The In Vitro Assessment of Antibacterial and Antioxidant Efficacy in Rosa damascena and Hypericum perforatum Extracts against Pathogenic Strains in the Interplay of Dental Caries, Oral Health, and Food Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.1.1. Extraction Method for Plants
2.1.2. Aqueous Extract
2.1.3. Ethanolic Extract (E)
2.1.4. Enzymatic Extract (ENZ)
2.2. The Quantification of the Biological and Antioxidant Activities of Plant Extracts
2.2.1. The Detection of Alkaloids
2.2.2. The Detection of Anthraquinones
2.2.3. The Detection of Terpenoids (Salkowski Test)
2.2.4. The Detection of Saponins (Foam Test)
2.2.5. The Detection of Tannins (Ferric Chloride Test—Braymer’s Test)
2.2.6. The Detection of Cardiac Glycosides (Keller–Kiliani Test)
2.2.7. Total Phenolic Concentrations
2.2.8. The Estimation of Total Flavonoid Content
2.2.9. Total Antioxidant Activity Assay (DPPH Free Radical Scavenging Assay)
2.2.10. Reducing Power Assay
2.3. The Determination of the Antibacterial Activity In Vitro
2.3.1. Tested Microbial Strains and Antibiotic Sensitivity Pattern
- -
- Staphylococcus aureus subsp. aureus, methicillin, and vancomycin resistant (source: dental caries area);
- -
- Methicillin-Resistant S. aureus, from raw milk;
- -
- Methicillin-Resistant S. aureus, from raw poultry;
- -
- Streptococcus mutans (source: oral cavity);
- -
- Streptococcus salivarius (source: oral cavity);
- -
- Fusobacterium nucleatum (source: oral cavity);
- -
- Porphyromonas gingivalis (source: oral cavity);
- -
- Prevotella intermedia (source: oral cavity);
- -
- Parvimonas micra (source: oral cavity);
- -
- Staphylococcus aureus subsp. aureus, reference strain ATCC 6538.
2.3.2. Antibiotic Susceptibility Assay
2.3.3. Diffusion Test in Agar
2.3.4. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration Determination
2.3.5. The Evaluation of the Anti-Biofilm Properties of the Studied Herbal Extractions
2.3.6. The Analysis of Time-Kill Kinetics
2.4. Statistical Analysis
3. Results
3.1. Screening the Phytochemical Pattern—The Antioxidant Activity of Plant Extracts
3.2. The Analysis of the Antimicrobial Activity of Plant Extracts
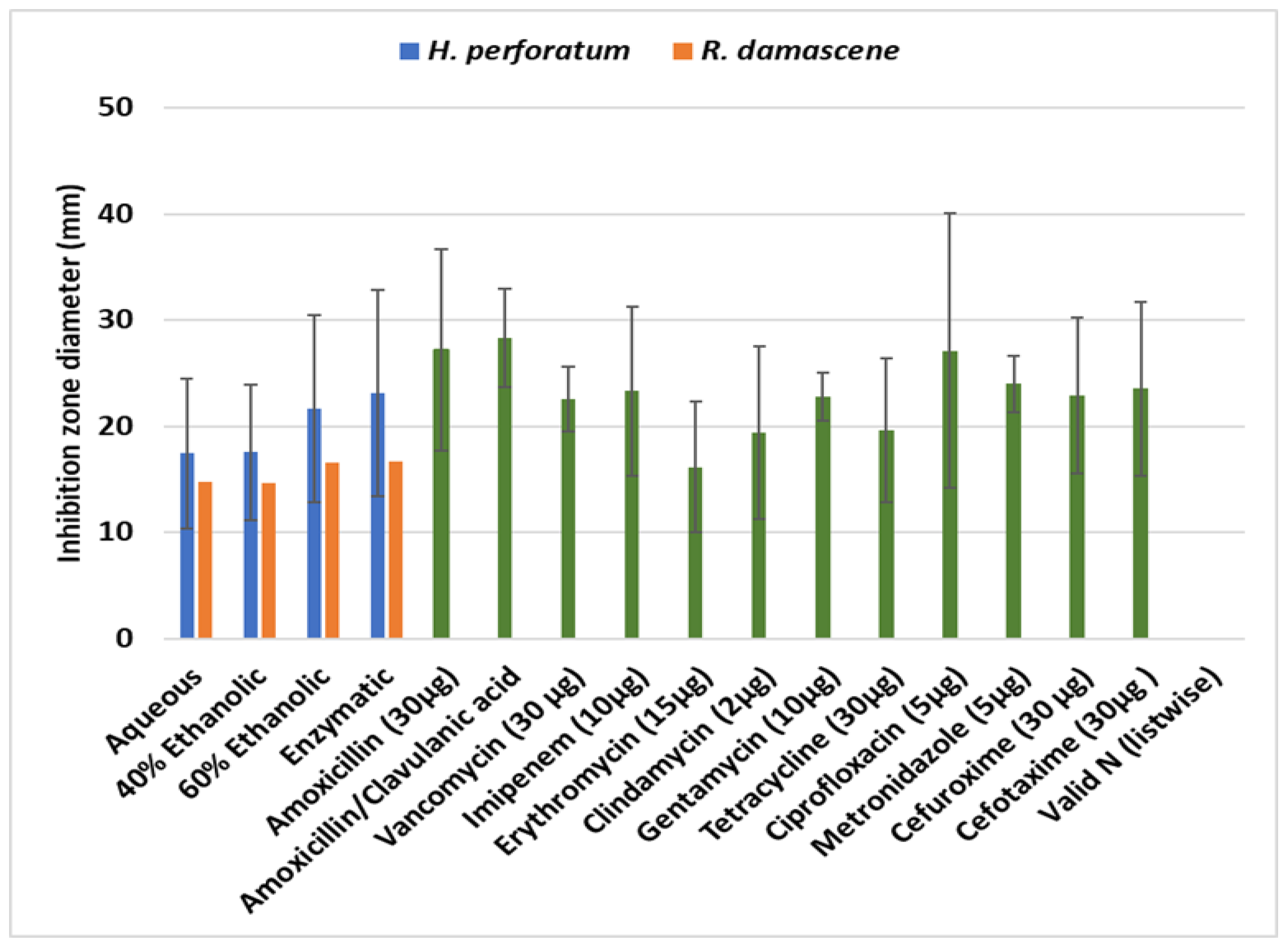
The Disk Diffusion Assays of the Herb Extracts
- -
- Antibiofilm effectiveness and inhibition zone results
- -
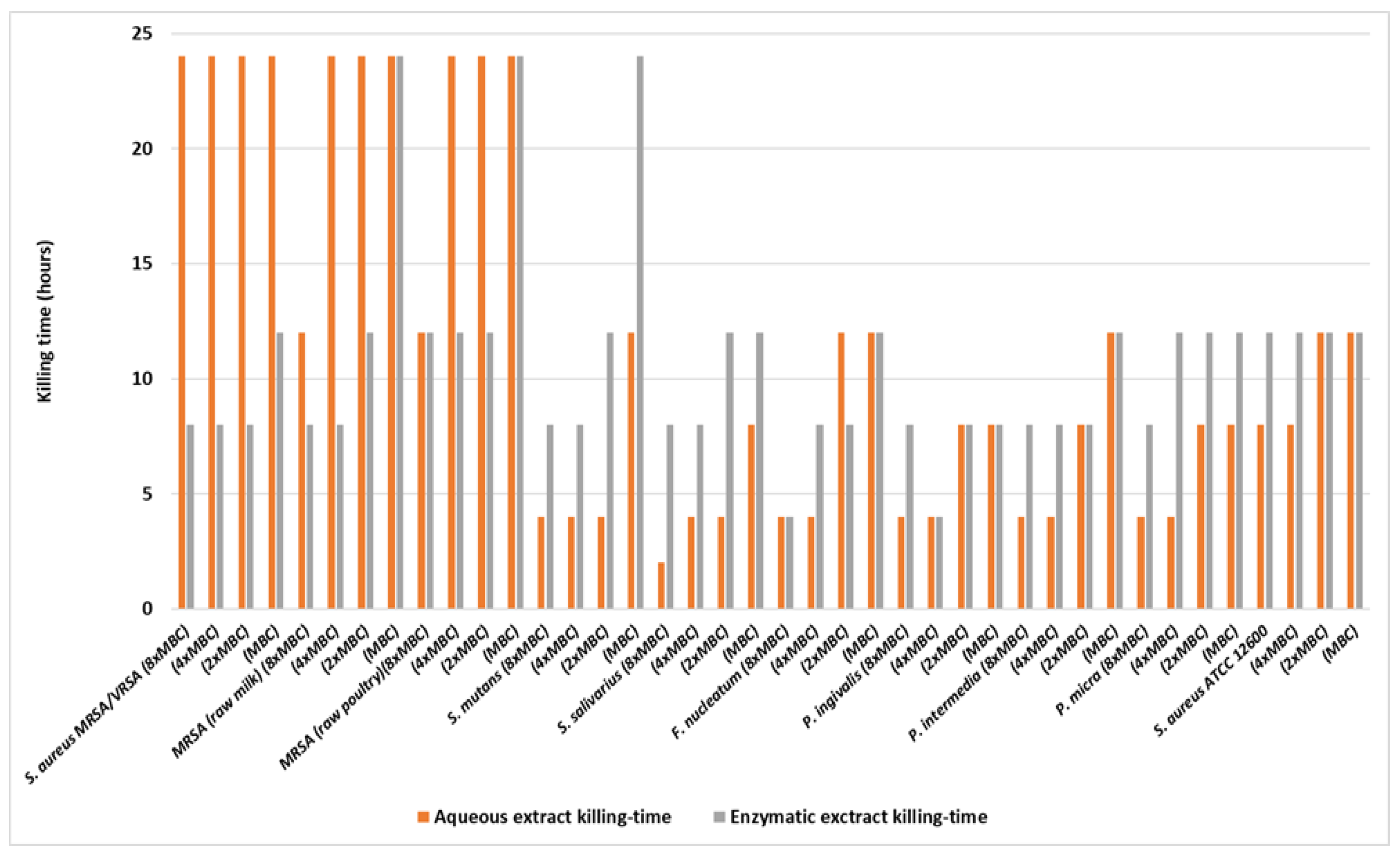
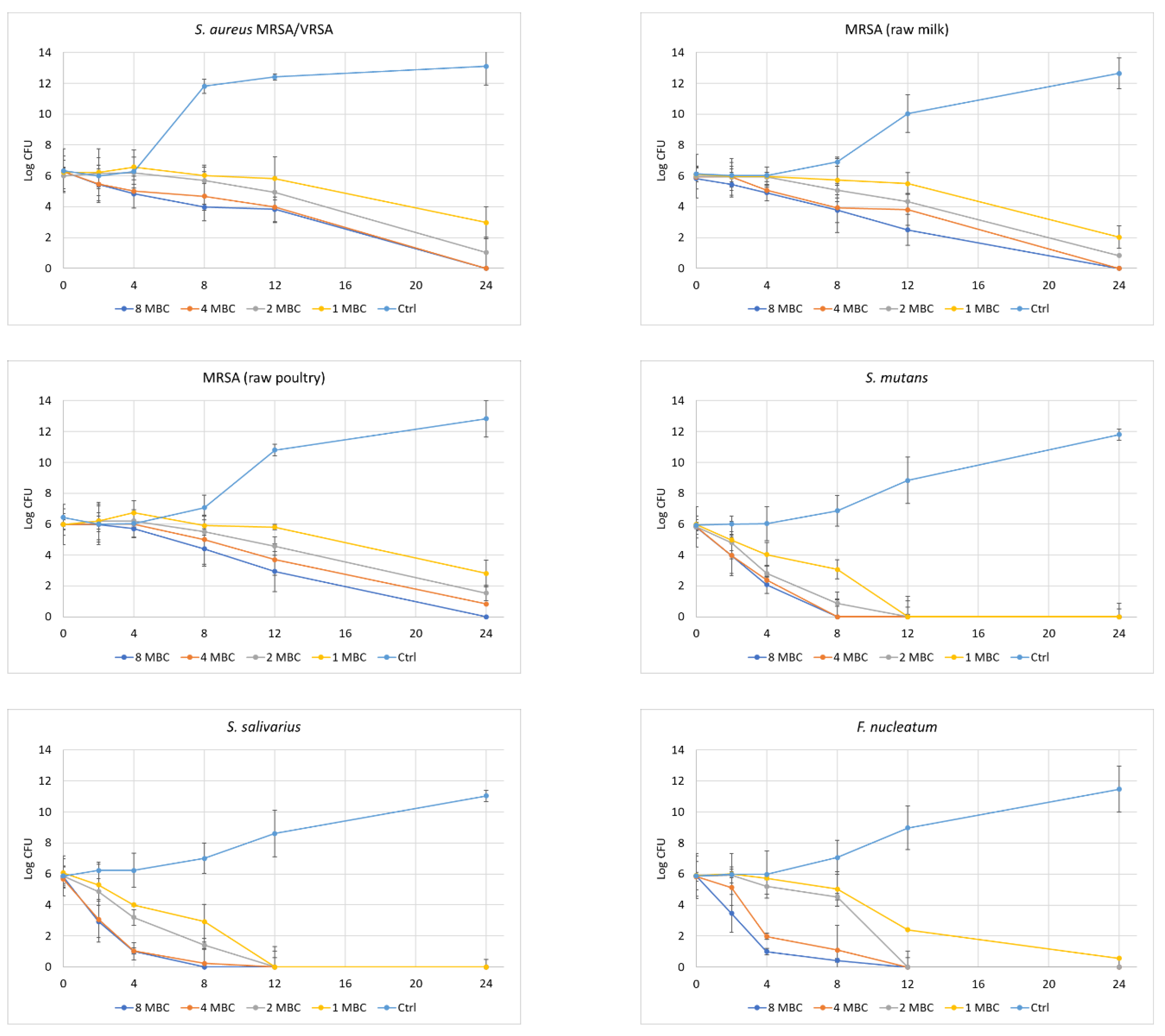
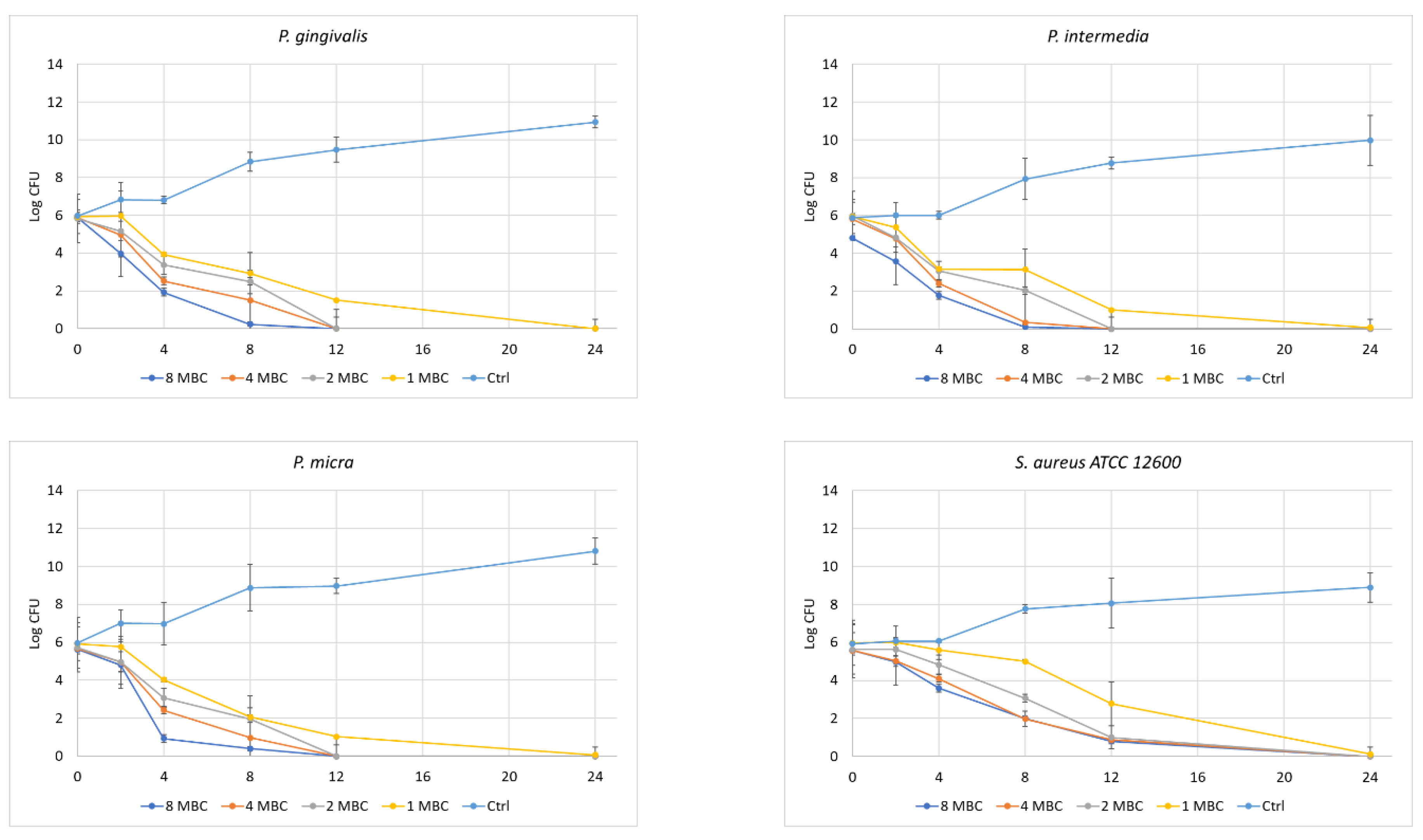
- Time of Kill Kinetics
4. Discussion
4.1. The Chemistry and Antioxidant Activities of Plant Extracts
4.1.1. General Aspects
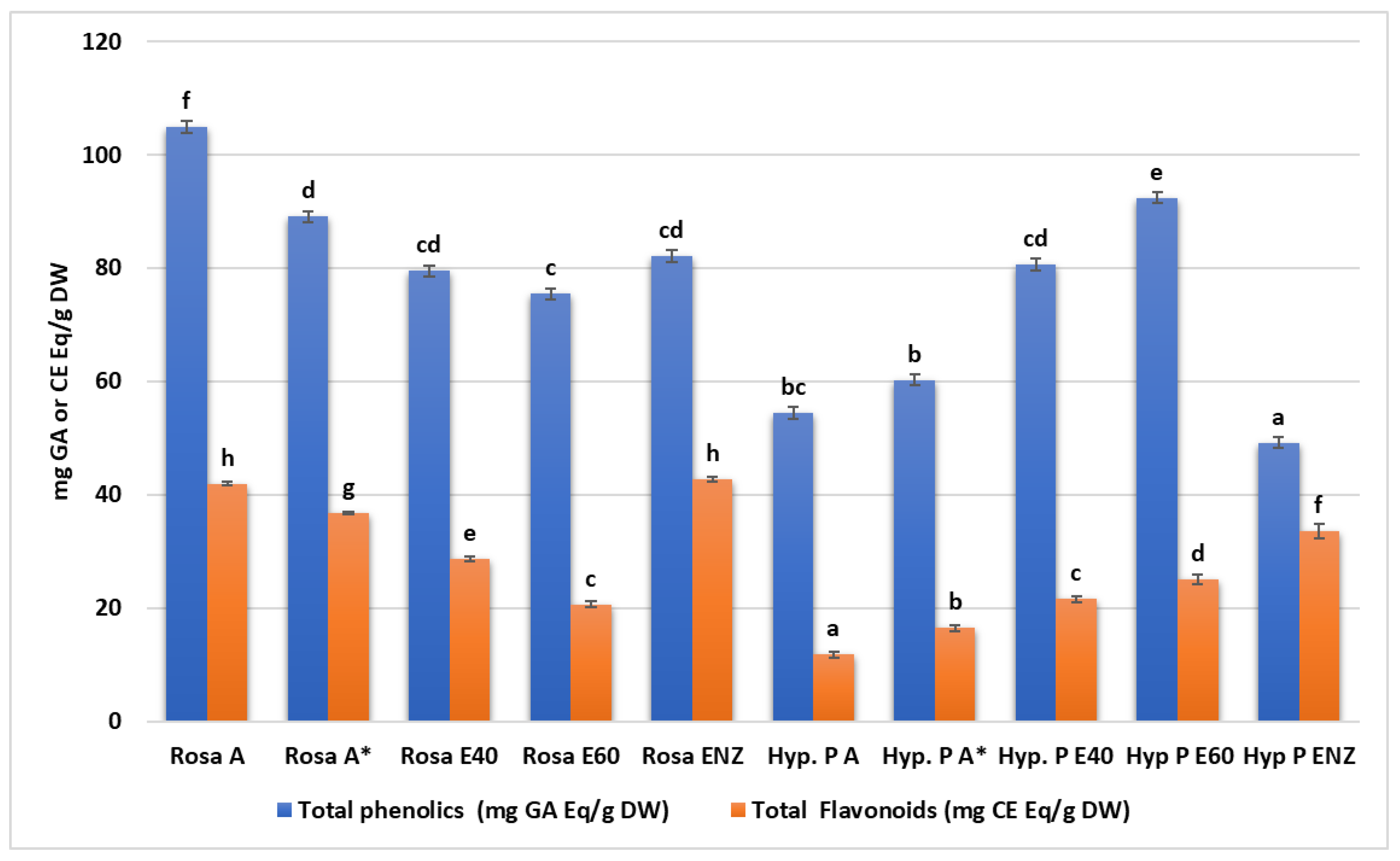
4.1.2. Total Phenolic and Flavonoid Content
4.1.3. The Evaluation of Antioxidant Capacity: DPPH Assay and Ferric Reducing Assay Power (FRAP)
4.2. The Antimicrobial and Antioxidant Capacity of the Studied Extracts
5. Limitations of the Study
6. Conclusions
- -
- The antibacterial effect of the studied plants extracts against oral pathogens was absolute in the kill-time kinetics, implying an increased antibacterial potential of these extracts.
- -
- Certain differences in the time-kill kinetics curve can be attributed to species-specific factors.
- -
- A similar elimination of the bacterial cells was also observed in the case of the foodborne bacteria, in time-kill kinetics.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Halberstein, R. Botanical Medicines for Oral Health. Nat. Prod. Commun. 2008, 3, 1813–1824. [Google Scholar] [CrossRef]
- Caselitz, P. Caries—Ancient plague of mankind. In Dental Anthropology; Alt, K., Rosing, F., Teschler-Nicola, M., Eds.; Springer: New York, NY, USA, 1998; pp. 203–226. [Google Scholar]
- Clarke, N.; Carey, S.; Sirkandi, W.; Hirsch, R.; Leppard, P. Periodontal disease in ancient populations. Am. J. Phys. Anthropol. 1986, 71, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Langsjoen, O. Diseases of the dentition. In The Cambridge Encyclopedia of Human Paleopathology; Aufderheide, A., Rodriguez-Martin, C., Eds.; Cambridge University Press: Cambridge, UK, 1998; pp. 393–412. [Google Scholar]
- Kennett, F. Folk Medicine; Crescent Books: New York, NY, USA, 1976. [Google Scholar]
- Halberstein, R.A. Medicinal plants: Historical and cross-cultural usage patterns. Ann. Epidemiol. 2005, 15, 686–699. [Google Scholar] [CrossRef]
- Emboden, W.A. Transcultural use of narcotic water lilies in ancient Egyptian and Mayan drug ritual. J. Ethnopharmacol. 1981, 3, 39–77. [Google Scholar] [CrossRef] [PubMed]
- Merlin, M.D. Archaeological evidence for the tradition of psychoactive plant use in the Old World. Econ. Bot. 2003, 57, 295–323. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 25, 17030. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Twetman, S.; Fisher, J.; Marsh, P.D. Understanding dental caries as a non-communicable disease. Br. Dent. J. 2021, 231, 749–753. [Google Scholar] [CrossRef]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, the Bad, and the Unknown. Front. Cell Infect. Microbiol. 2021, 7, 766944. [Google Scholar] [CrossRef]
