Factors and Mechanisms Affecting Arsenic Migration in Cultivated Soils Irrigated with Contained Arsenic Brackish Groundwater
Abstract
1. Introduction
2. Materials and Methods
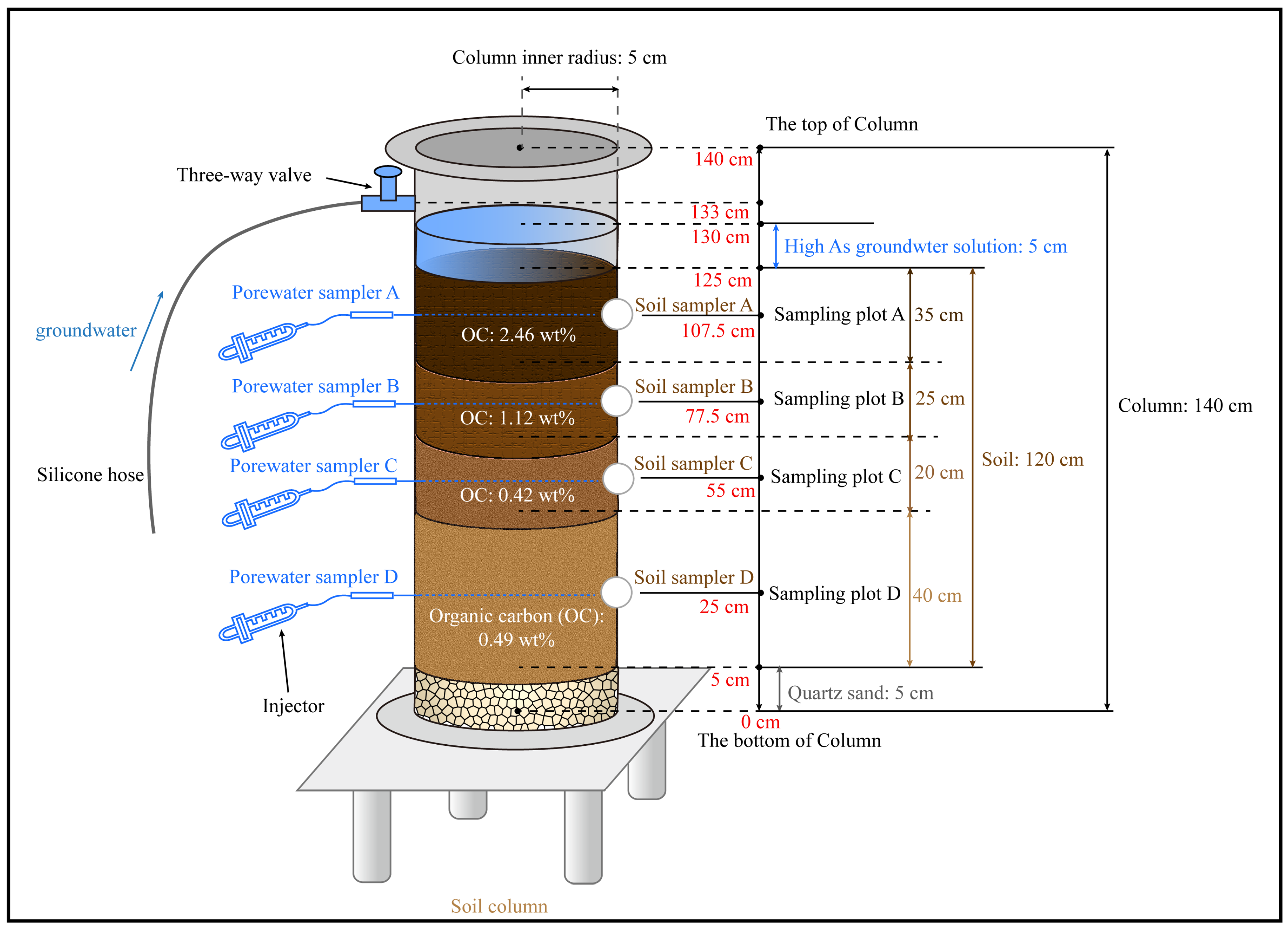
2.1. Column Experiments
2.2. Sample Analytical Methods
2.2.1. Aqueous Phase Analysis
2.2.2. Solid Phase Analysis
2.2.3. Illumina Novaseq Sequencing and Quality
2.3. Data Processing and Analysis
3. Results and Discussion
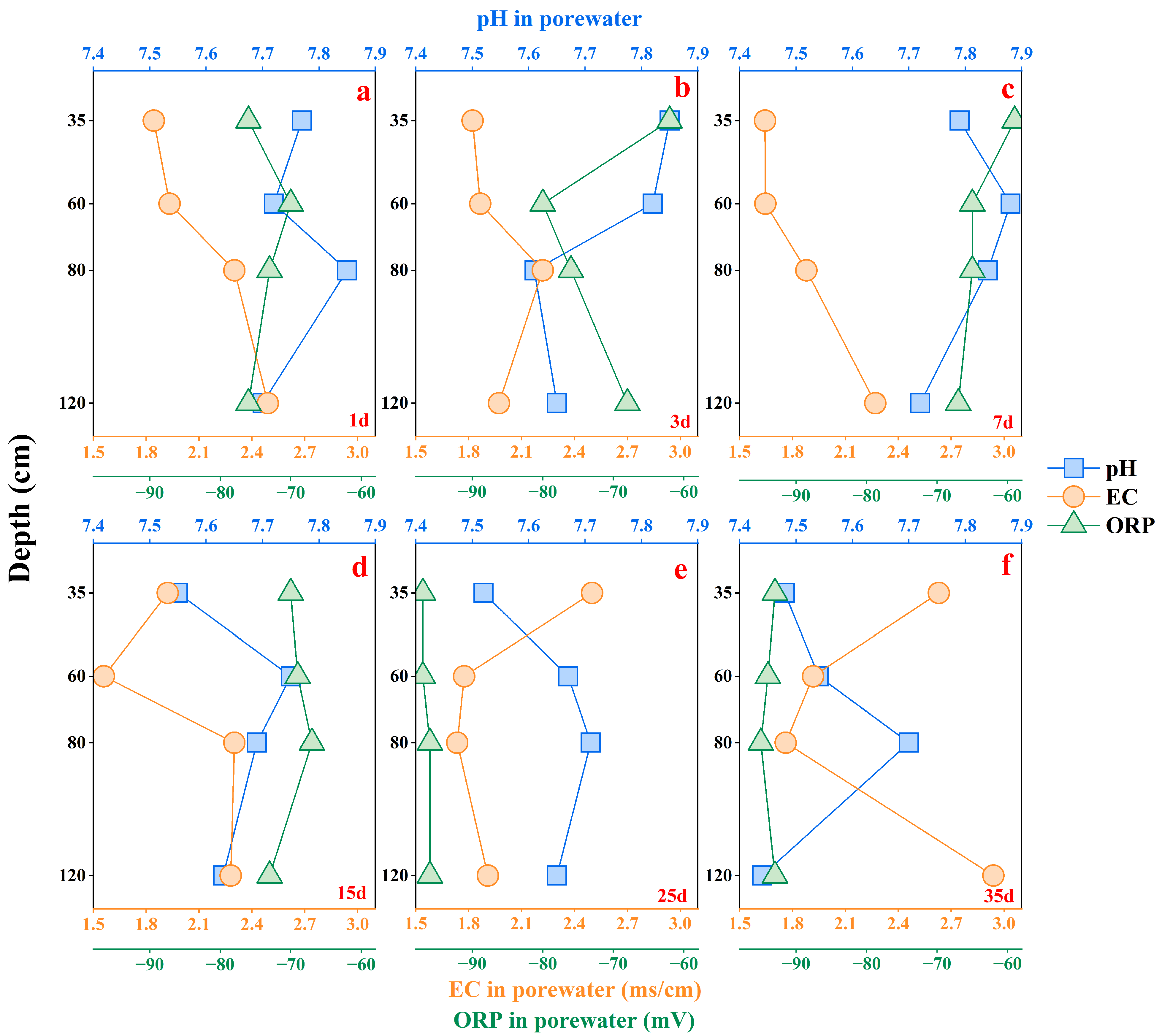
3.1. The Vertical and Temporal Changes of Soil Indexes After Leaching
3.1.1. Chemical and Biological Characteristics for Soil Porewater Samples
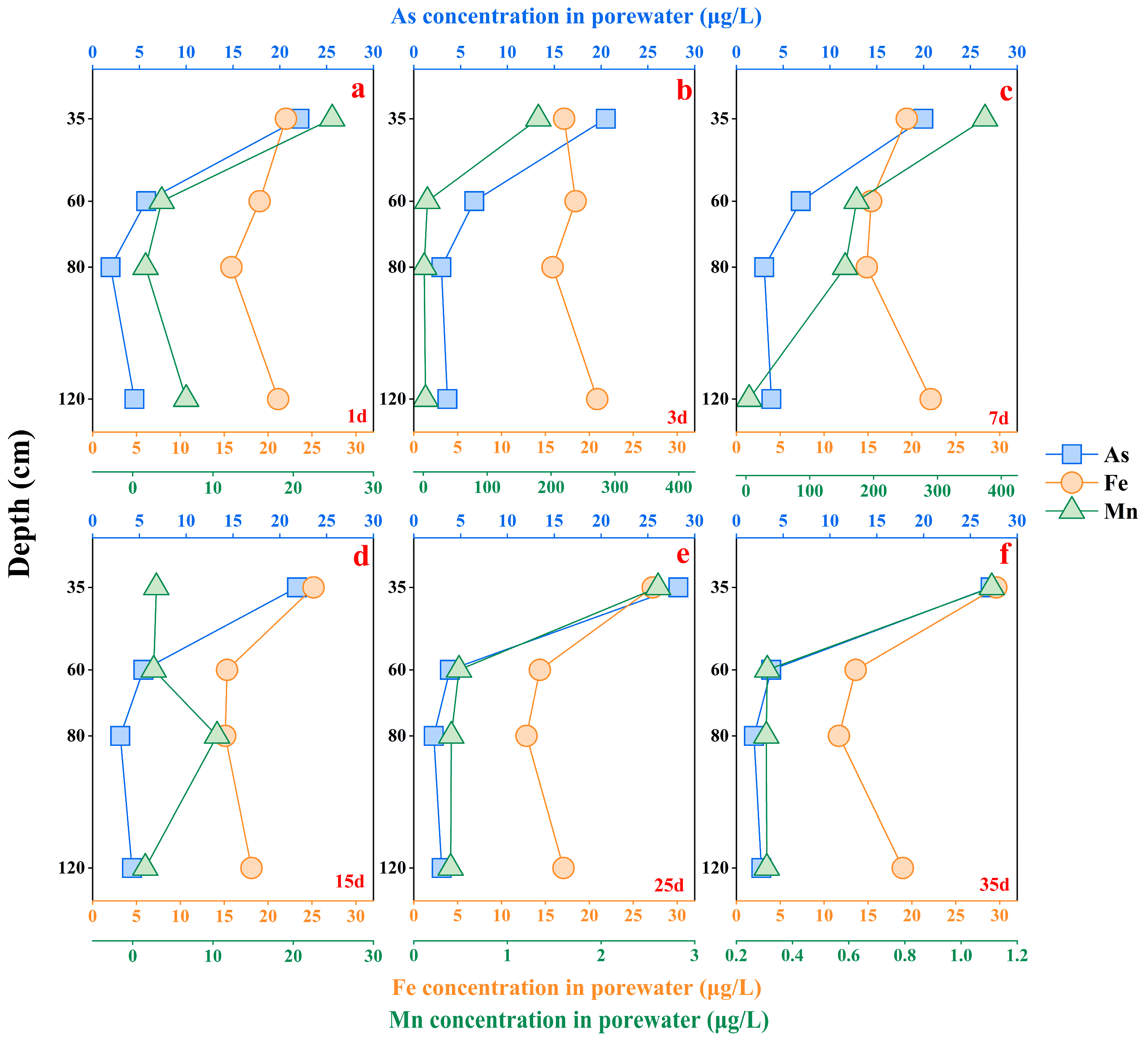
3.1.2. The Total As, Fe, and Mn Concentration for Soil Porewater Samples
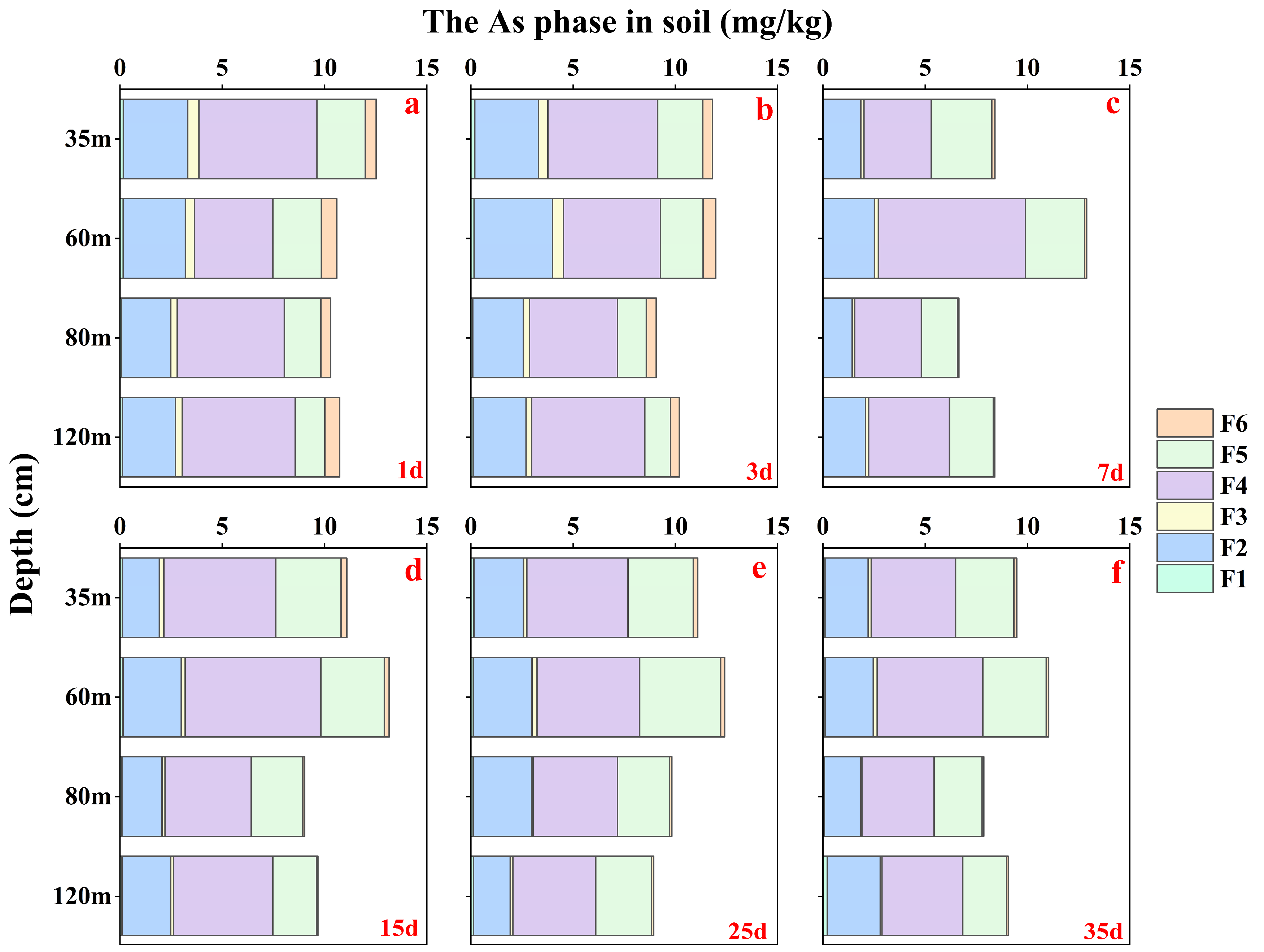
3.1.3. The As Content Based on Different Fractions for Soil Samples
3.2. The Sources of As for Soil Porewater
3.3. The Effect Factors for As Migration in Cultivated Soils
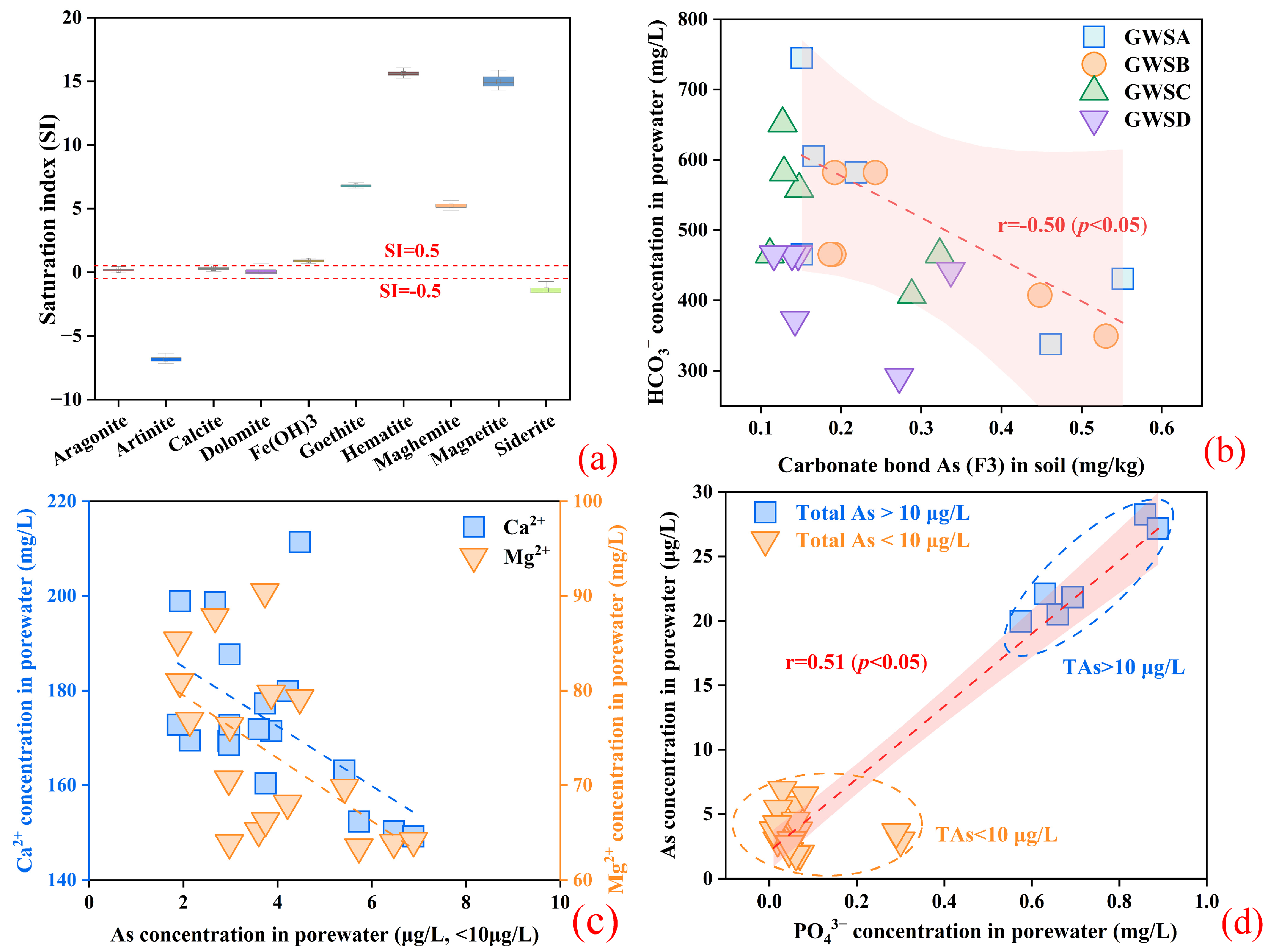
3.3.1. The Effect of Fe Oxides for As Migration
3.3.2. The Effect of Inorganic Ions for As Migration
3.3.3. The Main Controlling Factors for As Migration
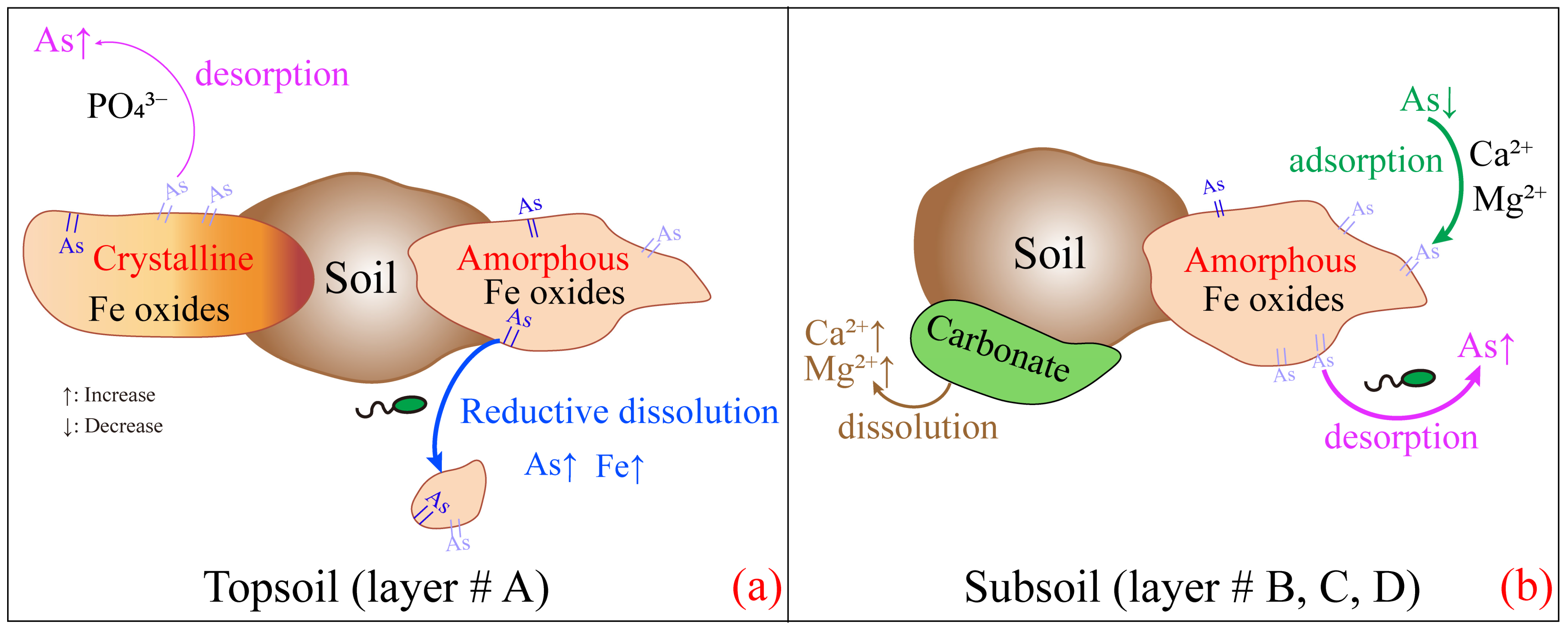
3.4. The Mechanisms of As Migration in Cultivated Soils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- KayZhang, S.; Zhang, J.; Niu, L.; Chen, Q.; Zhou, Q.; Xiao, N.; Man, J.; Ma, J.; Wei, C.; Zhang, S.; et al. Escalating arsenic contamination throughout Chinese soils. Nat. Sustain. 2024, 7, 766–775. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Šimůnek, J.; Shi, H.; Chen, N.; Hu, Q. Quantifying water and salt movement in a soil-plant system of a corn field using HYDRUS (2D/3D) and the stable isotope method. Agric. Water Manag. 2023, 288, 108492. [Google Scholar] [CrossRef]
- Sandil, S.; Óvári, M.; Dobosy, P.; Vetési, V.; Endrédi, A.; Takács, A.; Füzy, A.; Záray, G. Effect of arsenic-contaminated irrigation water on growth and elemental composition of tomato and cabbage cultivated in three different soils, and related health risk assessment. Environ. Res. 2021, 197, 111098. [Google Scholar] [CrossRef]
- Oloruntoba, A.; Omoniyi, A.O.; Shittu, Z.A.; Ajala, R.O.; Kolawole, S.A. Heavy metal contamination in soils, water, and food in nigeria from 2000–2019: A systematic review on methods, pollution level and policy implications. Water Air Soil Pollut. 2024, 235, 586. [Google Scholar] [CrossRef]
- Kaya, C.; Uğurlar, F.; Ashraf, M.; Hou, D.; Kirkham, M.B.; Bolan, N. Microbial consortia-mediated arsenic bioremediation in agricultural soils: Current status, challenges, and solutions. Sci. Total Environ. 2024, 917, 170297. [Google Scholar] [CrossRef]
