The Networked Interaction between Probiotics and Intestine in Health and Disease: A Promising Success Story
Abstract
1. Introduction: Probiotics as Novel Solutions to Old Problems
2. Bacterial Probiotic Strains
3. Probiotics and Their Metabolites
4. Probiotics and Food Products
5. Exploring the Role of Probiotics in Managing Intestinal Diseases
5.1. Crohn’s Disease
5.2. Ulcerative Colitis
5.3. Infectious Colitis
5.4. Celiac Disease
5.5. Irritable Bowel Syndrome (IBS)
5.6. Colorectal Cancer (CRC)
6. Exploring the Axis Administration of Probiotics—Human Gut (In Health and Diseases)
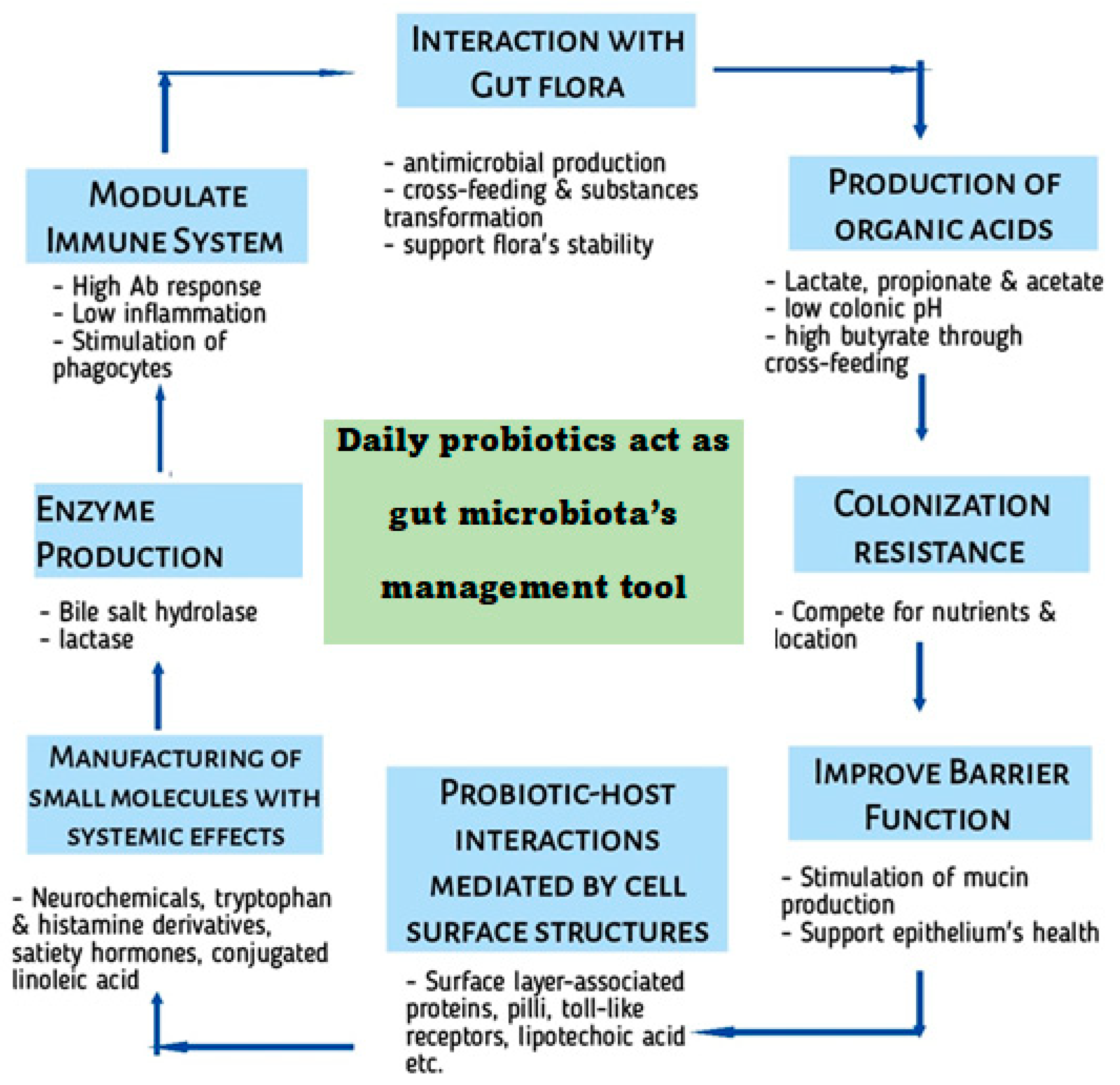
6.1. Several Metabolites Production
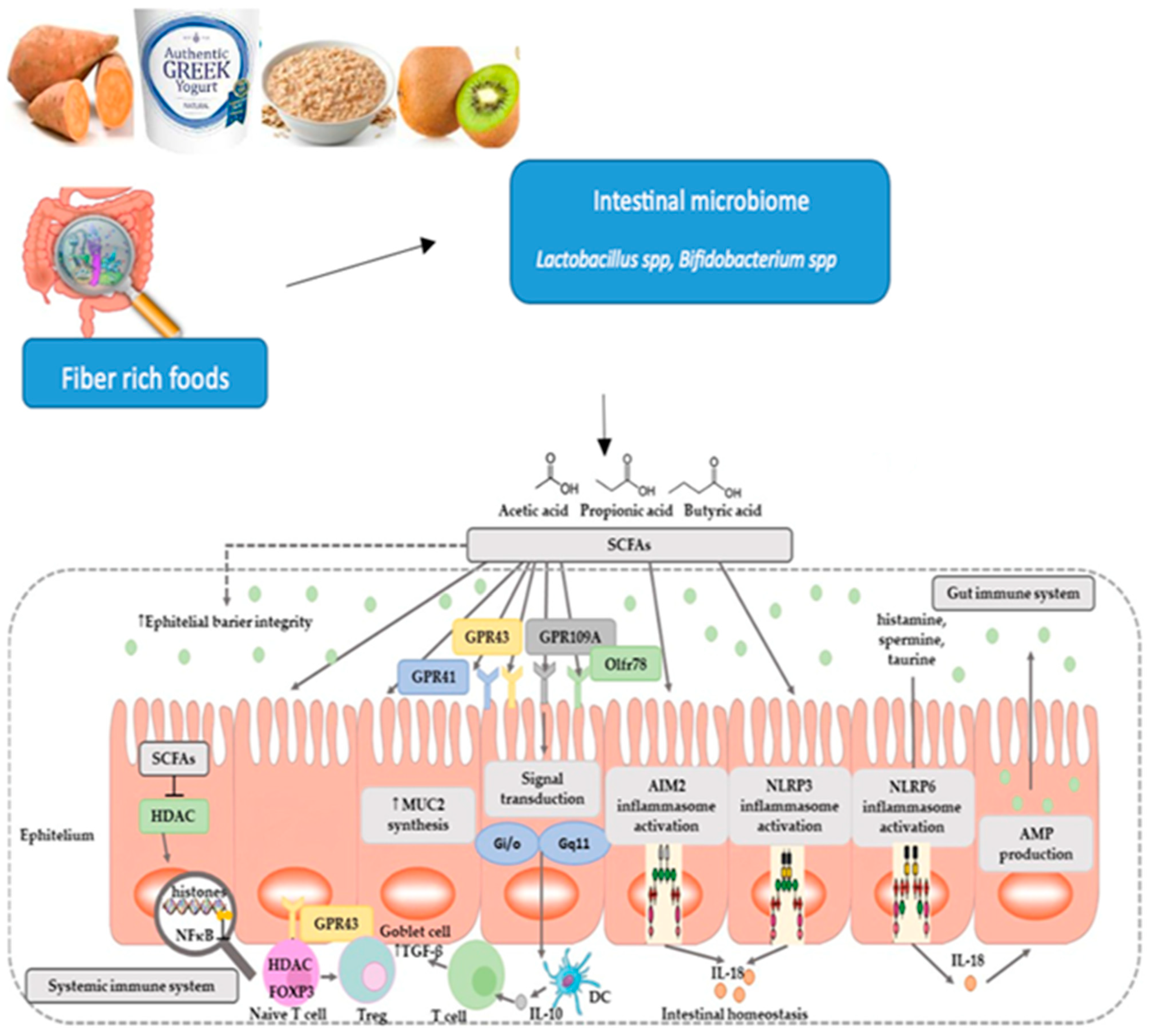
