A Case Series Report on the Effect of Tofacitinib on Joint Inflammation and Gut Microbiota Composition in Psoriatic Arthritis Patients Naive to Biologic Agents
Abstract
:1. Introduction
2. Methods
2.1. Patients and Study Design
- Health and clinical history;
- Clinical examination and assessment of disease activity [DAPSA, ASDAS, BASDAI, LEI (Leeds Enthesitis Index), and PASI (Psoriasis Area Severity Index)];
- Ultrasonography (US) of target joints and enthesis, as reported in the Section 2.4.
2.2. Cytokines Analysis
2.3. Next-Generation Sequencing of Bacterial 16S rRNA Gene
2.4. Ultrasonography
3. Statistical Analysis
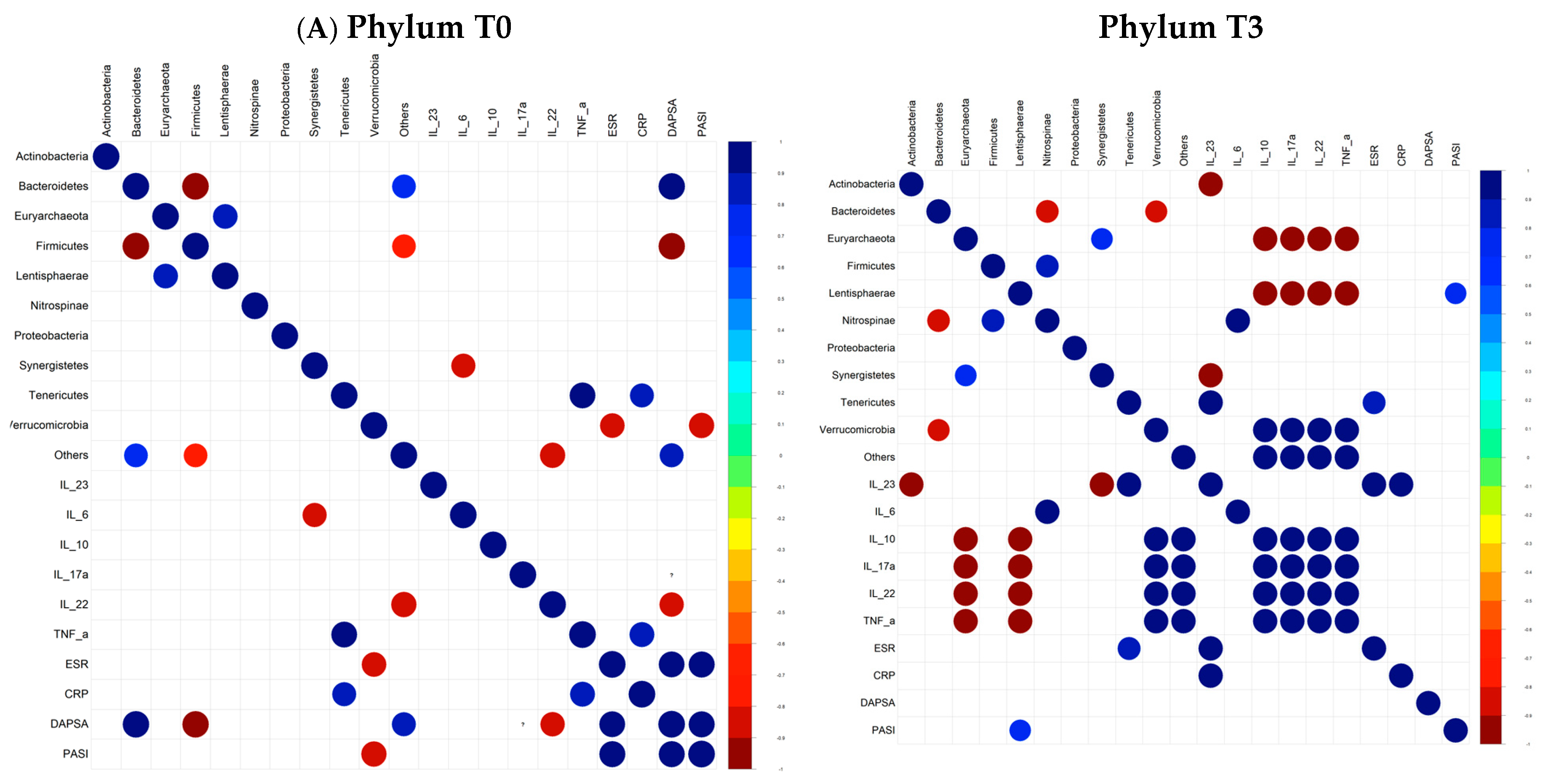
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Rodríguez, J.C.; Infante-Cano, M.; García-Muñoz, C.; Matias-Soto, J.; Martinez-Calderon, J. Psoriatic arthritis with psychological comorbidities: An overview of systematic reviews on incidence, prevalence, and geographic disparities. Rheumatol. Int. 2024, 44, 2337–2355. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Barrett, J.; Schentag, C.T.; Farewell, V.T.; Gladman, D.D. Axial psoriatic arthritis: Update on a longterm prospective study. J. Rheumatol. 2009, 36, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Furtunescu, A.R.; Georgescu, S.R.; Tampa, M.; Matei, C. Inhibition of the JAK-STAT Pathway in the Treatment of Psoriasis: A Review of the Literature. Int. J. Mol. Sci. 2024, 25, 4681. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, W.A.; Quraishi, M.N.; Iqbal, T.H. Gut microbiome and autoimmune disorders. Clin. Exp. Immunol. 2022, 209, 161–174. [Google Scholar] [CrossRef]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022, 13, 1007165. [Google Scholar] [CrossRef]
- Thye, A.Y.; Bah, Y.R.; Law, J.W.; Tan, L.T.; He, Y.W.; Wong, S.H.; Thurairajasingam, S.; Chan, K.G.; Lee, L.H.; Letchumanan, V. Gut-Skin Axis: Unravelling the Connection between the Gut Microbiome and Psoriasis. Biomedicines 2022, 10, 1037. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, J.; Zhang, W.; Doherty, M.; Zhang, Y.; Xie, H.; Li, W.; Wang, N.; Lei, G.; Zeng, C. Gut dysbiosis in rheumatic diseases: A systematic review and meta-analysis of 92 observational studies. EBioMedicine 2022, 80, 104055. [Google Scholar] [CrossRef]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef]
