Insights on the Pooled Prevalence and Global Distribution of Leptospirosis in Goats: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Design
2.2. Article Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Risk of Bias Assessment
2.5. Data Extraction
2.6. Data Analysis
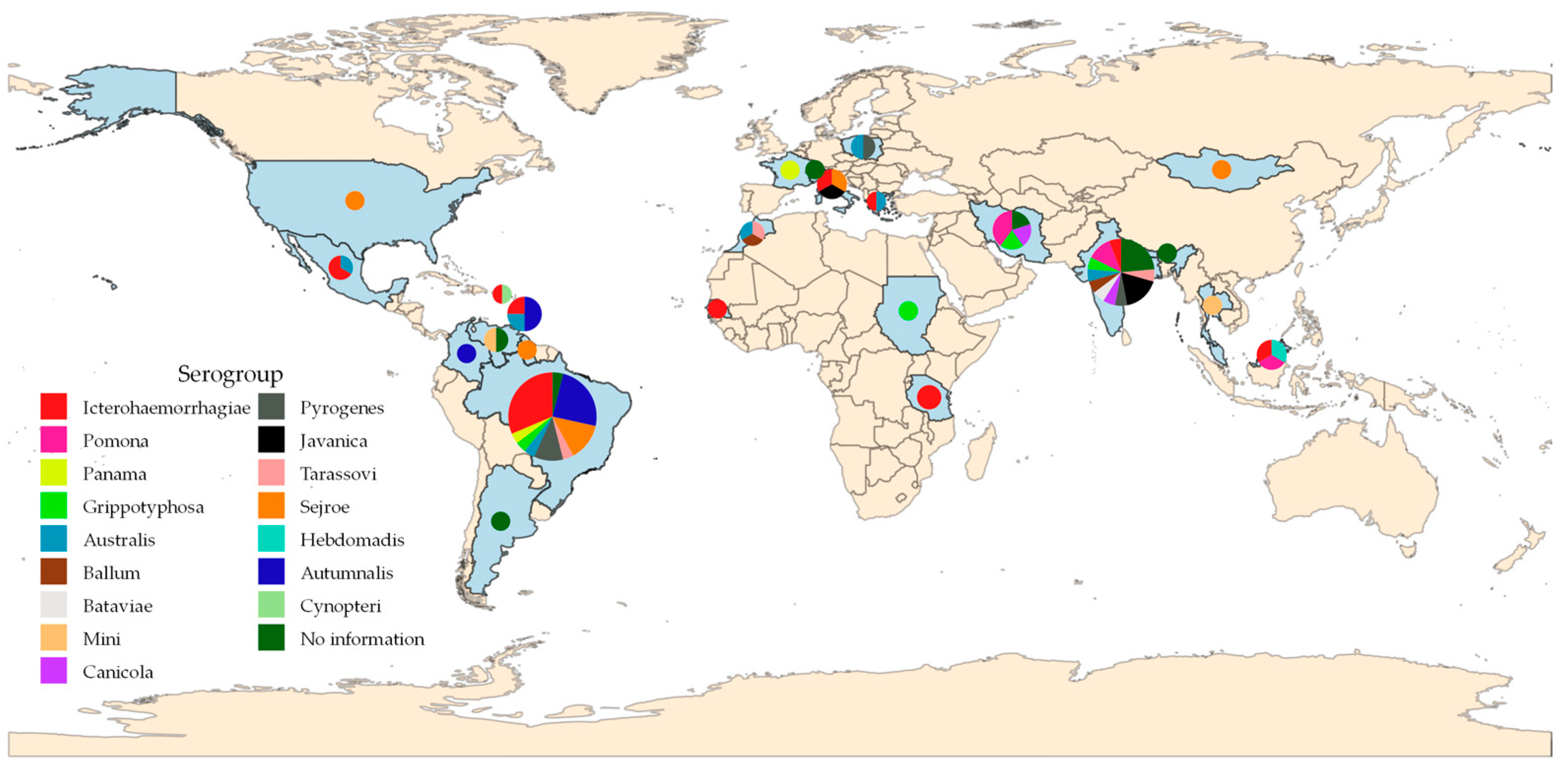
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karpagam, K.B.; Ganesh, B. Leptospirose: A Neglected Tropical Zoonotic Infection of Public Health Importance—An Updated Review. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Haji Hajikolaei, M.R.; Rezaei, S.; Ghadrdan Mashhadi, A.R.; Ghorbanpoor, M. The Role of Small Ruminants in the Epidemiology of Leptospirosis. Sci. Rep. 2022, 12, 2148. [Google Scholar] [CrossRef] [PubMed]
- Aymée, L.; Di Azevedo, M.I.N.; Melo, J.D.S.L.; Balaro, M.F.A.; Martins, G.M.S.; Consalter, A.; Leite, J.D.S.; Carvalho, C.F.A.; Lilenbaum, W. Leptospira noguchii associated to reproductive disease in ruminants. Transbound. Emerg. Dis. 2022, 69, 3103–3108. [Google Scholar] [CrossRef]
- Ali, S.; Zhao, Z.; Zhen, G.; Kang, J.Z.; Yi, P.Z. Reproductive Problems in Small Ruminants (Sheep and Goats): A Substantial Economic Loss in the World. Large Anim. Rev. 2019, 25, 215–223. Available online: https://www.largeanimalreview.com/index.php/lar/issue/view/6 (accessed on 20 September 2022).
