Microecological Shifts in the Rhizosphere of Perennial Large Trees and Seedlings in Continuous Cropping of Poplar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Sampling and Counting Culturable Microorganisms
2.3. Edaphic Properties Determination in Rhizosphere Soil
2.4. Isolation and PCR Amplification of DNA
2.5. Real-Time (q)PCR
2.6. Illumina MiSeq Sequencing
2.7. Processing of Sequencing Data
2.8. Statistical Analyses
3. Results
3.1. Changes in Edaphic Properties from March 2018 to October 2020
3.2. Differences in the Quantities of Microbes in Rhizosphere Soils of Poplars
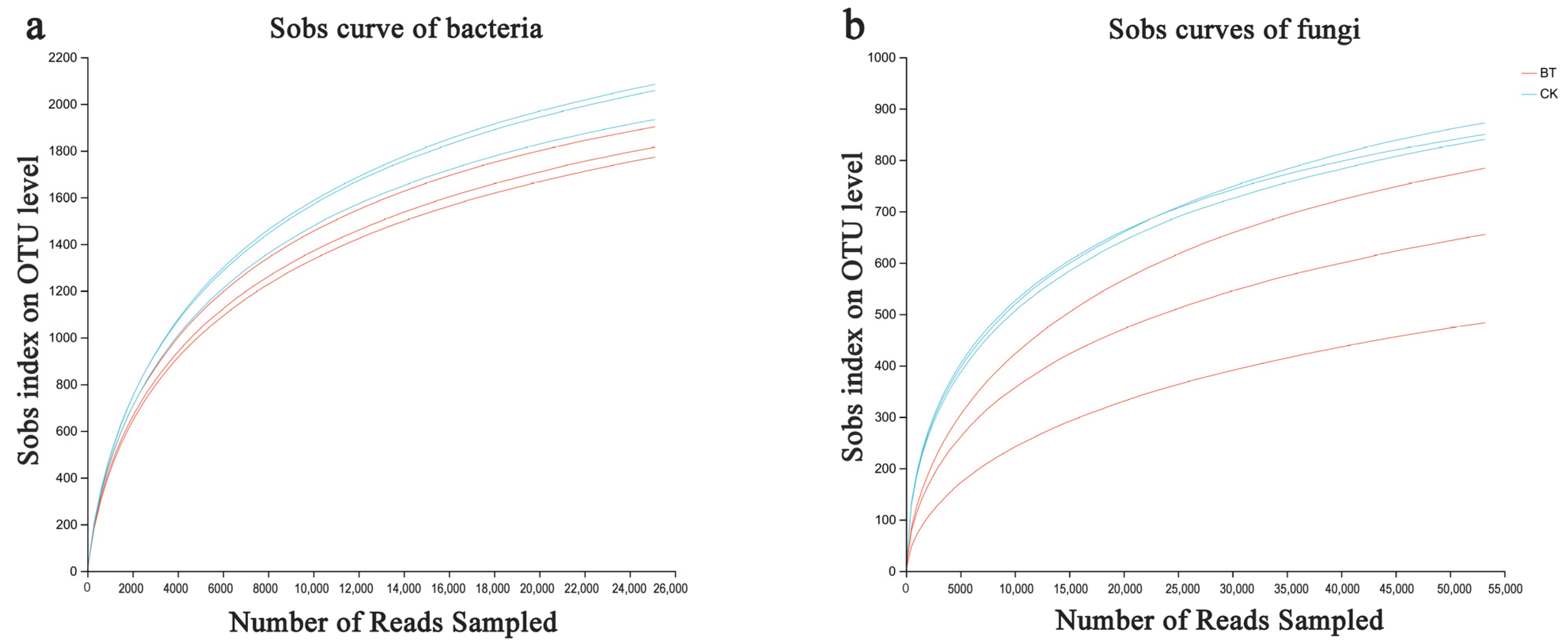
3.3. Sequencing Quality Evaluation
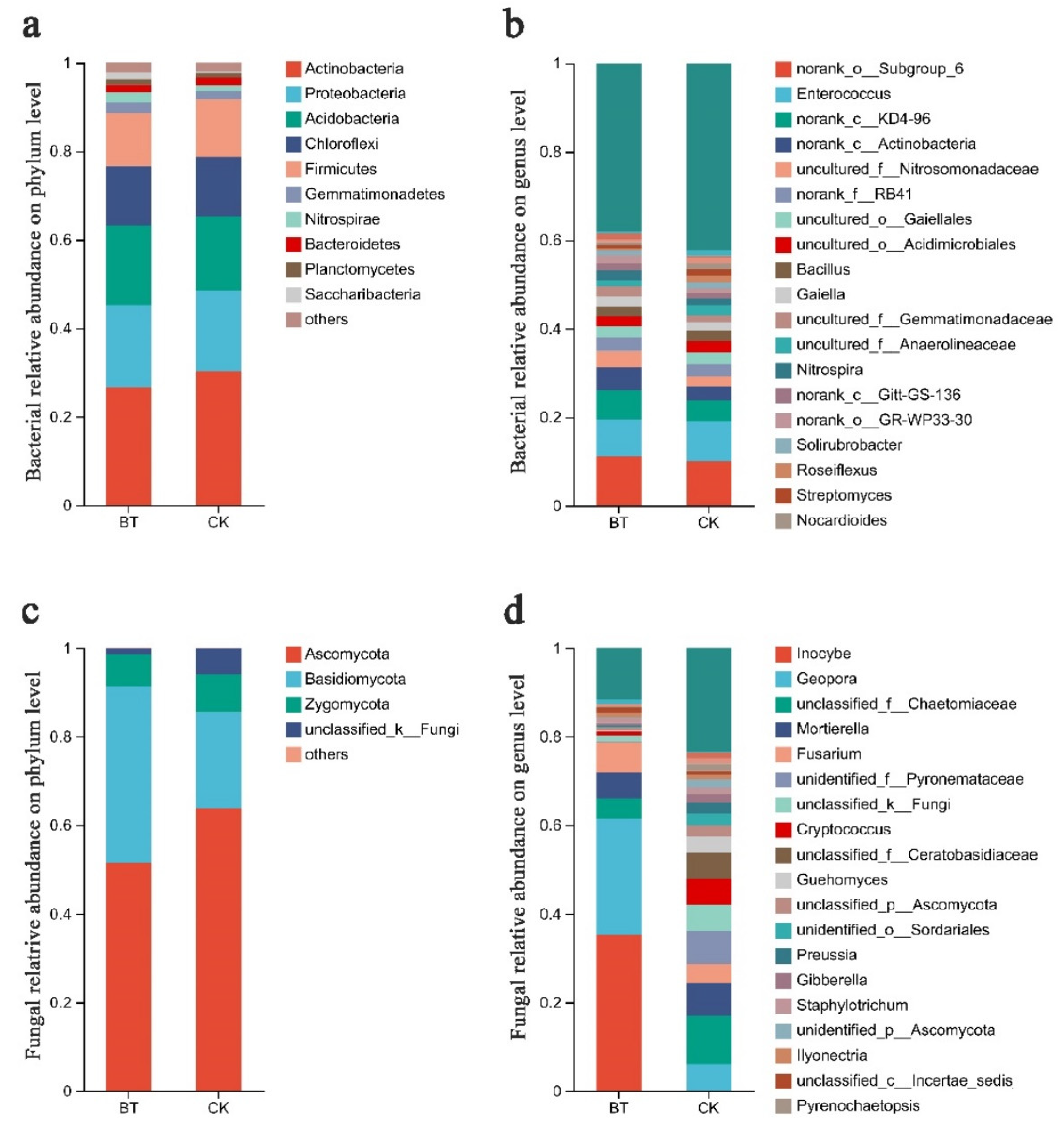
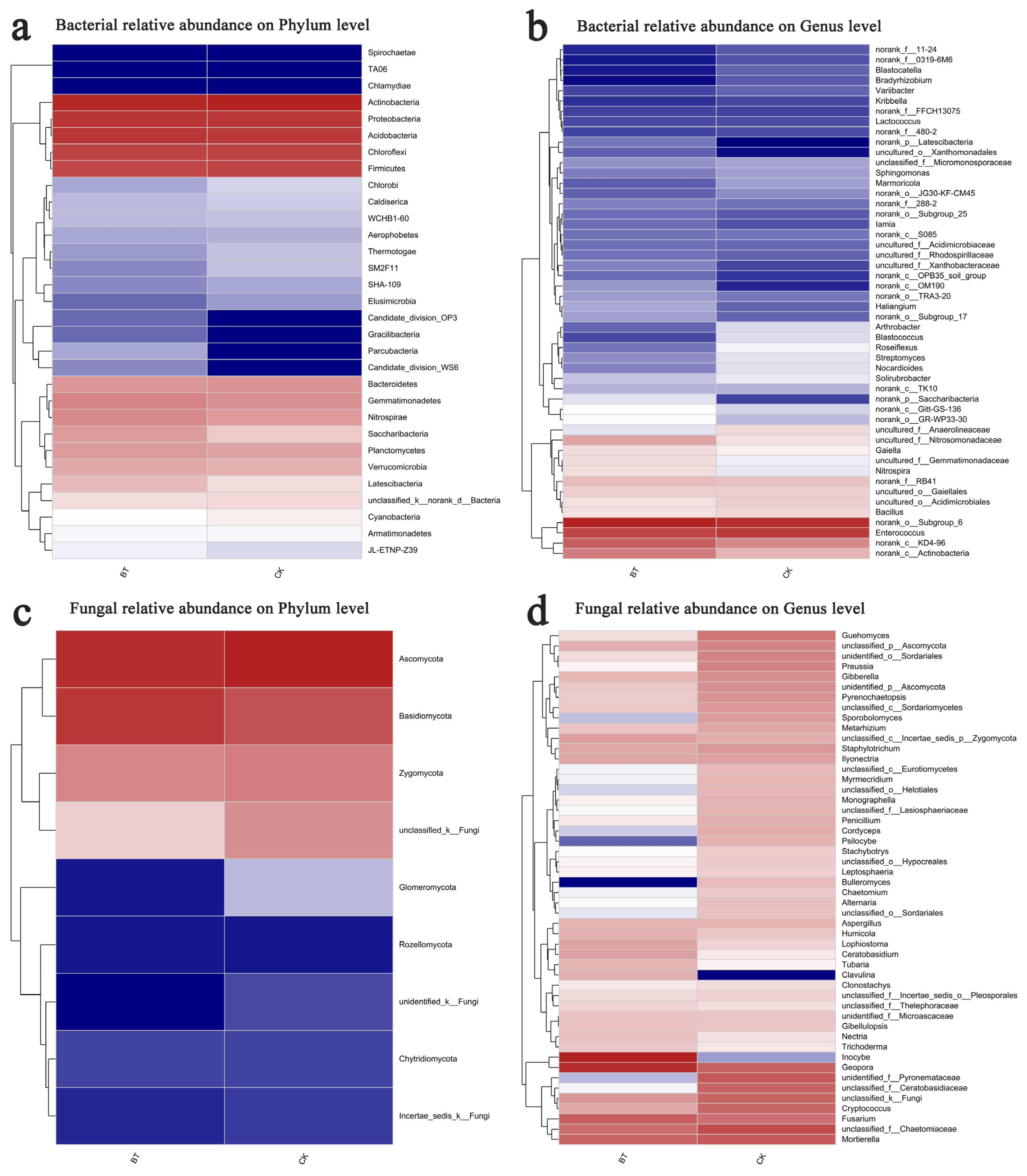
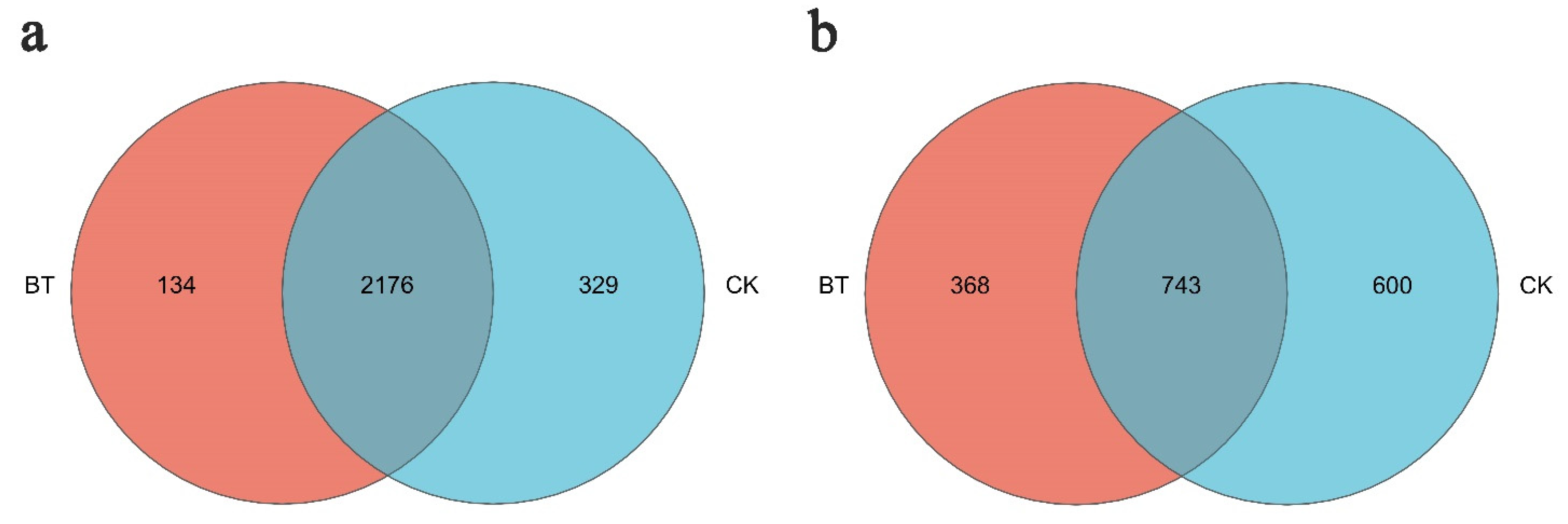
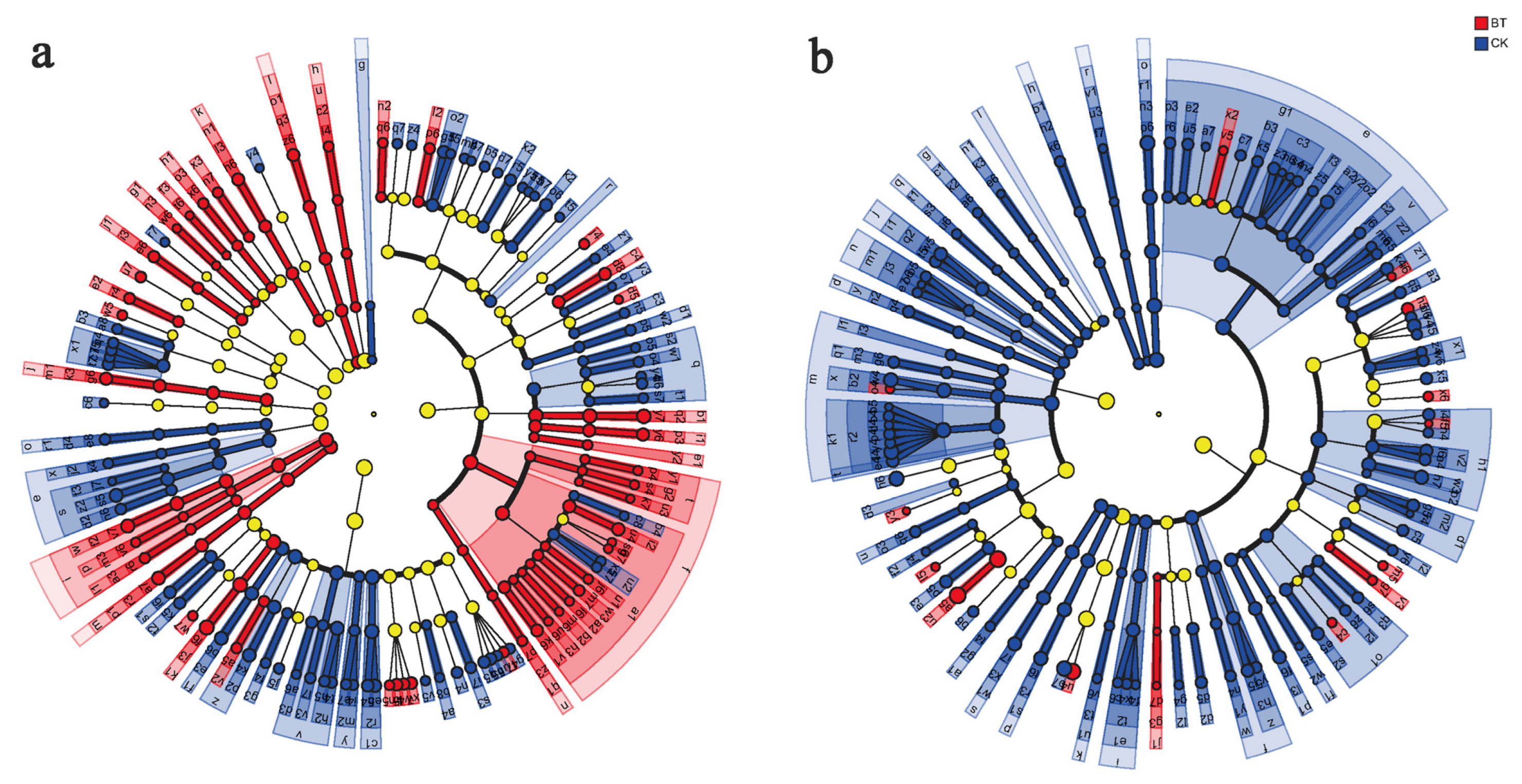
3.4. Differences in Community Composition and Structure
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maleki, S.S.; Mohammadi, K.; Movahedi, A.; Wu, F.; Ji, K.S. Increase in Cell Wall Thickening and Biomass Production by Overexpression of PmCesA2 in Poplar. Front. Plant Sci. 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Ding, C.; Huang, Q.; Zhang, W.; Yang, C.; Liang, D.; Fan, R.; Su, X. Transcriptome profiling revealed diverse gene expression patterns in poplar (Populus × euramericana) under different planting densities. PLoS ONE 2019, 14, e0217066. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.; Sun, X.; Wang, P.; Dou, H.; Yang, Z.; Wang, Y. A method for generating genome edited plant lines from CRISPR-transformed Shanxin poplar plants. Plant Sci. 2023, 333, 111732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qin, G.; Zhai, Z.; Zhou, S.; Tang, L.; Tian, Y. Diverse Understory Vegetation Alleviates Nitrogen Competition with Crop Trees in Poplar Plantations. Forests 2021, 12, 705. [Google Scholar] [CrossRef]
