Prevalence and Antimicrobial Susceptibility of Foodborne Pathogens from Raw Livestock Meat in China, 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Identification of Pathogens
2.3. AMR Testing
2.4. Statistical Analysis
3. Results
3.1. Prevalence of L. monocytogenes, Salmonella, and DEC in Raw Livestock Meat
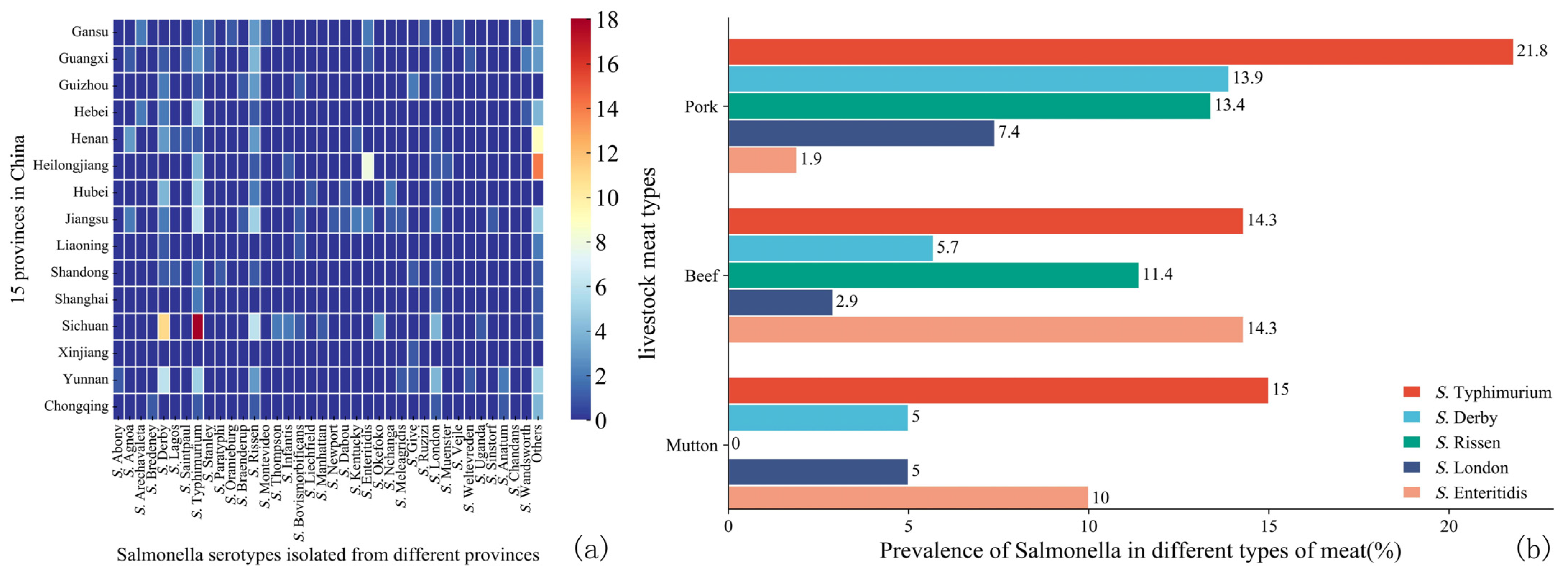
3.2. Serotypes and AMR of Salmonella in Raw Livestock Meat
3.2.1. Serotypes of Salmonella
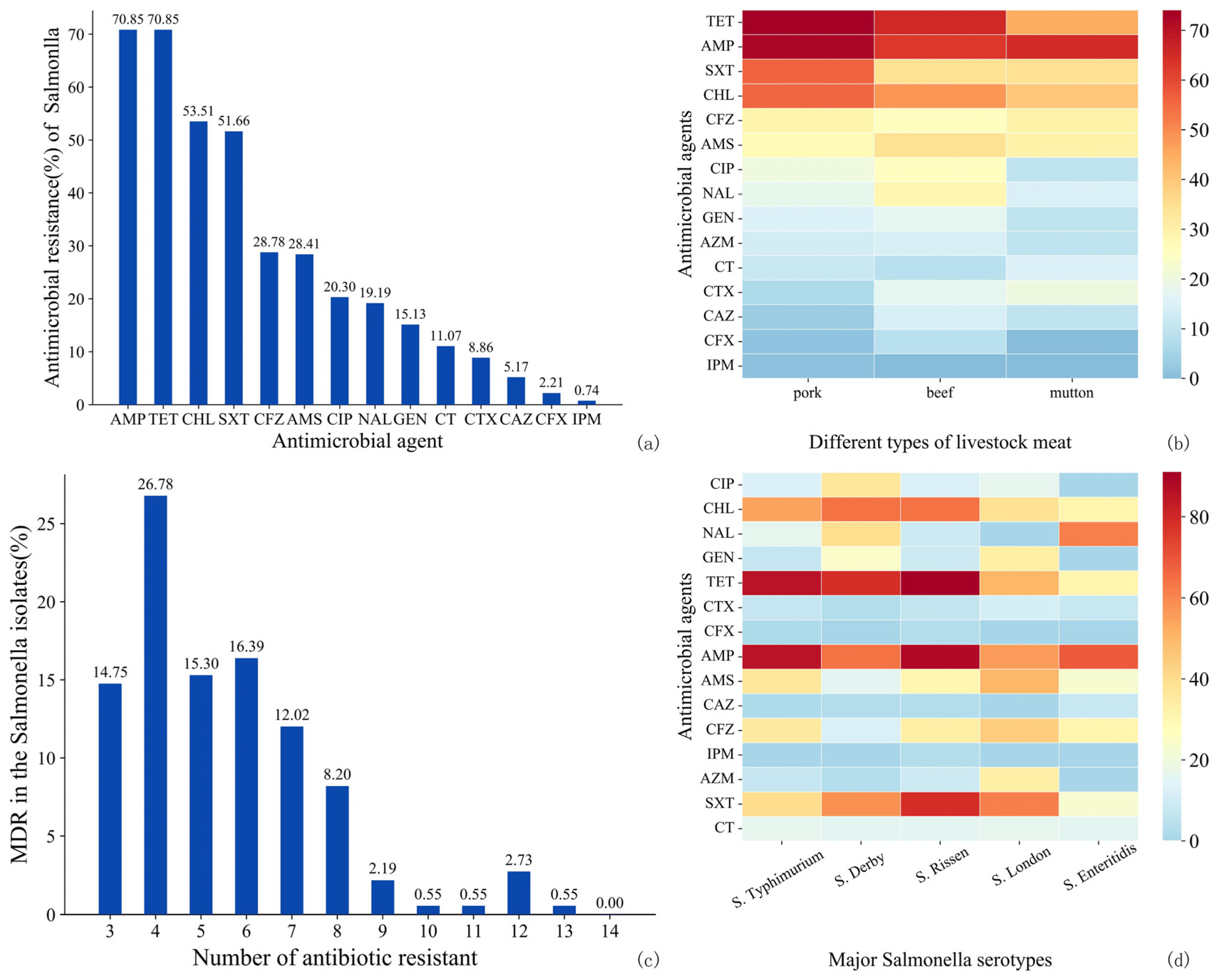
3.2.2. AMR of Salmonella
3.3. Pathotypes and AMR of DEC in Raw Livestock Meat
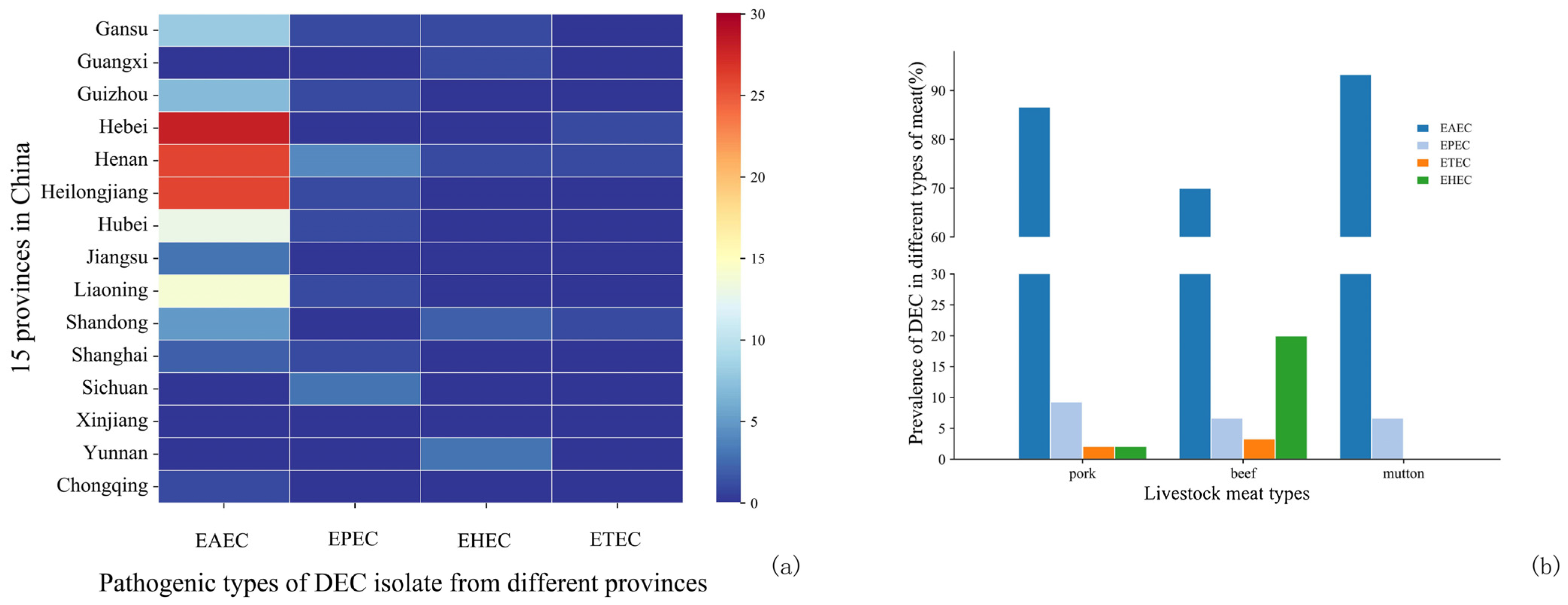
3.3.1. Pathotypes of DEC
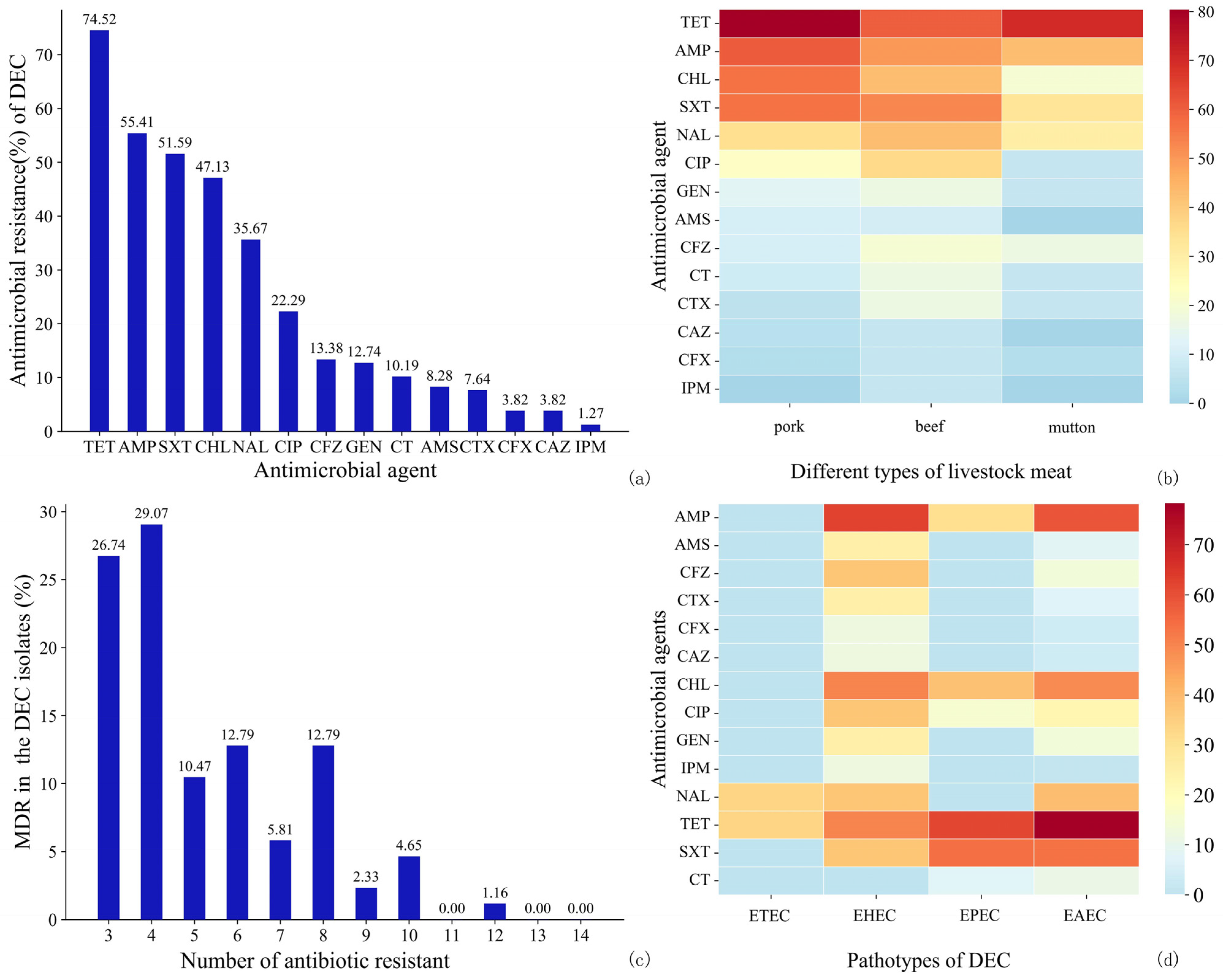
3.3.2. AMR of DEC
4. Discussion
4.1. Prevalence of Foodborne Pathogens in Raw Livestock Meat
4.2. Serotypes of Salmonella and Pathotypes of DEC
4.3. AMR of Salmonella and DEC
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- He, S.; Shi, X. Microbial Food Safety in China: Past, Present, and Future. Foodborne Pathog. Dis. 2021, 18, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Warmate, D.; Onarinde, B.A. Food safety incidents in the red meat industry: A review of foodborne disease outbreaks linked to the consumption of red meat and its products, 1991 to 2021. Int. J. Food Microbiol. 2023, 398, 110240. [Google Scholar] [CrossRef]
- Bélanger, P.; Tanguay, F.; Hamel, M.; Phypers, M. An overview of foodborne outbreaks in Canada reported through Outbreak Summaries: 2008-2014. Can. Commun. Dis. Rep. Relev. Des Mal. Transm. Au Can. 2015, 41, 254–262. [Google Scholar] [CrossRef]
- Bryan, F.L. Foodborne Diseases in the United States Associated with Meat and Poultry. J. Food Prot. 1980, 43, 140–150. [Google Scholar] [CrossRef]
- Jeffer, S.B.; Kassem, I.I.; Kharroubi, S.A.; Abebe, G.K. Analysis of Food Safety Management Systems in the Beef Meat Processing and Distribution Chain in Uganda. Foods 2021, 10, 2244. [Google Scholar] [CrossRef]