- Costello, M.E.; Ciccia, F.; Willner, D.; Warrington, N.; Robinson, P.C.; Gardiner, B.; Marshall, M.; Kenna, T.J.; Triolo, G.; Brown, M.A. Brief Report: Intestinal Dysbiosis in Ankylosing Spondylitis. Arthritis Rheumatol. 2015, 67, 686–691. [Google Scholar] [CrossRef]
- Stebbings, S.; Munro, K.; Simon, M.A.; Tannock, G.; Highton, J.; Harmsen, H.; Welling, G.; Seksik, P.; Dore, J.; Grame, G.; et al. Comparison of the faecal microflora of patients with ankylosing spondylitis and controls using molecular methods of analysis. Rheumatology 2002, 41, 1395–1401. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G. Update on the reciprocal interference between immunosuppressive therapy and gut microbiota after kidney transplantation. World J. Transplant. 2024, 14, 90194. [Google Scholar] [CrossRef] [PubMed]
- Picchianti-Diamanti, A.; Panebianco, C.; Salemi, S.; Sorgi, M.L.; Di Rosa, R.; Tropea, A.; Sgrulletti, M.; Salerno, G.; Terracciano, F.; D’Amelio, R.; et al. Analysis of Gut Microbiota in Rheumatoid Arthritis Patients: Disease-Related Dysbiosis and Modifications Induced by Etanercept. Int. J. Mol. Sci. 2018, 19, 2938. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Andriulli, A.; Pazienza, V. Pharmacomicrobiomics: Exploiting the drug-microbiota interactions in anticancer therapies. Microbiome 2018, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Pasini, E.; Copeland, J.; Angeli, M.; Husain, S.; Kumar, D.; Renner, E.; Teterina, A.; Allard, J.; Guttman, D.S.; et al. Impact of Immunosuppression on the Metagenomic Composition of the Intestinal Microbiome: A Systems Biology Approach to Post-Transplant Diabetes. Sci. Rep. 2017, 7, 10277. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak-Staruch, I.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Alterations of the Skin and Gut Microbiome in Psoriasis and Psoriatic Arthritis. Int. J. Mol. Sci. 2021, 22, 3998. [Google Scholar] [CrossRef]