- Martins, G.; Penna, B.; Hamond, C.; Leite, R.C.K.; Silva, A.; Ferreira, A.; Brandão, F.; Oliveira, F.; Lilenbaum, W. Leptospirosis as the Most Frequent Infectious Disease Impairing Productivity in Small Ruminants in Rio de Janeiro, Brazil. Trop. Anim. Health Prod. 2012, 44, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.R.; Barnabé, N.N.C.; Júnior, J.P.A.; Malossi, C.D.; Ullmann, L.S.; Costa, D.F.; Silva, M.L.C.R.; Higino, S.S.S.; Azevedo, S.S.; Alves, C.J. Investigation of the Presence of Leptospira Interrogans in Urinary and Genital Tracts of Male Goats Raised in the Semiarid Region of Brazil. Small Rumin. Res. 2023, 218, 106880. [Google Scholar] [CrossRef]
- Soares, R.R.; Barnabé, N.N.C.; Silva, M.L.C.R.; Costa, D.F.; Júnior, J.P.A.; Malossi, C.D.; Ullmann, L.S.; Higino, S.S.S.; Azevedo, S.S.; Alves, C.J. Detection of Leptospira spp. in Genitourinary Tract of Female Goats Managed in the Brazilian Semiarid. Microb. Pathogen. 2022, 172, 105763. [Google Scholar] [CrossRef]
- Balamurugan, V.; Thirumalesh, S.R.A.; Sridevi, R.; Govindaraj, G.; Nagalingam, M.; Hemadri, D.; Gajendragad, M.R.; Rahman, H. Microscopic Agglutination Test Analysis Identifies Prevalence of Intermediate Species Serovars in Ruminants in Endemic States of India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2016, 86, 469–475. [Google Scholar] [CrossRef]
- Viana, M.P.; Silva, J.D.; Lima, A.M.C.; Alves, F.S.F.; Pinheiro, R.R.; Costa, D.F.; Silva, G.C.P.; Calado, L.G.L.P.; Azevedo, S.S.; Alves, C.J.D. Epidemiological and Geospatial Characterization of Goat Leptospirosis in Northeast Region of Brazil. Small Rumin. Res. 2022, 206, 106589. [Google Scholar] [CrossRef]
- Ellis, W.A. Animal Leptospirosis. In Leptospira and Leptospirosis; Adler, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 387, pp. 99–137. [Google Scholar] [CrossRef]
- World Organization for Animal Health (WOAH, Founded as OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Leptospirosis. 2021. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/A_summry.htm (accessed on 10 June 2024).
- Goris, M.G.A.; Hartskeerl, R.A. Leptospirosis Serodiagnosis by the Microscopic Agglutination Test. Curr. Prot. Microbiol. 2014, 32, Unit.12E. [Google Scholar] [CrossRef]
- Di Azevedo, M.I.N.; Lilenbaum, W. An Overview on the Molecular Diagnosis of Animal Leptospirosis. Lett. Appl. Microbiol. 2021, 72, 496–508. [Google Scholar] [CrossRef]
- Mgode, G.F.; Mhamphi, G.G.; Massawe, A.W.; Machang’u, R.S. Leptospira Seropositivity in Humans, Livestock and Wild Animals in a Semi-Arid Area of Tanzania. Pathogens 2021, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Cranford, H.M.; Taylor, M.; Browne, A.S.; Alt, D.P.; Anderson, T.; Hamond, C.; Hornsby, R.L.; LeCount, K.; Schlater, L.; Stuber, T.; et al. Exposure and Carriage of Pathogenic Leptospira in Livestock in St. Croix, U.S. Virgin Islands. Trop. Med. Infect. Dis. 2021, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Roqueplo, C.; Kodjo, A.; Demoncheaux, J.-P.; Scandola, P.; Bassene, H.; Diatta, G.; Sokhna, C.; Raoult, D.; Davoust, B.; Mediannikov, O. Leptospirosis, One Neglected Disease in Rural Senegal. Vet. Med. Sci. 2019, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Benkirane, A.; Noury, S.; Hartskeerl, R.A.; Goris, M.G.A.; Ahmed, A.; Nally, J.E. Preliminary Investigations on the Distribution of Leptospira Serovars in Domestic Animals in North-West Morocco. Transbound. Emerg. Dis. 2016, 63, e178–e184. [Google Scholar] [CrossRef]
- Desvars, A.; Naze, F.; Benneveau, A.; Cardinale, E.; Michault, A. Endemicity of Leptospirosis in Domestic and Wild Animal Species from Reunion Island (Indian Ocean). Epidemiol. Infec. 2013, 141, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Anan’ina, I.V.; Korenberg, E.I.; Tserennorov, D.; Savel’eva, O.V.; Batjav, D.; Otgonbaatar, D.; Enkhbold, N.; Tsend, E.; Erdenechimeg, B. Detection of leptospirosis infection in certain wild and domestic animals in Mongolia. Zh. Mikrobiol. Epidemiol. Immunobiol. 2011, 36–39. Available online: https://pubmed.ncbi.nlm.nih.gov/22145347/ (accessed on 20 September 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological Guidance for Systematic Reviews of Observational Epidemiological Studies Reporting Prevalence and Cumulative Incidence Data. Int. J. Evid.-Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Arkew, M.; Asmerom, H.; Gemechu, K.; Tesfa, T. Global Prevalence of Anemia Among Type 2 Diabetic Adult Patients: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Obes. 2023, 16, 2243–2254. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- De Albuquerque, I.R.R.; Gois, G.C.; Campos, F.S. Profile Goat Herd Health in Senhor of Bonfim, Bahia. Acta Vet. Brasilica 2014, 8, 144–149. Available online: https://www.bvs-vet.org.br/vetindex/periodicos/acta-veterinaria-brasilica/8-(2014)-2/ (accessed on 20 September 2022).