- Rosas-Castor, J.M.; Guzmán-Mar, J.L.; Alfaro-Barbosa, J.M.; Hernández-Ramírez, A.; Pérez-Maldonado, I.N.; Caballero-Quintero, A.; Hinojosa-Reyes, L. Evaluation of the transfer of soil arsenic to maize crops in suburban areas of San Luis Potosi, Mexico. Sci. Total Environ. 2014, 497–498, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gulz, P.A.; Gupta, S.-K.; Schulin, R. Arsenic accumulation of common plants from contaminated soils. Plant Soil 2005, 272, 337–347. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Yao, D.; Wang, X.; Gao, Y. Bowl effect of irreversible primary salinization driven by geology in Hetao irrigation area, China. Sci. Total Environ. 2024, 920, 170834. [Google Scholar] [CrossRef]
- Malakar, A.; Ray, C.; D’Alessio, M.; Shields, J.; Adams, C.; Stange, M.; Weber, K.A.; Snow, D.D. Interplay of legacy irrigation and nitrogen fertilizer inputs to spatial variability of arsenic and uranium within the deep vadose zone. Sci. Total Environ. 2023, 897, 165299. [Google Scholar] [CrossRef]
- Li, C.; Carrijo, D.R.; Nakayama, Y.; Linquist, B.A.; Green, P.G.; Parikh, S.J. Impact of alternate wetting and drying irrigation on arsenic uptake and speciation in flooded rice systems. Agric. Ecosyst. Environ. 2019, 272, 188–198. [Google Scholar] [CrossRef]
- Sengupta, S.; Bhattacharyya, K.; Mandal, J.; Bhattacharya, P.; Halder, S.; Pari, A. Deficit irrigation and organic amendments can reduce dietary arsenic risk from rice: Introducing machine learning-based prediction models from field data. Agric. Ecosyst. Environ. 2021, 319, 107516. [Google Scholar] [CrossRef]
- Gillispie, E.C.; Sowers, T.D.; Duckworth, O.W.; Polizzotto, M.L. Soil pollution due to irrigation with arsenic-contaminated groundwater: Current state of science. Curr. Pollut. Rep. 2015, 1, 1–12. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Natural attenuation processes for remediation of arsenic contaminated soils and groundwater. J. Hazard. Mater. 2006, 138, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.D.; Postma, D.; Jakobsen, R.; Larsen, O. Fast transformation of iron oxyhydroxides by the catalytic action of aqueous Fe(II). Geochim. Cosmochim. Acta 2005, 69, 3967–3977. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Sun, J.; Chillrud, S.N.; Mailloux, B.J.; Stute, M.; Singh, R.; Dong, H.; Lepre, C.J.; Bostick, B.C. Enhanced and stabilized arsenic retention in microcosms through the microbial oxidation of ferrous iron by nitrate. Chemosphere 2016, 144, 1106–1115. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Li, J.; Yu, Q.; Wu, Y.; Su, C.; Duan, M. Effect of irrigation on Fe(III)–SO42− redox cycling and arsenic mobilization in shallow groundwater from the Datong basin, China: Evidence from hydrochemical monitoring and modeling. J. Hydrol. 2015, 523, 128–138. [Google Scholar] [CrossRef]
- Pedersen, H.D.; Postma, D.; Jakobsen, R. Release of arsenic associated with the reduction and transformation of iron oxides. Geochim. Cosmochim. Acta 2006, 70, 4116–4129. [Google Scholar] [CrossRef]
- Benner, S.G.; Hansel, C.M.; Wielinga, B.W.; Barber, T.M.; Fendorf, S. Reductive dissolution and biomineralization of iron hydroxide under dynamic flow conditions. Environ. Sci. Technol. 2002, 36, 1705–1711. [Google Scholar] [CrossRef]
