The Efficacy of a Mix of Probiotics (Limosilactobacillus reuteri LMG P-27481 and Lacticaseibacillus rhamnosus GG ATCC 53103) in Preventing Antibiotic-Associated Diarrhea and Clostridium difficile Infection in Hospitalized Patients: Single-Center, Open-Label, Randomized Trial
Abstract
1. Introduction
2. Endpoints of the Study
3. Patients and Methods
3.1. Inclusion and Exclusion Criteria
- -
- Age > 18 years
- -
- Patients who provided signed informed consent to participate in the study
- -
- Proven or suspected bacterial infection with a need for antibiotic therapy
- -
- Oral or parenteral antibiotic administration for a duration of at least 5 days
- -
- Age < 18 years
- -
- Sepsis or severe generalized infection with shock
- -
- Acute pancreatitis
- -
- Known immunodeficiency or chronic gastrointestinal disease
- -
- Oncology patients undergoing active chemotherapy
- -
- Severe neurological pathology or inability to verbalize
- -
- Enteral or parenteral nutrition
- -
- Gastrointestinal malformation or gastrointestinal or abdominal surgery
- -
- Acute or chronic diarrhea already present at the start of antibiotic therapy
- -
- Use of any probiotic at enrollment or within the previous four weeks
- -
- Antibiotic therapy started more than 24 h before enrollment
- -
- No signed informed consent
3.2. Randomization
3.3. Statistical Sample Calculation
4. Statistical Analysis
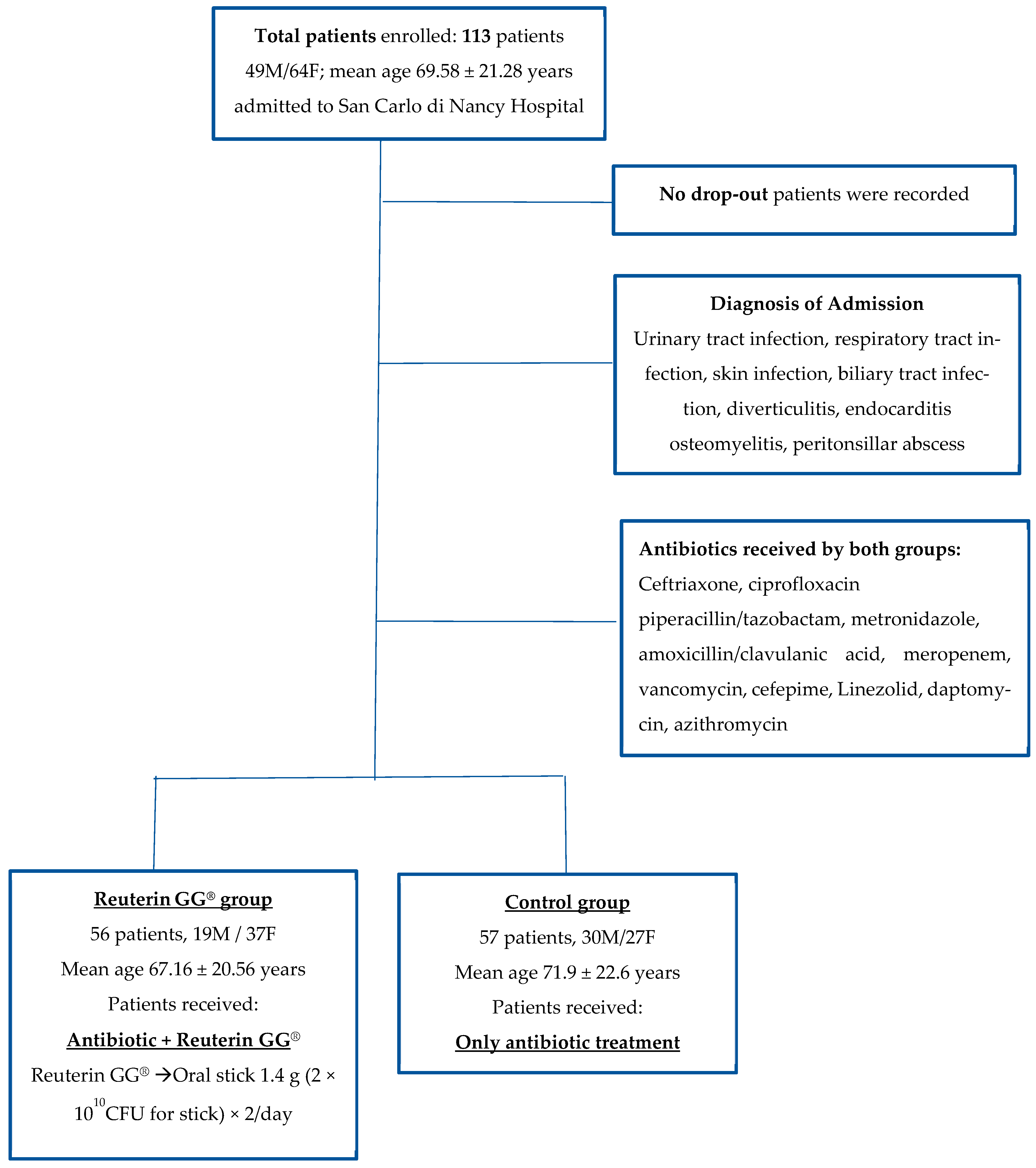
5. Methods
6. Results
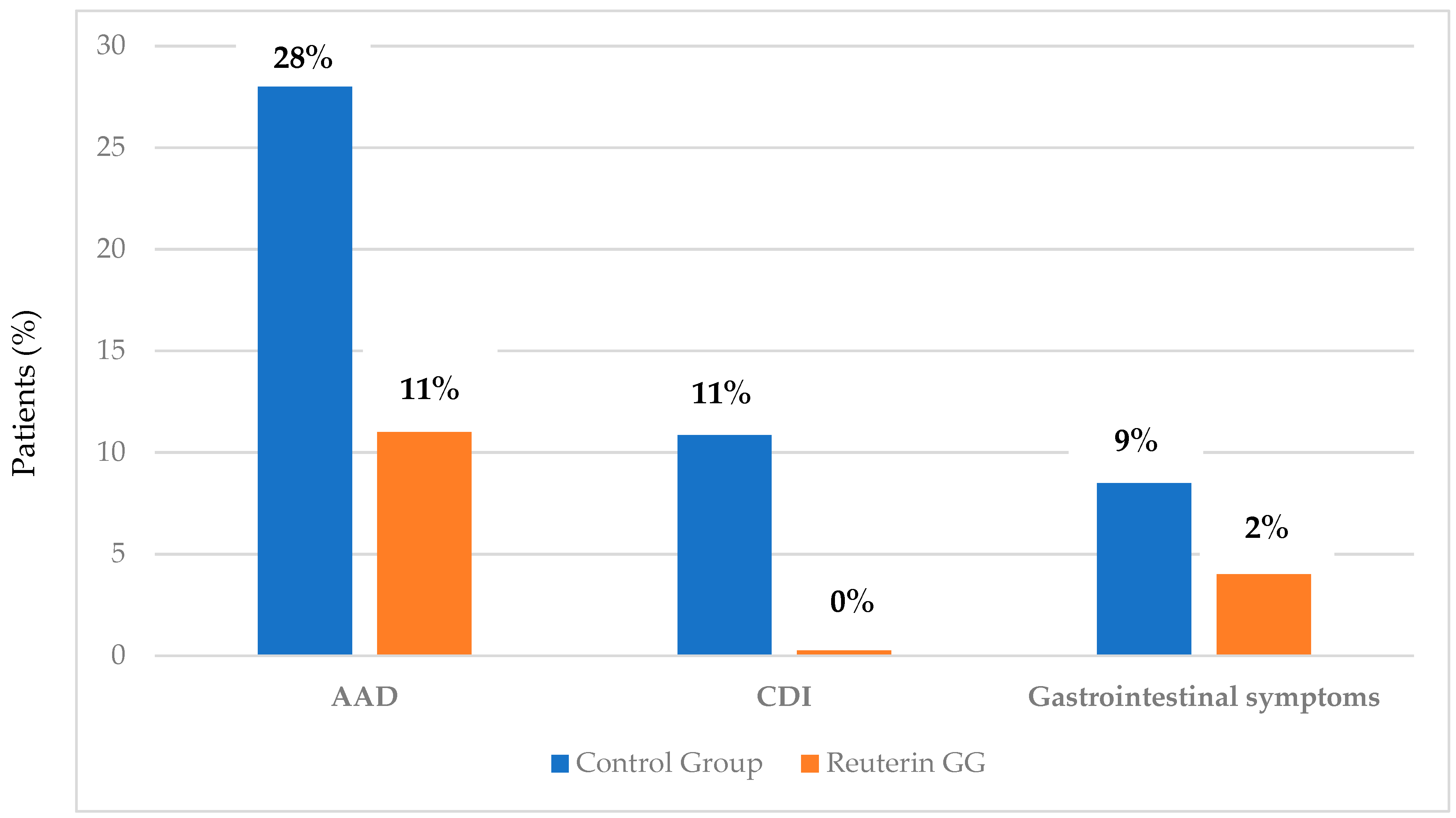
6.1. Antibiotic-Associated Diarrhea
6.2. Clostridium Difficile Infection (CDI)
6.3. Gastrointestinal Manifestations: Diarrhea and Vomiting
6.4. Abdominal Pain and the Number of Daily Bowel Movements
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McFarland, L.V. Antibiotic-associated diarrhea: Epidemiology, trends and treatment. Future Microbiol. 2008, 3, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, M.; Szajewska, H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: A review of the current evidence. Eur. J. Pediatr. 2014, 173, 1327–1337. [Google Scholar]
- Guandalini, S. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2011, 45, S149–S153. [Google Scholar] [CrossRef]
