Cd-Resistant Plant Growth-Promoting Rhizobacteria Bacillus siamensis R27 Absorbed Cd and Reduced Cd Accumulation in Lettuce (Lactuca sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rhizosphere Samples and Isolation of Cd-Resistant Bacteria
2.2. Characterization of Cd Tolerance
2.3. Selection of Cd-Resistant PGPR
2.4. Bacterial Identification
2.5. Removal Effect of R27 Strain on Cd2+
2.6. SEM, TEM, and EDX Analysis
2.7. FTIR Analysis
2.8. Pot Experiment
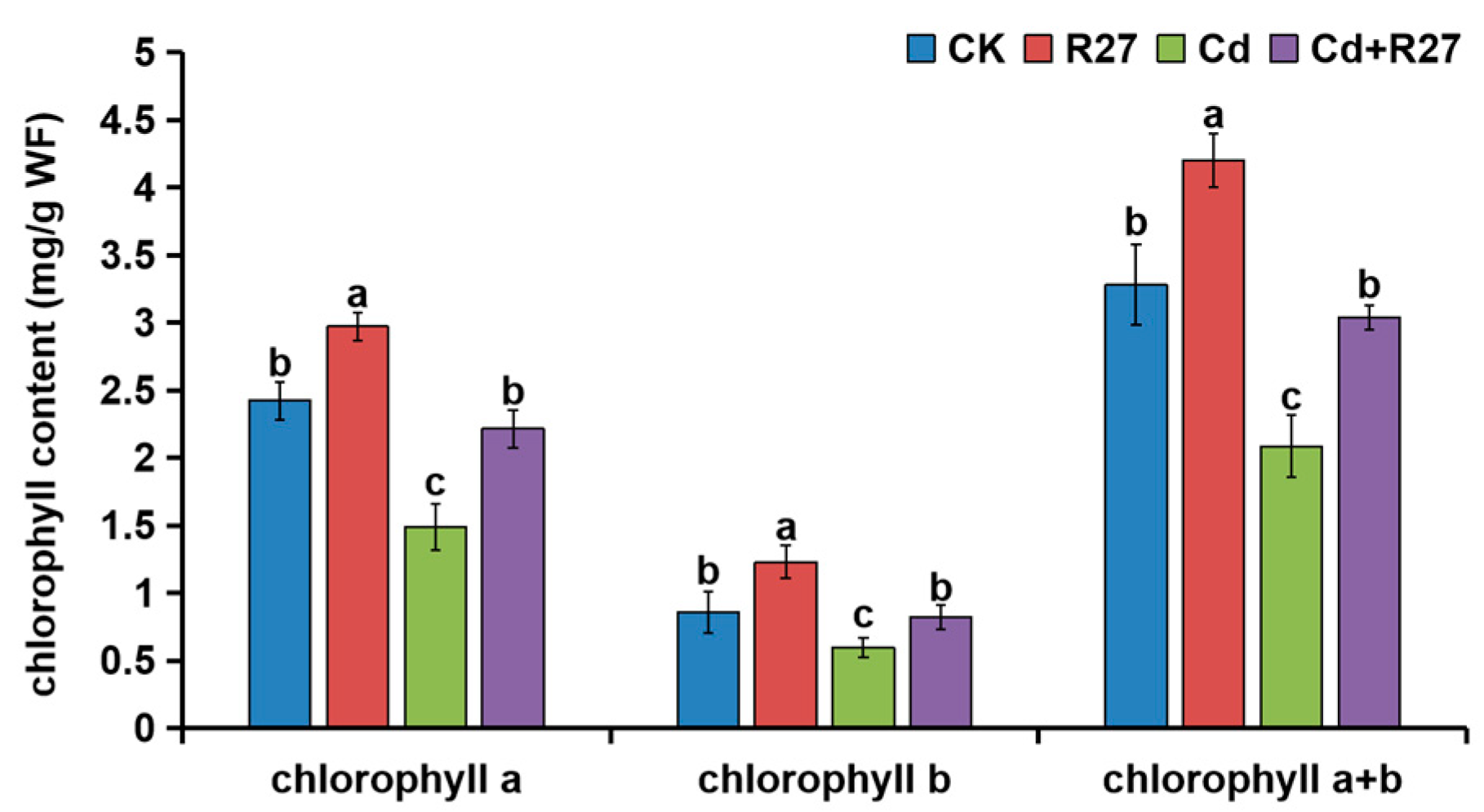
2.9. Chlorophyll Contents
2.10. Measurement of Antioxidant Activities
2.11. Transcript Analyses by qRT-PCR
2.12. Measurement of Proline Content
2.13. Determination of Cd Contents in Plant Tissues
2.14. Statistical Analysis
3. Results
3.1. Isolation of Cd-Resistant PGPR from Metal-Contaminated Soil
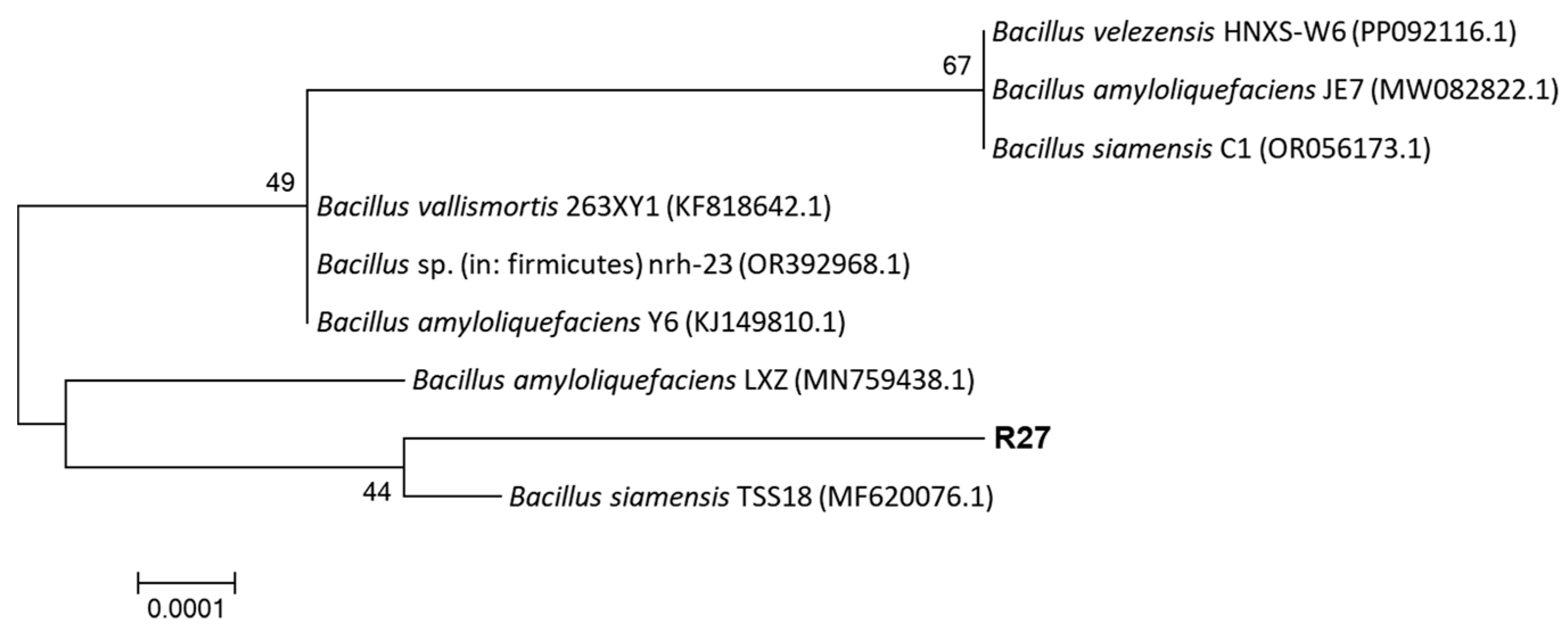
3.2. Identification of R27 Strain
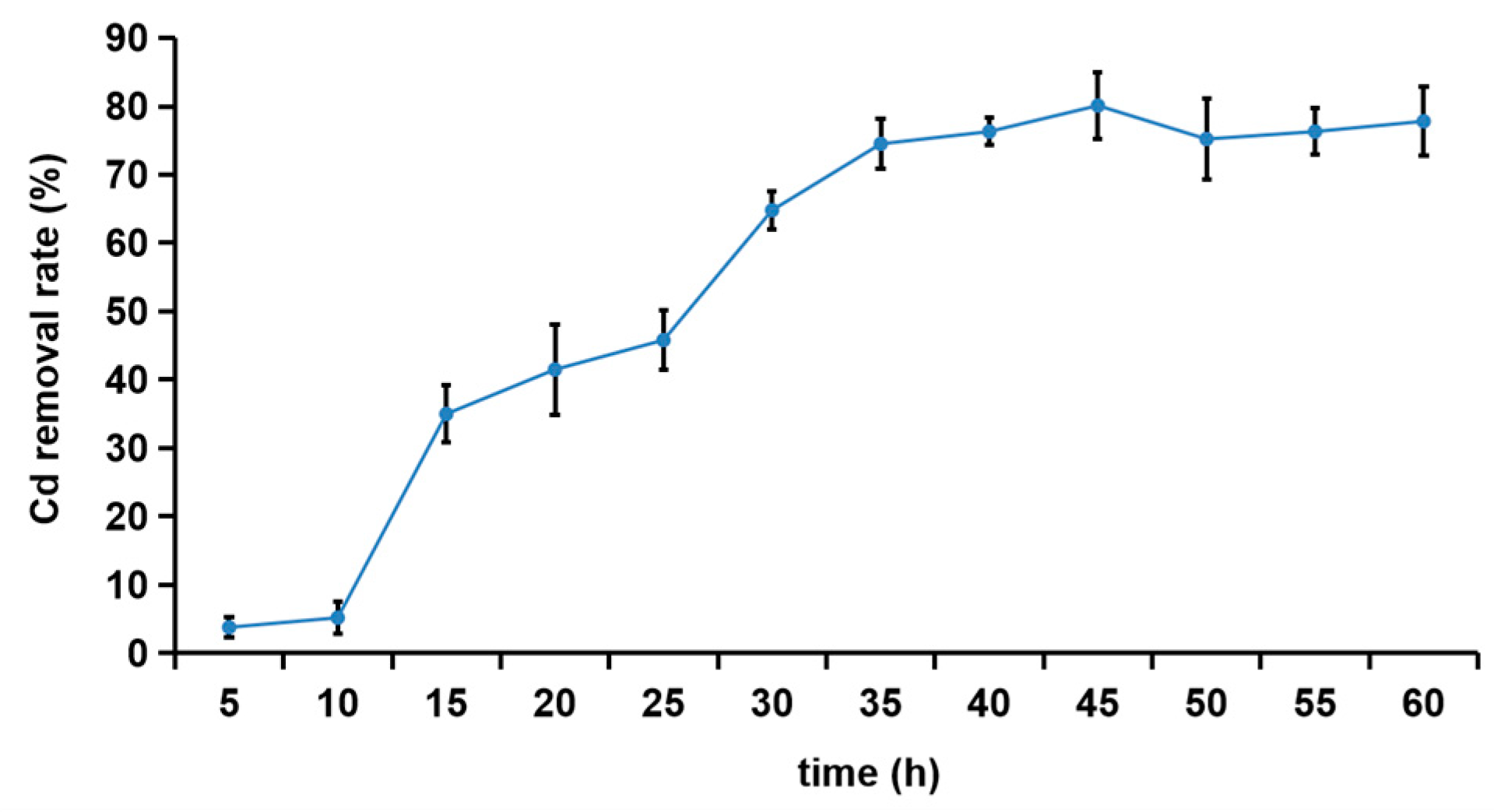
3.3. Removal Effect of Cd2+ by R27 Strain
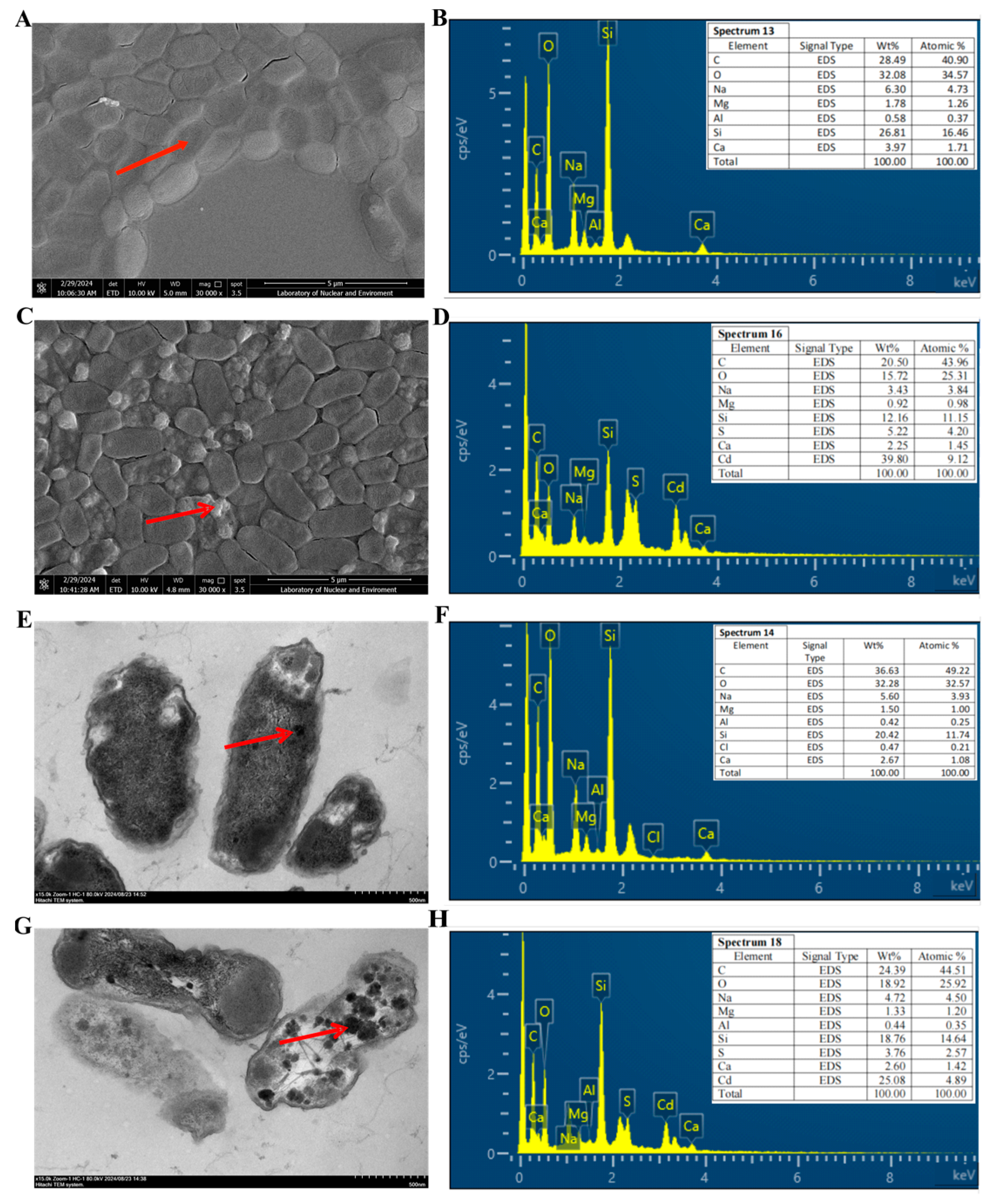
3.4. Adsorption Characteristic Analysis
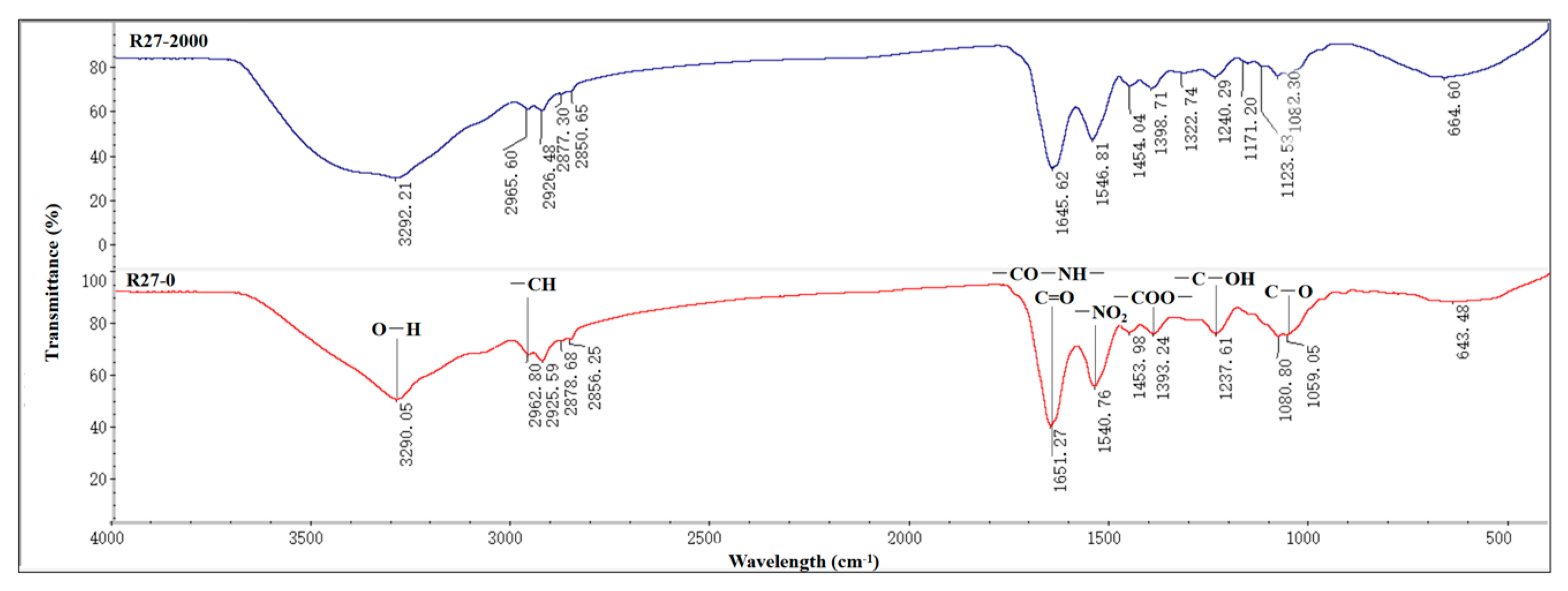
3.5. FTIR Spectroscopy of R 27 Strain
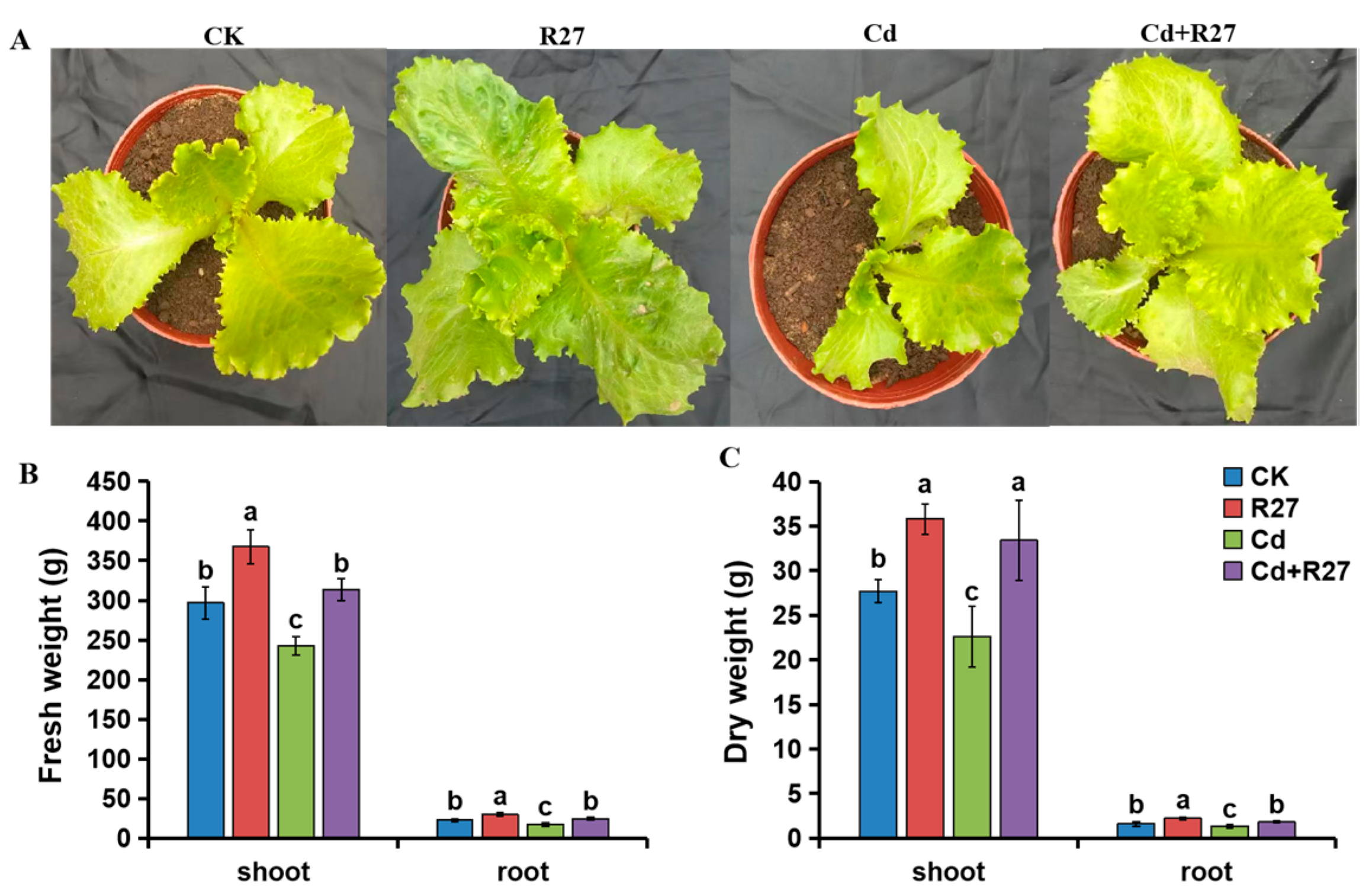
3.6. Effect of R27 Strain on Growth of Lettuce Seedlings
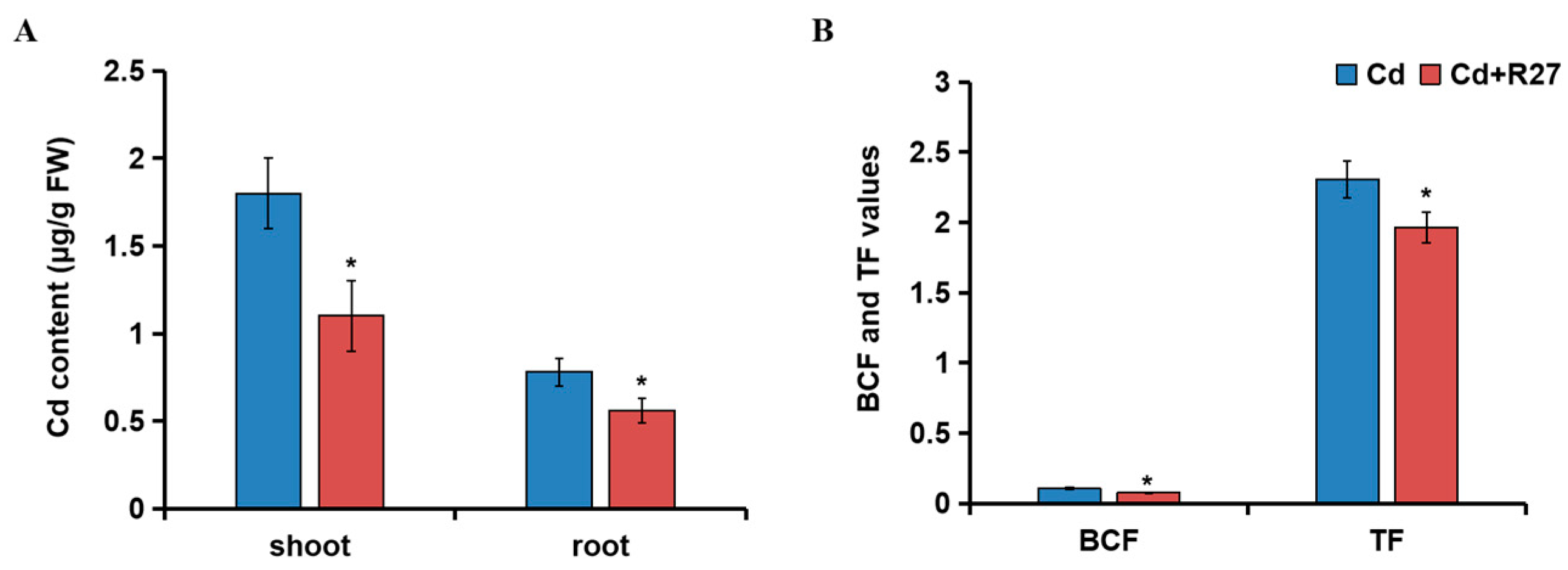
3.7. Effect of R27 Strain on Cd Accumulation, BCF, and TF
3.8. Effect of the R27 Strain on the Expression of Genes
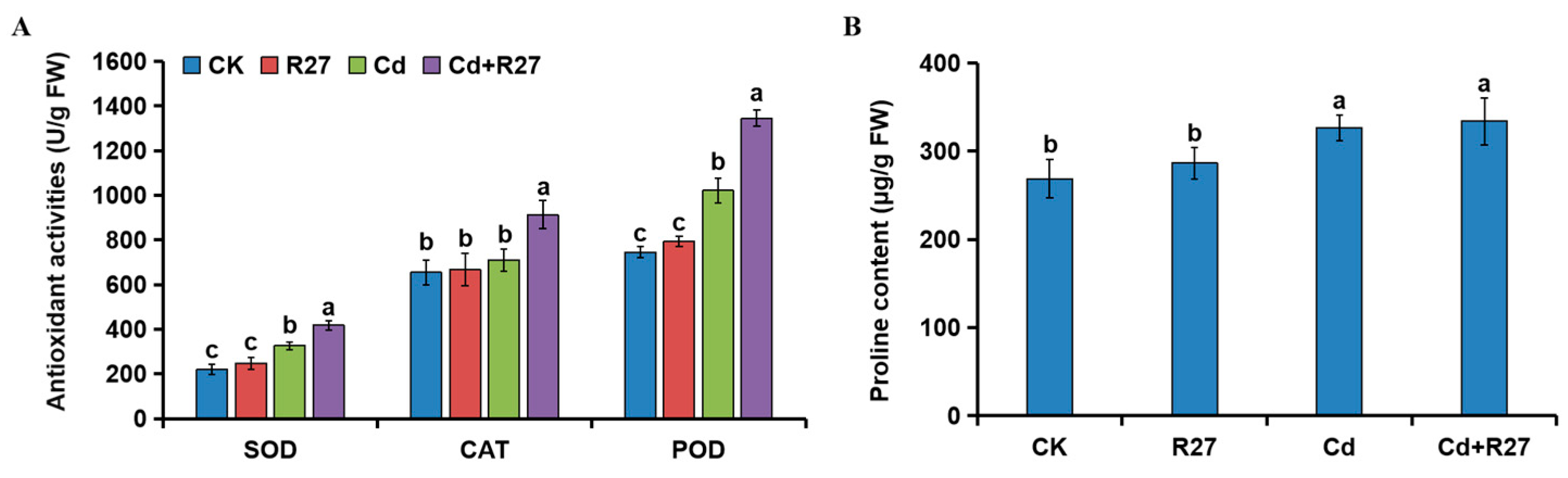
3.9. Effect of R27 on Antioxidant Activities and Proline Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schreinemachers, P.; Simmons, E.B.; Wopereis, M.C.S. Tapping the economic and nutritional power of vegetables. Glob. Food Secur. 2018, 16, 36–45. [Google Scholar] [CrossRef]