- Azam, M.S.; Shafiquzzaman, M.; Haider, H. Arsenic release dynamics of paddy field soil during groundwater irrigation and natural flooding. J. Environ. Manag. 2023, 343, 118204. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.Y.; Li, F.; Liu, T.; Wu, W.; Liu, C.; Liu, C.; Zhang, X. Enhanced immobilization of arsenic and cadmium in a paddy soil by combined applications of woody peat and Fe(NO3)3: Possible mechanisms and environmental implications. Sci. Total Environ. 2019, 649, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, J.W.; Schaefer, M.V.; Benner, S.G.; Fendorf, S. Reactivity and speciation of mineral-associated arsenic in seasonal and permanent wetlands of the Mekong Delta. Geochim. Cosmochim. Acta 2015, 171, 143–155. [Google Scholar] [CrossRef]
- Kocar, B.D.; Fendorf, S. Thermodynamic Constraints on Reductive Reactions Influencing the Biogeochemistry of Arsenic in Soils and Sediments. Environ. Sci. Technol. 2009, 43, 4871–4877. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-X.; Wang, Y.-J.; Liu, C.; Wang, L.-H.; Yang, K.; Zhou, D.-M.; Li, W.; Sparks, D.L. Effect of iron oxide reductive dissolution on the transformation and immobilization of arsenic in soils: New insights from X-ray photoelectron and X-ray absorption spectroscopy. J. Hazard. Mater. 2014, 279, 212–219. [Google Scholar] [CrossRef]
- Parsons, C.T.; Couture, R.-M.; Omoregie, E.O.; Bardelli, F.; Greneche, J.-M.; Roman-Ross, G.; Charlet, L. The impact of oscillating redox conditions: Arsenic immobilisation in contaminated calcareous floodplain soils. Environ. Pollut. 2013, 178, 254–263. [Google Scholar] [CrossRef]
- Liu, J.; Ye, L.; Jing, C. Active microbial arsenic methylation in saline-alkaline paddy soil. Sci. Total Environ. 2023, 865, 161077. [Google Scholar] [CrossRef]
- Patel, M.; Parida, A.K. Salinity alleviates the arsenic toxicity in the facultative halophyte Salvadora persica L. by the modulations of physiological, biochemical, and ROS scavenging attributes. J. Hazard. Mater. 2021, 401, 123368. [Google Scholar] [CrossRef]
- Parvez, S.; Abbas, G.; Shahid, M.; Amjad, M.; Hussain, M.; Asad, S.A.; Imran, M.; Naeem, M.A. Effect of salinity on physiological, biochemical and photostabilizing attributes of two genotypes of quinoa (Chenopodium quinoa Willd.) exposed to arsenic stress. Ecotoxicol. Environ. Saf. 2020, 187, 109814. [Google Scholar] [CrossRef]
- Wang, X.; Liu, T.; Li, F.; Li, B.; Liu, C. Effects of Simultaneous Application of Ferrous Iron and Nitrate on Arsenic Accumulation in Rice Grown in Contaminated Paddy Soil. ACS Earth Space Chem. 2018, 2, 103–111. [Google Scholar] [CrossRef]
- Coby, A.J.; Picardal, F.; Shelobolina, E.; Xu, H.; Roden, E.E. Repeated anaerobic microbial redox cycling of iron. Appl. Environ. Microbiol. 2011, 77, 6036–6042. [Google Scholar] [CrossRef]