- Theriot, C.M.; Young, V.B. Microbial and metabolic interactions between the gastrointestinal tract and Clostridium difficile infection. Gut Microbes 2014, 5, 86–95. [Google Scholar] [CrossRef]
- Doron, S.; Gorbach, S.L. Probiotics: Their role in the treatment and prevention of disease. Expert. Rev. Anti Infect. Ther. 2006, 4, 261–275. [Google Scholar] [PubMed]
- Coté, G.A.; Buchman, A.L. Antibiotic-associated diarrhoea. Expert. Opin. Drug Saf. 2006, 5, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Probiotics in human medicine. Gut 1991, 32, 439–442. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef]
- Salvatore, S.; Pensabene, L.; Borrelli, O.; Saps, M.; Thapar, N.; Concolino, D.; Staiano, A.; Vandenplas, Y. Mind the gut: Probiotics in paediatric neurogastroenterology. Benef. Microbes 2018, 9, 883–898. [Google Scholar] [CrossRef]
- Romano, C.; Ferrau, V.; Cavataio, F.; Iacono, G.; Spina, M.; Lionetti, E.; Comisi, F.; Famiani, A.; Comito, D. Lactobacillus reuteri in children with functional abdominal pain (FAP). J. Paediatr. Child. Health 2014, 50, E68–E71. [Google Scholar] [CrossRef]
- Wilkins, T.; Sequoia, J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am. Fam. Physician 2017, 96, 170–178. [Google Scholar] [PubMed]
- Baù, M.; Moretti, A.; Bertoni, E.; Vazzoler, V.; Luini, C.; Agosti, M.; Salvatore, S. Risk and Protective Factors for Gastrointestinal Symptoms associated with Antibiotic Treatment in Children: A Population Study. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Hugon, P.; Dufour, J.C.; Colson, P.; Fournier, P.E.; Sallah, K.; Raoult, D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect. Dis. 2015, 15, 1211–1219. [Google Scholar] [PubMed]
- Sazawal, S.; Hiremath, G.; Dhingra, U.; Malik, P.; Deb, S.; Black, R.E. Efficacy of probiotics in prevention of acute diarrhoea: A meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect. Dis. 2006, 6, 374–382. [Google Scholar] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Cummings, J.H. Probiotics and prebiotics: Can regulating the activities of intestinal bacteria benefit health? West. J. Med. 1999, 171, 187–191. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Fallani, M.; Amarri, S.; Uusijarvi, A.; Adam, R.; Khanna, S.; Aguilera, M.; Gil, A.; Vieites, J.M.; Norin, E.; Young, D.; et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 2011, 157, 1385–1392. [Google Scholar]