- Omer, M.K.; Álvarez-Ordoñez, A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, O.A. A Systematic Review of Bacterial Foodborne Outbreaks Related to Red Meat and Meat Products. Foodborne Pathog. Dis. 2018, 15, 598–611. [Google Scholar] [CrossRef]
- The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. Eur. Food Saf. Auth. 2018, 16, e05500. [CrossRef]
- Fotopoulou, E.T.; Jenkins, C.; Painset, A.; Amar, C. Listeria monocytogenes: The silent assassin. J. Med. Microbiol. 2024, 73, 001800. [Google Scholar] [CrossRef]
- Rogalla, D.; Bomar, P.A. Listeria Monocytogenes; BTI—StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Félix, B.; Feurer, C.; Maillet, A.; Guillier, L.; Boscher, E.; Kerouanton, A.; Denis, M.; Roussel, S. Population Genetic Structure of Listeria monocytogenes Strains Isolated from the Pig and Pork Production Chain in France. Front. Microbiol. 2018, 9, 684. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Shen, J.; Zhang, G.; Yang, J.; Zhao, L.; Jiang, Y.; Guo, D.; Wang, X.; Zhi, S.; Xu, X.; Dong, Q.; et al. Prevalence, antibiotic resistance, and molecular epidemiology of Listeria monocytogenes isolated from imported foods in China during 2018 to 2020. Int. J. Food Microbiol. 2022, 382, 109916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; Butler, F.J.B. Risk assessment of pork products produced in China. Biosyst. Food Eng. Res. Rev. 2018, 48, 48–51. [Google Scholar]
- Yang, S.C.; Lin, C.H.; Aljuffali, I.A.; Fang, J.Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, G.; Huang, Y.; Zhang, J.; Cui, L.; Wu, Q. Prevalence and Characterization of Atypical Enteropathogenic Escherichia coli Isolated from Retail Foods in China. J. Food Prot. 2018, 81, 1761–1767. [Google Scholar] [CrossRef]
- Li, W.; Pires, S.M.; Liu, Z.; Ma, X.; Liang, J.; Jiang, Y.; Chen, J.; Liang, J.; Wang, S.; Wang, L.; et al. Surveillance of foodborne disease outbreaks in China, 2003–2017. Food Control 2020, 118, 107359. [Google Scholar] [CrossRef]