- Goldberg, S.; Johnston, C.T. Mechanisms of Arsenic Adsorption on Amorphous Oxides Evaluated Using Macroscopic Measurements, Vibrational Spectroscopy, and Surface Complexation Modeling. J. Colloid Interface Sci. 2001, 234, 204–216. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W. Arsenate and Arsenite Removal by Zerovalent Iron: Effects of Phosphate, Silicate, Carbonate, Borate, Sulfate, Chromate, Molybdate, and Nitrate, Relative to Chloride. Environ. Sci. Technol. 2001, 35, 4562–4568. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, E.J.; Sparks, D.L. Phosphate adsorption onto hematite: An in situ ATR-FTIR investigation of the effects of pH and loading level on the mode of phosphate surface complexation. J. Colloid Interface Sci. 2007, 308, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, N.; Sun, B.; Su, S.; Wang, Y.; Zhang, Y.; Wu, C.; Zeng, X. Contradictory tendency of As(V) releasing from Fe–As complexes: Influence of organic and inorganic anions. Chemosphere 2022, 286, 131469. [Google Scholar] [CrossRef]
- Ali, W.; Mushtaq, N.; Javed, T.; Zhang, H.; Ali, K.; Rasool, A.; Farooqi, A. Vertical mixing with return irrigation water the cause of arsenic enrichment in groundwater of district Larkana Sindh, Pakistan. Environ. Pollut. 2019, 245, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, V.; Saini, R.K.; Pant, N.; Singh, R.; Singh, A.; Kumar, S.; Singh, S.; Yadav, B.K.; Krishan, G.; et al. Floodplains landforms, clay deposition and irrigation return flow govern arsenic occurrence, prevalence and mobilization: A geochemical and isotopic study of the mid-Gangetic floodplains. Environ. Res. 2021, 201, 111516. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.L.; Nguyen, H.A.; Richter, O.; Pham, M.T.; Nguyen, V.P. Ecophysiological responses of young mangrove species Rhizophora apiculata (Blume) to different chromium contaminated environments. Sci. Total Environ. 2017, 574, 369–380. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Zhan, H.; Yang, S. Effect of long-term saline mulched drip irrigation on soil-groundwater environment in arid Northwest China. Sci. Total Environ. 2022, 820, 153222. [Google Scholar] [CrossRef]
- Pellegrini, E.; Contin, M.; Mazhar, S.; Bravo, C.; De Nobili, M. Flooding by sea and brackish waters enhances mobility of Cd, Zn and Pb from airborne dusts in coastal soils. Sci. Total Environ. 2024, 922, 171038. [Google Scholar] [CrossRef]
- Keon, N.E.; Swartz, C.H.; Brabander, D.J.; Harvey, C.; Hemond, H.F. Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environ. Sci. Technol. 2001, 35, 2778–2784. [Google Scholar] [CrossRef]
- Poulton, S.W.; Canfield, D.E. Development of a sequential extraction procedure for iron: Implications for iron partitioning in continentally derived particulates. Chem. Geol. 2005, 214, 209–221. [Google Scholar] [CrossRef]