- Cai, Z.; Lin, S.; Hu, S.; Zhao, L. Structure and Function of Oral Microbial Community in Periodontitis Based on Integrated Data. Front. Cell. Infect. Microbiol. 2021, 11, 663756. [Google Scholar] [CrossRef]
- Aoun, A.; Darwiche, F.; Al Hayek, S.; Doumit, J. The Fluoride Debate: The Pros and Cons of Fluoridation. Prev. Nutr. Food Sci. 2018, 23, 171–180. [Google Scholar] [CrossRef]
- Bernatová, S.; Samek, O.; Pilát, Z.; Serý, M.; Ježek, J.; Jákl, P.; Siler, M.; Krzyžánek, V.; Zemánek, P.; Holá, V.; et al. Following the mechanisms of bacteriostatic versus bactericidal action using Raman spectroscopy. Molecules 2013, 24, 13188–13199. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Levin, B.R. Proximate and ultimate causes of the bactericidal action of antibiotics. Nat. Rev. Microbiol. 2021, 19, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Groppo, F.C.; Ramacciato, J.C.; Motta, R.H.; Ferraresi, P.M.; Sartoratto, A. Antimicrobial activity of garlic against oral streptococci. Int. J. Dent. Hyg. 2007, 5, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kubra, I.R.; Jaganmohanrao, L. An overview on inventions related to ginger processing and products for food and pharmaceutical applications. Recent. Pat. Food Nutr. Agric. 2012, 1, 31–49. [Google Scholar]
- Gościniak, A.; Paczkowska-Walendowska, M.; Skotnicka, A.; Ruchała, M.A.; Cielecka-Piontek, J. Can Plant Materials Be Valuable in the Treatment of Periodontal Diseases? Practical Review. Pharmaceutics 2021, 17, 2185. [Google Scholar] [CrossRef]
- Megalaa, N.; Kayalvizhi, G.; Silas, A.J.; Sajeev, R.; Saravana Kumar, M.S. Role of herbal leaf extracts in caries prevention. Int. J. Cont. Med. Res. 2014, 1, 71–78. [Google Scholar]
- Şengün, D.N.; Karaca, İ.R.; Saraç, N.; Uğur, A.; Fırat, A.; Kaymaz, F.F.; Öztürk, H.S. Evaluation of the chemopreventive effects of Hypericum perforatum L. on DMBA-applied rat oral mucosa. Arch. Oral. Biol. 2021, 127, 105139. [Google Scholar] [CrossRef]
- Chandra Shekar, B.R.; Nagarajappa, R.; Suma, S.; Thakur, R. Herbal extracts in oral health care—A review of the current scenario and its future needs. Pharmacogn. Rev. 2015, 9, 87–92. [Google Scholar] [CrossRef]
- Cha, J.; Jeong, M.; Jeong, S.; Moon, S.; Kil, B.; Yun, S.; Lee, K.; Song, Y. Chemical composition and antimicrobial activity of the essential oil of Cryptomeria japonica. Phytother. Res. 2007, 21, 295–299. [Google Scholar] [CrossRef]
- Süntar, I.; Oyardı, O.; Akkol, E.K.; Ozçelik, B. Antimicrobial effect of the extracts from Hypericum perforatum against oral bacteria and biofilm formation. Pharm. Biol. 2016, 54, 1065–1070. [Google Scholar] [CrossRef]
- Khadem Nezhad, S.; Taghavi Zenouz, A.; Aghazadeh, M.; Samadi Kafil, H. Strong antimicrobial activity of Hypericum perforatum L. against oral isolates of Lactobacillus spp. Cell Mol. Biol. 2017, 30, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Voidarou, C.; Antoniadou, Μ.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Amargianitakis, M.; Antoniadou, M.; Rahiotis, C.; Varzakas, T. Probiotics, Prebiotics, Synbiotics and Dental Caries. New Perspectives, Suggestions, and Patient Coaching Approach for a Cavity-Free Mouth. Appl. Sci. 2021, 11, 5472. [Google Scholar] [CrossRef]
- Antoniadou, M.; Varzakas, T. Advances in Processing, Quality, Safety, Authenticity, Nutrition, Health, and Oral Health of Extra Virgin Olive Oil. Proceedings 2020, 70, 107. [Google Scholar] [CrossRef]
- Israili, Z.H. Antimicrobial properties of honey. Am. J. Ther. 2014, 21, 304–323. [Google Scholar] [CrossRef] [PubMed]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Alexopoulos, A.; Giorgi, E.; Tzora, A.; Skoufos, I.; Varzakas, T.; Bezirtzoglou, E. An In Vitro Study of Different Types of Greek Honey as Potential Natural Antimicrobials against Dental Caries and Other Oral Pathogenic Microorganisms. Case Study Simulation of Oral Cavity Conditions. Appl. Sci. 2021, 11, 6318. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Shabrmi, F.M.; Aly, S.M. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 12, 125–136. [Google Scholar]
- Bernardes, W.A.; Lucarini, R.; Tozatti, M.G.; Souza, M.G.; Silva, M.L.; Filho, A.A.; Martins, C.H.; Crotti, A.E.; Pauletti, P.M.; Groppo, M.; et al. Antimicrobial activity of Rosmarinus officinalis against oral pathogens: Relevance of carnosic acid and carnosol. Chem. Biodivers. 2010, 7, 1835–1840. [Google Scholar] [CrossRef]
- Smullen, J.; Finney, M.; Storey, D.M.; Foster, H.A. Prevention of artificial dental plaque formation in vitro by plant extracts. J. Appl. Microbiol. 2012, 113, 964–973. [Google Scholar] [CrossRef]
- Park, Y.S.; Jung, S.T.; Kang, S.G.; Heo, B.G.; Arancibia-Avila, P.; Toledo, F.; Drzewiecki, J.; Namiesnik, J.; Gorinstein, S. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008, 107, 640–648. [Google Scholar] [CrossRef]
- FDA. Food Additive Status List. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (accessed on 30 October 2023).