- Thio, H.B. The Microbiome in Psoriasis and Psoriatic Arthritis: The Skin Perspective. J. Rheumatol. Suppl. 2018, 94, 30–31. [Google Scholar] [CrossRef]
- Harrington, R.; Al Nokhatha, S.A.; Conway, R. JAK Inhibitors in Rheumatoid Arthritis: An Evidence-Based Review on the Emerging Clinical Data. J. Inflamm. Res. 2020, 13, 519–531. [Google Scholar] [CrossRef]
- Ighani, A.; Georgakopoulos, J.R.; Yeung, J. Tofacitinib for the treatment of psoriasis and psoriatic arthritis. G. Ital. Dermatol. Venereol. 2020, 155, 400–410. [Google Scholar] [CrossRef]
- Kerschbaumer, A.; Smolen, J.S.; Ferreira, R.J.O.; Bertheussen, H.; Baraliakos, X.; Aletaha, D.; McGonagle, D.G.; van der Heijde, D.; McInnes, I.B.; Esbensen, B.A.; et al. Efficacy and safety of pharmacological treatment of psoriatic arthritis: A systematic literature research informing the 2023 update of the EULAR recommendations for the management of psoriatic arthritis. Ann. Rheum. Dis. 2024, 83, 760–774. [Google Scholar] [CrossRef]
- Mease, P.; Hall, S.; FitzGerald, O.; Van der Heijde, D.; Merola, J.F.; Avila-Zapata, F.; Cieślak, D.; Graham, D.; Wang, C.; Menon, S.; et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N. Engl. J. Med. 2017, 377, 1537–1550. [Google Scholar] [CrossRef]
- Gladman, D.; Rigby, W.; Azevedo, V.F.; Behrens, F.; Blanco, R.; Kaszuba, A.; Kudlacz, E.; Wang, C.; Menon, S.; Hendrikx, T.; et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N. Engl. J. Med. 2017, 377, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Nash, P.; Mease, P.J.; Wu, J.; Coates, L.C.; Behrens, F.; Gladman, D.D.; Kivitz, A.J.; Wei, J.C.; Shirinsky, I.; Menon, S. Tofacitinib as monotherapy following methotrexate withdrawal in patients with psoriatic arthritis previously treated with open-label tofacitinib plus methotrexate: A randomised, placebo-controlled sub-study of OPAL Balance. Lancet Rheumatol. 2020, 3, E28–E39. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Young, P.; Fallon, L.; Mundayat, R.; Dina, O.; Blachley, T.; Middaugh, N.; Ogdie, A. Effectiveness of Tofacitinib in Patients Initiating Therapy for Psoriatic Arthritis: Results from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry. Rheumatol. Ther. 2024, 11, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Blondeaux, A.; Valibouze, C.; Speca, S.; Rousseaux, C.; Dubuquoy, C.; Blanquart, H.; Zerbib, P.; Desreumaux, P.; Foligné, B.; Titécat, M. Changes in HLA-B27 Transgenic Rat Fecal Microbiota Following Tofacitinib Treatment and Ileocecal Resection Surgery: Implications for Crohn’s Disease Management. Int. J. Mol. Sci. 2024, 25, 2164. [Google Scholar] [CrossRef] [PubMed]
- Favaron, A.; Abdalla, Y.; McCoubrey, L.E.; Nandiraju, L.P.; Shorthouse, D.; Gaisford, S.; Basit, A.W.; Orlu, M. Exploring the interactions of JAK inhibitor and S1P receptor modulator drugs with the human gut microbiome: Implications for colonic drug delivery and inflammatory bowel disease. Eur. J. Pharm. Sci. 2024, 200, 106845. [Google Scholar] [CrossRef]
- Bruyn, G.A.; Iagnocco, A.; Naredo, E.; Balint, P.V.; Gutierrez, M.; Hammer, H.B.; Collado, P.; Filippou, G.; Schmidt, W.A.; Jousse-Joulin, S.; et al. OMERACT Ultrasound Working Group. OMERACT Definitions for Ultrasonographic Pathologies and Elementary Lesions of Rheumatic Disorders 15 Years On. J. Rheumatol. 2019, 46, 1388–1393. [Google Scholar] [CrossRef]
- D’Agostino, M.A.; Schett, G.; López-Rdz, A.; Šenolt, L.; Fazekas, K.; Burgos-Vargas, R.; Maldonado-Cocco, J.; Naredo, E.; Carron, P.; Duggan, A.M.; et al. Response to secukinumab on synovitis using Power Doppler ultrasound in psoriatic arthritis: 12-week results from a phase III study, ULTIMATE. Rheumatology 2022, 61, 1867–1876. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, Y.; Liu, Q.; Zhang, C. Efficacy and safety of tofacitinib for chronic plaque psoriasis and psoriatic arthritis: A systematic review and meta-analysis of randomized controlled trials. Clin. Rheumatol. 2024, 43, 1605–1613. [Google Scholar] [CrossRef]
- Gossec, L.; Kerschbaumer, A.; Ferreira, R.J.O.; Aletaha, D.; Baraliakos, X.; Bertheussen, H.; Boehncke, W.H.; Esbensen, B.A.; McInnes, I.B.; McGonagle, D.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann. Rheum. Dis. 2024, 83, 706–719. [Google Scholar] [CrossRef]