- Jia, Y.; Guo, H.; Xi, B.; Jiang, Y.; Zhang, Z.; Yuan, R.; Yi, W.; Xue, X. Sources of groundwater salinity and potential impact on arsenic mobility in the western Hetao Basin, Inner Mongolia. Sci. Total Environ. 2017, 601–602, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Morelli, G.; Rimondi, V.; Benvenuti, M.; Medas, D.; Costagliola, P.; Gasparon, M. Experimental simulation of arsenic desorption from Quaternary aquifer sediments following sea water intrusion. Appl. Geochem. 2017, 87, 176–187. [Google Scholar] [CrossRef]
- Papazotos, P.; Vasileiou, E.; Perraki, M. Elevated groundwater concentrations of arsenic and chromium in ultramafic environments controlled by seawater intrusion, the nitrogen cycle, and anthropogenic activities: The case of the Gerania Mountains, NE Peloponnese, Greece. Appl. Geochem. 2020, 121, 104697. [Google Scholar] [CrossRef]
- Muehe, E.M.; Scheer, L.; Daus, B.; Kappler, A. Fate of Arsenic during Microbial Reduction of Biogenic versus Abiogenic As–Fe(III)–Mineral Coprecipitates. Environ. Sci. Technol. 2013, 47, 8297–8307. [Google Scholar] [CrossRef]
- Jiang, S.; Lee, J.-H.; Kim, D.; Kanaly, R.A.; Kim, M.-G.; Hur, H.-G. Differential arsenic mobilization from as-bearing ferrihydrite by iron-respiring shewanella strains with different arsenic-reducing activities. Environ. Sci. Technol. 2013, 47, 8616–8623. [Google Scholar] [CrossRef]
- Coker, V.S.; Gault, A.G.; Pearce, C.I.; van der Laan, G.; Telling, N.D.; Charnock, J.M.; Polya, D.A.; Lloyd, J.R. XAS and XMCD Evidence for species-dependent partitioning of arsenic during microbial reduction of ferrihydrite to magnetite. Environ. Sci. Technol. 2006, 40, 7745–7750. [Google Scholar] [CrossRef]
- Tufano, K.J.; Reyes, C.; Saltikov, C.W.; Fendorf, S. Reductive Processes controlling arsenic retention: Revealing the relative importance of iron and arsenic reduction. Environ. Sci. Technol. 2008, 42, 8283–8289. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.-A.; Hofacker, A.F.; Voegelin, A.; Kretzschmar, R. Temperature dependence and coupling of iron and arsenic reduction and release during flooding of a contaminated soil. Environ. Sci. Technol. 2010, 44, 116–122. [Google Scholar] [CrossRef]
- Kocar, B.D.; Borch, T.; Fendorf, S. Arsenic repartitioning during biogenic sulfidization and transformation of ferrihydrite. Geochim. Cosmochim. Acta 2010, 74, 980–994. [Google Scholar] [CrossRef]
- Dixit, S.; Hering, J.G. Comparison of Arsenic(V) and Arsenic(III) Sorption onto iron oxide minerals: implications for arsenic mobility. Environ. Sci. Technol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Kjeldsen, P.; Hansen, H.C.B.; Jakobsen, R. Transformation of natural ferrihydrite aged in situ in As, Cr and Cu contaminated soil studied by reduction kinetics. Appl. Geochem. 2014, 51, 293–302. [Google Scholar] [CrossRef]
- Ford, R.G. Rates of hydrous ferric oxide crystallization and the influence on coprecipitated arsenate. Environ. Sci. Technol. 2002, 36, 2459–2463. [Google Scholar] [CrossRef]
- Fendorf, S.; Eick, M.J.; Grossl, P.; Sparks, D.L. Arsenate and Chromate Retention Mechanisms on Goethite. 1. Surface Structure. Environ. Sci. Technol. 1997, 31, 315–320. [Google Scholar] [CrossRef]