- Anonymous. Can microbes save the planet? Nat. Biotechnol. 2023, 41, 735. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.B.-M.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 20 October 2023).
- Gerritsen, A.E.; Allen, P.F.; Witter, D.J.; Bronkhorst, E.M.; Creugers, N.H. Tooth loss and oral health-related quality of life: A systematic review and meta-analysis. Health Qual. Life Outcomes 2010, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Hobbs, F.D.R.; Huck, O.; Hummers, E.; et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: Consensus report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European arm of the World Organization of. J. Clin. Periodontol. 2003, 50, 819–841. [Google Scholar] [CrossRef] [PubMed]
- Montagne, M. Book Review: Powerful Medicines: The Benefits, Risks, and Costs of Prescription Drugs. Ann. Pharmacother. 2005, 39, 776–777. [Google Scholar] [CrossRef]
- Micozzi, M.; Messerole, L. Herbal medicine. In Fundamentals of Complementary and Integrative Medicine; Micozzi, M., Ed.; Saunders: St. Louis, MI, USA, 2006; Volume 3, pp. 164–180. [Google Scholar]
- Cendrowski, A.; Kraśniewska, K.; Przybył, J.L.; Zielińska, A.; Kalisz, S. Antibacterial and Antioxidant Activity of Extracts from Rose Fruits (Rosa rugosa). Molecules 2020, 25, 1365. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, M.R.; Irfanullah Sajid, M.; Zahra, Z. Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement. Altern. Med. 2018, 18, 43. [Google Scholar] [CrossRef]
- Shaikh, J.R.; Patil, M. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Haile, K.; Mehari, B.; Atlabachew, M.; Chandravanshi, B.S. Phenolic composition and antioxidant activities of cladodes of the two varieties of cactus pear (Opuntia ficus-indica) grown in Ethiopia. Bull. Chem. Soc. 2016, 30, 347–356. [Google Scholar] [CrossRef]
- Seifu, T.; Mehari, B.; Atlabachew, M.; Chandravanshi, B. Polyphenolic content and antioxidant activity of leaves of Urtica simensis grown in Ethiopia. Lat. Am. Appl. Res. 2017, 40, 35–40. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 16, 221. [Google Scholar] [CrossRef] [PubMed]
- Ayele, D.T.; Akele, M.L.; Melese, A.T. Analysis of total phenolic contents, flavonoids, antioxidant and antibacterial activities of Croton macrostachyus root extracts. BMC Chem. 2022, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, R.C.; Montagner, F.; Signoretti, F.G.; Almeida, G.C.; Gomes, B.P. Frequency, microbial interactions, and antimicrobial susceptibility of Fusobacterium nucleatum and Fusobacterium necrophorum isolated from primary endodontic infections. J. Endod. 2008, 34, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Carrol, D.H.; Chassagne, F.; Dettweiler, M.; Quave, C.L. Antibacterial activity of plant species used for oral health against Porphyromonas gingivalis. PLoS ONE 2020, 15, e0239316. [Google Scholar] [CrossRef] [PubMed]
- Al-Nawas, B.; Karbach, J. S3 Leitlinie: Odontogene Infektionen. Online Verfügbar Unter. 2017. Available online: https://wwwawmforg/uploads/tx_szleitlinien/007-006l_S3_Odontogene_Infektionen_2017-12pdf (accessed on 20 October 2023).
- Meinen, A.; Reuss, A.; Willrich, N.; Feig, M.; Noll, I.; Eckmanns, T.; Al-Nawas, B.; Markwart, R. Antimicrobial Resistance and the Spectrum of Pathogens in Dental and Oral-Maxillofacial Infections in Hospitals and Dental Practices in Germany. Front. Microbiol. 2021, 2, 676108. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.G.; Ingalagi, P.; Patil, S.; Patil, S.; Pattar, G. Antimicrobial susceptibility pattern of oral gram negative anaerobes from Indian subjects. Anaerobe 2021, 70, 102367. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard—CLSI Document M02-A11; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Clinical Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, 8th ed.; CLSI Document M07-A7; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Microdilution. EUCAST Discussion Document E. Def. 2003, 5.1. Clin. Microbiol. Infect. 2003, 9, 1–7. [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Veloso, D.J.; Abrão, F.; Martins, C.H.G.; Bronzato, J.D.; Gomes, B.P.F.A.; Higino, J.S.; Sampaio, F.C. Potential antibacterial and anti-halitosis activity of medicinal plants against oral bacteria. Arch. Oral Biol. 2020, 110, 104585. [Google Scholar] [CrossRef]
- Bagheri, R.; Bohlouli, S.; Maleki Dizaj, S.; Shahi, S.; Memar, M.Y.; Salatin, S. The Antimicrobial and Anti-Biofilm Effects of Hypericum perforatum Oil on Common Pathogens of Periodontitis: An In Vitro Study. Clin. Pract. 2022, 12, 1009–1019. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Weir, M.D.; Zhang, K.; Zhou, Y.; Xu, H.H.K.; Reynolds, M.A. Novel multifunctional dental bonding agent for class-V restorations to inhibit periodontal biofilms. RSC Adv. 2017, 7, 29004–29014. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Toma, L.; Provot, C.; Ascenzioni, F.; Sperduti, I.; Prignano, G.; Gallo, M.T.; Pimpinelli, F.; Bordignon, V.; Bernardi, T.; et al. Development of an in vitro Assay, Based on the BioFilm Ring Test®, for Rapid Profiling of Biofilm-Growing Bacteria. Front. Microbiol. 2016, 21, 1429. [Google Scholar] [CrossRef] [PubMed]
- Samreen Qais, F.A.; Ahmad, I. Anti-quorum sensing and biofilm inhibitory effect of some medicinal plants against gram-negative bacterial pathogens: In vitro and in silico investigations. Heliyon 2022, 8, e11113. [Google Scholar] [CrossRef] [PubMed]
- Ersanli, C.; Tzora, A.; Skoufos, I.; Fotou, K.; Maloupa, E.; Grigoriadou, K.; Voidarou, C.C.; Zeugolis, D.I. The Assessment of Antimicrobial and Anti-Biofilm Activity of Essential Oils against Staphylococcus aureus Strains. Antibiotics 2023, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, L.M.; Barbalata-Mandru, M.; Enache, A.A.; Rimbu, C.M.; Badea, G.I.; Aflori, M. Chitosan Membranes Containing Plant Extracts: Preparation, Characterization and Antimicrobial Properties. Int. J. Mol. Sci. 2023, 24, 8673. [Google Scholar] [CrossRef] [PubMed]
- Ngene, A.; Ohaegbu, C.; Idu, E.; Odo, E. Time-kill kinetics and antibacterial activity of ethanolic extract of Allium sativum. Microbes Infect. Dis. 2023. [Google Scholar] [CrossRef]
- Tent, P.A.; Juncar, R.I.; Onisor, F.; Bran, S.; Harangus, A.; Juncar, M. The pathogenic microbial flora and its antibiotic susceptibility pattern in odontogenic infections. Drug Metab. Rev. 2019, 51, 340–355. [Google Scholar] [CrossRef]
- Dwivedi, D.; Kushwah, T.; Kushwah, M.; Singh, V. Antibiotic susceptibility pattern against pathogenic bacteria causing Dental Caries. S. Asian J. Exp. Biol. 2011, 1, 31–35. [Google Scholar] [CrossRef]
- Veloo, A.C.M.; Seme, K.; Raangs, E.; Rurenga, P.; Singadji, Z.; Wekema-Mulder, G.; Van Winkelhoff, A.J. Antibiotic susceptibility profiles of oral pathogens. Int. J. Antimicrob. Agents 2012, 40, 450–454. [Google Scholar] [CrossRef]
- Jepsen, K.; Falk, W.; Brune, F.; Fimmers, R.; Jepsen, S.; Bekeredjian-Ding, I. Prevalence and antibiotic susceptibility trends of periodontal pathogens in the subgingival microbiota of German periodontitis patients: A retrospective surveillance study. J. Clin. Periodontol. 2021, 48, 1216–1227. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Łubek-Nguyen, A.; Ziemichód, W.; Olech, M. Application of Enzyme-Assisted Extraction for the Recovery of Natural Bioactive Compounds for Nutraceutical and Pharmaceutical Applications. Appl. Sci. 2022, 12, 3232. [Google Scholar] [CrossRef]
- Liu, W.; Yin, D.; Li, N.; Hou, X.; Wang, D.; Li, D.; Liu, J. Influence of Environmental Factors on the Active Substance Production and Antioxidant Activity in Potentilla fruticosa L. and Its Quality Assessment. Sci. Rep. 2016, 4, 28591. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, N.; Imran, M.; Mohammed Basheeruddin Asdaq, S.; Imam Rabbani, S.; Ali Alanazi, F.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M. Total phenolic, flavonoid contents, and biological activities of stem extracts of Astragalus spinosus (Forssk.) Muschl. grown in Northern Border Province, Saudi Arabia. Saudi J. Biol. Sci. 2022, 29, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 27, 2041. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 19, 100217. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Kováč, J.; Slobodníková, L.; Trajčíková, E.; Rendeková, K.; Mučaji, P.; Sychrová, A.; Bittner Fialová, S. Therapeutic Potential of Flavonoids and Tannins in Management of Oral Infectious Diseases—A Review. Molecules 2022, 24, 158. [Google Scholar] [CrossRef]