- Storey, M.; Anderson, P. Total fruit and vegetable consumption increases among consumers of frozen fruit and vegetables. Nutrition 2018, 46, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Yu, C.; Yan, C.; Liu, Y.; Liu, Y.; Jia, Y.; Lavelle, D.; An, G.; Zhang, W.; Zhang, L.; Han, R.; et al. Upregulation of a KN1 homolog by transposon insertion promotes leafy head development in lettuce. Proc. Natl. Acad. Sci. USA 2020, 117, 33668–33678. [Google Scholar] [CrossRef]
- Franca, F.; Albuuerque, A.M.A.; Almeida, A.C.; Silveira, P.B.; Filho, C.A.; Hazin, C.A.; Honorato, E.V. Heavy metals deposited in the culture of lettuce (Lactuca sativa L.) by the influence of vehicular traffic in Pernambuco, Brazil. Food Chem. 2017, 215, 171–176. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Zhong, Q.; Peijnenburg, W.; Vijver, M.G. Feasibility of Chinese cabbage (Brassica bara) and lettuce (Lactuca sativa) cultivation in heavily metals-contaminated soil after washing with biodegradable chelators. J. Clean Prod. 2018, 197, 479–490. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Tang, X.; Fan, X.; Yang, S.; Yao, L.; Li, Y.; Han, H. Isolation of urease-producing bacteria and their effects on reducing Cd and Pb accumulation in lettuce (Lactuca sativa L.). Environ. Sci. Pollut. Res. 2020, 27, 8707–8718. [Google Scholar] [CrossRef]
- Adhikari, S.; Ghosh, S.; Azahar, I.; Adhikari, A.; Shaw, A.K.; Konar, S.; Roy, S.; Hossain, Z. Sulfate improves cadmium tolerance by limiting cadmium accumulation, modulation of sulfur metabolism and antioxidant defense system in maize. Environ. Exp. Bot. 2018, 153, 143–162. [Google Scholar] [CrossRef]
- Intawongse, M.; Dean, J.R. Uptake of heavy metals by vegetable plants grown on contaminated soil and their bioavailability in the human gastrointestinal tract. Food Addit. Contam. 2006, 23, 36–48. [Google Scholar] [CrossRef]