- Aijuka, M.; Buys, E.M.J.F.M. Persistence of foodborne diarrheagenic Escherichia coli in the agricultural and food production environment: Implications for food safety and public health. Food Microbiol. 2019, 82, 363–370. [Google Scholar] [CrossRef]
- Enciso-Martínez, Y.; González-Aguilar, G.A.; Martínez-Téllez, M.A.; González-Pérez, C.J.; Valencia-Rivera, D.E.; Barrios-Villa, E.; Ayala-Zavala, J.F. Relevance of tracking the diversity of Escherichia coli pathotypes to reinforce food safety. Int. J. Food Microbiol. 2022, 374, 109736. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Food Safety and Inspection Service. Raw Pork Products Exploratory Sampling Project (RPPESP). Available online: https://mdpi-res.com/data/mdpi_references_guide_v9.pdf (accessed on 24 October 2024).
- Abbasi, E.A.-O.; Mondanizadeh, M.A.-O.; van Belkum, A.A.-O.; Ghaznavi-Rad, E.A.-O. Multi-Drug-Resistant Diarrheagenic Escherichia coli Pathotypes in Pediatric Patients with Gastroenteritis from Central Iran. Infect. Drug Resist. 2020, 13, 1387–1396. [Google Scholar] [CrossRef]
- Aboyans, V.; Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Moraga, P.; GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar]
- UNICEF. Diarrhoea-UNICEF DATA. Diarrhoea Remains a Leading Killer of Young Children, Despite the Availability of a Simple Treatment Solution. Available online: https://data.unicef.org/topic/child-health/diarrhoeal-disease/ (accessed on 24 October 2024).
- Chiyangi, H.; Muma, J.B.; Malama, S.; Manyahi, J.; Abade, A.; Kwenda, G.; Matee, M.I. Identification and antimicrobial resistance patterns of bacterial enteropathogens from children aged 0–59 months at the University Teaching Hospital, Lusaka, Zambia: A prospective cross sectional study. BMC Infect. Dis. 2017, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, J.; Li, Y.; Xie, S.; Jiang, Y.; Wu, Y.; Ye, Y.; Yang, H.; Mo, H.; Situ, C.; et al. The 12 Gastrointestinal Pathogens Spectrum of Acute Infectious Diarrhea in a Sentinel Hospital, Shenzhen, China. Front. Microbiol. 2016, 7, 1926. [Google Scholar] [CrossRef] [PubMed]