- Ku, Y.; Li, W.; Mei, X.; Yang, X.; Cao, C.; Zhang, H.; Cao, L.; Li, M. Biological Control of Melon Continuous Cropping Obstacles: Weakening the Negative Effects of the Vicious Cycle in Continuous Cropping Soil. Microbiol. Spectr. 2022, 10, e0177622. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, P.; Yang, C.; Feng, Z.; Feng, H.; Zhang, Y.; Zhao, L.; Zhou, J.; Zhu, H.; Wei, F. Soil bacterial community response to continuous cropping of cotton. Front. Microbiol. 2023, 14, 1125564. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhou, Q.; Zhang, T. Effects of continuous cropping Jiashi muskmelon on rhizosphere microbial community. Front. Microbiol. 2022, 13, 1086334. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Qian, J.; Wei, F.; Zhang, G.; Wang, Y.; Wei, G.; Hu, Z.; Dong, L.; Chen, S. Composition and function of rhizosphere microbiome of Panax notoginseng with discrepant yields. Chin. Med. 2020, 15, 85. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, G.; Cheng, Y.; Shi, P.; Yang, C.; Yang, H.; Xu, Z. Soil acidification in continuously cropped tobacco alters bacterial community structure and diversity via the accumulation of phenolic acids. Sci. Rep. 2019, 9, 12499. [Google Scholar] [CrossRef]
- De Corato, U. Towards new soil management strategies for improving soil quality and ecosystem services in sustainable agriculture: Editorial overview. Sustainability 2020, 12, 9398. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Frąc, M. Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Q.; Wang, L.; Hu, J.; Xue, H.; Han, D.; Xing, Z.; Ruan, Z. Structure and Function Analysis of Cultivated Meconopsis integrifolia Soil Microbial Community Based on High-Throughput Sequencing and Culturability. Biology 2023, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Li, Z.-Z.; Zhao, Q.; Huang, X.-Y.; Huang, K.-F. Effect of continuous cropping on the rhizosphere soil and growth of common buckwheat. Plant Prod. Sci. 2020, 23, 81–90. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous cropping alters multiple biotic and abiotic indicators of soil health. Soil. Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Maguire, V.G.; Bordenave, C.D.; Nieva, A.S.; Llames, M.E.; Colavolpe, M.B.; Gárriz, A.; Ruiz, O.A. Soil bacterial and fungal community structure of a rice monoculture and rice-pasture rotation systems. Appl. Soil. Ecol. 2020, 151, 103535. [Google Scholar] [CrossRef]