- Manning, B.A.; Fendorf, S.E.; Goldberg, S. Surface structures and stability of arsenic(iii) on goethite: spectroscopic evidence for inner-sphere complexes. Environ. Sci. Technol. 1998, 32, 2383–2388. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, L.; Su, S.; Wang, Y.; Wu, C.; Zeng, X.; Sun, B. Stability of Fe–As composites formed with As(V) and aged ferrihydrite. J. Environ. Sci. 2021, 100, 43–50. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, X.; Wang, Y.; Li, L.; Sun, Y.; Wang, Y.; Zeng, X. The stability of poorly crystalline arsenical ferrihydrite after long-term soil suspension incubation. Chemosphere 2022, 291, 132844. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Xu, L.; Wang, X.; Demopoulos, G.P. Infrared spectroscopic and X-ray diffraction characterization of the nature of adsorbed arsenate on ferrihydrite. Geochim. Cosmochim. Acta 2007, 71, 1643–1654. [Google Scholar] [CrossRef]
- Hu, S.; Lu, Y.; Peng, L.; Wang, P.; Zhu, M.; Dohnalkova, A.C.; Chen, H.; Lin, Z.; Dang, Z.; Shi, Z. Coupled kinetics of ferrihydrite transformation and as(v) sequestration under the effect of humic acids: A mechanistic and quantitative study. Environ. Sci. Technol. 2018, 52, 11632–11641. [Google Scholar] [CrossRef]
- Burton, E.D.; Johnston, S.G.; Bush, R.T. Microbial sulfidogenesis in ferrihydrite-rich environments: Effects on iron mineralogy and arsenic mobility. Geochim. Cosmochim. Acta 2011, 75, 3072–3087. [Google Scholar] [CrossRef]
- Makris, K.C.; Harris, W.G.; O’Conno, G.A.; Obreza, T.A. Phosphorus immobilization in micropores of drinking-water treatment residuals: implications for long-term stability. Environ. Sci. Technol. 2004, 38, 6590–6596. [Google Scholar] [CrossRef] [PubMed]
- Axe, L.; Trivedi, P. Intraparticle Surface diffusion of metal contaminants and their attenuation in microporous amorphous Al, Fe, and Mn oxides. J. Colloid Interface Sci. 2002, 247, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-N.; Zhang, J.-W.; Wu, N.; Xia, Z.-H.; Liu, L.; Zhao, Z.-Q. Co-occurrence of elevated arsenic and fluoride concentrations in Wuliangsu Lake: Implications for the genesis of poor-quality groundwater in the (paleo-)Huanghe (Yellow River) catchment, China. Water Res. 2024, 258, 121767. [Google Scholar] [CrossRef] [PubMed]
- Bolanz, R.M.; Wierzbicka-Wieczorek, M.; Čaplovičová, M.; Uhlík, P.; Göttlicher, J.; Steininger, R.; Majzlan, J. Structural incorporation of As5+ into hematite. Environ. Sci. Technol. 2013, 47, 9140–9147. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.; Jessen, S.; Hue, N.T.M.; Duc, M.T.; Koch, C.B.; Viet, P.H.; Nhan, P.Q.; Larsen, F. Mobilization of arsenic and iron from Red River floodplain sediments, Vietnam. Geochim. Cosmochim. Acta 2010, 74, 3367–3381. [Google Scholar] [CrossRef]
- O’Reilly, S.E.; Strawn, D.G.; Sparks, D.L. Residence time effects on arsenate adsorption/desorption mechanisms on goethite. Soil Sci. Soc. Am. J. 2001, 65, 67–77. [Google Scholar] [CrossRef]