- Rafiq, M.T.; Aziz, R.; Yang, X.; Xiao, W.; Stoffella, P.J.; Saghir, A.; Azam, M.; Li, T. Phytoavailability of cadmium (Cd) to Pak choi (Brassica chinensis L.) grown in Chinese soils: A model to evaluate the impact of soil Cd pollution on potential dietary toxicity. PLoS ONE 2014, 9, e111461. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, T.; Liu, L.; Ouyang, X. Impact of soil heavy metal pollution on food safety in China. PLoS ONE 2015, 10, e0135182. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Amna, K.M.A.; Javed, M.T.; Hayat, K.; Farooq, M.A.; Ali, A.; Ali, M.; Manghwar, H.; Jan, F.; Chaudhary, H.J. Individual and combinatorial application of Kocuria rhizophila and citric acid on phytoextraction of multi-metal contaminated soils by Glycine max L. Environ. Exp. Bot. 2019, 159, 23–33. [Google Scholar] [CrossRef]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef]
- Singh, S.; Singh, V.P.; Prasad, S.M.; Sharma, S.; Ramawat, N.; Dubey, N.K.; Chauhan, D.K. Interactive effect of silicon (Si) and salicylic acid (SA) in maize seedlings and their mechanisms of cadmium (Cd) toxicity alleviation. J. Plant Growth Regul. 2019, 38, 1587–1597. [Google Scholar] [CrossRef]
- Kumar, M.; Giri, V.P.; Pandey, S.; Gupta, A.; Bajpai, A.B.; Jenkins, S.; Siddique, K.H.M. Plant-growth-promoting rhizobacteria emerging as an effective bioinoculant to improve the growth, production, and stress tolerance of vegetable crops. Int. J. Mol. Sci. 2021, 22, 12245. [Google Scholar] [CrossRef]
- Glick, B. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Shahid, M.; Javed, M.T.; Masood, S.; Akram, M.S.; Azeem, M.; Ali, Q.; Gilani, R.; Basit, F.; Abid, A.; Lindberg, S. Serratia sp. CP-13 augments the growth of cadmium (Cd)-stressed Linum usitatissimum L. by limited Cd uptake, enhanced nutrient acquisition and antioxidative potential. J. Appl. Microbiol. 2019, 126, 1708–1721. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, S.; Golchin, A.; Shahryari, F.; Alamdari, P. PGPR inoculation of a contaminated soil affects plant growth and phytoavailability of Cd and Pb. Arch. Agron. Soil Sci. 2022, 68, 579–596. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Ma, C.; Wu, F.; Jin, X.; Dini-Andreote, F.; Wei, Z. Biochar amendment reduces cadmium uptake by stimulating cadmium-resistant PGPR in tomato rhizosphere. Chemosphere 2022, 307, 136138. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, Z.; Zhang, X.; Wang, G.; Li, R.; Qu, J.; Jin, Y. Alleviation of Cd phytotoxicity and enhancement of rape seedling growth by plant growth–promoting bacterium Enterobacter sp. Zm-123. Environ. Sci. Pollut. Res. 2020, 27, 33192–33203. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Javed, M.T.; Tanwir, K.; Akram, M.S.; Tazeen, S.K.; Saleem, M.H.; Masood, S.; Mujtaba, S.; Chaudhary, H.J. Plant growth-promoting Bacillus sp. strain SDA-4 confers Cd tolerance by physio-biochemical improvements, better nutrient acquisition and diminished Cd uptake in Spinacia oleracea L. Physiol. Mol. Biol. Plants 2020, 26, 2417–2433. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, F.; Rashid, A.; Mahmood, T.; Dawson, L. Effect of DTPA on Cd solubility in soil-accumulation and subsequent toxicity to lettuce. Chemosphere 2013, 90, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef]
- Harris, J.K.; Kelley, S.T.; Pace, N.R. New perspective on uncultured bacterial phylogenetic division OP11. Appl. Environ. Microbiol. 2004, 70, 845–849. [Google Scholar] [CrossRef]
- Duan, Y.; Pang, Z.; Yin, S.; Xiao, W.; Hu, H.; Xie, J. Screening and analysis of antifungal strains Bacillus subtilis JF-4 and B. amylum JF-5 for the biological control of fusarium wilt of banana. J. Fungi 2023, 9, 886. [Google Scholar] [CrossRef]