- Resta-Lenert, S.; Barrett, K.E. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 2003, 52, 988–997. [Google Scholar] [PubMed]
- Hamilton-Miller, J.M.; Gibson, G.R.; Bruck, W. Some insights into the derivation and early uses of the word ‘probiotic’. Br. J. Nutr. 2003, 90, 845. [Google Scholar] [CrossRef] [PubMed]
- Valeur, N.; Engel, P.; Carbajal, N.; Connolly, E.; Ladefoged, K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl. Environ. Microbiol. 2004, 70, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [PubMed]
- Rosander, A.; Connolly, E.; Roos, S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl. Environ. Microbiol. 2008, 74, 6032–6040. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef]
- Capurso, L. First part: The intestinal microbiota. Recenti Prog. Med. 2016, 107, 257–266. [Google Scholar]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar]
- Evans, J.M.; Morris, L.S.; Marchesi, J.R. The gut microbiome: The role of a virtual organ in the endocrinology of the host. J. Endocrinol. 2013, 218, R37–R47. [Google Scholar]
- Nord, C.E.; Edlund, C. Impact of antimicrobial agents on human intestinal microflora. J. Chemother. 1990, 2, 218–237. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, Y.; Wang, Y.; Wang, P.; Song, H.; Wang, F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE 2019, 14, e0218384. [Google Scholar] [CrossRef]
- Rashid, M.U.; Zaura, E.; Buijs, M.J.; Keijser, B.J.; Crielaard, W.; Nord, C.E.; Weintraub, A. Determining the Long-term Effect of Antibiotic Administration on the Human Normal Intestinal Microbiota Using Culture and Pyrosequencing Methods. Clin. Infect. Dis. 2015, 60, S77–S84. [Google Scholar] [PubMed]
- Yang, L.; Bajinka, O.; Jarju, P.O.; Tan, Y.; Taal, A.M.; Ozdemir, G. The varying effects of antibiotics on gut microbiota. AMB Express 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [PubMed]
- Bartlett, J.G. Clinical practice. Antibiotic-associated diarrhea. N. Engl. J. Med. 2002, 346, 334–339. [Google Scholar] [CrossRef]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef]