- Radu, T.; Subacz, J.L.; Phillippi, J.M.; Barnett, M.O. Effects of dissolved carbonate on arsenic adsorption and mobility. Environ. Sci. Technol. 2005, 39, 7875–7882. [Google Scholar] [CrossRef]
- Polizzotto, M.L.; Harvey, C.F.; Li, G.; Badruzzman, B.; Ali, A.; Newville, M.; Sutton, S.; Fendorf, S. Solid-phases and desorption processes of arsenic within Bangladesh sediments. Chem. Geol. 2006, 228, 97–111. [Google Scholar] [CrossRef]
- Postma, D.; Larsen, F.; Minh Hue, N.T.; Duc, M.T.; Viet, P.H.; Nhan, P.Q.; Jessen, S. Arsenic in groundwater of the Red River floodplain, Vietnam: Controlling geochemical processes and reactive transport modeling. Geochim. Cosmochim. Acta 2007, 71, 5054–5071. [Google Scholar] [CrossRef]
- Thi Hoa Mai, N.; Postma, D.; Thi Kim Trang, P.; Jessen, S.; Hung Viet, P.; Larsen, F. Adsorption and desorption of arsenic to aquifer sediment on the Red River floodplain at Nam Du, Vietnam. Geochim. Cosmochim. Acta 2014, 142, 587–600. [Google Scholar] [CrossRef]
- Stachowicz, M.; Hiemstra, T.; van Riemsdijk, W.H. Arsenic–bicarbonate interaction on goethite particles. Environ. Sci. Technol. 2007, 41, 5620–5625. [Google Scholar] [CrossRef] [PubMed]
- Saalfield, S.L.; Bostick, B.C. Synergistic effect of calcium and bicarbonate in enhancing arsenate release from ferrihydrite. Geochim. Cosmochim. Acta 2010, 74, 5171–5186. [Google Scholar] [CrossRef]
- Fakhreddine, S.; Dittmar, J.; Phipps, D.; Dadakis, J.; Fendorf, S. Geochemical triggers of arsenic mobilization during managed aquifer recharge. Environ. Sci. Technol. 2015, 49, 7802–7809. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Weng, L.; Li, Y.; Chen, Y.; Ma, J. Redox-dependent effects of phosphate on arsenic speciation in paddy soils. Environ. Pollut. 2020, 264, 114783. [Google Scholar] [CrossRef]
- Neidhardt, H.; Rudischer, S.; Eiche, E.; Schneider, M.; Stopelli, E.; Duyen, V.T.; Trang, P.T.K.; Viet, P.H.; Neumann, T.; Berg, M. Phosphate immobilisation dynamics and interaction with arsenic sorption at redox transition zones in floodplain aquifers: Insights from the Red River Delta, Vietnam. J. Hazard. Mater. 2021, 411, 125128. [Google Scholar] [CrossRef]
- Muehe, E.M.; Morin, G.; Scheer, L.; Pape, P.L.; Esteve, I.; Daus, B.; Kappler, A. Arsenic(V) incorporation in vivianite during microbial reduction of arsenic(v)-bearing biogenic Fe(III) (oxyhydr)oxides. Environ. Sci. Technol. 2016, 50, 2281–2291. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, H.; Zhou, W.; Hu, S.; Zhang, B.; Dang, Z.; Liu, C. Effect of phosphate on ferrihydrite transformation and the associated arsenic behavior mediated by sulfate-reducing bacterium. J. Hazard. Mater. 2023, 448, 130863. [Google Scholar] [CrossRef]
- Schoepfer, V.A.; Burton, E.D.; Johnston, S.G.; Kraal, P. Phosphate loading alters schwertmannite transformation rates and pathways during microbial reduction. Sci. Total Environ. 2019, 657, 770–780. [Google Scholar] [CrossRef]