- Schestakow, A.; Guth, M.S.; Eisenmenger, T.A.; Hannig, M. Evaluation of Anti-Biofilm Activity of Mouthrinses Containing Tannic Acid or Chitosan on Dentin In Situ. Molecules 2021, 26, 1351. [Google Scholar] [CrossRef]
- Ghimeray, A.K.; Jung, U.S.; Lee, H.Y.; Kim, Y.H.; Ryu, E.K.; Chang, M.S. In Vitro Antioxidant, Collagenase Inhibition, and in Vivo Anti-Wrinkle Effects of Combined Formulation Containing Punica granatum, Ginkgo biloba, Ficus carica, and Morus alba Fruits Extract. Clin. Cosmet. Investig. Derm. 2015, 8, 389–396. [Google Scholar] [CrossRef]
- Henning, S.M.; Yang, J.; Lee, R.-P.; Huang, J.; Hsu, M.; Thames, G.; Gilbuena, I.; Long, J.; Xu, Y.; Park, E.H.; et al. Pomegranate Juice and Extract Consumption Increases the Resistance to UVB-Induced Erythema and Changes the Skin Microbiome in Healthy Women: A Randomized Controlled Trial. Sci. Rep. 2019, 9, 14528. [Google Scholar] [CrossRef] [PubMed]
- Chroho, M.; Bouymajane, A.; Oulad El Majdoub, Y.; Cacciola, F.; Mondello, L.; Aazza, M.; Zair, T.; Bouissane, L. Phenolic Composition, Antioxidant and Antibacterial Activities of Extract from Flowers of Rosa damascena from Morocco. Separations 2022, 9, 247. [Google Scholar] [CrossRef]
- Santos, T.N.; Costa, G.; Ferreira, J.P.; Liberal, J.; Francisco, V. Antioxidant, Anti-Inflammatory, and Analgesic Activities of Agrimonia eupatoria L. Infus. Evid. Based Complement. Altern. Med. 2017, 13, 8309894. [Google Scholar]
- Piotrowicz, Z.; Tabisz, Ł.; Waligórska, M.; Pankiewicz, R.; Łęska, B. Phenol-rich alternatives for Rosa x damascena Mill. Efficient phytochemical profiling using different extraction methods and colorimetric assays. Sci. Rep. 2021, 11, 23883. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yu, X.; Maninder, M.; Xu, B. Total phenolics and antioxidants profiles of commonly consumed edible flowers in China. Int. J. Food Prop. 2018, 21, 1524–1540. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Fattahi, M. Essential oil, total phenolic, flavonoids, anthocyanins, carotenoids and antioxidant activity of cultivated Damask Rose (Rosa damascena) from Iran: With chemotyping approach concerning morphology and composition. Sci. Hortic. 2020, 288, 110341. [Google Scholar] [CrossRef]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crops Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dräger, G.; Walter, J.G.; Scheper, T. Identification of Major Constituents of Hypericum perforatum L. Extracts in Syria by Development of a Rapid, Simple, and Reproducible HPLC-ESI-Q-TOF MS Analysis and Their Antioxidant Activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef]
- Makarova, K.; Sajkowska-Kozielewicz, J.J.; Zawada, K.; Olchowik-Grabarek, E.; Ciach, M.A.; Gogolewski, K.; Dobros, N.; Ciechowicz, P.; Freichels, H.; Gambin, A. Harvest time affects antioxidant capacity, total polyphenol and flavonoid content of Polish St John’s wort’s (Hypericum perforatum L.) flowers. Sci. Rep. 2021, 17, 3989. [Google Scholar] [CrossRef]
- Kakouri, E.; Trigas, P.; Daferera, D.; Skotti, E.; Tarantilis, P.A.; Kanakis, C. Chemical Characterization and Antioxidant Activity of Nine Hypericum Species from Greece. Antioxidants 2023, 12, 899. [Google Scholar] [CrossRef]
- Mileva, M.; Ilieva, Y.; Jovtchev, G.; Gateva, S.; Zaharieva, M.M.; Georgieva, A.; Dimitrova, L.; Dobreva, A.; Angelova, T.; Vilhelmova-Ilieva, N.; et al. Rose Flowers-A Delicate Perfume or a Natural Healer? Biomolecules 2021, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Taneva, I.; Petkova, N.; Dimov, I.; Ivanov, I.; Denev, P. Characterization of rose hip (Rosa canina L.) fruits extracts and evaluation of their in vitro antioxidant activity. J. Pharmacogn. Phytochem. 2016, 5, 35–38. [Google Scholar]
- Franco, D.; Pinelo, M.; Sineiro, J.; Núñez, M.J. Processing of Rosa rubiginosa: Extraction of oil and antioxidant substances. Bioresour. Technol. 2007, 98, 3506–3512. [Google Scholar] [CrossRef] [PubMed]
- Ilbay, Z.; Sahin, S.; Kırbaslar, S.I. Investigation of polyhenolic content of rose hip (Rosa canina L.) tea extracts: A comparative study. Foods 2013, 2, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Filomena, C.; Chiara, T.; Marcello, N.; Giancarlo, S.; Francesco, M. Hypericum perforatum: Influences of the habitat on chemical composition, photo-induced cytotoxicity, and antiradical activity. Pharm. Biol. 2014, 52, 909–918. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral. Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’Addona, A. Oral microbiota in human health and disease: A perspective. Exp. Biol. Med. 2020, 248, 1288–1301. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 28, 1014. [Google Scholar] [CrossRef]
- Alibi, S.; Crespo, D.; Navas, J. Plant-Derivatives Small Molecules with Antibacterial Activity. Antibiotics 2021, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Alsafi, A.; Alkaabi, S.J. Aqueous Rosa damascena extract: Antibacterial activity and its role of adhesion to human epithelial cells in vitro. Cell Biochem. Funct. 2023, 41, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Mohd Amin, I.; Mohamad Zain, N.; Ahmad, V.N.; Mat Daud, D.N.; Abdullah Sani, N. Antimicrobial and Antibiofilm Activities of the Methanol Extracts of Rosa Damascena against S. aureus. In Proceedings of the 9th Dental Students’ Scientific Symposium—Proceeding Book; Universiti Teknologi MARA: Sungai Buloh, Malaysia, 2019; Available online: https://ir.uitm.edu.my/id/eprint/30163/1/K_INDAH%20MOHD%20AMIN%20DSSS%20B%2019.pdf (accessed on 24 November 2023).
- Shokouhinejad, N.; Emaneini, M.; Aligholi, M.; Jabalameli, F. Antimicrobial effect of Rosa damascena extract on selected endodontic pathogens. J. Calif. Dent. Assoc. 2010, 38, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Tsai, T.H.; Chien, Y.C.; Lee, C.W.; Tsai, P.J. In vitro antimicrobial activities against cariogenic streptococci and their antioxidant capacities: A comparative study of green tea versus different herbs. Food Chem. 2008, 110, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Aliasghari, A.; Rabbani, M.; Khoroushi, M.; Emami, H. In-Vitro Effect of Alcoholic Extract of Rosa damascene Extract on Cariogenic streptococci. J. Isfahan Med. Sch. 2015, 33, 326–335. Available online: https://jims.mui.ac.ir/article_14590.html?lang=en (accessed on 20 October 2023).
- Ramezanalizadeh, F.; Rabbani, M.; Khoroushi, M.; Aliasghari, A. In vitro assessment of antibacterial activity of pomegranate vinegar and rose water compared with Persica mouthwash against Oral Bacteria. J. Iran. Dent. Assoc. 2015, 27, 150–157. Available online: http://jida.ir/article-1-1809-en.html (accessed on 20 October 2023).
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid.-Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Stoyanova, N.; Nachev, N.; Spasova, M. Innovative Bioactive Nanofibrous Materials Combining Medicinal and Aromatic Plant Extracts and Electrospinning Method. Membranes 2023, 13, 840. [Google Scholar] [CrossRef]
- Schempp, C.M.; Pelz, K.; Wittmer, A.; Schöpf, E.; Simon, J.C. Antibacterial activity of hyperforin from St John’s wort, against multiresistant Staphylococcus aureus and gram-positive bacteria. Lancet 1999, 353, 2129. [Google Scholar] [CrossRef]
- Vollmer, A.; Al-Ahmad, A.; Argyropoulou, A.; Thurnheer, T.; Hellwig, E.; Attin, T.; Vach, K.; Wittmer, A.; Ferguson, K.; Skaltsounis, A.L.; et al. Antimicrobial photoinactivation using visible light plus water-filtered infrared-a (vis + wira) and Hypericum perforatum modifies in situ oral biofilms. Sci. Rep. 2019, 9, 20325. [Google Scholar] [CrossRef]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 2019, 71, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Lasik, M.; Nowak, J.; Stachowiak, B.; Czarnecki, Z. Evaluation of the antagonistic properties of natural antibacterial substance extracted from herbs: Poster presentation. Eurobiotech 2007, 54, 10. [Google Scholar]