- Coates, L.C.; Soriano, E.R.; Corp, N.; Bertheussen, H.; Callis Duffin, K.; Campanholo, C.B.; Chau, J.; Eder, L.; Fernández-Ávila, D.G.; FitzGerald, O.; et al. GRAPPA Treatment Recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol. 2022, 18, 465–479. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Spinelli, F.R.; Garufi, C.; Mancuso, S.; Alessandri, C.; Di Franco, M.; Orefice, V.; Pacucci, V.A.; Pirone, C.; Priori, R.; et al. The role of musculoskeletal ultrasound in predicting the response to JAK inhibitors: Results from a monocentric cohort. Clin. Exp. Rheumatol. 2022, 40, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Germanò, G.; Macchioni, P.; Maranini, B.; Ciancio, G.; Bonazza, S.; Govoni, M.; Salvarani, C. Ultrasound response to tofacitinib in patients with rheumatoid arthritis: Data from a multicenter 24 weeks prospective study. Front. Med. 2022, 9, 990317. [Google Scholar] [CrossRef] [PubMed]
- Mpakogiannis, K.; Fousekis, F.S.; Christodoulou, D.K.; Katsanos, K.H.; Narula, N. The current role of Tofacitinib in acute severe ulcerative colitis in adult patients: A systematic review. Dig. Liver Dis. 2023, 55, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hsu, C.Y.; He, H.R.; Chiang, W.Y.; Lin, S.H.; Huang, Y.L.; Kuo, Y.H.; Su, Y.J. Gut microbiota differences between psoriatic arthritis and other undifferentiated arthritis: A pilot study. Medicine 2022, 101, e29870. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.X.; Chang, M.J.; Qiao, J.; Wang, C.H.; Li, X.F.; Yu, Q.; He, P.F. Characteristics of the Gut Microbiome and Its Relationship with Peripheral CD4+ T Cell Subpopulations and Cytokines in Rheumatoid Arthritis. Front. Microbiol. 2022, 13, 799602. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, L.; Sun, T.; Guo, K.; Geng, S. Dysbiosis of gut microbiota and its correlation with dysregulation of cytokines in psoriasis patients. BMC Microbiol. 2021, 21, 78. [Google Scholar] [CrossRef]
- Balmant, B.D.; Fonseca, D.C.; Prudêncio, A.P.A.; Rocha, I.M.; Callado, L.; Alves, J.T.M.; Torrinhas, R.S.M.M.; Borba, E.F.; Waitzberg, D.L. Megamonas funiformis, Plasma Zonulin, and Sodium Intake Affect C3 Complement Levels in Inactive Systemic Lupus Erythematosus. Nutrients 2023, 15, 1999. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Sun, W.; Wang, M.; Li, M. Characteristics of gut microbiota in patients with primary Sjögren’s syndrome in Northern China. PLoS ONE 2022, 17, e0277270. [Google Scholar] [CrossRef]