6.2. Impact of Diet on Gut Microbiota
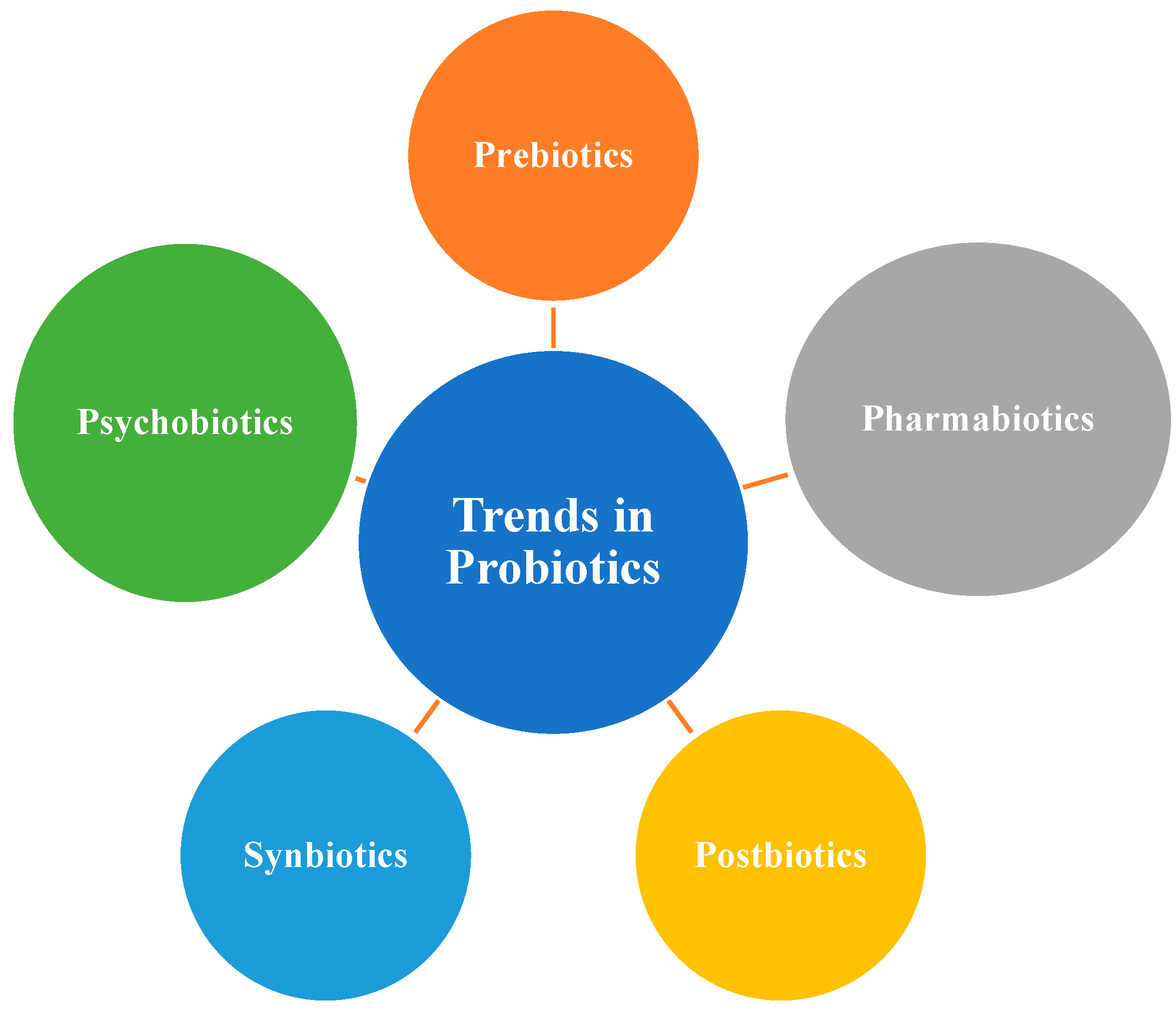
7. Probiotics/Symbiotics/Postbiotics
8. Selection Criteria of Probiotics in Food
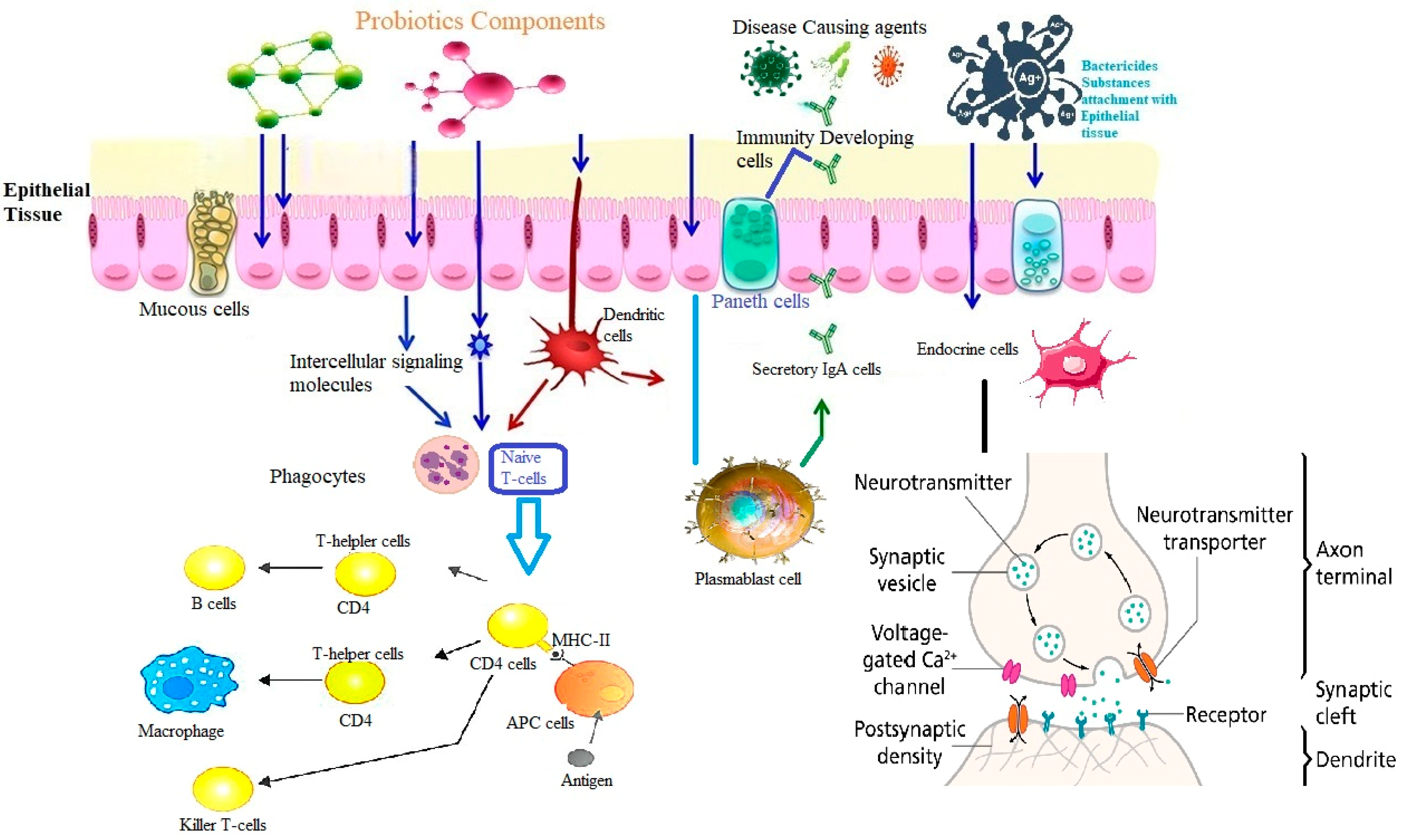
9. Probiotics and Immune System
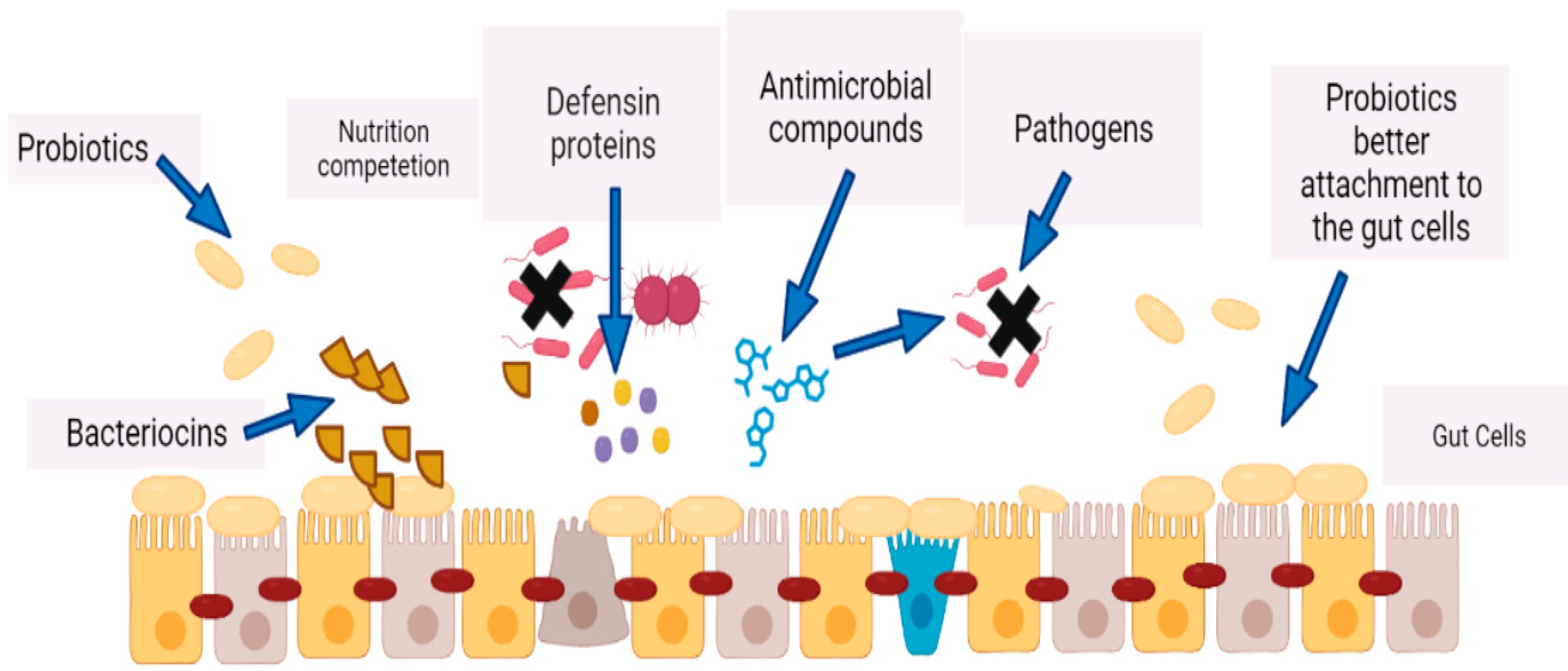
- Restoration of Gut Microbiota Homeostasis: By restoring healthy gut bacteria, probiotics can increase resistance to pathogens and stimulate the immune system.
- Modulation of Intestinal Barrier Function: Probiotics can improve gut barrier function and reduce intestinal permeability.
- Production of Short-Chain Fatty Acids: Short-chain fatty acids produced by probiotics regulate the immune system, have an antimicrobial effect and an anti-inflammatory effect.