- Hartmann, M.; Berditsch, M.; Hawecker, J.; Ardakani, M.F.; Gerthsen, D.; Ulrich, A.S. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob. Agents Chemother. 2010, 54, 3132–3142. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Ashraf, M.; Iram, A. Drought stress induced changes in some organic substances in nodules and other plant parts of two potential legumes differing in salt tolerance. Flora 2005, 200, 535–546. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, X.; Tian, X.; Yue, M. Effects of CO2 laser pretreatment on drought stress resistance in wheat. J. Photochem. Photobiol. B. 2007, 90, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Patel, P.R.; Shaikh, S.S.; Sayyed, R.Z. Dynamism of PGPR in bioremediation and plant growth promotion in heavy metal contaminated soil. Indian J. Exp. Biol. 2016, 54, 286–290. [Google Scholar]
- Gupta, R.; Khan, F.; Alqahtani, F.M.; Hashem, M.; Ahmad, F. Plant growth-promoting Rhizobacteria (PGPR) assisted bioremediation of Heavy Metal Toxicity. Appl. Biochem. Biotechnol. 2024, 196, 2928–2956. [Google Scholar] [CrossRef]
- Verma, S.; Bhatt, P.; Verma, A.; Mudila, H.; Prasher, P.; Rene, E.R. Microbial technologies for heavy metal remediation: Effect of process conditions and current practices. Clean Technol. Environ. Policy 2023, 25, 1485–1507. [Google Scholar] [CrossRef]
- Chi, Y.; Huang, Y.; Wang, J.; Chen, X.; Chu, S.; Hayat, K.; Xu, Z.; Xu, H.; Zhou, P.; Zhang, D. Two plant growth promoting bacterial Bacillus strains possess different mechanisms in adsorption and resistance to cadmium. Sci. Total Environ. 2020, 741, 140422. [Google Scholar] [CrossRef]
- Siripornadulsil, S.; Siripornadulsil, W. Cadmium-tolerant bacteria reduce the uptake of cadmium in rice: Potential for microbial bioremediation. Ecotoxicol. Environ. Saf. 2013, 94, 94–103. [Google Scholar] [CrossRef]