- Crespo, E.; García, A.; Rivero, J.; Gómez, A. Seroprevalence of Leptospirosis in Goat of Faría Parish, Miranda Municipality, Zulia State-Venezuela. Rev. Cient. FCV-LUZ 2013, 23, 287–292. Available online: https://www.redalyc.org/articulo.oa?id=95926991003 (accessed on 20 September 2022).
- Schnydrig, P.; Vidal, S.; Brodard, I.; Frey, C.; Posthaus, H.; Perreten, V.; Rodriguez-Campos, S. Bacterial, Fungal, Parasitological and Pathological Analyses of Abortions in Small Ruminants from 2012–2016. Schweiz. Arch. Tierheilkd 2017, 159, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Turner, L.H.; Harrison, J.L.; Broom, J.C. Animal Leptospirosis in Malaya: 1. Methods, Zoogeographical Background, and Broad Analysis of Results. Bull World Health Organ. 1961, 24, 5–21. Available online: https://pubmed.ncbi.nlm.nih.gov/20604085/ (accessed on 20 September 2022). [PubMed]
- Maleki, S.; Zakian, A.; Abdollahpour, G. Seroepidemiology of Leptospira Interrogans Infection in Ruminants of Lorestan Province: A Cross-Sectional Study. J. Vet. Res. 2021, 75, 486–497. [Google Scholar] [CrossRef]
- Araújo Neto, J.O.; Alves, C.J.; Azevedo, S.S.; Silva, M.L.C.R.; Batista, C.S.A. Seroprevalence of leptospirosis in goats of the Seridó Oriental microregion, Rio Grande do Norte State, Northeastern Brazil, and risk factors research. Braz. J. Vet. Res. Anim. Sci. 2010, 47, 150–155. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/lil-559366 (accessed on 20 September 2022). [CrossRef]
- Ramin, A.; Abdollahpour, G.; Hosseinzadeh, A.; Azizzadeh, F.; Ramin, P.; Klalili, Y.; Sanajo, D.; Iran Nezhad, S. Comparison of Anti-Leptospira Antibodies by Microscopic Agglutination Test in Ruminants and Equines of Urmia, Iran. Vet. Res. Forum 2023, 14, 229–235. [Google Scholar] [CrossRef]
- Guzman-Barragan, B.L.; Martínez-Rodríguez, L.C.; Tobón-Torreglosa, J.C.; Tafur-Gómez, G.A. Seroprevalence and Risk Factors for Leptospira spp. in Small Ruminants of Semi-Arid Zone in Northeastern Colombia. Trop. Anim. Health Prod. 2021, 54, 10. [Google Scholar] [CrossRef]
- Saranya, P.; Goswami, C.; Sumathi, K.; Balasundareshwaran, A.H.; Bothammal, P.; Dutta, L.J.; Muralitharan, G.; Bora, D.P.; Natarajaseenivasan, K. Prevalence of Leptospirosis among Animal Herds of North Eastern Provinces of India. Comp. Immunol. Microbiol. Infect. Dis. 2021, 79, 101698. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.R.A.; de Souza Lima, G.M.; da Silva, J.D.; da Costa, D.F.; dos Santos, F.A.; dos Santos Higino, S.S.; de Azevedo, S.S.; Alves, C.J. Epidemiological Characterization and Risk Factors Associated with Leptospirosis and Brucellosis in Small Ruminants Sold at Animal Fair in the Sertão Region of Pernambuco State, a Semiarid Region of Northeastern Brazil. Semina Ciências Agrárias 2017, 38, 1933. [Google Scholar] [CrossRef]
- Campos, Â.P.; Miranda, D.F.H.; Rodrigues, H.W.S.; da Silva Carneiro Lustosa, M.; Martins, G.H.C.; Mineiro, A.L.B.B.; Castro, V.; Azevedo, S.S.; de Sousa Silva, S.M.M. Seroprevalence and Risk Factors for Leptospirosis in Cattle, Sheep, and Goats at Consorted Rearing from the State of Piauí, Northeastern Brazil. Trop. Anim. Health Prod. 2017, 49, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, A.K.S.; Chideroli, R.T.; Benitez, A.D.N.; Caldart, E.T.; Evers, F.; Fortes, M.S.; Ferreira, F.P.; Monteiro, K.C.; Giordano, L.G.P.; Freire, R.L.; et al. Cross-Sectional Study of Leptospira spp. and Brucela Abortus in Goat Herds from Paraná State, Brazil. Acta Sci. Vet. 2017, 45, 9. [Google Scholar] [CrossRef]
- Rizzo, H.; Silva, T.R.D.; Carvalho, J.S.; Marinho, F.A.; Santos, H.D.A.; Silva, W.S.; Alemán, M.A.R.; Pinheiro, J.W.; Castro, V. Frequency of and Risk Factors Associated to Leptospira spp. Seropositivity in Goats in the State of Sergipe, Northeastern Brazil. Ciênc. Rural 2017, 47, e20160845. [Google Scholar] [CrossRef]