- Boosting Immune Cells: Probiotics stimulate the production of immune cells that help defend the body against harmful pathogens.
- Reducing Inflammation: Research suggests that probiotics can help reduce inflammation throughout the body, which is linked to many chronic diseases.
- Protecting Against Infections: Probiotics have been shown to help reduce the risk of infections and may even be effective in treating certain types of infections.
10. Therapeutic Interventions Based on Probiotics
10.1. Gut Microbiota and Gut–Brain Axis (GBA) Signaling
10.2. Dietary Interventions Targeting the Gut Microbiome
10.3. Probiotics as Medications
10.4. Fecal Microbiota Transplantation (FMT)
10.5. Microfluidic Technology
11. Safety of Probiotics
12. Summarizing the Current and Future Perspectives
12.1. Clinical Research and Evidence
12.2. Future Challenges and Limitations
12.2.1. Strain Specificity
12.2.2. Quality Control and Standardization
12.2.3. Survival in the Gastrointestinal Tract
12.2.4. Understanding Mechanisms of Action
12.2.5. Personalized Approaches
12.2.6. Regulatory Framework
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, P.; Lv, J.; Yan, X.; Bai, L.; Zhang, L. Microfluidics: Insights into Intestinal Microorganisms. Microorganisms 2023, 11, 1134. [Google Scholar] [CrossRef]
- Senchukova, M.A. Microbiota of the gastrointestinal tract: Friend or foe? World J Gastroenterol. 2023, 29, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Gut Bacteria in Health and Disease. Gastroenterol. Hepatol. 2013, 9, 560–569. [Google Scholar]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Mitev, K.; Taleski, V. Association between the gut microbiota and obesity. Open Access Maced. J. Med. Sci. 2019, 7, 2050–2056. [Google Scholar]
- Budden, K.F.; Shukla, S.D.; Rehman, S.F.; Bowerman, K.L.; Keely, S.; Hugenholtz, P.; Armstrong-James, D.P.H.; Adcock, I.M.; Chotirmall, S.H.; Chung, K.F.; et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir. Med. 2019, 7, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Lourido, L.; Blanco, F.J.; Ruiz-Romero, C. Defining the proteomic landscape of rheumatoid arthritis: Progress and prospective clinical applications. Expert Rev. Proteom. 2017, 14, 431–444. [Google Scholar] [CrossRef]
- Horta-Baas, G.; Romero-Figueroa, M.D.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal Dysbiosis and rheumatoid arthritis: A link between gut microbiota and the patho-genesis of rheumatoid arthritis. J. Immunol. Res. 2017, 2017, 4835189. [Google Scholar] [CrossRef]
- Robles-Alonso, V.; Guarner, F. Progreso en el conocimiento de la microbiota intestinal humana. Nutr. Hosp. 2013, 28, 553–557. [Google Scholar]
- Caricilli, A.M. Intestinal barrier: A gentlemen’s agreement between microbiota and immunity. World J. Gastrointest. Pathophysiol. 2014, 5, 18–32. [Google Scholar]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Nookaew, I.; Petranovic, D.; Nielsen, J. Prospects for Systems Biology and Modeling of the Gut Microbiome. Trends Biotechnol. 2011, 29, 251–258. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002, 22, 283–307. [Google Scholar]
- Li, W.; Deng, Y.; Chu, Q.; Zhang, P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019, 447, 41–47. [Google Scholar] [CrossRef]
- Sultan, S.; El-Mowafy, M.; Elgaml, A.; Ahmed, T.A.E.; Hassan, H.; Mottawea, W. Metabolic influences of gut microbiota Dysbiosis on inflammatory bowel disease. Front. Physiol. 2021, 12, 715506. [Google Scholar]
- Niu, Y.; Liu, W.; Fan, X.; Wen, D.; Wu, D.; Wang, H.; Liu, Z.; Li, B. Beyond cellulose: Pharmaceutical potential for bioactive plant polysaccharides in treating disease and gut dysbiosis. Front. Microbiol. 2023, 14, 1183130. [Google Scholar] [CrossRef]
- Round June, L.; Palm Noah, W. Causal Effects of the Microbiota on Immune-Mediated Diseases. Sci. Immunol. 2018, 3, eaao1603. [Google Scholar]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Clevers, H. Organoids and Organs-on-Chips: Insights into Human Gut-Microbe Interactions. Cell Host Microbe 2021, 29, 867–878. [Google Scholar] [CrossRef]