- Khan, Z.; Rehman, A.; Hussain, S.Z.; Nisar, M.A.; Zulfiqar, S.; Shakoori, A.R. Cadmium resistance and uptake by bacterium, Salmonella enterica 43C, isolated from industrial effluent. Amb Express 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Q.; Lin, Y.; Hou, Y.; Deng, Z.; Liu, W.; Wang, H.; Xia, M. Biochemical and genetic basis of cadmium biosorption by Enterobacter ludwigii LY6, isolated from industrial contaminated soil. Environ. Pollut. 2020, 264, 114637. [Google Scholar] [CrossRef]
- Chen, Y.; Chao, Y.; Li, Y.; Lin, Q.; Bai, J.; Tang, L.; Wang, S.; Ying, R.; Qiua, R. Survival strategies of the plant-associated bacterium Enterobacter sp. strain EG16 under cadmium stress. Appl. Environ. Microbiol. 2016, 82, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Pramanik, K.; Sarkar, A.; Ghosh, P.K.; Soren, T.; Maiti, T.K. Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol. Environ. Saf. 2018, 156, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nie, Z.; He, L.; Wang, Q.; Sheng, X. Isolation of As-tolerant bacteria and their potentials of reducing As and Cd accumulation of edible tissues of vegetables in metal (loid)-contaminated soils. Sci. Total Environ. 2017, 579, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, C.M.; He, Z.; Sheng, X.; He, L. Effect of heavy metal-tolerant spore-forming bacteria on the cadmium and lead uptake of pepper. J. Agro Environ. Sci. 2018, 37, 1086–1093. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Zhao, J.; Guan, F.; Yao, D.; Wu, N.; Tian, J. The endophytic bacterium Bacillus koreensis 181-22 promotes rice growth and alleviates cadmium stress under cadmium exposure. Appl. Microbiol. Biotechnol. 2021, 105, 8517–8529. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Lin, Z.; Teng, D.; Zhao, Y.; Chen, S.; Hu, X. Screening of cadmium resistant bacteria and their growth promotion of Sorghum bicolor (L.) Moench Under Cadmium Stress. Ecotoxicol. Environ. Saf. 2024, 272, 116012. [Google Scholar] [CrossRef]