- Beveridge, T.J. Structures of Gram-Negative Cell Walls and Their Derived Membrane Vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Staleva, P.; Petkova, Z.; Ivanova, V.; Evstatieva, Y.; Nikolova, D.; Rasheva, I.; Atanasov, N.; Topouzova-Hristova, T.; Veleva, R.; et al. Phytochemical Profile, Antioxidant Potential, Antimicrobial Activity, and Cytotoxicity of Dry Extract from Rosa damascena Mill. Molecules 2023, 28, 7666. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Rosa damascena as Holy Ancient Herb with Novel Applications. J. Tradit. Complement. Med. 2016, 6, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological Effects of Rosa damascena. Iran. J. Basic. Med. Sci. 2011, 14, 295–307. [Google Scholar] [PubMed]
- Gochev, V.; Wlcek, K.; Buchbauer, G.; Stoyanova, A.; Dobreva, A.; Schmidt, E.; Jirovetz, L. Comparative Evaluation of Antimicrobial Activity and Composition of Rose Oils from Various Geographic Origins, in Particular Bulgarian Rose Oil. Nat. Prod. Commun. 2008, 3, 1063–1068. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahasneh, A.M. Antimicrobial, Cytotoxicity and Phytochemical Screening of Jordanian Plants Used in Traditional Medicine. Molecules 2010, 15, 1811–1824. [Google Scholar] [CrossRef]
- Denkova, Z.R.; Denkova-Kostova, R.S.; Vasileva, I.N.; Slavov, A.M. Antimicrobial Activity of Plant Extracts of Rose By-Products from the Essential Oil Industry against Saprophytic and Pathogenic Microorganisms. Bulg. Chem. Commun. 2022, 54, 95–101. [Google Scholar]
- Basim, E.; Basim, H. Antibacterial Activity of Rosa Damascena Essential Oil. Fitoterapia 2003, 74, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, Y.; Dimitrova, L.; Georgieva, A.; Vilhelmova-Ilieva, N.; Zaharieva, M.M.; Kokanova-Nedialkova, Z.; Dobreva, A.; Nedialkov, P.; Kussovski, V.; Kroumov, A.D.; et al. In Vitro Study of the Biological Potential of Wastewater Obtained after the Distillation of Four Bulgarian Oil-Bearing Roses. Plants 2022, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, N.; Tansho-Nagakawa, S.; Miyazaki, C.; Shimomura, K.; Ono, Y.; Abe, S. Inhibition of Neutrophil Adhesion and Antimicrobial Activity by Diluted Hydrosol Prepared from Rosa damascena. Biol. Pharm. Bull. 2017, 40, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible Flowers as Sources of Phenolic Compounds with Bioactive Potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Özkan, G.; Săgdiç, O.; Baydar, N.G.; Baydar, H. Note: Antioxidant and Antibacterial Activities of Rosa Damascena Flower Extracts. Food Sci. Technol. Int. 2004, 10, 277–281. [Google Scholar] [CrossRef]
- Hoseinpour, H.; Peel, S.A.F.; Rakhshandeh, H.; Forouzanfar, A.; Taheri, M.; Rajabi, O.; Saljoghinejad, M.; Sohrabi, K. Evaluation of Rosa Damascena Mouthwash in the Treatment of Recurrent Aphthous Stomatitis: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Quintessence Int. 2011, 42, 483–491. [Google Scholar] [PubMed]
- Abdel-Hameed, E.-S. Total Phenolics and Antioxidant Activity of Defatted Fresh Taif Rose, Saudi Arabia. Br. J. Pharm. Res. 2012, 2, 129–140. [Google Scholar] [CrossRef]
- Slavov, A.; Denev, P.; Panchev, I.; Shikov, V.; Nenov, N.; Yantcheva, N.; Vasileva, I. Combined Recovery of Polysaccharides and Polyphenols from Rosa damascena Wastes. Ind. Crops Prod. 2017, 100, 85–94. [Google Scholar] [CrossRef]
- Dragoev, S.; Vlahova-Vangelova, D.; Balev, D.; Bozhilov, D.; Dagnon, S. Valorization of Waste By-Products of Rose Oil Production as Feedstuff Phytonutrients. Bulg. J. Agric. Sci. 2021, 27, 209–219. [Google Scholar] [CrossRef]
- Dina, E.; Sklirou, A.D.; Chatzigeorgiou, S.; Manola, M.S.; Cheilari, A.; Louka, X.P.; Argyropoulou, A.; Xynos, N.; Skaltsounis, A.L.; Aligiannis, N.; et al. An Enriched Polyphenolic Extract Obtained from the By-Product of Rosa damascena Hydrodistillation Activates Antioxidant and Proteostatic Modules. Phytomedicine 2021, 93, 153757. [Google Scholar] [CrossRef]
- Herranz-López, M.; Fernández-Arroyo, S.; Pérez-Sanchez, A.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Menéndez, J.A.; Alonso-Villaverde, C.; Segura-Carretero, A.; Joven, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis: Perspectives and implications. Phytomedicine 2012, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Saad, B.; Kmail, A.; Haq, S.Z.H. Anti-Diabesity Middle Eastern Medicinal Plants and Their Action Mechanisms. Hindawi Evid.-Based Complement. Altern. Med. 2022, 2022, 2276094. [Google Scholar] [CrossRef] [PubMed]
- Sherif, M.M.; Elshikh, H.H.; Abdel-Aziz, M.M.; Elaasser, M.M.; Yosri, M. In Vitro Antibacterial and Phytochemical Screening of Hypericum perforatum Extract as Potential Antimicrobial Agents against Multi-Drug-Resistant (MDR) Strains of Clinical Origin. BioMed Res. Int. 2023, 2023, 6934398. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, E.C.; Boeren, S.; Exarchou, V.; Troganis, A.N.; Vervoort, I.P. Gerothanassis, Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007, 68, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, N.; Lechtenberg, M.; Petereit, F.; Sendker, J.; Hensel, A. Isolation and quantification of oligomeric and polymeric procyanidins in the aerial parts of St. John’s wort (Hypericum perforatum). Planta Medica 2015, 81, 1175–1181. [Google Scholar] [PubMed]
- Belwal, T.; Devkota, H.P.; Singh, M.K. St. John’s wort (Hypericum perforatum). In Non-Vitamin and Non-Mineral Nutritional Supplements; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Kakouri, E.; Daferera, D.; Trigas, P.; Charalambous, D.; Pantelidou, M.; Tarantilis, P.A.; Kanakis, C.D. Comparative Study of the Antibacterial Activity, Total Phenolic and Total Flavonoid Content of Nine Hypericum Species Grown in Greece. Appl. Sci. 2023, 13, 3305. [Google Scholar] [CrossRef]
- Besra, M.; Kumar, V. In vitro investigation of antimicrobial activities of ethnomedicinal plants against dental caries pathogens. 3 Biotech 2018, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: A systematic review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Wölfle, E.U.; Seelinger, G.; Schempp, C.M. Topical application of St. John’s wort (Hypericum perforatum). Planta Medica 2014, 80, 109–120. [Google Scholar]
- Jarzębski, M.; Smułek, W.; Baranowska, H.M.; Masewicz, Ł.; Kobus-Cisowska, J.; Ligaj, M.; Kaczorek, E. Characterization of St. John’s wort (Hypericum perforatum L.) and the impact of filtration process on bioactive extracts incorporated into carbohydrate-based hydrogels. Food Hydrocoll. 2020, 104, 105748. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Zirak, N.; Shafiee, M.; Soltani, G.; Mirzaei, M.; Sahebkar, A. Hypericum perforatum in the treatment of psychiatric and neurodegenerative disorders: Current evidence and potential mechanisms of action. J. Cell. Physiol. 2019, 234, 8496–8508. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Menegazzi, M.; Masiello, P.; Novelli, M. Anti-tumor activity of Hypericum perforatum L. and hyperforin through modulation of inflammatory signaling, ROS generation and proton dynamics. Antioxidants 2020, 10, 18. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species—A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef]
- Seyis, F.; Yurteri, E.; Özcan, A.; Cirak, C. Altitudinal impacts on chemical content and composition of Hypericum perforatum, a prominent medicinal herb. S. Afr. J. Bot. 2020, 135, 391–403. [Google Scholar] [CrossRef]

| Phytochemical Compounds | Plants Extracts | |||||||
|---|---|---|---|---|---|---|---|---|
| Roses (Rosa damascene) | Hypericum perforatum | |||||||
| A | Ε40 | Ε60 | ΕΝΖ | A | Ε40 | Ε60 | ΕΝΖ | |
| Alkaloids | + | + | + | + | + | + | + | + |
| Anthraquinones | − | − | − | + | + | + | + | + |
| Terpenoids (Salkowski’s test) | + | + | + | + | + | + | + | |
| Steroids | + | + | + | + | − | − | − | − |
| Saponins | + | − | − | + | − | − | − | + |
| Flavonoids (alkaline reagent test) | + | + | + | + | + | + | + | + |
| Tannins (ferric chloride test) | + | + | + | + | + | + | + | + |
| Glycosides (Keller–Kiliani test) | + | + | + | + | + | + | + | + |
| Plant Extracts | Total Phenolics (mg Gallic Acid Equivalent/g of Dried Sample) | Total Flavonoids (mg Catechin Equivalent per/g of Dried Sample) |