- AboNahas, H.H.; Darwish, A.M.; Abd El-kareem, H.F.; AboNahas, Y.H.; Mansour, S.A.; Korra, Y.H.; Sayyed, R.Z.; Abdel-Azeem, A.M.; Saied, E.M. Trust Your Gut: The Human Gut Microbiome in Health and Disease. In Microbiome-Gut-Brain Axis; Sayyed, R.Z., Khan, M., Eds.; Springer: Singapore, 2022; pp. 53–96. [Google Scholar]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western diet and the immune system: An inflammatory connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The gut microbiota-masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Littman, D.R. Segmented filamentous bacteria take the stage. Mucosal Immunol. 2010, 3, 209–212. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Cui, H.L.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cells 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Huguet, M.A.; Belloc, B.; Domínguez-Cajal, M. Small and Large Intestine (I): Malabsorption of Nutrients. Nutrients 2021, 13, 1254. [Google Scholar] [CrossRef] [PubMed]
- Enujiugha, V.N.; Badejo, A.A. Probiotic potentials of cereal-based beverages. Crit. Rev. Food Sci. Nutr. 2017, 57, 790–804. [Google Scholar] [CrossRef]
- Sarwar, A.; Aziz, T.; Al-Dalali, S.; Zhao, X.; Zhang, J.; ud Din, J.; Chen, C.; Cao, Y.; Yang, Z. Physicochemical and Microbiological Properties of Synbiotic Yogurt Made with Probiotic Yeast Saccharomyces boulardii in Combination with Inulin. Foods 2019, 8, 468. [Google Scholar] [CrossRef]
- Talwalkar, A.; Miller, C.W.; Kailasapathy, K.; Nguyen, M.H. Effect of packaging materials and dissolved oxygen on the survival of probiotic bacteria in yoghurt. Int. J. Food Sci. Technol. 2004, 39, 605–611. [Google Scholar] [CrossRef]
- Aziz, T.; Naveed, M.; Sarwar, A.; Makhdoom, S.I.; Mughal, M.S.; Ali, U.; Yang, Z.; Shahzad, M.; Sameeh, M.Y.; Alruways, M.W.; et al. Functional Annotation of Lactiplantibacillus plantarum 13-3 as a Potential Starter Probiotic Involved in the Food Safety of Fermented Products. Molecules 2022, 27, 5399. [Google Scholar]
- Probiotics in Food—Health and Nutritional Properties and Guidelines for Evaluation. 2006. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 5 January 2024).
- Mack, D.R. Probiotics: Mixed messages. Can. Fam. Physician 2005, 51, 1455–1457. [Google Scholar]
- Astuti, R.I.; Prastya, M.E.; Wulan, R.; Anam, K.; Meryandini, A. Current trends, and future perspective of probiotic yeasts research in Indonesia. FEMS Yeast Res. 2023, 23, foad013. [Google Scholar] [CrossRef]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.-Y.; Zhang, H.; Sun, Z. Targeting gut microbiota and metabolism as the major probiotic mechanism—An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Lee, Y.; Lew, C.C.H.; Ortiz-Reyes, A.; Patel, J.J.; Wong, Y.J.; Loh, C.T.I.; Martindale, R.G.; Heyland, D.K. Benefits and harm of probiotics and synbiotics in adult critically ill patients. A systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Clin. Nutr. 2023, 42, 519–553. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Y.; Liu, D.; Li, J.; Sun, J.; Wei, Q.; Zhao, Y.; Pandol, S.J.; Li, L. Porous Microcapsules Encapsulating β Cells Generated by Microfluidic Electrospray Technology for Diabetes Treatment. NPG Asia Mater. 2022, 14, 39. [Google Scholar]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of the Mediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. Ser. A 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of Probiotics in Human World: A Nonstop Source of Benefactions till the End of Time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- Bai, P.; Ouyang, Q. Probiotics and inflammatory bowel diseases. Postgrad. Med. J. 2006, 82, 376–382. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, T.; Ding, H.; Chen, J.; Li, B.; Zhang, Q.; Yang, S.; Zhang, S.; Guan, W. Exploring the Benefits of Probiotics in Gut Inflammation and Diarrhea—From an Antioxidant Perspective. Antioxidants 2023, 12, 1342. [Google Scholar] [CrossRef]
- Sugihara, N.; Okada, Y.; Tomioka, A.; Ito, S.; Tanemoto, R.; Nishii, S.; Mizoguchi, A.; Inaba, K.; Hanawa, Y.; Horiuchi, K.; et al. Probiotic Yeast from Miso Ameliorates Stress-Induced Visceral Hypersensitivity by Modulating the Gut Microbiota in a Rat Model of Irritable Bowel Syndrome. Gut Liver 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Khan, S.; Mehdizadeh, M.; Nirmal, N.P.; Khanashyam, A.C.; Fernando, I.; Jatmiko, Y.D.; Afiyanti, M.; Bansal, S.; Adli, D.N.; et al. Consumer Studies Focus on Prebiotics, Probiotics, and Synbiotics in Food Packaging: A Review. Curr. Food Sci. Technol. Rep. 2023, 1, 13–29. [Google Scholar] [CrossRef]
- Malik, J.K.; Ahmad, A.H.; Kalpana, S.; Prakash, A.; Gupta, R.C. Chapter 57—Synbiotics: Safety and Toxicity Considerations. In Nutraceuticals; Gupta, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 811–822. [Google Scholar] [CrossRef]