- Song, X.; Pan, Y.; Li, L.; Wu, X.; Wang, Y. Composition and diversity of rhizosphere fungal community in Coptis chinensis Franch. continuous cropping fields. PLoS ONE 2018, 13, e0193811. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.; van der Putten, W. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 205–511. [Google Scholar] [CrossRef]
- Yao, Y.; Yao, X.; An, L.; Bai, Y.; Xie, D.; Wu, K. Rhizosphere Bacterial Community Response to Continuous Cropping of Tibetan Barley. Front. Microbiol. 2020, 11, 551444. [Google Scholar] [CrossRef]
- Li, Y.; Chi, J.; Ao, J.; Gao, X.; Liu, X.; Sun, Y.; Zhu, W. Effects of different continuous cropping years on bacterial community and diversity of cucumber rhizosphere soil in solar-greenhouse. Curr. Microbiol. 2021, 78, 2380–2390. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Ren, X.; Chen, B.; Zhang, Y.; Shen, C.; Wang, F.; Wu, D. Long-term greenhouse cucumber production alters soil bacterial community structure. J. Soil. Sci. Plant Nutr. 2020, 20, 306–321. [Google Scholar] [CrossRef]
- Liu, W.; Wang, N.; Yao, X.; He, D.; Sun, H.; Ao, X.; Wang, H.; Zhang, H.; St Martin, S.; Xie, F.; et al. Continuous-cropping-tolerant soybean cultivars alleviate continuous cropping obstacles by improving structure and function of rhizosphere microorganisms. Front. Microbiol. 2022, 13, 1048747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Maltais-Landry, G.; Liao, H.-L. How soil biota regulate C cycling and soil C pools in diversified crop rotations. Soil. Biol. Biochem. 2021, 156, 108219. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S. Responses of soil microbial community structure and greenhouse gas fluxes to crop rotations that include winter cover crops. Geoderma 2021, 385, 114843. [Google Scholar] [CrossRef]
- Chen, D.; Wang, M.; Wang, G.; Zhou, Y.; Yang, X.; Li, J.; Zhang, C.; Dai, K. Functional organic fertilizers can alleviate tobacco (Nicotiana tabacum L.) continuous cropping obstacle via ameliorating soil physicochemical properties and bacterial community structure. Front. Bioeng. Biotechnol. 2022, 10, 1023693. [Google Scholar] [CrossRef]
- Bonito, G.; Hameed, K.; Ventura, R.; Krishnan, J.; Schadt, C.W.; Vilgalys, R. Isolating a functionally relevant guild of fungi from the root microbiome of Populus. Fungal Ecol. 2016, 22, 35–42. [Google Scholar] [CrossRef]