- Santos, L.F.; Guimarães, M.F.; de Souza, G.O.; da Silva, I.W.G.; Santos, J.R.; Azevedo, S.S.; Labruna, M.B.; Heinemann, M.B.; Horta, M.C. Seroepidemiological Survey on Leptospira spp. Infection in Wild and Domestic Mammals in Two Distinct Areas of the Semi-Arid Region of Northeastern Brazil. Trop. Anim. Health Prod. 2017, 49, 1715–1722. [Google Scholar] [CrossRef]
- Cortizo, P.; Loureiro, A.P.; Martins, G.; do Rodrigues, P.R.; Faria, B.P.; Lilenbaum, W.; Deminicis, B.B. Risk Factors to Incidental Leptospirosis and Its Role on the Reproduction of Ewes and Goats of Espírito Santo State, Brazil. Trop. Anim. Health Prod. 2015, 47, 231–235. [Google Scholar] [CrossRef]
- Topazio, J.; Tonin, A.A.; Machado, G.; Noll, J.C.G.; Ribeiro, A.; Moura, A.B.; Carmo, G.M.; Grosskopf, H.M.; Martins, J.L.R.; Badke, M.R.T.; et al. Antibodies to Leptospira Interrogans in Goats and Risk Factors of the Disease in Santa Catarina (West Side), Brazil. Res. Vet. Sci. 2015, 99, 53–57. [Google Scholar] [CrossRef]
- Santos, J.P.; Lima-Ribeiro, A.M.C.; Oliveira, P.R.; dos Santos, M.P.; Ferreira, A.J.; Medeiros, A.A.; Tavares, T.C.F. Seroprevalence and Risk Factors for Leptospirosis in Goats in Uberlândia, Minas Gerais, Brazil. Trop. Anim. Health Prod. 2012, 44, 101–106. [Google Scholar] [CrossRef]
- Lilenbaum, W.; Varges, R.; Medeiros, L.; Cordeiro, A.G.; Cavalcanti, A.; Souza, G.N.; Richtzenhain, L.; Vasconcellos, S.A. Risk Factors Associated with Leptospirosis in Dairy Goats under Tropical Conditions in Brazil. Res. Vet. Sci. 2008, 84, 14–17. [Google Scholar] [CrossRef]
- Valeris-Chacín, R.; Boscán-Duque, L.; Urdaneta-Pacheco, R.; Chango-Villasmil, J.; Torres-Rodríguez, P.; Quintero-Moreno, A.; Arzalluz-Fischer, A.; Sánchez-Villalobos, A. Seroprevalence of Leptospirosis and Brucellosis in Goat Farms from Mauroa County, Falcon State, Venezuela. Rev. Cient. FCV-LUZ 2012, 22, 231–237. Available online: https://www.redalyc.org/pdf/959/95922219006.pdf (accessed on 20 September 2022).
- Suarez, V.H.; Martínez, G.M.; Olmos, L.H.; Arapa, C.; Cortez, H.S.; Rojas, M.C.; Brihuega, B.F.; Santillán, G.; Álvarez, I.; Goz, M.L. Problemas sanitarios de las majadas caprinas en los sistemas familiares de los valles calchaquies (Payogasta, Salta). Revista FAVE. Sección Cienc. Vet. 2020, 19, 40–49. [Google Scholar] [CrossRef]
- Suepaul, S.M.; Carrington, C.V.; Campbell, M.; Borde, G.; Adesiyun, A.A. Seroepidemiology of Leptospirosis in Livestock in Trinidad. Trop. Anim. Health Prod. 2011, 43, 367–375. [Google Scholar] [CrossRef]
- Motie, A.; Myers, D.M. Leptospirosis in Sheep and Goats in Guyana. Trop. Anim. Health Prod. 1986, 18, 113–114. [Google Scholar] [CrossRef]
- Carvalho, R.R.M.; Paz, L.N.; Dias, C.S.; Nocera, G.A.; de Mesquita, A.J.P.; Pinna, M.H. Serological Survey of Leptospirosis, Brucellosis, and Lentivirus in Herds of Small Ruminants in Recôncavo Baiano, Bahia, Brazil. Braz. J. Vet. Res. Anim. Sci. 2021, 58, e180290. [Google Scholar] [CrossRef]
- Miller, B.A.; Lu, C.D. Current status of global dairy goat production: An overview. Asian-Australas. J. Anim. Sci. 2019, 32, 1219–1232. [Google Scholar] [CrossRef]
- Bahaman, A.R.; Ibrahim, A.L.; Adam, H. Serological Prevalence of Leptospiral Infection in Domestic Animals in West Malaysia. Epidemiol. Infect. 1987, 99, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, V.; Alamuri, A.; Kumar, K.V.; Varghese, B.; Govindaraj, G.; Hemadri, D.; Roy, P. Prevalence of Anti-Leptospiral Antibodies and Frequency Distribution of Leptospira Serovars in Small Ruminants in Enzootic South Peninsular India. Vet. World 2021, 14, 2023–2030. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations Statistical Databases and Agriculture (FAO). 2019. Available online: http://faostat.fao.org/ (accessed on 20 November 2023).