- Blanchi, J.; Goret, J.; Mégraud, F. Clostridium difficile Infection: A Model for Disruption of the Gut Microbiota Equilibrium. Dig. Dis. 2016, 34, 217–220. [Google Scholar]
- Rupnik, M.; Wilcox, M.H.; Gerding, D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 526–536. [Google Scholar]
- Zanella Terrier, M.C.; Simonet, M.L.; Bichard, P.; Frossard, J.L. Recurrent Clostridium difficile infections: The importance of the intestinal microbiota. World J. Gastroenterol. 2014, 20, 7416–7423. [Google Scholar] [CrossRef]
- Mileti, E.; Matteoli, G.; Iliev, I.D.; Rescigno, M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: Prediction for in vivo efficacy. PLoS ONE 2009, 4, e7056. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, R.; Miniello, V.; Magistà, A.M.; De Canio, A.; Bucci, N.; Gagliardi, F.; Lionetti, E.; Castellaneta, S.; Polimeno, L.; Peccarisi, L.; et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics 2010, 126, e1445–e1452. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.T.; Maw, A.; Lyubov, L.; Pino, A.; Matthew, A.S.; Evans, E.T. Timely Use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review With Meta-Regression Analysis. Gastroenterology 2017, 152, 1889–1900. [Google Scholar]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 12, CD006095. [Google Scholar]
- Bruzzese, E.; Fedele, M.C.; Bruzzese, D.; Viscovo, S.; Giannattasio, A.; Mandato, C.; Siani, P.; Guarino, A. Randomised clinical trial: A Lactobacillus GG and micronutrient-containing mixture is effective in reducing nosocomial infections in children, vs. placebo. Aliment. Pharmacol. Ther. 2016, 44, 568–575. [Google Scholar] [CrossRef]
- Savino, F.; Cordisco, L.; Tarasco, V.; Palumeri, E.; Calabrese, R.; Oggero, R.; Roos, S.; Matteuzzi, D. Lactobacillus reuteri DSM 17938 in infantile colic: A randomized, double-blind, placebo-controlled trial. Pediatrics 2010, 126, e526–e533. [Google Scholar]
- Savino, F.; Montanari, P.; Galliano, I.; Daprà, V.; Bergallo, M. (ATCC 53103) for the Management of Infantile Colic: A Randomized Controlled Trial. Nutrients 2020, 12, 1693. [Google Scholar] [CrossRef]