- Walkly, A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification to the chromic acid titration method. Soil. Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Olsen, S.R. Miscellaneous Paper Institute for Agricultural Research Samaru. In Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Dept. of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Peng, W.; Bo, C.; Hua, Z. High throughput sequencing analysis of bacterial communities in soils of a typical Poyang Lake wetland. Acta Ecol. Sin. 2017, 37, 1650–1658. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.H.; Lin, J.Y.; Liu, L.S.; Huang, Z.X. Analysis of Microbial Diversity in the Fermented Grains of Maotai-flavor Liquor Using High-throughput Sequencing. J. Fujian Norm. Univ. 2017, 33, 51–59. [Google Scholar]
- Hao, Y.T.; Wu, S.G.; Xiong, F.; Tran, N.T.; Jakovlić, I.; Zou, H.; Li, W.X.; Wang, G.T. Succession and Fermentation Products of Grass Carp (Ctenopharyngodon idellus) Hindgut Microbiota in Response to an Extreme Dietary Shift. Front. Microbiol. 2017, 8, 1585. [Google Scholar] [CrossRef]
- Gao, M.; Qiu, T.; Sun, Y.; Wang, X. The abundance and diversity of antibiotic resistance genes in the atmospheric environment of composting plants. Environ. Int. 2018, 116, 229. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Christopher, Q.; Rob, K. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194. [Google Scholar] [CrossRef]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. Isme J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T. The UNITE database for molecular identification of fungi—Recent updates and future perspectives. New Phytol. 2010, 186, 281. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, P.; Wang, Y.; Li, W.; Cui, X.; Zhou, J.; Peng, F.; Dai, L. Earthworm activity optimized the rhizosphere bacterial community structure and further alleviated the yield loss in continuous cropping lily (Lilium lancifolium Thunb.). Sci. Rep. 2021, 11, 20840. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Zhang, M.-Y.; Hou, S.-Y.; Chen, J.; Wu, Y.-Y.; Zhang, Y.