- Paine, A.; Brookes, P.S.; Bhattacharya, S.; Li, D.; De La Luz Garcia-Hernandez, M.; Tausk, F.; Ritchlin, C. Dysregulation of Bile Acids, Lipids, and Nucleotides in Psoriatic Arthritis Revealed by Unbiased Profiling of Serum Metabolites. Arthritis Rheumatol. 2023, 75, 53–63. [Google Scholar] [CrossRef]
- Freidin, M.B.; Stalteri, M.A.; Wells, P.M.; Lachance, G.; Baleanu, A.F.; Bowyer, R.C.E.; Kurilshikov, A.; Zhernakova, A.; Steves, C.J.; Williams, F.M.K. An association between chronic widespread pain and the gut microbiome. Rheumatology 2021, 60, 3727–3737. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Hablot, J.; Ferhat, M.; Lavelle, A.; Salem, F.; Taieb, M.; Medvedovic, J.; Kindt, N.; Reboul, P.; Cailotto, F.; Jouzeau, J.Y.; et al. Tofacitinib treatment alters mucosal immunity and gut microbiota during experimental arthritis. Clin. Transl. Med. 2020, 10, e163. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Mannaa, M.; Kim, Y.; Kim, J.; Kim, G.T.; Seo, Y.S. Comparative Analysis of Fecal Microbiota Composition Between Rheumatoid Arthritis and Osteoarthritis Patients. Genes 2019, 10, 748. [Google Scholar] [CrossRef]
- Alpizar-Rodriguez, D.; Lesker, T.R.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 590–593. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Gómez, J.; Gómez, J.; Delgado, S.; Requena-López, S.; Queiro-Silva, R.; Margolles, A.; Coto, E.; Sánchez, B.; Coto-Segura, P. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br. J. Dermatol. 2019, 181, 1287–1295. [Google Scholar] [CrossRef]

| Patients’ Characteristics | |
|---|---|
| Total (n) | 7 |
| Age, median (IQR) years | 52.00 (33.00–56.00) |
| Gender, Female (n/tot) | 57.14% (4/7) |
| Comorbidities, yes (n/tot) | 57.14% (4/7) |
| BMI, median (IQR) | 25.7 (23–26.6) |
| Smoking habits, yes (n/tot) | 28.57% (2/7) |
| Disease duration, median (IQR) years | 10.34 (0.4–13) |
