2 days. If known macrolide resistance, moxifloxacin, 400 mg OD PO for 7 days [113]. |
| Mpox | Prodromal fever and lymphadenopathy. Rash. Rectal pain, mucopurulent discharge, and painful defecation. | Oedematous, erythematous, and friable mucosa with ulceration. | NAAT via skin lesion or rectal swab. | Symptomatic management. In severe cases, tecovirimat, 600 mg BD for 14 days [132]. |
| Inflammatory Proctitis | Infective Proctitis | |
|---|---|---|
| Biological sex | Male = Female | Predominately male |
| Sexuality | Any | Predominately gay or bisexual men, or transgender women |
| HIV seropositive status | Rare | Common |
| Recent unprotected receptive anal sex | Unrelated | Very common |
| Time from symptom onset to presentation | Weeks to several months | Days to short weeks |
| Rectal pain | Uncommon (common in perianal Crohn’s disease) | Very common |
| Mucopurulent discharge | Uncommon | Common |
| Diagnostic test | No | Yes |
| Improves with antimicrobials | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, R.; Patel, K.; Poullis, A.; Pollok, R.; Honap, S. Separating Infectious Proctitis from Inflammatory Bowel Disease—A Common Clinical Conundrum. Microorganisms 2024, 12, 2395. https://doi.org/10.3390/microorganisms12122395
Hall R, Patel K, Poullis A, Pollok R, Honap S. Separating Infectious Proctitis from Inflammatory Bowel Disease—A Common Clinical Conundrum. Microorganisms. 2024; 12(12):2395. https://doi.org/10.3390/microorganisms12122395
Chicago/Turabian StyleHall, Richard, Kamal Patel, Andrew Poullis, Richard Pollok, and Sailish Honap. 2024. "Separating Infectious Proctitis from Inflammatory Bowel Disease—A Common Clinical Conundrum" Microorganisms 12, no. 12: 2395. https://doi.org/10.3390/microorganisms12122395
APA StyleHall, R., Patel, K., Poullis, A., Pollok, R., & Honap, S. (2024). Separating Infectious Proctitis from Inflammatory Bowel Disease—A Common Clinical Conundrum. Microorganisms, 12(12), 2395. https://doi.org/10.3390/microorganisms12122395