-X. Insights into Streptomyces spp. isolated from the rhizospheric soil of Panax notoginseng: Isolation, antimicrobial activity and biosynthetic potential for polyketides and non-ribosomal peptides. BMC Microbiol. 2020, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, D.; Yang, Y.; Pan, Y.; Zhao, D.; Zhu, J.; Zhang, L.; Yang, Z. Dissecting the effect of continuous cropping of potato on soil bacterial communities as revealed by high-throughput sequencing. PLoS ONE 2020, 15, e0233356. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, A.; Zachow, C.; Müller, H.; Grand, A.; Temme, N.; Tilcher, R.; Berg, G. Understanding the Impact of Cultivar, Seed Origin, and Substrate on Bacterial Diversity of the Sugar Beet Rhizosphere and Suppression of Soil-Borne Pathogens. Front. Plant Sci. 2020, 11, 560869. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Song, N. Biochar and vermicompost improve the soil properties and the yield and quality of cucumber (Cucumis sativus L.) grown in plastic shed soil continuously cropped for different years. Agric. Ecosyst. Environ. 2021, 315, 107425. [Google Scholar] [CrossRef]
- Jiao, S.; Peng, Z.; Qi, J.; Gao, J.; Wei, G. Linking Bacterial-Fungal Relationships to Microbial Diversity and Soil Nutrient Cycling. mSystems 2021, 6, e01052-20. [Google Scholar] [CrossRef]
- Wu, F.; Wang, X. Effect of Monocropping and Rotation on Soil Microbial Community Diversity and Cucumber Yield, Quality Under Protected Cultivation. Sci. Agric. Sin. 2007, 761, 555–561. [Google Scholar] [CrossRef]
- Paudel, B.R.; Carpenter-Boggs, L.; Higgins, S. Influence of brassicaceous soil amendments on potentially beneficial and pathogenic soil microorganisms and seedling growth in Douglas-fir nurseries. Appl. Soil. Ecol. 2016, 105, 91–100. [Google Scholar] [CrossRef]
- Patkowska, E. Biostimulants Managed Fungal Phytopathogens and Enhanced Activity of Beneficial Microorganisms in Rhizosphere of Scorzonera (Scorzonera hispanica L.). Agriculture 2021, 11, 347. [Google Scholar] [CrossRef]