- Milios, K.T.; Drosinos, E.H.; Zoiopoulos, P.E.J.F.C. Food Safety Management System validation and verification in meat industry: Carcass sampling methods for microbiological hygiene criteria–A review. Food Control 2014, 43, 74–81. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Hou, H.; Lu, Y.; Yu, J.; Mao, L.; Mao, L.; Sun, Z. Characteristics of diarrheagenic Escherichia coli among children under 5 years of age with acute diarrhea: A hospital based study. BMC Infect. Dis. 2018, 18, 63. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Q.; Zhang, J.; Zhu, X. Occurrence and Characterization of Enteropathogenic Escherichia coli (EPEC) in Retail Ready-to-Eat Foods in China. Foodborne Pathog. Dis. 2016, 13, 49–55. [Google Scholar] [CrossRef]
- Nørrung, B.; Andersen, J.; Buncic, S. Main Concerns of Pathogenic Microorganisms in Meat; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–29. [Google Scholar]
- Fegan, N.; Jenson, I. The role of meat in foodborne disease: Is there a coming revolution in risk assessment and management? Meat Sci. 2018, 144, 22–29. [Google Scholar] [CrossRef]
- Liu, J.; Bai, L.; Li, W.; Han, H.; Fu, P.; Ma, X.; Bi, Z.; Yang, X.; Zhang, X.; Zhen, S.; et al. Trends of foodborne diseases in China: Lessons from laboratory-based surveillance since 2011. Front. Med. 2018, 12, 48–57. [Google Scholar] [CrossRef]
- GB 4789.17-2003; Microbiological Examination of Food Hygiene-Examination of Meat and Meat Products. Standardization Administration of China: Beijing, China, 2003.
- GB 4789.30-2016; National Food Safety Standard Food Microbiology Detection Listeria monocytogenes. Standardization Administration of China: Beijing, China, 2016.
- GB 4789.4-2016; National Food Safety Standard Food Microbiology Detection Salmonella. Standardization Administration of China: Beijing, China, 2016.