- Álvarez, M.A.L.; Escatell, G.S.; Arzate, J.J.M.; Ibarra, J.M.O.; Rivera, E.M.L. Anticuerpos contra Leptospira spp. en caprinos lecheros en Guanajuato, México. Rev. Investig. Vet. Perú 2018, 29, 611–618. [Google Scholar] [CrossRef]
- Anderson, T.; Hamond, C.; Haluch, A.; Toot, K.; Nally, J.E.; LeCount, K.; Schlater, L.K. Animals Exposed to Leptospira Serogroups Not Included in Bacterins in the United States and Puerto Rico. Trop. Med. Infect. Dis. 2023, 8, 183. [Google Scholar] [CrossRef]
- Shiokawa, K.; Welcome, S.; Kenig, M.; Lim, B.; Rajeev, S. Epidemiology of Leptospira Infection in Livestock Species in Saint Kitts. Trop. Anim. Health Prod. 2019, 51, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Czopowicz, M.; Kaba, J.; Smith, L.; Szalus-Jordanow, O.; Nowicki, M.; Witkowski, L.; Frymus, T. Leptospiral Antibodies in the Breeding Goat Population of Poland. Vet. Rec. 2011, 169, 230. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.D.; da Costa Barnabé, N.N.; Soares, R.R.; de Azevedo, S.S.; Limeira, C.H.; Alves, C.J. Efficacy of Leptospirosis Vaccination in Small Ruminants: Systematic Review and Meta-Analysis. Small Rumin. Res. 2023, 220, 106931. [Google Scholar] [CrossRef]
- Ciceroni, L.; Lombardo, D.; Pinto, A.; Ciarrocchi, S.; Simeoni, J. Prevalence of Antibodies to Leptospira Serovars in Sheep and Goats in Alto Adige-South Tyrol. J. Vet. Med. B. Infect. Dis. Vet. Public Health 2000, 47, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, S.; Figarolli, B.M.; D’Incau, M. Serological Surveillance of Leptospirosis in Italy: Two year National Data (2010–2011). Vet. Italiana 2016, 52, 129–139. [Google Scholar] [CrossRef]
- Vihol, P.D.; Patel, J.M.; Patel, J.H.; Prasad, M.C.; Kalyani, I.H.; Brahmkshtri, B.P. Caprine Leptospirosis: Hematobiochemical and Urinalyses Studies. Vet. World 2016, 9, 337–341. [Google Scholar] [CrossRef]
- Shigidi, M.T.A. Animal Leptospirosis in the Sudan. Br. Vet. J. 1974, 130, 528–531. [Google Scholar] [CrossRef]
- Silva, F.J.; Santos, C.E.P.; Silva, T.R.; Silva, G.C.P.; Loffler, S.G.; Brihuega, B.; Alarcon, M.F.F.; Curci, V.C.M.; Mathias, L.A. Pesquisa de leptospiras e de anticorpos contra leptospiras em animais e humanos de propriedades rurais nos biomas brasileiros Pantanal e Caatinga. Braz. J. Vet. Res. Anim. Sci. 2015, 52, 234. [Google Scholar] [CrossRef]
- Bertelloni, F.; Cilia, G.; Turchi, B.; Pinzauti, P.; Cerri, D.; Fratini, F. Epidemiology of Leptospirosis in North-Central Italy: Fifteen Years of Serological Data (2002–2016). Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 14–22. [Google Scholar] [CrossRef]
- Picardeau, M. Diagnosis and Epidemiology of Leptospirosis. Méd. Mal. Infect. 2013, 43, 1–9. [Google Scholar] [CrossRef]
- Damude, D.F.; Jones, C.J.; Myers, D.M. A Study of Leptospirosis among Animals in Barbados W.I. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, C.L.R.M.; Bezerra, C.S.; Morais, D.A.; Silva, M.L.C.R.; Nogueira, D.B.; Costa, D.F.; Santos, C.S.A.B.; Higino, S.S.S.; Alves, C.J.; Azevedo, S.S. Seroprevalence and Predominant Serogroups of Leptospira sp. In Serological Tests of Ruminants in Northeastern Brazil. Semina Cienc. Agrar. 2019, 40, 1513–1522. [Google Scholar] [CrossRef]
- Higino, S.S.S.; Santos, F.A.; Costa, D.F.; Santos, C.S.A.B.; Silva, M.L.C.R.; Alves, C.J.; Azevedo, S.S. Flock-Level Risk Factors Associated with Leptospirosis in Dairy Goats in a Semiarid Region of Northeastern Brazil. Prev. Vet. Med. 2013, 109, 158–161. [Google Scholar] [CrossRef]
- Higino, S.S.S.; Alves, C.J.; Santos, C.S.A.B.; Vasconcellos, S.A.; Silva, M.L.C.R.; Brasil, A.W.L.; Pimenta, C.L.R.M.; Azevedo, S.S. Prevalence of Leptospirosis in Dairy Goats in the Semiarid Region of Paraíba State. Pesqui. Veterinária Bras. 2012, 32, 199–203. [Google Scholar] [CrossRef]
- Lilenbaum, W.; de Souza, G.N.; Ristow, P.; Moreira, M.C.; Fráguas, S.; Cardoso, V.D.S.; Oelemann, W.M.R.A. Serological Study on Brucella Abortus, Caprine Arthritis-Encephalitis Virus and Leptospira in Dairy Goats in Rio de Janeiro, Brazil. Vet. J. 2007, 173, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Arent, Z.; Gilmore, C.; Brem, S.; Ellis, W.A. Molecular Studies on European Equine Isolates of Leptospira Interrogans Serovars Bratislava and Muenchen. Infect. Genet. Evol. 2015, 34, 26–31. Available online: https://pubmed.ncbi.nlm.nih.gov/26165505/ (accessed on 20 September 2023). [CrossRef] [PubMed]
- Abdollahpour, G.; Ramin, A.; Khalili, Y. Serological evaluation of leptospira serotypes using microscopic agglutination test in Urmia goats. J. Anim. Sci. Res. 2014, 24, 71–81. [Google Scholar]
- Dhivahar, M.; Ambily, R.; Joseph, S.; Shyma, V.H.; Reshma, P.S.; Mini, M. Seroprevalence of Leptospirosis among Aborted Goats in Kerala. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1403–1407. [Google Scholar] [CrossRef]
- Vallée, E.; Ridler, A.L.; Heuer, C.; Collins-Emerson, J.M.; Benschop, J.; Wilson, P.R. Effectiveness of a Commercial Leptospiral Vaccine on Urinary Shedding in Naturally Exposed Sheep in New Zealand. N. Z. Vet. J. 2017, 35, 1362–1368. [Google Scholar] [CrossRef]
- Costa, D.F.; Silva, M.L.C.R.; Martins, G.; Dantas, A.F.M.; Melo, M.A.; Azevedo, S.S.; Lilenbaum, W.; Alves, C.J. Susceptibility among Breeds of Sheep Experimentally Infected with Leptospirainterrogans Pomona Serogroup. Microb. Pathogen. 2018, 122, 79–83. [Google Scholar] [CrossRef]
- Vihol, P.; Patel, J.M.; Patel, J.H.; Raval, J.K.; Varia, R.D.; Makwana, P.M. Pathomorphological study on leptospirosis in slaughtered goats. Pharma Innov. J. 2020, 9, 84–87. Available online: https://www.thepharmajournal.com/special-issue?year=2020&vol=9&issue=9S&ArticleId=5184 (accessed on 20 September 2023).