- Tanwir, K.; Javed, M.T.; Abbas, S.; Shahid, M.; Akram, M.S.; Chaudhary, H.J.; Iqbal, M. Serratia sp. CP-13 alleviates Cd toxicity by morpho-physio-biochemical improvements, antioxidative potential and diminished Cd uptake in Zea mays L. cultivars differing in Cd tolerance. Ecotoxicol. Environ. Saf. 2021, 208, 111584. [Google Scholar] [CrossRef]
- Awan, S.A.; Ilyas, N.; Khan, I.; Raza, M.A.; Rehman, A.U.; Rizwan, M.; Rastogi, A.; Tariq, R.; Brestic, M. Bacillus siamensis reduces cadmium accumulation and improves growth and antioxidant defense system in two wheat (Triticum aestivum L.) varieties. Plants 2020, 9, 878. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. Ros as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2102, 217037. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Naveed, M.; Mitter, B.; Sessitsch, A. Cadmiumtolerant bacteria induce metal stress tolerance in cereals. Environ. Sci. Pollut. Res. 2014, 21, 11054–11065. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.A.; Syed, J.H.; Eqani, S.A.M.A.S.; Munis, M.F.H.; Chaudhary, H.J. Effect of plant growth-promoting rhizobacteria inoculation on cadmium (Cd) uptake by Eruca sativa. Environ. Sci. Pollut. Res. 2015, 22, 9275–9283. [Google Scholar] [CrossRef]
- Chmielowska-Bak, J.; Lefèvre, I.; Lutts, S.; Kulik, A.; Deckert, J. Effect of cobalt chloride on soybean seedlings subjected to cadmium stress. Acta Soc. Bot. Pol. 2014, 83, 201–207. [Google Scholar] [CrossRef]

| Isolate | MIC (mg/L) | Phosphate Solubilization (mg/L) | IAA Synthesis (mg/L) | Siderophores (mg/L) |
|---|---|---|---|---|
| R5 | 500 | 257 ± 15.66 | 4.54 ± 0.28 | 8.37 ± 1.14 |
| R12 | 1500 | - | 23.76 ± 1.76 | - |