- GB 4789.6-2016; National Food Safety Standard Food Microbiology Detection Escherichia coli. Standardization Administration of China: Beijing, China, 2016.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PE, USA, 2021. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows; Version 26.0; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- Zhang, H.; Luo, X.; Aspridou, Z.; Misiou, O.; Dong, P.; Zhang, Y. The Prevalence and Antibiotic-Resistant of Listeria monocytogenes in Livestock and Poultry Meat in China and the EU from 2001 to 2022: A Systematic Review and Meta-Analysis. Foods 2023, 12, 769. [Google Scholar] [CrossRef]
- Li, W.; Bai, L.; Fu, P.; Han, H.; Liu, J.; Guo, Y. The Epidemiology of Listeria monocytogenes in China. Foodborne Pathog. Dis. 2018, 15, 459–466. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, W.; Sun, T.; Gorris, L.G.M.; Wang, X.; Liu, B.; Dong, Q. The prevalence of Listeria monocytogenes in meat products in China: A systematic literature review and novel meta-analysis approach. Int. J. Food Microbiol. 2020, 312, 108358. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z. Overview of Food Safety Situation in China. In Food Safety in China: Science, Technology, Management and Regulation; Wiley: New York, NY, USA, 2017; pp. 15–28. [Google Scholar]
- Cavalcanti, A.A.C.; Limeira, C.H.; Siqueira, I.N.; Lima, A.C.; Medeiros, F.J.P.; Souza, J.G.; Medeiros, N.G.A.; Oliveira Filho, A.A.; Melo, M.A. The prevalence of Listeria monocytogenes in meat products in Brazil: A systematic literature review and meta-analysis. Res. Vet. Sci. 2022, 145, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-j.; Zhang, X.; Wang, X.-y.; Su, Y.-l.; Li, P.; Wang, S.J.F.C. Isolation and characterization of Listeria monocytogenes in Chinese food obtained from the central area of China. Food Control 2017, 74, 9–16. [Google Scholar] [CrossRef]
- Little, C.L.; Richardson, J.F.; Owen, R.J.; de Pinna, E.; Threlfall, E.J. Campylobacter and Salmonella in raw red meats in the United Kingdom: Prevalence, characterization and antimicrobial resistance pattern, 2003-2005. Food Microbiol. 2008, 25, 538–543. [Google Scholar] [CrossRef]
- Carraturo, F.; Gargiulo, G.; Giorgio, A.; Aliberti, F.; Guida, M. Prevalence, Distribution, and Diversity of Salmonella spp. in Meat Samples Collected from Italian Slaughterhouses. J. Food Sci. 2016, 81, M2545–M2551. [Google Scholar] [CrossRef]
- FSAI. Microbiological Safety of Raw Minced Beef and Beef Burgers on Retail Sale in Ireland (11NS1); FSAI: Dublin, Ireland, 2013. [Google Scholar]
- Bishop, H.; Evans, J.; Eze, J.I.; Webster, C.; Humphry, R.W.; Beattie, R.; White, J.; Couper, J.; Allison, L.; Brown, D.; et al. Bacteriological Survey of Fresh Minced Beef on Sale at Retail Outlets in Scotland in 2019: Three Foodborne Pathogens, Hygiene Process Indicators, and Phenotypic Antimicrobial Resistance. J. Food Prot. 2022, 85, 1370–1379. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union one health 2019 zoonoses report. Efsa J. 2021, 19, e06406. [Google Scholar]
- Nimri, L.; Abu Al-Dahab, F.; Batchoun, R. Foodborne bacterial pathogens recovered from contaminated shawarma meat in northern Jordan. J. Infect. Dev. Ctries. 2014, 8, 1407–1414. [Google Scholar] [CrossRef]
- Iyer, A.; Kumosani, T.; Yaghmoor, S.; Barbour, E.; Azhar, E.; Harakeh, S. Escherichia coli and Salmonella spp. in meat in Jeddah, Saudi Arabia. J. Infect. Dev. Ctries. 2013, 7, 812–818. [Google Scholar] [CrossRef]
- Bantawa, K.; Rai, K.; Subba Limbu, D.; Khanal, H. Food-borne bacterial pathogens in marketed raw meat of Dharan, eastern Nepal. BMC Res. Notes 2018, 11, 618. [Google Scholar] [CrossRef]
- Fallah, N.; Ghaemi, M.; Ghazvini, K.; Rad, M.; Jamshidi, A. Occurrence, pathotypes, and antimicrobial resistance profiles of diarrheagenic Escherichia coli strains in animal source food products from public markets in Mashhad, Iran. Food Control 2021, 121, 107640. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Q.; Zhang, J.; Huang, J.; Chen, L.; Wu, S.; Zeng, H.; Wang, J.; Chen, M.; Wu, H.; et al. Prevalence, Bacterial Load, and Antimicrobial Resistance of Salmonella Serovars Isolated from Retail Meat and Meat Products in China. Front. Microbiol. 2019, 10, 2121. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Chen, H.; Geng, J.; Wu, R.A.; Wang, X.; Ding, T. Prevalence, serovar distribution, and antibiotic resistance of Salmonella spp. isolated from pork in China: A systematic review and meta-analysis. Int. J. Food Microbiol. 2022, 361, 109473. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Qu, D.; Zhang, X.; Shen, J.; Cui, S.; Shi, Y.; Xi, M.; Sheng, M.; Zhi, S.; Meng, J. Prevalence and characterization of Salmonella serovars in retail meats of marketplace in Shaanxi, China. Int. J. Food Microbiol. 2010, 141, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef]