- Tarrah, A.; da Silva Duarte, V.; de Castilhos, J.; Pakroo, S.; Lemos Junior, W.J.F.; Luchese, R.H.; Guerra, A.F.; Rossi, R.C.; Ziegler, D.R.; Corich, V.; et al. Probiotic potential and biofilm inhibitory activity of Lactobacillus casei group strains isolated from infant feces. J. Funct. Foods 2019, 54, 489–497. [Google Scholar] [CrossRef]
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679. [Google Scholar] [CrossRef]
- Terciolo, C.; Dapoigny, M.; Andre, F. Beneficial effects of Saccharomyces boulardii CNCM I-745 on clinical dis-orders associated with intestinal barrier disruption. Clin. Exp. Gastroenterol. 2019, 12, 67–82. [Google Scholar]
- Bajinka, O.; Tan, Y.; Abdelhalim, K.A.; Özdemir, G.; Qiu, X. Extrinsic factors influencing gut microbes, the immediate consequences and restoring eubiosis. AMB Express 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Ramasamy, U.; Meyer, D.; Pullens, G.; Venema, K.; Faas, M.M.; Schols, H.A.; de Vos, P. Immune modulation by different types of β2→ 1-fructans is toll-like receptor dependent. PLoS ONE 2013, 8, e68367. [Google Scholar] [CrossRef]
- Sadrin, S.; Sennoune, S.; Gout, B.; Marque, S.; Moreau, J.; Zinoune, K.; Grillasca, J.; Pons, O.; Maixent, J. A 2-strain mixture of Lactobacillus acidophilus in the treatment of irritable bowel syndrome: A placebo-controlled randomized clinical trial. Dig. Liver Dis. 2020, 52, 534–540. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Qiao, N.; Wittouck, S.; Mattarelli, P.; Zheng, J.; Lebeer, S.; Felis, G.E.; Gänzle, M.G. After the storm—Perspectives on the taxonomy of Lactobacillaceae. JDS Commun. 2022, 3, 222–227. [Google Scholar] [CrossRef]
- Steele, C. Lactobacillus rhamnosus GG: A review of clinical use and efficacy. Nutr. Med. J. 2022, 1, 70–116. [Google Scholar]
- Li, S.N.; Tang, S.H.; Ren, R.; Gong, J.X.; Chen, Y.M. Metabolomic profile of milk fermented with Streptococcus thermophilus cocultured with Bifidobacterium animalis ssp. lactis, Lactiplantibacillus plantarum, or both during storage. J. Dairy Sci. 2021, 104, 8493–8505. [Google Scholar]
- Szajewska, H. Functional foods and acute gastrointestinal infections. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Functional Foods. In Functional Foods, 2nd ed.; Saarela, M., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 129–152. [Google Scholar] [CrossRef]
- Lopez-Escalera, S.; Lund, M.L.; Hermes, G.D.; Choi, B.S.; Sakamoto, K.; Wellejus, A. In Vitro Screening for Probiotic Properties of Lactobacillus and Bifidobacterium Strains in Assays Relevant for Non-Alcoholic Fatty Liver Disease Prevention. Nutrients 2022, 15, 2361. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, T.; Ma, D.; Shi, P.; Zhang, H.; Li, J.; Sun, Z. Probiotics Bifidobacterium lactis M8 and Lactobacillus rhamnosus M9 prevent high blood pressure via modulating the gut microbiota composition and host metabolic products. mSystems 2023, 8, e0033123. [Google Scholar] [CrossRef]
- Cukrowska, B.; Bierła, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The relationship between the infant gut microbiota and allergy. The role of Bifidobacterium breve and prebiotic oligosaccharides in the activation of anti-allergic mechanisms in early life. Nutrients 2020, 12, 946. [Google Scholar]
- Lee, J.E.; Lee, E. The Probiotic Effects of the Saccharomyces cerevisiae 28-7 Strain Isolated from Nuruk in a DSS-Induced Colitis Mouse Model. J. Microbiol. Biotechnol. 2022, 32, 877–884. [Google Scholar] [CrossRef]
- Martinović, A.; Cocuzzi, R.; Arioli, S.; Mora, D. Streptococcus thermophilus: To Survive, or Not to Survive the Gastrointestinal Tract, That Is the Question! Nutrients 2020, 12, 2175. [Google Scholar] [CrossRef]
- Taj, R.; Masud, T.; Sohail, A.; Sammi, S.; Naz, R.; Sharma Khanal, B.K.; Nawaz, M.A. In vitro screening of EPS-producing Streptococcus thermophilus strains for their probiotic potential from Dahi. Food Sci. Nutr. 2022, 10, 2347–2359. [Google Scholar] [CrossRef]
- Secher, T.; Kassem, S.; Benamar, M.; Bernard, I.; Boury, M.; Barreau, F.; Oswald, E.; Saoudi, A. Oral administration of the probiotic strain Escherichia coli Nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental auto-immune encephalomyelitis-induced intestinal barrier dysfunction. Front. Immunol. 2017, 8, 1096. [Google Scholar] [CrossRef]