|---|---|---|
| R. damascene | ||
| Rosa A | 104.92 ± 6.05 c | 41.97 ± 0.34 d |
| Rosa A* | 89.1 ± 2.2 b | 36.81 ± 0.26 c |
| Rosa E40 | 79.47 ± 2.38 a | 28.73 ± 0.45 b |
| Rosa E60 | 75.47 ± 1.79 a | 20.68 ± 0.5 a |
| Rosa ENZ | 82.12 ± 2.95 ab | 42.78 ± 0.41 d |
| H. perforatum | ||
| Hyp. P A | 54.45 ± 3.03 ab | 11.78 ± 0.62 a |
| Hyp. P A* | 60.26 ± 0.94 b | 16.47 ± 0.58 b |
| Hyp. P E40 | 80.61 ± 6.85 c | 21.56 ± 0.62 c |
| Hyp P E60 | 92.39 ± 2.06 d | 25.06 ± 0.87 d |
| Hyp P ENZ | 49.2 ± 0.83 a | 33.63 ± 1.3 e |
| Plant Extracts | Concentration (μg/mL) | |||||
|---|---|---|---|---|---|---|
| 500 | 300 | 100 | 50 | 10 | 1 | |
| R. damascene | ||||||
| Rosa A | 86.05 ± 0.14 c | 83.26 ± 0.26 b | 72.32 ± 0.4 d | 45.09 ± 0.9 d | 12.85 ± 0.27 d | 4.3 ± 0.25 d |
| Rosa A* | 85.11 ± 0.12 c | 85.41 ± 0.96 b | 62.13 ± 0.82 c | 43.09 ± 0.54 c | 11.26 ± 0.55 c | 3.84 ± 0.25 d |
| Rosa E40 | 65.85 ± 0.53 b | 61.18 ± 0.24 ab | 50.68 ± 0.15 b | 41.93 ± 0.57 bc | 14.05 ± 0.19 e | 2.96 ± 0.1 c |
| Rosa E60 | 64.45 ± 0.37 a | 42.49 ± 31.3 a | 39.32 ± 0.3 a | 28.07 ± 0.17 a | 7.67 ± 0.56 a | 1.99 ± 0.54 b |
| Rosa ENZ | 65.03 ± 0.88 ab | 59.4 ± 1.02 ab | 51.33 ± 0.57 b | 41.31 ± 0.57 b | 9.22 ± 0.32 b | 0.87 ± 0.11 a |
| H. perforatum | ||||||
| Hyp. P A | 61 ± 1.4 b | 58.6 ± 0.86 b | 39.51 ± 0.68 c | 31.53 ± 0.6 b | 10.65 ± 0.22 c | 3.5 ± 0.42 b |
| Hyp. P A* | 67.87 ± 0.45 c | 53.11 ± 0.77 a | 37.32 ± 0.4 b | 24.02 ± 0.49 a | 3.38 ± 0.39 a | 0.67 ± 0.24 a |
| Hyp. P E40 | 87.95 ± 0.36 d | 82.81 ± 0.77 c | 77.16 ± 0.25 d | 43.66 ± 0.76 c | 11.05 ± 0.19 c | 4.22 ± 0.29 b |
| Hyp P E60 | 89.16 ± 0.37 d | 87.44 ± 0.48 d | 78.99 ± 0.12 e | 54.43 ± 0.57 d | 16.66 ± 0.36 d | 11.32 ± 0.48 c |
| Hyp P ENZ | 55.29 ± 0.52 a | 52.38 ± 0.5 a | 29.02 ± 0.48 a | 25.12 ± 0.46 a | 6.15 ± 0.09 b | 1.42 ± 0.16 a |
| Plant Extracts | Concentration (μg/mL) | |||||
|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 150 | 200 | 250 | |
| R. damascene | ||||||
| Rosa A | 0.18 ± 0 a | 0.26 ± 0.01 a | 0.32 ± 0.01 a | 0.75 ± 0 a | 0.91 ± 0.01 a | 1.02 ± 0 a |
| Rosa A* | 0.2 ± 0 b | 0.34 ± 0 b | 0.68 ± 0.01 c | 0.88 ± 0.01 b | 1 ± 0.01 b | 1.22 ± 0.01 b |
| Rosa E40 | 0.2 ± 0 b | 0.48 ± 0 d | 0.68 ± 0.01 c | 1.18 ± 0 c | 1.31 ± 0.01 d | 1.46 ± 0 c |
| Rosa E60 | 0.21 ± 0 c | 0.49 ± 0.01 d | 0.71 ± 0 d | 1.22 ± 0 d | 1.35 ± 0 e | 1.54 ± 0.01 e |
| Rosa ENZ | 0.18 ± 0 a | 0.35 ± 0.01 c | 0.65 ± 0 b | 1.18 ± 0.01 c | 1.22 ± 0 c | 1.47 ± 0.01 d |
| H. perforatum | ||||||
| Hyp. P A | 0.5 ± 0.57 d | 0.28 ± 0.01 b | 0.35 ± 0 b | 0.74 ± 0.01 b | 1.02 ± 0.01 b | 1.22 ± 0 b |
| Hyp. P A* | 0.23 ± 0.01 c | 0.44 ± 0.01 c | 0.79 ± 0.01 c | 0.92 ± 0.01 c | 1.22 ± 0.01 c | 1.44 ± 0.01 c |
| Hyp. P E40 | 0.21 ± 0.01 b | 0.22 ± 0 a | 0.32 ± 0.01 a | 0.44 ± 0 a | 0.65 ± 0 a | 0.89 ± 0.01 a |
| H-yp P E60 | 0.19 ± 0.01 a | 0.22 ± 0.01 a | 0.32 ± 0.01 a | 0.45 ± 0 a | 0.66 ± 0.01 a | 0.88 ± 0.01 a |
| Hyp P ENZ | 0.23 ± 0 a,c | 0.52 ± 0.01 d | 0.78 ± 0.01 c | 1.33 ± 0.01 d | 1.45 ± 0.01 d | 1.61 ± 0.01 d |
| Reference | ||||||
| Gallic acid (GA) | 0.18 ± 0.01 | 0.43 ± 0.01 | 1.82 ± 0.01 | 1.85 ± 0.01 | 1.88 ± 0 | 1.88 ± 0.01 |
| Ascorbic acid (AA) | 0.19 ± 0 | 0.42 ± 0.01 | 0.69 ± 0.01 | 1.13 ± 0.01 | 1.66 ± 0.01 | 1.89 ± 0.01 |
| Pathogen | Disk Content (%) | A (mm) | E40 (mm) | E60 (mm) | Enz (mm) |
|---|---|---|---|---|---|
| S. aureus MRSA/VRSA | 10 | 10.7 ± 0.7 a | 12.2 ± 0.3 a | 14.3 ± 0.9 b | 14 ± 0.8 b |
| 20 | 14.6 ± 0.4 a | 14.9 ± 0.2 a | 14.2 ± 0.3 a | 21.9 ± 0.6 b | |
| 50 | 17.5 ± 0.6 a | 21.5 ± 0.6 a | 18.4 ± 0.6 b | 31.4 ± 1.1 c | |
| 100 | 23.1 ± 2 a | 25 ± 5.2 ab | 31.7 ± 0.6 b | 48.2 ± 1 c | |
| MRSA (raw milk) | 10 | 9.4 ± 0.4 a | 8.3 ± 0.4 a | 12.4 ± 0.4 b | 12.2 ± 0.7 b |
| 20 | 15 ± 1.6 a | 12.2 ± 0.4 ab | 14.1 ± 0.6 b | 15.7 ± 0.8 b | |
| 50 | 17.9 ± 0.1 a | 15 ± 0.3 b | 18.6 ± 0.5 b | 20.5 ± 0.6 c | |
| 100 | 21.7 ± 0.8 a | 19.5 ± 0.6 b | 22.8 ± 1.2 b | 29.2 ± 0.2 c | |
| MRSA (raw poultry) | 10 | 11.2 ± 1 a | 11.7 ± 0.4 ab | 12.9 ± 0.3 b | 10.5 ± 0.4 a |
| 20 | 17.3 ± 0.5 b | 15.8 ± 0.7 a | 18 ± 0.2 b | 20.5 ± 0.6 c | |
| 50 | 28.2 ± 0.9 c | 24.9 ± 1.3 ab | 24.8 ± 0.7 a | 27.1 ± 0.2 bc | |
| 100 | 35.2 ± 0 b | 30.5 ± 0.5 a | 30.1 ± 0.7 a | 40.7 ± 0.5 c | |
| S. mutans | 10 | 9.2 ± 0.6 a | 11 ± 0.7 b | 12.1 ± 0.8 bc | 13.7 ± 0.4 c |
| 20 | 13.4 ± 0.5 a | 12 ± 0.4 a | 15.1 ± 0.3 b | 20.1 ± 0.9 c | |
| 50 | 18.3 ± 0.6 a | 18.9 ± 6 a | 20.4 ± 0.4 a | 30.4 ± 0.5 b | |
| 100 | 26.6 ± 1.5 a | 22.4 ± 5.6 a | 28.6 ± 0.6 a | 37.9 ± 0.4 b | |
| S. salivarius | 10 | 10.7 ± 0.6 a | 11.9 ± 0.6 ab | 13.3 ± 0.4 b | 12.6 ± 0.5 b |
| 20 | 14.6 ± 0.5 a | 14.4 ± 0.6 a | 19.1 ± 0.1 b | 18.2 ± 0.3 b | |
| 50 | 19 ± 0.1 a | 20.7 ± 0.5 a | 28 ± 0.2 b | 27.4 ± 0.8 b | |
| 100 | 24.3 ± 0.9 a | 27.2 ± 1.1 b | 36.7 ± 1.6 c | 38.2 ± 0.3 c | |
| P. gingivalis | 10 | 13.7 ± 0.5 c | 10.7 ± 0.5 a | 12 ± 0.2 b | 12.7 ± 0.5 bc |
| 20 | 18.3 ± 0.5 b | 12.8 ± 0.3 a | 14 ± 0.7 a | 19.1 ± 1 b | |
| 50 | 28.7 ± 0.2 c | 15.5 ± 0.7 a | 17.6 ± 0.4 b | 28 ± 0.5 c | |
| 100 | 39.3 ± 0.9 b | 18.6 ± 0.4 a | 20 ± 0.2 a | 39.3 ± 1.1 b | |
| F. nucleatum | 10 | 10.7 ± 0.4 a | 12.6 ± 0.3 b | 14.7 ± 0.4 c | 12.3 ± 0.7 b |
| 20 | 13.6 ± 0.8 a | 18.2 ± 0.5 b | 21.9 ± 0.2 c | 18 ± 0.2 b | |
| 50 | 18.4 ± 0.4 a | 27.8 ± 1.1 b | 34.3 ± 0.9 c | 28.3 ± 0.8 b | |
| 100 | 21 ± 0.1 a | 33 ± 0.8 b | 43.1 ± 1.6 d | 38.8 ± 0.8 c | |
| P. intermedia | 10 | 8.9 ± 0.2 a | 10.3 ± 0.5 b | 12.2 ± 0.3 c | 11.3 ± 0.4 c |
| 20 | 10.9 ± 0.4 a | 13.1 ± 0.3 a | 18.9 ± 0.3 b | 17.9 ± 0.5 b | |
| 50 | 17.1 ± 0.3 a | 18.9 ± 1 a | 31.6 ± 1.3 c | 23.7 ± 1 b | |
| 100 | 22.8 ± 0.6 a | 23.2 ± 0.3 a | 39.2 ± 0.4 c | 28.6 ± 0.9 b | |
| P. micra | 10 | 9 ± 0.7 a | 10.5 ± 0.4 b | 12.5 ± 0.4 c | 11.5 ± 0.3 bc |
| 20 | 13.2 ± 0.3 a | 15 ± 0.5 b | 19 ± 0.5 c | 18 ± 0.5 c | |
| 50 | 19.2 ± 0.5 a | 21.5 ± 0.6 b | 30.5 ± 0.6 d | 26 ± 0.3 c | |
| 100 | 16.2 ± 5.2 a | 28.7 ± 0.9 b | 40.5 ± 0.6 c | 28.2 ± 6.1 b | |
| S. aureus ATCC 12600 (Ref) | 10 | 9.2 ± 0.3 a | 11.6 ± 0.4 bc | 12.3 ± 0.4 c | 10.9 ± 0.2 b |
| 20 | 13.9 ± 0.5 a | 13.5 ± 0.3 a | 18.2 ± 0.6 b | 14.2 ± 0.3 a | |
| 50 | 16.9 ± 0.1 a | 16.5 ± 0.3 a | 21.2 ± 1 c | 19.5 ± 0.3 b | |
| 100 | 18.3 ± 0.3 a | 20 ± 0.7 a | 26.6 ± 2.7 b | 26.8 ± 0.6 b |
| Pathogen | Aqueous | Ethanolic | Aq/Eth Mix | Enzymatic |
|---|---|---|---|---|
| S. aureus MRSA/VRSA | 0.972 ** | 0.907 ** | 0.842 ** | 0.972 ** |
| MRSA (raw milk) | 0.972 ** | 0.973 ** | 0.972 ** | 0.972 ** |
| MRSA (raw poultry) | 0.972 ** | 0.972 ** | 0.972 ** | 0.972 ** |
| S. mutans | 0.972 ** | 0.928 ** | 0.972 ** | 0.972 ** |
| S. salivarius | 0.972 ** | 0.972 ** | 0.972 ** | 0.972 ** |
| P. gingivalis | 0.972 ** | 0.972 ** | 0.972 ** | 0.972 ** |
| F. nucleatum | 0.972 ** | 0.972 ** | 0.972 ** | 0.972 ** |
| P. intermedia | 0.972 ** | 0.972 ** | 0.972 ** | 0.972 ** |
| P. micra | 0.712 * | 0.972 ** | 0.972 ** | 0.842 ** |
| S. aureus ATCC 12600 (Ref) | 0.972 ** | 0.972 ** | 0.972 ** | 0.972 ** |
| Pathogen | Disk Content (%) | A(mm) | E40 (mm) | E60 (mm) | Enz (mm) |
|---|---|---|---|---|---|
| S. aureus MRSA/VRSA | 10 | 6.7 ± 0.6 a | 10 ± 0.7 b | 10.5 ± 0.5 b | 6 ± 0 a |
| 20 | 11.3 ± 0.4 b | 12.6 ± 0.2 c | 15 ± 0.4 d | 8.5 ± 0.6 a | |
| 50 | 13.4 ± 0.4 b | 17.5 ± 0.5 c | 18.9 ± 0.5 d | 12.2 ± 0.3 a | |
| 100 | 17.6 ± 0.3 a | 18.5 ± 0.3 a | 22.8 ± 2 b | 17.9 ± 0.4 a | |
| MRSA (raw milk) | 10 | 7.6 ± 0.6 a | 12.5 ± 0.4 b | 12.6 ± 0.3 b | 7.4 ± 0.5 a |
| 20 | 11.9 ± 0.7 a | 15 ± 0.2 b | 18.6 ± 0.3 c | 12 ± 0.4 a | |
| 50 | 14.8 ± 0.5 a | 18 ± 0.3 b | 23.1 ± 0.8 c | 14.6 ± 0.2 a | |
| 100 | 17.6 ± 0.3 a | 24.3 ± 0.4 b | 28.7 ± 0.6 c | 17.4 ± 0.4 a | |
| MRSA (raw poultry) | 10 | 6.4 ± 0.4 a | 9.1 ± 0.7 c | 11.4 ± 0.7 d | 7.8 ± 0.5 b |
| 20 | 10.6 ± 0.1 a | 10.6 ± 0.2 a | 16.2 ± 0.6 b | 10.7 ± 0.4 a | |
| 50 | 14.9 ± 0.2 a | 16.2 ± 0.3 b | 20.6 ± 0.5 c | 15.2 ± 0.4 a | |
| 100 | 17.4 ± 0.4 a | 18.3 ± 0.6 a | 29.1 ± 0.8 b | 17.7 ± 0.3 a | |
| S. mutans | 10 | 10.8 ± 0.8 c | 8.6 ± 0.3 a | 9.8 ± 0.4 b | 12.1 ± 0.5 d |
| 20 | 16.4 ± 0.4 c | 10.7 ± 0.3 a | 11.9 ± 0.5 b | 17.1 ± 0.2 c | |
| 50 | 20.7 ± 0.8 c | 15.2 ± 0.3 a | 16.7 ± 0.5 b | 21.5 ± 0.7 c | |
| 100 | 29.3 ± 0.9 c | 16.7 ± 0.2 a | 18.5 ± 0.5 b | 30.8 ± 0.6 d | |