- Guo, Q.; Goldenberg, J.Z.; Humphrey, C.; El Dib, R.; Johnston, B.C. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2019, 30, CD004827. [Google Scholar] [CrossRef]
- Szajewska, H.; Canani, R.B.; Guarino, A.; Hojsak, I.; Indrio, F.; Goudoever, J.B.; Weizman, Z. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Children. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 495–506. [Google Scholar] [CrossRef]
- Sagheddu, V.; Uggeri, F.; Belogi, L.; Remollino, L.; Brun, P.; Bernabè, G.; Castagliuolo, I.; Elli, M. The Biotherapeutic Potential of Lactobacillus reuteri Characterized Using a Target-Specific Selection Process. Front. Microbiol. 2020, 15, 532. [Google Scholar] [CrossRef]
| Total Patients = 113 | Group A (Reuterin GG®) = 56 | Group B (Control Group) = 57 | p-Value |
|---|---|---|---|
| F/M | 37/19 | 27/30 | ns |
| Mean Age ± DS | 67.16 ± 20.56 | 71.9 ± 22.6 | ns |
| Comorbidities (Y/N) | 33 (58.9%)/23 (41%) | 42 (73.6%)/15 (26.3%) | ns |
| Diabetes | 13 (23.2%) | 8 (14%) | |
| Hypertension | 30 (53.5%) | 37 (64.9%) | |
| Ischemic Cardiopathy | 11 (19.6%) | 15 (26.3%) | |
| Renal Failure | 6(10.7%) | 6(10.5%) | |
| COPD (Chronic Obstructive Pulmonary Disease) | 7 (12.5%) | 5 (8.7%) | |
| Diagnosis of Admission | ns | ||
| Urinary tract infection | 11 (19.6%) | 11 (19.2%) | |
| Respiratory tract infection | 20 (35.7%) | 9(15.7%) | |
| Skin infection | 0 (0%) | 2 (3.5%) | |
| Biliary tract infection | 19 (33.9%) | 19 (33.3%) | |
| Diverticulitis | 4 (7.1%) | 10 (17.5%) | |
| Endocarditis | 2 (3.5%) | 0 (0%) | |
| Osteomyelitis | 0 (0%) | 5 (8.7%) | |
| Peritonsillar Abscess | 0 (0%) | 1 (1.7%) |
| Total Patients = 113 Type of Antibiotics | Group A (Reuterin GG®) = 56 | Group B (Control Group) = 57 | p-Value |
|---|---|---|---|
| Ceftriaxone | 27 (48.2%) | 26 (45.6%) | ns |
| Ciprofloxacin | 1 (1.7%) | 1 (1.7%) | |
| Piperacillin/Tazobactam | 15 (26.7%) | 18 (31.5%) | |
| Metronidazole | 9 (16%) | 8 (14%) | |
| Amoxicillin/Clavulanic Acid | 1 (1.78%) | 3 (5.2%) | |
| Meropenem | 6 (10.7%) | 7 (12.2%) | |
| Vancomycin | 1 (1.7%) | 9 (15.7%) | |
| Cefepime | 0 (0%) | 1 (1.7%) | |
| Linezolid | 2 (3.5%) | 2 (3.5%) | |