- Gu, S.; Xiong, X.; Tan, L.; Deng, Y.; Du, X.; Yang, X.; Hu, Q. Soil microbial community assembly and stability are associated with potato (Solanum tuberosum L.) fitness under continuous cropping regime. Front. Plant Sci. 2022, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Aguirre de Cárcer, D. A conceptual framework for the phylogenetically constrained assembly of microbial communities. Microbiome 2019, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Whitman, W.B.; Coleman, D.C.; Chiu, C.-Y. Effects of Reforestation on the Structure and Diversity of Bacterial Communities in Subtropical Low Mountain Forest Soils. Front. Microbiol. 2018, 9, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Besaury, L.; Martinet, L.; Mühle, E.; Clermont, D.; Rémond, C. Streptomyces silvae sp. nov., isolated from forest soil. Int. J. Syst. Evol. Microbiol. 2021, 71, 005147. [Google Scholar] [CrossRef]
- Roh, S.G.; Lee, C.; Kim, M.K.; Kang, H.J.; Kim, Y.S.; Kim, M.J.; Malik, A.; Kim, S.B. Nocardioides euryhalodurans sp. nov., Nocardioides seonyuensis sp. nov. and Nocardioides eburneiflavus sp. nov., isolated from soil. Int. J. Syst. Evol. Microbiol. 2020, 70, 2682–2689. [Google Scholar] [CrossRef]
- Wang, M.; Xue, J.; Ma, J.; Feng, X.; Ying, H.; Xu, H. Streptomyces lydicus M01 Regulates Soil Microbial Community and Alleviates Foliar Disease Caused by Alternaria alternata on Cucumbers. Front. Microbiol. 2020, 11, 942. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Mingma, R.; Duangmal, K.; Thamchaipenet, A.; Trakulnaleamsai, S.; Matsumoto, A.; Takahashi, Y. Streptomyces oryzae sp. nov., an endophytic actinomycete isolated from stems of rice plant. J. Antibiot. 2015, 68, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.S.; Souza, D.T.; Zucchi, T.D.; Pansa, C.C.; de Figueiredo Vasconcellos, R.L.; Crevelin, E.J.; de Moraes, L.A.; Melo, I.S. Streptomyces atlanticus sp. nov., a novel actinomycete isolated from marine sponge Aplysina fulva (Pallas, 1766). Antonie Van Leeuwenhoek 2016, 109, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, G.; Chen, T.; Hou, Y.; Yang, S.; Zhang, K.-Q.; Yang, J. Nicotine-degrading microorganisms and their potential applications. Appl. Microbiol. Biotechnol. 2015, 99, 3775–3785. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.K.; Srivastava, A.; Singh, V.P. Bacterial degradation of nitrophenols and their derivatives. J. Hazard. Mater. 2014, 266, 42–59. [Google Scholar] [CrossRef] [PubMed]