| S. salivarius | 10 | 8.4 ± 0.8 a | 10.8 ± 0.6 b | 11.7 ± 0.4 b | 9.3 ± 0.3 a |
| 20 | 12.9 ± 0.6 a | 13.1 ± 0.8 a | 17.4 ± 0.5 b | 12.8 ± 0.5 a | |
| 50 | 16.4 ± 0.5 a | 16.3 ± 0.5 a | 22.2 ± 0.7 c | 17.8 ± 0.3 b | |
| 100 | 18.5 ± 0.6 a | 18.5 ± 0.5 a | 27.5 ± 5.5 b | 18.5 ± 0.4 a | |
| P. gingivalis | 10 | 11.2 ± 0.8 c | 7.6 ± 0.5 a | 10.2 ± 0.2 b | 12.2 ± 0.6 c |
| 20 | 15.2 ± 0.5 c | 11.9 ± 0.3 a | 14 ± 0.7 b | 18.2 ± 0.3 d | |
| 50 | 20.6 ± 0.5 c | 14.1 ± 0.3 a | 17 ± 0.2 b | 28.1 ± 1 d | |
| 100 | 32.3 ± 1.9 b | 17.5 ± 0.3 a | 18.6 ± 0.4 a | 33.8 ± 1.2 b | |
| F. nucleatum | 10 | 6.1 ± 0.1 a | 9.8 ± 0.4 b | 11.9 ± 0.6 c | 9.8 ± 0.4 b |
| 20 | 9.3 ± 0.6 a | 10.4 ± 0.4 b | 17.2 ± 0.2 c | 16.7 ± 0.4 c | |
| 50 | 12.4 ± 0.6 a | 13.9 ± 0.2 a | 24.1 ± 1.4 c | 19.6 ± 0.8 b | |
| 100 | 16 ± 0.2 a | 16.8 ± 0.2 a | 28.9 ± 0.9 b | 30.3 ± 0.8 c | |
| P. intermedia | 10 | 9.6 ± 0.2 b | 8.3 ± 0.6 a | 11.7 ± 0.5 c | 9 ± 0.1 ab |
| 20 | 13.7 ± 0.2 b | 12 ± 0.4 a | 19 ± 0.4 c | 12.5 ± 0.7 a | |
| 50 | 19 ± 0.2 b | 17.3 ± 0.4 a | 28.4 ± 0.6 c | 16.7 ± 0.4 a | |
| 100 | 24.5 ± 0.5 c | 19.1 ± 0.8 b | 31.7 ± 0.6 d | 17.9 ± 0.1 a | |
| P. micra | 10 | 9.5 ± 0.6 a | 10.6 ± 0.6 b | 11 ± 0.5 bc | 12 ± 0.5 c |
| 20 | 12.4 ± 0.4 a | 15.3 ± 0.5 b | 16.2 ± 0.3 b | 19.3 ± 0.5 c | |
| 50 | 18.1 ± 1 a | 21.6 ± 1.2 b | 20.8 ± 0.5 b | 29.3 ± 0.5 c | |
| 100 | 21.2 ± 0.9 a | 27.8 ± 1.1 b | 30.9 ± 0.3 c | 35.4 ± 1.1 d | |
| S. aureus ATCC 12600 (Ref) | 10 | 9.4 ± 0.5 a | 10.9 ± 1.1 b | 10.5 ± 0.6 ab | 11.1 ± 0.1 c |
| 20 | 12.9 ± 0.5 a | 14.9 ± 0.1 b | 18.1 ± 0.6 c | 18 ± 0.2 c | |
| 50 | 17.5 ± 0.6 a | 18.3 ± 1 a | 26.7 ± 0.7 c | 22.2 ± 1 b | |
| 100 | 19.8 ± 0.4 a | 21 ± 0.4 a | 30.7 ± 0.4 b | 31.8 ± 2 b |
| Pathogen | Aqueous | Ethanolic | Aq/Eth Mix | Enzymatic |
|---|---|---|---|---|
| S. aureus MRSA/VRSA | 0.972 ** | 0.972 ** | 0.950 ** | 0.865 ** |
| MRSA (raw milk) | 0.885 ** | 0.897 ** | 0.854 ** | 0.885 ** |
| MRSA (raw poultry) | 0.973 ** | 0.973 ** | 0.919 ** | 0.973 ** |
| S. mutans | 0.972 ** | 0.973 ** | 0.972 ** | 0.972 ** |
| S. salivarius | 0.973 ** | 0.972 ** | 0.972 ** | 0.972 ** |
| P. gingivalis | 0.973 ** | 0.972 ** | 0.928 ** | 0.972 ** |
| F. nucleatum | 0.972 ** | 0.972 ** | 0.972 ** | 0.972 ** |
| P. intermedia | 0.972 ** | 0.972 ** | 0.972 ** | 0.972 ** |
| P. micra | 0.901 ** | 0.972 ** | 0.972 ** | 0.972 ** |
| S. aureus ATCC 12600 (Ref) | 0.972 ** | 0.972 ** | 0.978 ** | 0.972 ** |
| Minimum Inhibitory Concentration (mg/mL) | Minimum Bactericidal Concentration (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pathogen | A | E40 | E60 | Enz | A | E40 | E60 | Enz |
| S. aureus MRSA/VRSA | 3.1 ± 0 4 | 1.6 ± 0 3 | 0.8 ± 0 2 | 0.4 ± 0 1 | 4.2 ± 1.8 c | 6.3 ± 0 d | 1.6 ± 0 b | 0.8 ± 0 a |
| MRSA (raw milk) | 6.3 ± 0 3 | 6.3 ± 0 3 | 3.1 ± 0 2 | 0.8 ± 0 1 | 6.3 ± 0 b | 6.3 ± 0 b | 6.3 ± 0 b | 0.8 ± 0 a |
| MRSA (raw poultry) | 0.8 ± 0 2 | 1.6 ± 0 3 | 0.8 ± 0 2 | 0.4 ± 0 1 | 0.8 ± 0 b | 3.1 ± 0 c | 0.8 ± 0 b | 0.4 ± 0 a |
| S. mutans | 3.1 ± 0 2 | 6.3 ± 0 3 | 3.1 ± 0 2 | 0.4 ± 0 1 | 6.3 ± 0 b | 6.3 ± 0 b | 6.3 ± 0 b | 0.4 ± 0 a |
| S. salivarius | 3.1 ± 0 4 | 1.6 ± 0 3 | 0.8 ± 0 2 | 0.4 ± 0 1 | 6.3 ± 0 d | 3.1 ± 0 c | 0.8 ± 0 b | 0.4 ± 0 a |
| P. gingivalis | 0.4 ± 0 1 | 6.3 ± 0 2 | 6.3 ± 0 2 | 0.4 ± 0 1 | 0.8 ± 0 b | 12.5 ± 0 d | 10.4 ± 3.6 c | 0.4 ± 0 a |
| F. nucleatum | 3.1 ± 0 3 | 1.6 ± 0 2 | 0.4 ± 0 1 | 0.4 ± 0 1 | 6.3 ± 0 d | 3.1 ± 0 c | 0.7 ± 0.2 b | 0.4 ± 0 a |
| P. intermedia | 6.3 ± 0 4 | 3.1 ± 0 3 | 0.8 ± 0 1 | 1.6 ± 0 2 | 12.5 ± 0 d | 8.3 ± 3.6 c | 3.1 ± 0 b | 1.6 ± 0 a |
| P. micra | 3.1 ± 0 3 | 3.1 ± 0 3 | 0.8 ± 0 1 | 1.6 ± 0 2 | 6.3 ± 0 b | 6.3 ± 0 b | 1.6 ± 0 a | 1.6 ± 0 a |
| S. aureus ATCC 12600 (Ref) | 3.1 ± 0 2 | 3.1 ± 0 2 | 1.6 ± 0 1 | 1.6 ± 0 1 | 6.3 ± 0 c | 6.3 ± 0 c | 3.1 ± 0 b | 3.1 ± 0 a |
| Minimum Inhibitory Concentration (mg/mL) | Minimum Bactericidal Concentration (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pathogen | A | E40 | E60 | Enz | A | E40 | E60 | Enz |
| S. aureus MRSA/VRSA | 2.6 ± 0.9 1 | 12.5 ± 0 | 6.3 ± 0 | 2.1 ± 0.9 1 | 5.2 ± 1.8 a | 25 ± 0 b | 25 ± 0 b | 6.3 ± 0 a |
| MRSA (raw milk) | 41.7 ± 14.4 4 | 25 ± 0 3 | 5.2 ± 1.8 1 | 12.5 ± 02 2 | 70 ± 52 d | 50 ± 0 c | 6.3 ± 0 a | 25 ± 0 b |
| MRSA (raw poultry) | 16.7 ± 7.2 2 | 12.5 ± 0 1 | 12.5 ± 0 1 | 25 ± 0 3 | 25 ± 0 c | 6.3 ± 0 a | 12.5 ± 0 b | 50 ± 0 d |
| S. mutans | 12.5 ± 0 3 | 8.3 ± 3.6 2 | 3.1 ± 0 1 | 3.1 ± 0 1 | 12.5 ± 0 c | 6.3 ± 0 b | 6.3 ± 0 b | 3.1 ± 0 a |
| S. salivarius | 25 ± 0 4 | 12.5 ± 0 3 | 3.1 ± 0 1 | 6.3 ± 0 2 | 50 ± 0 d | 25 ± 0 c | 3.1 ± 0 a | 12.5 ± 0 b |
| P. gingivalis | 3.1 ± 0 1 | 12.5 ± 0 2 | 12.5 ± 0 2 | 3.1 ± 0 1 | 6.3 ± 0 b | 16.7 ± 7 a | 12.5 ± 0 c | 4.2 ± 1.8 a |
| F. nucleatum | 0.8 ± 0 2 | 1.6 ± 0 3 | 0.4 ± 0 1 | 3.1 ± 0 4 | 1.6 ± 0 b | 1.6 ± 0 b | 0.8 ± 0 a | 3.1 ± 0 c |
| P. intermedia | 0.8 ± 0 2 | 1.6 ± 0 3 | 0.4 ± 0 1 | 3.1 ± 0 4 | 3.1 ± 0 b | 3.1 ± 0 b | 0.8 ± 0 a | 6.3 ± 0 c |
| P. micra | 25 ± 0 3 | 12.5 ± 0 2 | 12.5 ± 0 2 | 0.4 ± 0 1 | 50 ± 0 d | 25 ± 0 c | 16.7 ± 7.2 b | 0.7 ± 0.2 a |
| S. aureus ATCC 12600 (Ref) | 3.1 ± 0 2 | 3.1 ± 0 2 | 2.1 ± 0.9 2 | 0.8 ± 0 1 | 12.5 ± 0 d | 6.3 ± 0 c | 3.1 ± 0 b | 1.6 ± 0 a |
| Extract/Herbal | N | Mean ± SD | |
|---|---|---|---|
| Aqueous | Hypericum perforatum | 120 | 17.43 ± 7.03 b |
| Rosa damascene | 120 | 14.76 ± 6.02 a | |
| Ethanolic | Hypericum perforatum | 120 | 17.55 ± 6.37 b |
| Rosa damascene | 120 | 14.62 ± 4.59 a | |
| Aqueous/Ethanolic Mix | Hypericum perforatum | 120 | 21.64 ± 8.81 c |
| Rosa damascene | 120 | 16.58 ± 5.01 a | |
| Enzymatic | Hypericum perforatum | 120 | 23.08 ± 9.73 b |
| Rosa damascene | 120 | 16.66 ± 6.64 a | |
| Antibiofilm Agent | Corr. Coefficient, (2-Tailed Sig.), Sample N |
|---|---|
| Aqueous extract | 0.155, (<0.05), 198 |
| Ethanolic extract | 0.111 (>0.05), 199 |
| Aqueous/Ethanolic Mix extract | 0.014, (>0.05), 197 |
| Enzymatic extract | −0.091, (>0.05, 197 |
| Clidamycin | 0.761, (<0.001), 96 |
| Ciprofloxacin | 0.732, (>0.05), 92 |
| Gentamycin | 0.712, (<0.001), 92 |
| Imipenem | 0.778, (<0.001), 196 |
| Vancomycin | 0.638, (<0.001), 100 |
| Metronidazol | 0.775, (<0.001), 100 |
| Amoxicillin/Clavulanic Acid | 0.812, (<0.001), 100 |
| Pathogen | MBC | Aqueous Extract | Enzymatic Extract | Control (No Extract) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 2 h | 4 h | 8 h | 12 h | 24 h | 0 h | 2 h | 4 h | 8 h | 12 h | 24 h | 0 h | 2 h | 4 h | 8 h | 12 h | 24 h | ||
| S. aureus MRSA/VRSA | 8 | 6.27 ± 0.21 | 5.43 ± 1.02 | 4.83 ± 0.91 | 3.97 ± 0.21 | 3.83 ± 0.8 | 0 ± 0 | 5.77 ± 1.29 | 4.57 ± 1.52 | 4.57 ± 0.42 | 2.23 ± 1.4 | 0 ± 0 | 0 ± 0 | 6.27 ± 1.42 | 5.9 ± 0.69 | 5.97 ± 0.31 | 11.77 ± 0.47 | 12.37 ± 0.21 | 13.1 ± 1.21 |
| 4 | 6.27 ± 0.29 | 5.47 ± 1.18 | 5 ± 0.2 | 4.67 ± 1.59 | 3.97 ± 1.02 | 0 ± 0 | 5.83 ± 0.21 | 4.87 ± 0.6 | 4.63 ± 0.9 | 2.4 ± 0.44 | 0 ± 0 | 0 ± 0 | |||||||
| 2 | 6 ± 0.69 | 6.17 ± 0.4 | 6.2 ± 1.3 | 5.7 ± 1.21 | 4.93 ± 0.67 | 1.03 ± 0.74 | 5.83 ± 0.31 | 4.97 ± 0.47 | 4.83 ± 0.38 | 2.93 ± 0.21 | 0.5 ± 0 | 0 ± 0 | |||||||
| 1 | 6.23 ± 1.06 | 6.23 ± 1.51 | 6.57 ± 1.1 | 6.03 ± 0.51 | 5.83 ± 1.4 | 2.97 ± 1.03 | 5.83 ± 0.78 | 5.63 ± 0.6 | 5.63 ± 1.38 | 3.37 ± 0.68 | 1.8 ± 0.61 | 0 ± 0 | |||||||
| MRSA (milk) | 8 | 5.83 ± 0.68 | 5.43 ± 0.81 | 4.9 ± 0.52 | 3.77 ± 0.81 | 2.5 ± 1.01 | 0 ± 0 | 5.83 ± 0.21 | 5.3 ± 1.51 | 2.93 ± 0.7 | 2.47 ± 0.78 | 0 ± 0 | 0 ± 0 | 6.13 ± 0.5 | 6.03 ± 0.42 | 6.03 ± 0.21 | 6.9 ± 0.3 | 10.03 ± 1.23 | 12.63 ± 0.99 |
| 4 | 5.93 ± 1.4 | 5.93 ± 0.68 | 5.07 ± 0.29 | 3.93 ± 0.31 | 3.8 ± 0.52 | 0 ± 0 | 5.83 ± 1.24 | 5.37 ± 0.12 | 3.37 ± 0.29 | 2.8 ± 1.4 | 0 ± 0 | 0 ± 0 | |||||||
| 2 | 5.97 ± 0.49 | 5.93 ± 0.9 | 5.93 ± 0.61 | 5.07 ± 1.38 | 4.33 ± 0.7 | 0.83 ± 0.72 | 5.83 ± 0.25 | 5.73 ± 0.92 | 4.57 ± 1.12 | 3.93 ± 0.29 | 0 ± 0 | 0 ± 0 | |||||||
| 1 | 6.1 ± 0.26 | 5.97 ± 0.25 | 5.97 ± 0.58 | 5.73 ± 1.39 | 5.5 ± 0.5 | 2.03 ± 1.39 | 5.93 ± 0.9 | 5.77 ± 1.11 | 5 ± 0.17 | 4.77 ± 1.5 | 4.03 ± 0.5 | 0 ± 0 | |||||||
| MRSA (poultry) | 8 | 5.97 ± 0.7 | 5.97 ± 1.32 | 5.7 ± 0.56 | 4.4 ± 1.14 | 2.93 ± 1.31 | 0 ± 0 | 5.83 ± 0.59 | 4.93 ± 0.6 | 4.1 ± 0.1 | 2.93 ± 1.1 | 0 ± 0 | 0 ± 0 | 6.43 ± 0.55 | 6 ± 0.52 | 6.03 ± 0.9 | 7.07 ± 0.81 | 10.8 ± 0.36 | 12.83 ± 1.19 |