- Volf, J.; Stepanova, H.; Matiasovic, J.; Kyrova, K.; Sisak, F.; Havlickova, H.; Leva, L.; Faldyna, M.; Rychlik, I. Salmonella enterica serovar Typhimurium and Enteritidis infection of pigs and cytokine signalling in palatine tonsils. Vet. Microbiol. 2012, 156, 127–135. [Google Scholar] [CrossRef]
- Boyen, F.; Pasmans, F.; Van Immerseel, F.; Morgan, E.; Adriaensen, C.; Hernalsteens, J.P.; Decostere, A.; Ducatelle, R.; Haesebrouck, F. Salmonella Typhimurium SPI-1 genes promote intestinal but not tonsillar colonization in pigs. Microbes Infect. 2006, 8, 2899–2907. [Google Scholar] [CrossRef]
- Castagna, S.M.F.; Schwarz, P.; Canal, C.W.; Cardoso, M. Presença de Salmonella sp. no trato intestinal e em tonsilas/linfonodos submandibulares de suínos ao abate. Arq. Bras. De Med. Veterinária E Zootec. 2004, 56, 300–306. [Google Scholar] [CrossRef]
- Ng, K.C.; Rivera, W.L. Antimicrobial Resistance of Salmonella enterica Isolates from Tonsil and Jejunum with Lymph Node Tissues of Slaughtered Swine in Metro Manila, Philippines. ISRN Microbiol. 2014, 2014, 364265. [Google Scholar] [CrossRef]
- Van Parys, A.; Boyen, F.; Volf, J.; Verbrugghe, E.; Leyman, B.; Rychlik, I.; Haesebrouck, F.; Pasmans, F. Salmonella Typhimurium resides largely as an extracellular pathogen in porcine tonsils, independently of biofilm-associated genes csgA, csgD and adrA. Vet. Microbiol. 2010, 144, 93–99. [Google Scholar] [CrossRef]
- Botteldoorn, N.; Heyndrickx, M.; Rijpens, N.; Grijspeerdt, K.; Herman, L. Salmonella on pig carcasses: Positive pigs and cross contamination in the slaughterhouse. J. Appl. Microbiol. 2003, 95, 891–903. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomes-Neves, E.; Antunes, P.; Tavares, A.; Themudo, P.; Cardoso, M.F.; Gärtner, F.; Costa, J.M.; Peixe, L. Salmonella cross-contamination in swine abattoirs in Portugal: Carcasses, meat and meat handlers. Int. J. Food Microbiol. 2012, 157, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Abdlla, Y.; Alsanjary, R. The molecular identification of diarrheagenic Escherichia coli (DEC) isolated from meat and meat products. Iraqi J. Vet. Sci. 2022, 37, 9–15. [Google Scholar] [CrossRef]
- Tian, L.Z.N.; Duan, B.; Yang, Y.; Zhao, Z.; Yang, W.; Wang, H.; Li, J. Analysis of microbial contamination of fresh animal and poultry meat in China’s market—Based on China Knowledge Network Database. Shandong Anim. Husb. Vet. Med. 2022, 43, 36–41+44. [Google Scholar]
- Zhang, S.; Zhu, X.; Wu, Q.; Zhang, J.; Xu, X.; Li, H. Prevalence and characterization of Escherichia coli O157 and O157:H7 in retail fresh raw meat in South China. Ann. Microbiol. 2015, 65, 1993–1999. [Google Scholar] [CrossRef]
- Khan, S.B.; Zou, G.; Xiao, R.; Cheng, Y.; Rehman, Z.U.; Ali, S.; Memon, A.M.; Fahad, S.; Ahmad, I.; Zhou, R. Prevalence, quantification and isolation of pathogenic shiga toxin Escherichia coli O157:H7 along the production and supply chain of pork around Hubei Province of China. Microb. Pathog. 2018, 115, 93–99. [Google Scholar] [CrossRef]
- Broadway, P.R.; Brooks, J.C.; Mollenkopf, D.F.; Calle, M.A.; Loneragan, G.H.; Miller, M.F.; Carroll, J.A.; Sanchez, N.C.B.; Wittum, T.E. Prevalence and Antimicrobial Susceptibility of Salmonella Serovars Isolated from U.S. Retail Ground Pork. Foodborne Pathog. Dis. 2021, 18, 219–227. [Google Scholar] [CrossRef]
- Ripon, R.K.; Motahara, U.; Ahmed, A.; Devnath, N.; Mahua, F.A.; Hashem, R.B.; Ishadi, K.S.; Alam, A.; Sujan, M.S.H.; Sarker, M.S. Exploring the prevalence of antibiotic resistance patterns and drivers of antibiotics resistance of Salmonella in livestock and poultry-derived foods: A systematic review and meta-analysis in Bangladesh from 2000 to 2022. JAC-Antimicrob. Resist. 2023, 5, dlad059. [Google Scholar] [CrossRef]
- Inbaraj, S.; Agrawal, R.K.; Thomas, P.; Mohan, C.; Agarwal, R.K.S.; Verma, M.R.; Chaudhuri, P. Antimicrobial resistance in Indian isolates of non typhoidal Salmonella of livestock, poultry and environmental origin from 1990 to 2017. Comp. Immunol. Microbiol. Infect. Dis. 2022, 80, 101719. [Google Scholar] [CrossRef]