- King, G.M.; Weber, C.F. Distribution, diversity and ecology of aerobic CO-oxidizing bacteria. Nat. Rev. Microbiol. 2007, 5, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.R.; Kim, S.W.; Adhikari, M.; Um, Y.H.; Kim, H.S.; Kim, C.; Lee, H.B.; Lee, Y.S. Three New Records of Mortierella Species Isolated from Crop Field Soil in Korea. Mycobiology 2015, 43, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhang, Z.; Wang, X.; Zhou, Z.; Chen, D.; Zeng, H.; Zhao, S.; Chen, L.; Hu, Y.; Zhang, C.; et al. Diversity and Contributions to Nitrogen Cycling and Carbon Fixation of Soil Salinity Shaped Microbial Communities in Tarim Basin. Front. Microbiol. 2018, 9, 431. [Google Scholar] [CrossRef]
- Challacombe, J.F.; Hesse, C.N.; Bramer, L.M.; McCue, L.A.; Lipton, M.; Purvine, S.; Nicora, C.; Gallegos-Graves, L.V.; Porras-Alfaro, A.; Kuske, C.R. Genomes and secretomes of Ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genom. 2019, 20, 976. [Google Scholar] [CrossRef]
- Heitmann, N.; Glemnitz, M.; Birkhofer, K.; Müller, M.E.H. Unselective Transport of Phytopathogenic Fusarium Fungi from Litter and Soil by Ground-Dwelling Arthropods Links Semi-Natural and Agricultural Habitats. Microorganisms 2022, 10, 335. [Google Scholar] [CrossRef]
- Shi, G.; Sun, H.; Calderón-Urrea, A.; Jia, X.; Yang, H.; Su, G. Soil Fungal Diversity Loss and Appearance of Specific Fungal Pathogenic Communities Associated with the Consecutive Replant Problem (CRP) in Lily. Front. Microbiol. 2020, 11, 1649. [Google Scholar] [CrossRef]
- Sui, J.; Yang, J.; Li, C.; Zhang, L.; Hua, X. Effects of a Microbial Restoration Substrate on Plant Growth and Rhizosphere Microbial Community in a Continuous Cropping Poplar. Microorganisms 2023, 11, 486. [Google Scholar] [CrossRef] [PubMed]

| Organic Carbon (g/kg) | Available P (mg/kg) | Available K (mg/kg) | Total N (mg/kg) | pH | ||
|---|---|---|---|---|---|---|
| March 2018 | BT | 7.41 ± 0.05 a | 23.31 ± 0.07 b | 121.24 ± 1.08 b | 695.31 ± 3.24 b | 7.21 ± 0.01 a |
| CK | 6.05 ± 0.07 c | 35.63 ± 2.33 a | 132.28 ± 1.44 a | 765.82 ± 7.43 a | 7.23 ± 0.02 a | |