- Alamuri, A.; Veena, S.; Vinod Kumar, K.; Kalyani, I.H.; Rahman, H.; Shome, B.R.; Balamurugan, V. Changing Trend in the Prevalence and Emergence of Leptospira Serogroup-Specific Antibodies in Livestock in Gujarat, India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 1145–1151. [Google Scholar] [CrossRef]
- Parveen, S.M.A.; Suganyaa, B.; Sathya, M.S.; Margreat, A.A.P.; Sivasankari, K.; Shanmughapriya, S.; Hoffman, N.E.; Natarajaseenivasan, K. Leptospirosis Seroprevalence Among Blue Metal Mine Workers of Tamil Nadu, India. Am. J. Trop. Med. Hyg. 2016, 95, 38–42. [Google Scholar] [CrossRef]
- Natarajaseenivasan, K.; Ratnam, S. Isolation of Leptospira Javanica from Sheep. Indian J. Anim. Sci. 1999, 69, 759–761. [Google Scholar]
- Adler, B.; De La Peña-Moctezuma, A. Leptospira and Leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.N.; Shah, S.; Ahmad, F.M. Laboratory diagnosis of leptospirosis. J. Postgrad. Med. 2005, 51, 195–200. Available online: https://pubmed.ncbi.nlm.nih.gov/16333192/ (accessed on 10 January 2024).
- Sharma, P.K.; Yadav, M. Confidence Interval: Advantages, Disadvantages and the Dilemma of Interpretation. Rev. Recent Clin. Trials 2023, 19, 76–80. Available online: https://pubmed.ncbi.nlm.nih.gov/38099533/ (accessed on 10 January 2024). [CrossRef]
- Migliavaca, C.B.; Stein, C.; Colpani, V.; Barker, T.H.; Ziegelmann, P.K.; Munn, Z.; Falavigna, M. Meta-Analysis of Prevalence: I2 Statistic and How to Deal with Heterogeneity. Res. Synth. Methods 2022, 13, 363–367. [Google Scholar] [CrossRef]
- Araújo, H.G.; Limeira, C.H.; Aquino, V.V.F.; Vilela, V.L.R.; Alves, C.J.; Higino, S.S.S.; Santos, C.S.A.B.; Azevedo, S.S. Global Seropositivity of Swine Leptospirosis: Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2023, 5, 158. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Loannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rucker, G.; Harbord, R.M.; Schimid, C.H.; et al. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Lean, I.J.; Rabiee, A.R.; Duffield, T.F.; Dohoo, I.R. Invited Review: Use of Meta-Analysis in Animal Health and Reproduction: Methods and Applications. J. Dairy Sci. 2009, 92, 3545–3565. [Google Scholar] [CrossRef] [PubMed]
- Christley, R.; French, N. Validity in epidemiological studies. In Veterinary Epidemiology, 4th ed.; Thrusfield, M., Christley, R., Eds.; John Wiley and Sons Ltd.: Oxford, UK, 2018; pp. 383–400. [Google Scholar]
- Galvão, C.M.M.D.Q.; Oliveira, P.R.F.D.; Cavalcanti, A.L.D.A.; Nogueira, D.B.; Azevedo, S.S.D.; Ramos, R.A.D.N.; Mota, R.A. Occurrence of IgG Antibodies against Toxoplasma Gondii, Neospora Caninum, and Leptospira spp. in Goats and Sheep from an Indigenous Village in Pernambuco, Brazil. Rev. Bras. De Parasitol. Veterinária 2023, 32, e000423. [Google Scholar] [CrossRef]
- Hamond, C.; Martins, G.; Loureiro, A.P.; Pestana, C.; Lawson-Ferreira, R.; Medeiros, M.A.; Lilenbaum, W. Urinary PCR as an Increasingly Useful Tool for an Accurate Diagnosis of Leptospirosis in Livestock. Vet. Res. Commun. 2014, 38, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Gaytán-Camarillo, F.; Rico-Chávez, O.; Palomares-Resendiz, E.G.; Gutiérrez-Hernández, J.L.; Díaz-Aparicio, E.; Herrera-López, E. Spatial Autocorrelation and Co-Occurrence of Six Serovarieties of Leptospira in Goat Herds of the State of Guanajuato, Mexico. Braz. J. Microbiol. 2021, 52, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.A.; Castro, J.R.; Moreira, R.Q.; Bombonato, N.G.; Soares, P.M.; Lima, A.M.C. Anti- Leptospira spp. Antibodies in Several Animal Species on the Same Farm. Biosci. J. 2016, 32, 202–207. [Google Scholar] [CrossRef]
- Benkirane, A.; Essamkaoui, S.; El Idrissi, A.; Lucchese, L.; Natale, A. A Sero-Survey of Major Infectious Causes of Abortion in Small Ruminants in Morocco. Vet. Ital. 2015, 51, 25–30. [Google Scholar] [CrossRef]
- Soares, R.R.; Barnabé, N.N.C.; Nogueira, D.B.; Silva, L.S.C.; Araújo Júnior, J.P.; Malossi, C.D.; Ullmann, L.S.; Costa, D.F.; Silva, M.L.C.R.; Higino, S.S.S.; et al. Serological, molecular and bacteriological approaches for detecting Leptospira sp. carrier rams maintained in semiarid conditions. Acta Trop. 2021, 213, 105759. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions [Online], Version 6.5, Cochrane. 2024. Available online: http://Handbook.Cochrane.Org/ (accessed on 15 November 2024).