- Li, M.C.; Wang, F.; Li, F. Identification and molecular characterization of antimicrobial-resistant shiga toxin-producing Escherichia coli isolated from retail meat products. Foodborne Pathog. Dis. 2011, 8, 489–493. [Google Scholar] [CrossRef]
- Manafi, L.; Aliakbarlu, J.; Dastmalchi Saei, H. Antibiotic resistance and biofilm formation ability of Salmonella serotypes isolated from beef, mutton, and meat contact surfaces at retail. J. Food Sci. 2020, 85, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Pławińska-Czarnak, J.; Wódz, K.; Kizerwetter-Świda, M.; Bogdan, J.; Kwieciński, P.; Nowak, T.; Strzałkowska, Z.; Anusz, K. Multi-Drug Resistance to Salmonella spp. When Isolated from Raw Meat Products. Antibiotics 2022, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; M’Ikanatha, N.M.; Nyirabahizi, E.; McDermott, P.F.; Tate, H. Antimicrobial resistance in non-Typhoidal Salmonella from retail poultry meat by antibiotic usage-related production claims—United States, 2008–2017. Int. J. Food Microbiol. 2021, 342, 109044. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, R.; Akhtari, L. Antimicrobial resistance and integron gene cassette arrays in commensal Escherichia coli from human and animal sources in IRI. Gut Pathog. 2016, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Overdevest, I.; Willemsen, I.; Rijnsburger, M.; Eustace, A.; Xu, L.; Hawkey, P.; Heck, M.; Savelkoul, P.; Vandenbroucke-Grauls, C.; van der Zwaluw, K.; et al. Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerg. Infect. Dis. 2011, 17, 1216–1222. [Google Scholar] [CrossRef]
- Wang, L.; Nakamura, H.; Kage-Nakadai, E.; Hara-Kudo, Y.; Nishikawa, Y. Prevalence, antimicrobial resistance and multiple-locus variable-number tandem-repeat analysis profiles of diarrheagenic Escherichia coli isolated from different retail foods. Int. J. Food Microbiol. 2017, 249, 44–52. [Google Scholar] [CrossRef]
- Yue, S.; Zhang, Z.; Liu, Y.; Zhou, Y.; Wu, C.; Huang, W.; Chen, N.; Zhu, Z. Phenotypic and molecular characterizations of multidrug-resistant diarrheagenic E. coli of calf origin. Anim. Dis. 2021, 1, 14. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, Q.; Hu, H.; Zhang, J.; Huang, H. Prevalence, antimicrobial resistance and genetic diversity of Yersinia enterocolitica isolated from retail frozen foods in China. FEMS Microbiol. Lett. 2015, 362, fnv197. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Yang, L.; Wu, X.; Wu, Y.; Shao, B. Prevalence of Escherichia coli and Antibiotic Resistance in Animal-Derived Food Samples—Six Districts, Beijing, China, 2020. China CDC Wkly. 2021, 3, 999. [Google Scholar] [CrossRef]
- McDermott, P.F.; Tyson, G.H.; Kabera, C.; Chen, Y.; Li, C.; Folster, J.P.; Ayers, S.L.; Lam, C.; Tate, H.P.; Zhao, S. Whole-Genome Sequencing for Detecting Antimicrobial Resistance in Nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016, 60, 5515–5520. [Google Scholar] [CrossRef]
- Taylor, E.A.; Ossa-Trujillo, C.; Vinasco, J.; Jordan, E.R.; García Buitrago, J.A.; Hagevoort, R.; Norman, K.N.; Lawhon, S.A.-O.; Piñeiro, J.M.; Levent, G.; et al. Use of critically important antimicrobial classes early in life may adversely impact bacterial resistance profiles during adult years: Potential co-selection for plasmid-borne fluoroquinolone and macrolide resistance via extended-spectrum beta-lactam use in dairy cattle. Lett. Appl. Microbiol. 2021, 72, 220–224. [Google Scholar] [PubMed]
- Wang, Y.; Chen, Q.; Cui, S.; Xu, X.; Zhu, J.; Luo, H.; Wang, D.; Li, F. Enumeration and characterization of Salmonella isolates from retail chicken carcasses in Beijing, China. Foodborne Pathog. Dis. 2014, 11, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gu, D.; Wang, F.; Liu, B.; Li, J.; Kang, X.; Meng, C.; Jiao, X.; Pan, Z.A.-O.X. Prevalence and Characteristics of Salmonella spp. from a Pig Farm in Shanghai, China. Foodborne Pathog. Dis. 2021, 18, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Oniciuc, E.-A.; Likotrafiti, E.; Alvarez-Molina, A.; Prieto, M.; López, M.; Alvarez-Ordóñez, A. Food processing as a risk factor for antimicrobial resistance spread along the food chain. Curr. Opin. Food Sci. 2019, 30, 21–26. [Google Scholar] [CrossRef]