| ESR, median (IQR) mm/hr | 8.00 (2.00–68.00) |
| CRP, median (IQR) mg/dL | 0.40 (0.10–4.00) |
| Swollen joints, mean (SD) | 3.1 (2.1) |
| Tender joints, mean (SD) | 7 (4.4) |
| LEI, mean (SD) | 1 (0.9) |
| PASI, mean (SD) | 3.86 (3.9) |
| DAPSA, mean (SD)BASDAI, mean (SD)ASDAS, mean (SD) | 25.8 (5.6)7.00 (0.14)4.35 (0.1) |
| Monotherapy (n/tot) | 42.85% (3/7) |
| GLOESS score, mean (SD) | 7.00 (7.7) |
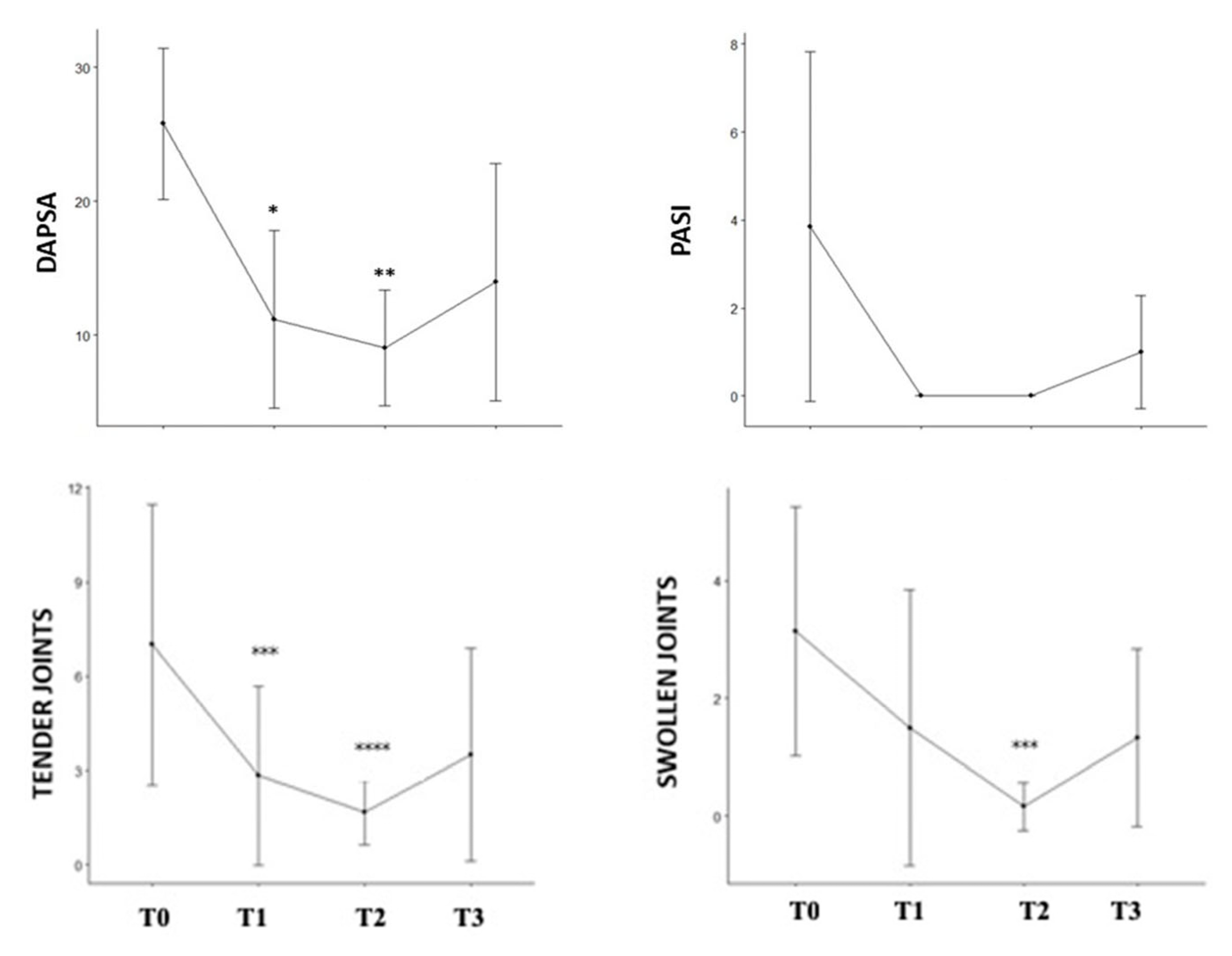
| T0 | T1 | T2 | T3 | p-Value * | |
|---|---|---|---|---|---|
| DAPSA, mean (SD) | 25.80 (5.65) | 9.40(5.64) | 8.40 (4.51) | 13.97 (8.88) | 0.001 |
| ASDAS, mean (SD) | 4.35 (1.34) | 2.40 (NA) | 4.20 (NA) | 2.50 (NA) | - |
| BASDAI, mean (SD) | 7.00 (0.14) | 1.60 (NA) | 4.20 (NA) | 3.10 (NA) | - |
| LEI, mean (SD) | 1 (0.90) | 0.50 (1.00) | 0.40 (0.89) | 0.9 (0.70) | 0.482 |
| PASI, mean (SD) | 3.86 (3.98) | 0.00 (0.00) | 0.00 (0.00) | 1.00 (1.29) | 0.030 |
| Tender joints, mean (SD) | 7.00 (4.47) | 1.40 (2.61) | 0.20 (0.45) | 3.50 (3.39) | 0.028 |
| Swollen joints, mean (SD) | 3.14 (2.12) | 1.40 (2.61) | 0.20 (0.45) | 1.33 (1.51) | 0.083 |
| GLOESS, mean (SD) | 7.00 (7.72) | 3.00 (3.37) | 1.57 (1.40) | 2.00 (2.77) | 0.122 |
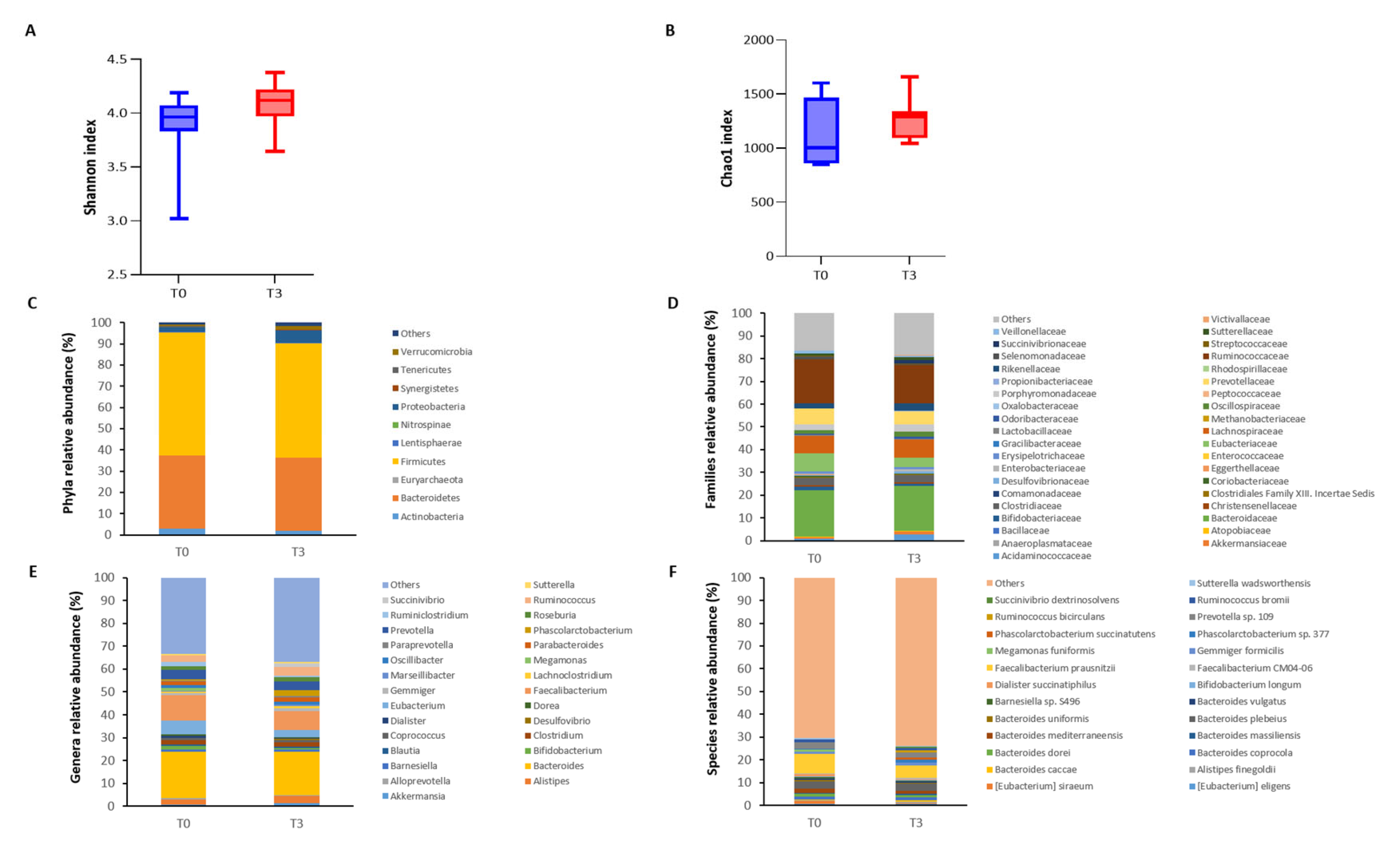
| Relative Abundance at T0 (%) | Relative Abundance at T3 (%) | |
|---|---|---|
| Increased at T3 | ||
| Lentisphaerae | 0.073 | 0.410 |
| Proteobacteria | 2.160 | 6.018 |
| Oxalobacteraceae | 0.009 | 0.048 |
| Desulfovibrionaceae | 0.258 | 0.730 |
| Phascolarctobacterium | 0.398 | 2.366 |
| Angelakisella | 0.015 | 0.049 |
| Bilophila | 0.067 | 0.189 |
| Anaeromassilibacillus | 0.014 | 0.044 |
| Coprococcus comes | 0.000 | 0.059 |
| Alistipes sp. NML05A004 | 0.000 | 0.045 |
| Sutterella massiliensis | 0.022 | 0.290 |
| Ruminococcus bicirculans | 0.242 | 0.997 |
| Butyricimonas sp. AT11 | 0.008 | 0.042 |
| Bilophila wadsworthia | 0.034 | 0.121 |
| Clostridium leptum | 0.012 | 0.047 |
| Parabacteroides distasonis | 0.153 | 0.497 |
| Decreased at T3 | ||
| Actinobacteria | 2.988 | 1.879 |
| Selenomonadaceae | 1.344 | 0.070 |
| Coriobacteraceae | 0.686 | 0.484 |
| Megamonas | 1.304 | 0.001 |
| Adelcreutzia | 0.051 | 0.012 |