| Daptomycin | 1 (1.7%) | 1 (1.7%) | |
| Azithromycin | 3 (5.3%) | 1 (1.7%) | |
| Number of antibiotics | ns | ||
| 1 | 45 (80.3%) | 40 (70.1%) | |
| 2 | 8 (14.2%) | 14 (24.5%) | |
| 3 | 3 (5.3%) | 3 (5.2%) | |
| Days of antibiotic treatment | 9 | 10 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saviano, A.; Petruzziello, C.; Cancro, C.; Macerola, N.; Petti, A.; Nuzzo, E.; Migneco, A.; Ojetti, V. The Efficacy of a Mix of Probiotics (Limosilactobacillus reuteri LMG P-27481 and Lacticaseibacillus rhamnosus GG ATCC 53103) in Preventing Antibiotic-Associated Diarrhea and Clostridium difficile Infection in Hospitalized Patients: Single-Center, Open-Label, Randomized Trial. Microorganisms 2024, 12, 198. https://doi.org/10.3390/microorganisms12010198
Saviano A, Petruzziello C, Cancro C, Macerola N, Petti A, Nuzzo E, Migneco A, Ojetti V. The Efficacy of a Mix of Probiotics (Limosilactobacillus reuteri LMG P-27481 and Lacticaseibacillus rhamnosus GG ATCC 53103) in Preventing Antibiotic-Associated Diarrhea and Clostridium difficile Infection in Hospitalized Patients: Single-Center, Open-Label, Randomized Trial. Microorganisms. 2024; 12(1):198. https://doi.org/10.3390/microorganisms12010198
Chicago/Turabian StyleSaviano, Angela, Carmine Petruzziello, Clelia Cancro, Noemi Macerola, Anna Petti, Eugenia Nuzzo, Alessio Migneco, and Veronica Ojetti. 2024. "The Efficacy of a Mix of Probiotics (Limosilactobacillus reuteri LMG P-27481 and Lacticaseibacillus rhamnosus GG ATCC 53103) in Preventing Antibiotic-Associated Diarrhea and Clostridium difficile Infection in Hospitalized Patients: Single-Center, Open-Label, Randomized Trial" Microorganisms 12, no. 1: 198. https://doi.org/10.3390/microorganisms12010198
APA StyleSaviano, A., Petruzziello, C., Cancro, C., Macerola, N., Petti, A., Nuzzo, E., Migneco, A., & Ojetti, V. (2024). The Efficacy of a Mix of Probiotics (Limosilactobacillus reuteri LMG P-27481 and Lacticaseibacillus rhamnosus GG ATCC 53103) in Preventing Antibiotic-Associated Diarrhea and Clostridium difficile Infection in Hospitalized Patients: Single-Center, Open-Label, Randomized Trial. Microorganisms, 12(1), 198. https://doi.org/10.3390/microorganisms12010198