- Zhai, W.; Tian, Y.; Shao, D.; Zhang, M.; Li, J.A.-O.; Song, H.; Sun, C.; Wang, Y.; Liu, D.; Zhang, Y. Fecal Carriage of Escherichia coli Harboring the tet(X4)-IncX1 Plasmid from a Tertiary Class-A Hospital in Beijing, China. Antibiotics 2022, 11, 1068. [Google Scholar] [CrossRef]
- Wu, Y.A.-O.; Zeng, Z. Antibiotic Residues, Antimicrobial Resistance and Intervention Strategies of Foodborne Pathogens. Antibiotics 2024, 13, 321. [Google Scholar] [CrossRef]
| Regions | Provinces | No. of Samples | No. of Samples for Different Categories | ||
|---|---|---|---|---|---|
| Pork | Beef | Mutton | |||
| Eastern | Shanghai | 232 | 117 | 63 | 52 |
| Jiangsu | 212 | 132 | 53 | 27 | |
| Shandong | 200 | 85 | 74 | 41 | |
| Western | Sichuan | 235 | 135 | 77 | 23 |
| Gansu | 165 | 74 | 56 | 35 | |
| Xinjiang | 104 | 30 | 38 | 36 | |
| Southern | Guangxi | 109 | 46 | 36 | 27 |
| Guizhou | 109 | 63 | 29 | 17 | |
| Yunnan | 159 | 78 | 48 | 33 | |
| Northern | Hebei | 215 | 92 | 81 | 42 |
| Liaoning | 130 | 58 | 38 | 34 | |
| Heilongjiang | 215 | 106 | 56 | 53 | |
| Central | Henan | 162 | 84 | 38 | 40 |
| Hubei | 168 | 68 | 61 | 39 | |
| Chongqing | 100 | 48 | 31 | 21 | |
| Total | 2515 | 1216 | 779 | 520 | |
| No. of Samples | L. monocytogenes | Salmonella | DEC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Positive | Prevalence Rate (%) | χ2, p Value | No. of Positive | Prevalence Rate (%) | χ2, p Value | No. of Positive | Prevalence Rate (%) | χ2, p Value | ||
| Meat categories | ||||||||||
| Pork | 1216 | 110 | 9.05 | χ2 = 0.884, p > 0.05 | 214 | 17.60 | χ2 = 124.62, p < 0.05 | 96 | 7.89 | χ2 = 14.466, p < 0.05 |
| Beef | 779 | 66 | 8.47 | 32 | 4.11 | 29 | 3.72 | |||
| Mutton | 520 | 52 | 10.00 | 19 | 3.65 | 30 | 5.77 | |||
| Storage states | ||||||||||
| Frozen | 444 | 55 | 12.39 | χ2 = 43.510, p < 0.05 | 25 | 5.63 | χ2 = 15.173, p < 0.05 | 12 | 2.70 | χ2 = 12.275, p < 0.05 |
| Chill | 582 | 84 | 14.43 | 60 | 10.31 | 35 | 6.01 | |||
| Fresh | 1489 | 89 | 5.98 | 180 | 12.09 | 108 | 7.25 | |||
| Regions | ||||||||||
| Northern | 560 | 70 | 12.50 | χ2 = 24.136, p < 0.05 | 49 | 8.75 | χ2 = 21.902, p < 0.05 | 71 | 12.68 | χ2 = 89.570, p < 0.05 |
| Eastern | 644 | 71 | 11.02 | 46 | 7.14 | 14 | 2.17 | |||
| Southern | 377 | 25 | 6.63 | 56 | 14.85 | 12 | 3.18 | |||
| Western | 504 | 25 | 4.96 | 68 | 13.49 | 13 | 2.58 | |||
| Central | 430 | 37 | 8.60 | 46 | 10.70 | 45 | 10.47 | |||
| Sampling sites | ||||||||||
| Farmer’s market | 1220 | 97 | 7.95 | χ2 = 12.466, p < 0.05 | 135 | 11.07 | χ2 = 9.011, p < 0.05 | 77 | 6.31 | χ2 = 2.400, p > 0.05 |
| Supermarket | 629 | 78 | 12.40 | 69 | 10.97 | 39 | 6.20 | |||
| Retail shop | 365 | 33 | 9.04 | 44 | 12.05 | 26 | 7.12 | |||
| Catering | 301 | 20 | 2.21 | 17 | 5.65 | 13 | 4.32 | |||
| Quarters * | ||||||||||
| First quarter | 154 | 8 | 5.19 | χ2 = 2.126, p > 0.05 | 8 | 5.19 | χ2 = 7.86, p < 0.05 | 18 | 11.69 | χ2 = 19.746, p < 0.05 |
| Second quarter | 484 | 35 | 7.23 | 60 | 12.40 | 50 | 10.33 | |||
| Third quarter | 460 | 26 | 5.65 | 61 | 13.26 | 20 | 4.35 | |||
| Fourth quarter | 391 | 20 | 5.12 | 51 | 13.04 | 20 | 5.12 | |||
| Total | 2515 | 228 | 9.06 | 265 | 10.54 | 155 | 6.16 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Yang, D.; Yang, Z.; Li, Y.; Yang, S.; Li, W.; Qiao, X.; Xue, C.; Chen, M.; Zhang, L.; et al. Prevalence and Antimicrobial Susceptibility of Foodborne Pathogens from Raw Livestock Meat in China, 2021. Microorganisms 2024, 12, 2157. https://doi.org/10.3390/microorganisms12112157
Ren X, Yang D, Yang Z, Li Y, Yang S, Li W, Qiao X, Xue C, Chen M, Zhang L, et al. Prevalence and Antimicrobial Susceptibility of Foodborne Pathogens from Raw Livestock Meat in China, 2021. Microorganisms. 2024; 12(11):2157. https://doi.org/10.3390/microorganisms12112157
Chicago/Turabian StyleRen, Xiang, Dajin Yang, Zushun Yang, Ying Li, Shuran Yang, Weiwei Li, Xin Qiao, Chengyu Xue, Min Chen, Limin Zhang, and et al. 2024. "Prevalence and Antimicrobial Susceptibility of Foodborne Pathogens from Raw Livestock Meat in China, 2021" Microorganisms 12, no. 11: 2157. https://doi.org/10.3390/microorganisms12112157
APA StyleRen, X., Yang, D., Yang, Z., Li, Y., Yang, S., Li, W., Qiao, X., Xue, C., Chen, M., Zhang, L., Yan, L., & Peng, Z. (2024). Prevalence and Antimicrobial Susceptibility of Foodborne Pathogens from Raw Livestock Meat in China, 2021. Microorganisms, 12(11), 2157. https://doi.org/10.3390/microorganisms12112157