- Ding, S.; Wang, Y.; Yang, M.; Shi, R.; Ma, T.; Cui, G.; Li, X. Distribution and speciation of arsenic in seasonally stratified reservoirs: Implications for biotransformation mechanisms governing interannual variability. Sci. Total Environ. 2022, 806, 150925. [Google Scholar] [CrossRef]
- Burton, E.D.; Johnston, S.G.; Kocar, B.D. Arsenic mobility during flooding of contaminated soil: The effect of microbial sulfate reduction. Environ. Sci. Technol. 2014, 48, 13660–13667. [Google Scholar] [CrossRef]
- Li, D.; He, H.; Yang, M.; Zhang, X.; Guan, T.; Dai, W.; Li, Y.; Shao, H.; Ding, S.; Li, X. Arsenic distribution and partitioning in multiple media in a typical catchment in the Qinghai-Tibetan plateau: A comparison between freshwater and saltwater lakes. Environ. Res. 2024, 246, 118132. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, T.; Yan, Y.; Xie, X.; Abass, O.K.; Liu, C.; Zhao, Z.; Wang, Z. Effects of Fe-S-As coupled redox processes on arsenic mobilization in shallow aquifers of Datong Basin, northern China. Environ. Pollut. 2018, 237, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yan, L.; Sun, S.; Pi, K.; Shi, J.; Wang, Y. Arsenic biogeochemical cycling association with basin-scale dynamics of microbial functionality and organic matter molecular composition. Water Res. 2024, 251, 121117. [Google Scholar] [CrossRef] [PubMed]
- Xiu, W.; Wu, M.; Nixon, S.L.; Lloyd, J.R.; Bassil, N.M.; Gai, R.; Zhang, T.; Su, Z.; Guo, H. genome-resolved metagenomic analysis of groundwater: Insights into arsenic mobilization in biogeochemical interaction networks. Environ. Sci. Technol. 2022, 56, 10105–10119. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, W.; Shi, R.; Li, X.; Zhao, Z.; Xia, Z.; Li, D.; Li, Y.; Cui, G.; Ding, S. Factors and Mechanisms Affecting Arsenic Migration in Cultivated Soils Irrigated with Contained Arsenic Brackish Groundwater. Microorganisms 2024, 12, 2385. https://doi.org/10.3390/microorganisms12122385
Dai W, Shi R, Li X, Zhao Z, Xia Z, Li D, Li Y, Cui G, Ding S. Factors and Mechanisms Affecting Arsenic Migration in Cultivated Soils Irrigated with Contained Arsenic Brackish Groundwater. Microorganisms. 2024; 12(12):2385. https://doi.org/10.3390/microorganisms12122385
Chicago/Turabian StyleDai, Wenjing, Rongguang Shi, Xiaodong Li, Zhiqi Zhao, Zihan Xia, Dongli Li, Yan Li, Gaoyang Cui, and Shiyuan Ding. 2024. "Factors and Mechanisms Affecting Arsenic Migration in Cultivated Soils Irrigated with Contained Arsenic Brackish Groundwater" Microorganisms 12, no. 12: 2385. https://doi.org/10.3390/microorganisms12122385
APA StyleDai, W., Shi, R., Li, X., Zhao, Z., Xia, Z., Li, D., Li, Y., Cui, G., & Ding, S. (2024). Factors and Mechanisms Affecting Arsenic Migration in Cultivated Soils Irrigated with Contained Arsenic Brackish Groundwater. Microorganisms, 12(12), 2385. https://doi.org/10.3390/microorganisms12122385