- Sabarinath, T.; Behera, S.K.; Deneke, Y.; Atif Ali, S.; Kaur, G.; Kumar, A.; Kumar, G.R.; Kumar, K.S.; Sinha, D.K.; Verma, M.R.; et al. Serological evidence of anti-Leptospira antibodies in goats in various agro climatic zones of India. Small Rumin. Res. 2018, 169, 74–80. [Google Scholar] [CrossRef]
- Rocha, L.M.S.R.; Faria, P.J.A.; Soares, R.R.; Araújo Júnior, J.P.; Malossi, C.D.; Ullmann, L.S.; Silva, M.L.C.R.; Higino, S.S.S.; Azevedo, S.S.; Alves, C.J. Leptospira spp. of the Urinary Tract of Female Carrier Goats in Semi-Arid Conditions. Act. Sci. Vet. 2022, 50, 1872. [Google Scholar]
- Rahman, M.S.A.; Bejo, S.K.; Zakaria, Z.; Hassan, L.; Roslan, M.A. Seroprevalence and distribution of Leptospiral Serovars in livestock (cattle, goats, and sheep) in Flood-Prone Kelantan, Malaysia. J. Vet. Res. 2021, 65, 53–58. [Google Scholar] [CrossRef]
- Shrestha, R.; McKenzie, J.S.; Gautam, M.; Adhikary, R.; Pandey, K.; Koirala, P.; Bahadur, B.C.G.; Miller, L.C.; Collins-Emerson, J.; Craig, S.B.; et al. Determinants of clinical leptospirosis in Nepal. Zoonoses Public Health 2018, 65, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Vihol, P.D.; Patel, J.M.; Patel, J.H.; Dabas, V.S.; Kalyani, I.H.; Chaudhari, C.F.; Patel, A.C. Identification of Pathogenic Leptospira spp. Carrier Goats Using Polymerase Chain Reaction (PCR). Int. J. Curr. Microbiol. App. Sci. 2017, 6, 2174–2183. [Google Scholar] [CrossRef]
- Rezaei, S.; Haji, H.M.; Ghadrdan, M.A.; Ghorbanpour, M.; Abdollahpour, G. Comparison of Leptospira interrogans infection in the goats and sheep. Iranian J. Vet. Med. 2017, 10, 113–119. Available online: https://www.cabdirect.org/cabdirect/abstract/20163241128 (accessed on 10 January 2024).
- Balamurugan, V.; Veena, S.; Thirumalesh, S.R.A.; Alamuri, A.; Sridevi, R.; Sengupta, P.P.; Govindaraj, G.; Nagalingam, M.; Hemadri, D.; Gajendragad, M.R.; et al. Distribution of Serogroup Specific Antibodies against Leptospirosis in Livestock in Odisha. Indian J. Anim. Sci. 2017, 87, 546–551. [Google Scholar] [CrossRef]
- Assenga, J.A.; Matemba, L.E.; Muller, S.K.; Mhamphi, G.G.; Kazwala, R.R. Predominant leptospiral serogroups circulating among humans, livestock and wildlife in Katavi-Rukwa ecosystem, Tanzania. PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, A.; Jagatheesan, P.R.; Reetha, T.L.; Henry, A.C.E. Sero-prevalence of leptospirosis in small ruminants in Pudukkottai District of Tamil Nadu. Indian J. Vet. Sci. Biotechnol 2014, 10, 23–24. Available online: https://acspublisher.com/journals/index.php/ijvsbt/article/view/2972 (accessed on 10 January 2024).