| 4 | 5.97 ± 1.3 | 6 ± 0.52 | 6 ± 0.5 | 5 ± 0.2 | 3.7 ± 0.61 | 0.83 ± 0.49 | 5.87 ± 0.4 | 5.07 ± 0.32 | 4.97 ± 1.2 | 3.2 ± 1.3 | 0 ± 0 | 0 ± 0 | |||||||
| 2 | 5.97 ± 0.59 | 6.2 ± 0.7 | 6.2 ± 0.1 | 5.5 ± 0.7 | 4.57 ± 1.29 | 1.53 ± 0.21 | 5.87 ± 0.21 | 5.77 ± 0.72 | 5 ± 1.22 | 3.93 ± 1.4 | 2.3 ± 1.51 | 0 ± 0 | |||||||
| 1 | 5.97 ± 0.32 | 6.2 ± 1.22 | 6.73 ± 0.78 | 5.9 ± 0.62 | 5.8 ± 0.17 | 2.8 ± 0.87 | 5.93 ± 1.4 | 5.87 ± 0.4 | 4.83 ± 0.61 | 3.97 ± 1.27 | 3.7 ± 1.31 | 0 ± 0 | |||||||
| S. mutans | 8 | 5.8 ± 0.6 | 3.97 ± 1.04 | 2.07 ± 0.93 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 5.93 ± 0.15 | 4.97 ± 1.11 | 4.13 ± 0.12 | 2.03 ± 0.93 | 0 ± 0 | 0 ± 0 | 5.93 ± 0.59 | 6 ± 0.52 | 6.03 ± 1.1 | 6.87 ± 0.99 | 8.83 ± 1.5 | 11.8 ± 0.36 |
| 4 | 5.83 ± 0.86 | 3.97 ± 0.51 | 2.37 ± 0.21 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 5.9 ± 1.11 | 5.07 ± 0.31 | 4.23 ± 0.12 | 2.07 ± 0.31 | 0 ± 0 | 0 ± 0 | |||||||
| 2 | 5.83 ± 1.51 | 4.8 ± 0.2 | 2.8 ± 0.44 | 0.87 ± 0.8 | 0 ± 0 | 0 ± 0 | 5.9 ± 0.2 | 5.83 ± 0.7 | 4.97 ± 0.12 | 3.4 ± 1.39 | 0 ± 0 | 0 ± 0 | |||||||
| 1 | 5.97 ± 0.31 | 4.97 ± 1.27 | 4.03 ± 0.12 | 3.07 ± 0.7 | 0 ± 0 | 0 ± 0 | 5.97 ± 1.1 | 6.1 ± 0.5 | 6.03 ± 0.06 | 5.7 ± 1.3 | 3 ± 0.1 | 1.1 ± 0.1 | |||||||
| S. salivarius | 8 | 5.8 ± 0.72 | 2.93 ± 0.59 | 1 ± 1.05 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 5.83 ± 1.18 | 6 ± 0.4 | 4.9 ± 0.53 | 2.07 ± 1.31 | 0 ± 0 | 0 ± 0 | 5.87 ± 0.61 | 6.23 ± 0.38 | 6.23 ± 0.9 | 7 ± 1.48 | 8.6 ± 0.7 | 11.03 ± 0.5 |
| 4 | 5.67 ± 1.21 | 3.07 ± 0.31 | 1.03 ± 0.31 | 0.23 ± 0.95 | 0 ± 0 | 0 ± 0 | 5.9 ± 0.3 | 5.97 ± 1.07 | 4.93 ± 0.4 | 2.07 ± 0.9 | 0 ± 0 | 0 ± 0 | |||||||
| 2 | 5.87 ± 0.21 | 4.87 ± 0.5 | 3.2 ± 1.31 | 1.4 ± 0.61 | 0 ± 0 | 0 ± 0 | 5.93 ± 1.48 | 6 ± 0.7 | 5.03 ± 0.71 | 3.87 ± 1.27 | 2 ± 0.1 | 0 ± 0 | |||||||
| 1 | 6.07 ± 0.9 | 5.3 ± 1.31 | 4 ± 0.1 | 2.93 ± 1.1 | 0 ± 0 | 0 ± 0 | 5.93 ± 0.67 | 6 ± 0.1 | 6.07 ± 0.38 | 4.83 ± 0.5 | 2.53 ± 0.15 | 0.9 ± 0.2 | |||||||
| P. gingivitis | 8 | 5.87 ± 0.12 | 3.47 ± 1.22 | 1 ± 0.2 | 0.43 ± 0.12 | 0 ± 0 | 0 ± 0 | 5.8 ± 1.11 | 3.03 ± 0.06 | 1.03 ± 0.49 | −0.03 ± 0.35 | 0 ± 0 | 0 ± 0 | 5.87 ± 1.44 | 5.97 ± 0.21 | 5.97 ± 1.52 | 7.07 ± 1.11 | 8.97 ± 1.4 | 11.47 ± 1.5 |
| 4 | 5.83 ± 0.12 | 5.13 ± 0.51 | 1.97 ± 0.95 | 1.1 ± 0.7 | 0 ± 0 | 0 ± 0 | 5.87 ± 1.1 | 4.47 ± 1.31 | 3.1 ± 1.05 | 1.8 ± 0.78 | 0 ± 0 | 0 ± 0 | |||||||
| 2 | 5.87 ± 0.71 | 5.93 ± 1.01 | 5.2 ± 0.1 | 4.53 ± 0.42 | 0 ± 0 | 0 ± 0 | 5.87 ± 0.61 | 5.87 ± 0.21 | 5.73 ± 1.07 | 1.97 ± 1.21 | 0 ± 0 | 0 ± 0 | |||||||
| 1 | 5.9 ± 0.1 | 6 ± 1.21 | 5.73 ± 1.16 | 5.03 ± 1.42 | 2.4 ± 1.13 | 0.57 ± 0.4 | 5.87 ± 1.52 | 5.83 ± 0.12 | 5.73 ± 0.57 | 2.97 ± 1.12 | 1.07 ± 0.93 | 0 ± 0 | |||||||
| F. nucleatum | 8 | 5.87 ± 0.81 | 3.97 ± 0.49 | 1.93 ± 0.32 | 0.23 ± 0.87 | 0 ± 0 | 0 ± 0 | 5.8 ± 0.26 | 4.07 ± 1.1 | 1.9 ± 1.5 | 0.03 ± 1.05 | 0 ± 0 | 0 ± 0 | 5.97 ± 0.31 | 6.83 ± 0.9 | 6.8 ± 0.2 | 8.83 ± 0.5 | 9.47 ± 0.67 | 10.93 ± 0.32 |
| 4 | 5.87 ± 1.15 | 4.97 ± 1.02 | 2.53 ± 0.38 | 1.5 ± 1.31 | 0 ± 0 | 0 ± 0 | 5.83 ± 1.08 | 4.1 ± 0.75 | 2.83 ± 0.47 | 1.03 ± 0.67 | 0 ± 0 | 0 ± 0 | |||||||
| 2 | 5.83 ± 0.4 | 5.17 ± 1.17 | 3.37 ± 0.9 | 2.5 ± 0.79 | 0 ± 0 | 0 ± 0 | 5.87 ± 0.31 | 4.83 ± 0.32 | 3.03 ± 0.29 | 1.93 ± 0.42 | 0 ± 0 | 0 ± 0 | |||||||
| 1 | 5.93 ± 0.85 | 5.97 ± 0.47 | 3.93 ± 0.32 | 2.93 ± 0.5 | 1.5 ± 0.1 | 0 ± 0 | 5.87 ± 0.21 | 5.87 ± 0.96 | 4.8 ± 0.1 | 2.83 ± 0.06 | 0 ± 0 | 0 ± 0 | |||||||
| P. intermedia | 8 | 4.8 ± 0.1 | 3.57 ± 0.61 | 1.77 ± 1.54 | 0.1 ± 0.1 | 0 ± 0 | 0 ± 0 | 5.8 ± 0.78 | 5.3 ± 1.2 | 3.83 ± 0.91 | 1.97 ± 0.5 | 0 ± 0 | 0 ± 0 | 5.87 ± 0.83 | 6 ± 0.1 | 6 ± 0.2 | 7.93 ± 1.08 | 8.77 ± 0.31 | 9.97 ± 1.32 |
| 4 | 5.8 ± 0.1 | 4.77 ± 1.51 | 2.4 ± 0.2 | 0.34 ± 0.05 | 0 ± 0 | 0 ± 0 | 5.77 ± 0.6 | 5.37 ± 0.64 | 3.93 ± 0.21 | 2.07 ± 0.5 | 0 ± 0 | 0 ± 0 | |||||||
| 2 | 5.97 ± 0.31 | 4.83 ± 0.32 | 3.07 ± 1.3 | 2.03 ± 1.29 | 0 ± 0 | 0 ± 0 | 5.83 ± 1.16 | 5.5 ± 0.9 | 4.37 ± 0.9 | 2.87 ± 1 | 0 ± 0 | 0 ± 0 | |||||||
| 1 | 5.93 ± 0.9 | 5.37 ± 1.12 | 3.17 ± 0.29 | 3.13 ± 1.3 | 1 ± 0.17 | 0.07 ± 0.01 | 5.83 ± 0.38 | 5.5 ± 1.28 | 4.9 ± 0.61 | 3.97 ± 1 | 1.7 ± 0.2 | 0 ± 0 | |||||||
| P. micra | 8 | 5.63 ± 0.12 | 4.8 ± 1.11 | 0.93 ± 0.4 | 0.4 ± 0.53 | 0 ± 0 | 0 ± 0 | 5.83 ± 1.29 | 5.57 ± 0.21 | 3.97 ± 1.1 | 3.8 ± 0.8 | 0 ± 0 | 0 ± 0 | 5.97 ± 1.33 | 7 ± 0.7 | 6.97 ± 1.1 | 8.87 ± 1.21 | 8.97 ± 0.4 | 10.8 ± 0.69 |
| 4 | 5.67 ± 1.5 | 4.97 ± 0.32 | 2.43 ± 1 | 0.97 ± 0.47 | 0 ± 0 | 0 ± 0 | 5.87 ± 0.59 | 5.53 ± 1.06 | 4.67 ± 0.67 | 3.97 ± 0.7 | 0 ± 0 | 0 ± 0 | |||||||
| 2 | 5.73 ± 0.76 | 4.97 ± 0.29 | 3.07 ± 0.23 | 1.97 ± 0.9 | 0 ± 0 | 0 ± 0 | 5.9 ± 0.6 | 5.87 ± 0.4 | 4.73 ± 1.33 | 4.03 ± 1.4 | 0 ± 0 | 0 ± 0 | |||||||
| 1 | 5.93 ± 0.42 | 5.77 ± 0.4 | 4.03 ± 1.19 | 2.07 ± 0.6 | 1.03 ± 0.38 | 0.08 ± 0.01 | 5.93 ± 1.18 | 5.9 ± 1.31 | 5.83 ± 0.81 | 5.57 ± 0.38 | 2.03 ± 0.32 | 0.9 ± 0.1 | |||||||
| S. aureus ATCC 12600 (Ref) | 8 | 5.57 ± 0.32 | 4.97 ± 0.9 | 3.6 ± 0.98 | 2 ± 0.98 | 0.8 ± 0.1 | 0 ± 0 | 0 ± 0 | 4.4 ± 0.2 | 4 ± 1.61 | 2.8 ± 1.1 | 0 ± 0 | 0 ± 0 | 5.93 ± 0.59 | 6.07 ± 0.78 | 6.07 ± 0.06 | 7.77 ± 0.23 | 8.07 ± 1.31 | 8.9 ± 0.78 |
| 4 | 5.57 ± 1.43 | 5.03 ± 0.29 | 4.1 ± 0.2 | 1.97 ± 0.4 | 0.87 ± 0.21 | 0 ± 0 | 0 ± 0 | 4.67 ± 0.12 | 4.07 ± 0.42 | 2.5 ± 0.1 | 0 ± 0 | 0 ± 0 | |||||||
| 2 | 5.63 ± 0.76 | 5.63 ± 1.4 | 4.83 ± 0.38 | 3.07 ± 1.3 | 1 ± 0.7 | 0 ± 0 | 0 ± 0 | 5.03 ± 1.52 | 4.53 ± 1 | 3.07 ± 0.49 | 0.3 ± 0.1 | 0 ± 0 | |||||||
| 1 | 5.97 ± 1.19 | 6 ± 0.26 | 5.6 ± 0.5 | 5 ± 0.1 | 2.77 ± 1.16 | 0.13 ± 0.01 | 5.93 ± 0.71 | 5.67 ± 1.1 | 5.63 ± 1.44 | 3.37 ± 1.19 | 1.03 ± 0.12 | 0 ± 0 | |||||||
| Pathogen | Concentration (×MBC) | Aqueous Extract Killing-Time (h) (Hours) | Enzymatic Extract Killing-Time (h) (Hours) |
|---|---|---|---|
| S. aureus MRSA/VRSA | 8 | 24 | 8 |
| 4 | 24 | 8 | |
| 2 | 24 | 8 | |
| 1 | 24 | 12 | |
| MRSA (raw milk) | 8 | 12 | 8 |
| 4 | 24 | 8 | |
| 2 | 24 | 12 | |
| 1 | 24 | 24 | |
| MRSA (raw poultry) | 8 | 12 | 12 |
| 4 | 24 | 12 | |
| 2 | 24 | 12 | |
| 1 | 24 | 24 | |
| S. mutans | 8 | 4 | 8 |
| 4 | 4 | 8 | |
| 2 | 4 | 12 | |
| 1 | 12 | 24 | |
| S. salivarius | 8 | 2 | 8 |
| 4 | 4 | 8 | |
| 2 | 4 | 12 | |
| 1 | 8 | 12 | |
| F. nucleatum | 8 | 4 | 4 |
| 4 | 4 | 8 | |
| 2 | 12 | 8 | |
| 1 | 12 | 12 | |
| P. gingivitis | 8 | 4 | 8 |
| 4 | 4 | 4 | |
| 2 | 8 | 8 | |
| 1 | 8 | 8 | |
| P. intermedia | 8 | 4 | 8 |
| 4 | 4 | 8 | |
| 2 | 8 | 8 | |
| 1 | 12 | 12 | |
| P. micra | 8 | 4 | 8 |
| 4 | 4 | 12 | |
| 2 | 8 | 12 | |
| 1 | 8 | 12 | |
| S. aureus ATCC 12600 | 8 | 8 | 12 |
| 4 | 8 | 12 | |
| 2 | 12 | 12 | |
| 1 | 12 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniadou, M.; Rozos, G.; Vaiou, N.; Zaralis, K.; Ersanli, C.; Alexopoulos, A.; Tzora, A.; Varzakas, T.; Voidarou, C. The In Vitro Assessment of Antibacterial and Antioxidant Efficacy in Rosa damascena and Hypericum perforatum Extracts against Pathogenic Strains in the Interplay of Dental Caries, Oral Health, and Food Microbiota. Microorganisms 2024, 12, 60. https://doi.org/10.3390/microorganisms12010060
Antoniadou M, Rozos G, Vaiou N, Zaralis K, Ersanli C, Alexopoulos A, Tzora A, Varzakas T, Voidarou C. The In Vitro Assessment of Antibacterial and Antioxidant Efficacy in Rosa damascena and Hypericum perforatum Extracts against Pathogenic Strains in the Interplay of Dental Caries, Oral Health, and Food Microbiota. Microorganisms. 2024; 12(1):60. https://doi.org/10.3390/microorganisms12010060
Chicago/Turabian StyleAntoniadou, Maria, Georgios Rozos, Natalia Vaiou, Konstantinos Zaralis, Caglar Ersanli, Athanasios Alexopoulos, Athina Tzora, Theodoros Varzakas, and Chrysoula (Chrysa) Voidarou. 2024. "The In Vitro Assessment of Antibacterial and Antioxidant Efficacy in Rosa damascena and Hypericum perforatum Extracts against Pathogenic Strains in the Interplay of Dental Caries, Oral Health, and Food Microbiota" Microorganisms 12, no. 1: 60. https://doi.org/10.3390/microorganisms12010060
APA StyleAntoniadou, M., Rozos, G., Vaiou, N., Zaralis, K., Ersanli, C., Alexopoulos, A., Tzora, A., Varzakas, T., & Voidarou, C. (2024). The In Vitro Assessment of Antibacterial and Antioxidant Efficacy in Rosa damascena and Hypericum perforatum Extracts against Pathogenic Strains in the Interplay of Dental Caries, Oral Health, and Food Microbiota. Microorganisms, 12(1), 60. https://doi.org/10.3390/microorganisms12010060