| Enterohabdus | 0.065 | 0.025 |
| Ruminoclostridium | 1.708 | 0.550 |
| Collinsella | 0.580 | 0.412 |
| Veillonella | 0.127 | 0.069 |
| Megamonas rupellensis | 0.090 | 0.000 |
| Megamonas funiformis | 0.997 | 0.001 |
| Bacteroides galacturonicus | 0.119 | 8.571 × 10−5 |
| Tyzzerella nexilis | 0.049 | 0.001 |
| Dialister sp. S7D | 0.096 | 0.027 |
| Eubacterium hallii | 0.185 | 0.078 |
| Parasutterella excrementihominis | 0.072 | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picchianti Diamanti, A.; Panebianco, C.; Di Gioia, V.; Bellofatto, I.A.; Salemi, S.; Di Rosa, R.; Sesti, G.; Nalli, G.; Salerno, G.; Finocchiaro, E.; et al. A Case Series Report on the Effect of Tofacitinib on Joint Inflammation and Gut Microbiota Composition in Psoriatic Arthritis Patients Naive to Biologic Agents. Microorganisms 2024, 12, 2387. https://doi.org/10.3390/microorganisms12122387
Picchianti Diamanti A, Panebianco C, Di Gioia V, Bellofatto IA, Salemi S, Di Rosa R, Sesti G, Nalli G, Salerno G, Finocchiaro E, et al. A Case Series Report on the Effect of Tofacitinib on Joint Inflammation and Gut Microbiota Composition in Psoriatic Arthritis Patients Naive to Biologic Agents. Microorganisms. 2024; 12(12):2387. https://doi.org/10.3390/microorganisms12122387
Chicago/Turabian StylePicchianti Diamanti, Andrea, Concetta Panebianco, Valeria Di Gioia, Ilaria Anna Bellofatto, Simonetta Salemi, Roberta Di Rosa, Giorgio Sesti, Gabriele Nalli, Gerardo Salerno, Etta Finocchiaro, and et al. 2024. "A Case Series Report on the Effect of Tofacitinib on Joint Inflammation and Gut Microbiota Composition in Psoriatic Arthritis Patients Naive to Biologic Agents" Microorganisms 12, no. 12: 2387. https://doi.org/10.3390/microorganisms12122387
APA StylePicchianti Diamanti, A., Panebianco, C., Di Gioia, V., Bellofatto, I. A., Salemi, S., Di Rosa, R., Sesti, G., Nalli, G., Salerno, G., Finocchiaro, E., & Laganà, B. (2024). A Case Series Report on the Effect of Tofacitinib on Joint Inflammation and Gut Microbiota Composition in Psoriatic Arthritis Patients Naive to Biologic Agents. Microorganisms, 12(12), 2387. https://doi.org/10.3390/microorganisms12122387