- Suwancharoen, D.; Chaisakdanugull, Y.; Thanapongtharm, W.; Yoshida, S. Serological survey of leptospirosis in livestock in Thailand. Epidemiol. Infect. 2013, 14, 2269–2277. [Google Scholar] [CrossRef]
- Hassanpour, A.; Asgarloo, S.; Imandar, M.; Mashayekhi, M.; Abdollahpour, G.R.; Safarmashaei, S. Seroepidemiologic study of goats leptospirosis in Khoy-Iran. J. Anim. Vet. Adv. 2012, 11, 229–233. [Google Scholar] [CrossRef]
- Krupakaran, R.P.; Porcheziyan, T.; Sivseelan, S. Seroprevalence of Leptospirosis in Domestic Animals of Karur District of Tamil Nadu. Vet. Pract. 2009, 10, 84–85. [Google Scholar]
- Krawczyk, M. Serological Evidence of Leptospirosis in Animals in Northern Poland. Vet. Rec. 2005, 156, 88–89. [Google Scholar] [CrossRef]
- Sunder, J.; Rai, R.B.; Kundu, A.; Chatterjee, R.N.; Senani, S.; Jeyakumar, S. Incidence and prevalence of livestock diseases of Andaman and Nicobar Islands. Indian J. Anim. Sci. 2005, 75, 1041–1043. [Google Scholar]
- Burriel, A.R.; Dalley, C.; Woodward, M.J. Prevalence of Leptospira Species among Farmed and Domestic Animals in Greece. Vet. Rec. 2003, 153, 146–148. [Google Scholar] [CrossRef]
- Schmidt, V.; Arosi, A.; Santos, A.R. Levantamento sorológico da leptospirose em caprinos leiteiros no Rio Grande do Sul, Brasil. Ciência Rural 2002, 32, 609–612. [Google Scholar] [CrossRef]
- Everard, C.O.; Fraser-Chanpong, G.M.; James, A.C.; Butcher, L.V. Serological studies on leptospirosis in livestock and chickens from Grenada and Trinidad. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 859–864. [Google Scholar] [CrossRef]
- Costa, D.F.; Silva, A.F.; Farias, A.E.M.; Brasil, A.W.L.; Santos, F.A.; Guilherme, R.F.; Azevedo, S.S.; Alves, C.J. Serological study of the Leptospira spp. infection in sheep and goats slaughtered in the State of Paraíba, semiarid of Northeastern Brazil. Semin. Ciências Agrárias 2016, 37, 819–828. [Google Scholar] [CrossRef]





| Author/Country | Statistically Significant Risk Factors |
|---|---|
| [31] Iran | Female gender |
| 1.5 to 4 years old | |
| [32] Colombia | Lack of quarantine measures of acquired animals |
| Housing deficiency for animal management | |
| [33] India | Use of tap water source in goat farms |
| Presence of pigs in goat farms | |
| Contact with other animals | |
| Other animals on the goat farm | |
| Deficiency of the vaccination program | |
| [34] Brazil | Intensive management systems |
| Practice of consort rearing with horses | |
| [35] Brazil | No veterinary services |
| Sheep herd over 52 animals | |
| [36] Brazil | Presence of wild animals such as deer and capybaras on the property |
| Presence of pigs on the property | |
| Production-type meat | |
| Sewer destination in a dry sump | |
| Shared use of pastures | |
| Slaughter of pigs, sheep, cattle, and goats | |
| Frequent occurrence of abortion | |
| [37] Brazil | Ingestion of still water, including supplies from buckets, wells, dams, and ponds |
| [38] Brazil | Age 1 to 3 years and older than 3 years |
| Preserved area | |
| [39] Brazil | Presence of waterholes |
| Semi-intensive breeding | |
| Reproductive failure with abortion | |
| [40] Brazil | Farms that provided concentrate as a supplement |
| Presence of contact between sheep and goats | |
| [41] Brazil | Adult goats |
| Defined races | |
| Intensive systems of goat production | |
| Properties using hired labor | |
| Contact among species | |
| [42] Brazil | Tropical climate |
| Frequency of professional veterinary supervision |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo Portela, R.; Limeira, C.H.; Bezerra, J.A.B.; Martins, C.M.; da Costa, D.F.; Santos, C.d.S.A.B.; Alves, C.J.; Azevedo, S.S.d. Insights on the Pooled Prevalence and Global Distribution of Leptospirosis in Goats: Systematic Review and Meta-Analysis. Microorganisms 2024, 12, 2391. https://doi.org/10.3390/microorganisms12122391
de Araújo Portela R, Limeira CH, Bezerra JAB, Martins CM, da Costa DF, Santos CdSAB, Alves CJ, Azevedo SSd. Insights on the Pooled Prevalence and Global Distribution of Leptospirosis in Goats: Systematic Review and Meta-Analysis. Microorganisms. 2024; 12(12):2391. https://doi.org/10.3390/microorganisms12122391
Chicago/Turabian Stylede Araújo Portela, Roseane, Clécio Henrique Limeira, José Artur Brilhante Bezerra, Camila Marinelli Martins, Diego Figueiredo da Costa, Carolina de Sousa Américo Batista Santos, Clebert José Alves, and Sérgio Santos de Azevedo. 2024. "Insights on the Pooled Prevalence and Global Distribution of Leptospirosis in Goats: Systematic Review and Meta-Analysis" Microorganisms 12, no. 12: 2391. https://doi.org/10.3390/microorganisms12122391
APA Stylede Araújo Portela, R., Limeira, C. H., Bezerra, J. A. B., Martins, C. M., da Costa, D. F., Santos, C. d. S. A. B., Alves, C. J., & Azevedo, S. S. d. (2024). Insights on the Pooled Prevalence and Global Distribution of Leptospirosis in Goats: Systematic Review and Meta-Analysis. Microorganisms, 12(12), 2391. https://doi.org/10.3390/microorganisms12122391