| October 2020 | BT | 7.35 ± 0.03 a | 24.07 ± 0.11 b | 125.31 ± 1.12 b | 701.23 ± 1.67 b | 7.19 ± 0.01 a |
| CK | 6.27 ± 0.04 b | 35.38 ± 2.77 a | 139.20 ± 2.03 a | 791.58 ± 3.77 a | 7.07 ± 0.04 b |
| Treatment | Quantity of Culturable Microbial | Quantity of Total Microbial | ||
|---|---|---|---|---|
| Bacterial × 107 (cfu/g Soil) | Fungal × 106 (cfu/g Soil) | Bacterial × 107 (Copies/μL) | Fungal × 104 (Copies/μL) | |
| BT | 3.25 ± 0.11 a | 4.76 ± 0.19 b | 1.01 ± 0.13 a | 1.97 ± 0.04 b |
| CK | 0.96 ± 0.17 b | 9.25 ± 0.49 a | 0.71 ± 0.02 b | 2.95 ± 0.10 a |
| Sample | Cutoff | ACE | Chao | Shannon | Simpson | Coverage | |
|---|---|---|---|---|---|---|---|
| Bacterial | BT | 0.03 | 2186.33 ± 43.93 b | 2194.97 ± 42.72 a | 6.05 ± 0.16 a | 0.012 ± 0.027 a | 0.982177 |
| CK | 0.03 | 2376.19 ± 100.38 a | 2378.34 ± 117.81 a | 6.22 ± 0.13 a | 0.012 ± 0.044 a | 0.981184 | |
| Fungal | BT | 0.03 | 893.98 ± 65.89 a | 883.48 ± 46.29 b | 2.87 ± 0.52 b | 0.30 ± 0.147 a | 0.996437 |
| CK | 0.03 | 1029.27 ±34.58 a | 1016.56 ± 27.96 a | 4.34 ± 0.02 a | 0.04 ± 0.005 b | 0.996280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, J.; Li, C.; Wang, Y.; Li, X.; Liu, R.; Hua, X.; Liu, X.; Qi, H. Microecological Shifts in the Rhizosphere of Perennial Large Trees and Seedlings in Continuous Cropping of Poplar. Microorganisms 2024, 12, 58. https://doi.org/10.3390/microorganisms12010058
Sui J, Li C, Wang Y, Li X, Liu R, Hua X, Liu X, Qi H. Microecological Shifts in the Rhizosphere of Perennial Large Trees and Seedlings in Continuous Cropping of Poplar. Microorganisms. 2024; 12(1):58. https://doi.org/10.3390/microorganisms12010058
Chicago/Turabian StyleSui, Junkang, Chenyu Li, Yinping Wang, Xiangyu Li, Rui Liu, Xuewen Hua, Xunli Liu, and Hui Qi. 2024. "Microecological Shifts in the Rhizosphere of Perennial Large Trees and Seedlings in Continuous Cropping of Poplar" Microorganisms 12, no. 1: 58. https://doi.org/10.3390/microorganisms12010058
APA StyleSui, J., Li, C., Wang, Y., Li, X., Liu, R., Hua, X., Liu, X., & Qi, H. (2024). Microecological Shifts in the Rhizosphere of Perennial Large Trees and Seedlings in Continuous Cropping of Poplar. Microorganisms, 12(1), 58. https://doi.org/10.3390/microorganisms12010058