| R15 | 1000 | 7.73 ± 1.43 | - | 1.35 ± 0.56 |
| R27 | 2000 | 385.11 ± 8.54 | 35.92 ± 2.15 | 3.34 ± 0.83 |
| R30 | 1000 | 143.78 ± 5.69 | 18.92 ± 3.33 | - |
| R32 | 1500 | - | 3.89 ± 0.98 | 6.44 ± 1.15 |
| Physiological and Biochemical Experiments | Result |
|---|---|
| Gram’s stain test | + |
| Starch hydrolysis test | + |
| Gelatin hydrolysis test | + |
| Indole reaction | + |
| Methyl red test | − |
| Oxidase reaction | + |
| Hydrogen peroxidase reaction | + |
| V-P reaction | + |
| Nitrate utilization test | + |
| H2S production | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Huang, Y.; Zheng, Q.; Zhan, M.; Hu, Z.; Ji, H.; Zhu, D.; Zhao, X. Cd-Resistant Plant Growth-Promoting Rhizobacteria Bacillus siamensis R27 Absorbed Cd and Reduced Cd Accumulation in Lettuce (Lactuca sativa L.). Microorganisms 2024, 12, 2321. https://doi.org/10.3390/microorganisms12112321
Liu S, Huang Y, Zheng Q, Zhan M, Hu Z, Ji H, Zhu D, Zhao X. Cd-Resistant Plant Growth-Promoting Rhizobacteria Bacillus siamensis R27 Absorbed Cd and Reduced Cd Accumulation in Lettuce (Lactuca sativa L.). Microorganisms. 2024; 12(11):2321. https://doi.org/10.3390/microorganisms12112321
Chicago/Turabian StyleLiu, Shaofang, Yushan Huang, Qinyuan Zheng, Mengting Zhan, Zhihong Hu, Hongjie Ji, Du Zhu, and Xia Zhao. 2024. "Cd-Resistant Plant Growth-Promoting Rhizobacteria Bacillus siamensis R27 Absorbed Cd and Reduced Cd Accumulation in Lettuce (Lactuca sativa L.)" Microorganisms 12, no. 11: 2321. https://doi.org/10.3390/microorganisms12112321
APA StyleLiu, S., Huang, Y., Zheng, Q., Zhan, M., Hu, Z., Ji, H., Zhu, D., & Zhao, X. (2024). Cd-Resistant Plant Growth-Promoting Rhizobacteria Bacillus siamensis R27 Absorbed Cd and Reduced Cd Accumulation in Lettuce (Lactuca sativa L.). Microorganisms, 12(11), 2321. https://doi.org/10.3390/microorganisms12112321