- Hu, R.; Lin, H.; Li, J.; Zhao, Y.; Wang, M.; Sun, X.; Min, Y.; Gao, Y.; Yang, M. Probiotic Escherichia coli Nissle 1917-derived outer membrane vesicles enhance immunomodulation and antimicrobial activity in RAW264.7 macrophages. BMC Microbiol. 2020, 20, 268. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, E.H.; Chen, R.P.; Dunny, G.M.; Hu, W.S.; Lee, K.T. Probiotic Bacillus Affects Enterococcus faecalis Antibiotic Resistance Transfer by Interfering with Pheromone Signaling Cascades. Appl. Environ. Microbiol. 2021, 87, e0044221. [Google Scholar] [CrossRef]
- Lee, K.; Kim, S.; Paik, D. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef]
- Abengózar, M.A.; Cebrian, R.; Saugar, J.M.; Garate, T.; Valdivia, E.; MartinezBueno, M.; Maqueda, M.; Rivas, L. Enterocin AS-48 as evidence for the use of bacteriocins as new leishmanicidal agents. Antimicrob. Agents Chemother. 2017, 61, e02288-16. [Google Scholar] [CrossRef] [PubMed]
- Im, E.J.; Lee, H.H.-Y.; Kim, M.; Kim, M.-K. Evaluation of Enterococcal Probiotic Usage and Review of Potential Health Benefits, Safety, and Risk of Antibiotic-Resistant Strain Emergence. Antibiotics 2023, 12, 1327. [Google Scholar] [CrossRef]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, S.; Mehta, V.; Ali El Enshasy, H.; Thevarajoo, S.; El Adawi, H.; Zeini, I.; Pham, K.; Varzakas, T.; Abomoelak, B. Efficacy of Probiotics-Based Interventions as Therapy for Inflammatory Bowel Disease: A Recent Update. Saudi J. Biol. Sci. 2022, 29, 3546–3567. [Google Scholar] [CrossRef] [PubMed]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M.-H. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F.A.; et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 879–913. [Google Scholar] [CrossRef]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics—Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Milner, E.; Stevens, B.; An, M.; Lam, V.; Ainsworth, M.; Dihle, P.; Stearns, J.; Dombrowski, A.; Rego, D.; Segars, K. Utilizing Probiotics for the Prevention and Treatment of Gastrointestinal Diseases. Front. Microbiol. 2021, 12, 689958. [Google Scholar] [CrossRef]
- Sharma, R.; Bhaskar, B.; Sanodiya, B.S.; Thakur, G.S.; Jaiswal, P.; Yadav, N.; Sharma, A.; Bisen, P.S. Probiotic Efficacy and Potential of Streptococcus thermophilus modulating human health: A synoptic review. IOSR J. Pharm. Biol. Sci. 2014, 9, 52–58. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.H.F.; Krych, L.; Nielsen, D.S.; Vogensen, F.K.; Hansen, L.H.; Sørensen, S.J.; Buschard, K.; Hansen, A.K. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 2012, 55, 2285–2294. [Google Scholar] [CrossRef]
- Navab-Moghadam, F.; Sedighi, M.; Khamseh, M.E.; Alaei-Shahmiri, F.; Talebi, M.; Razavi, S.; Amirmozafari, N. The association of type II diabetes with gut microbiota composition. Microb. Pathog. 2017, 110, 630–636. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 356589. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Tibbs, T.N.; Lopez, L.R.; Arthur, J.C. The influence of the microbiota on immune development, chronic inflammation, and cancer in the context of aging. Microb. Cell 2019, 6, 324. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Krishnan, S.; Alden, N.; Lee, K. Pathways and Functions of Gut Microbiota Metabolism Impacting Host Physiology. Curr. Opin. Biotechnol. 2015, 36, 137. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. For Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Alesa, D.I.; Alshamrani, H.M.; Alzahrani, Y.A.; Alamssi, D.N.; Alzahrani, N.S.; Almohammadi, M.E. The role of gut microbiome in the pathogenesis of psoriasis and the therapeutic effects of probiotics. J. Fam. Med. Prim. Care 2019, 8, 3496. [Google Scholar]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nagpal, R.; Verma, V.; Kumar, A.; Kaur, N.; Hemalatha, R.; Gautam, S.K.; Singh, B. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr. Rev. 2013, 71, 23–34. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, M.; Pu, J.; Zhu, Z.; Gao, Z.; Zhou, Q.; Gu, Q.; Li, P. Probiotics and Their Metabolites Ameliorate Inflammatory Bowel Disease: A Critical Review. Infect. Microbes Dis. 2021, 3, 4–13. [Google Scholar] [CrossRef]
- Yan, F.; Liu, L.; Dempsey, P.J.; Tsai, Y.-H.; Raines, E.W.; Wilson, C.L.; Cao, H.; Cao, Z.; Liu, L.; Polk, D.B. A Lactobacillus rhamnosus GG-derived Soluble Protein, p40, Stimulates Ligand Release from Intestinal Epithelial Cells to Transactivate Epidermal Growth Factor Receptor. J. Biol. Chem. 2013, 288, 30742–30751. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Caicedo, R.; Neu, J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J. Nutr. 2005, 135, 1752–1756. [Google Scholar] [CrossRef]
- Madsen, K.; Cornish, A.; Soper, P.; McKaigney, C.; Jijon, H.; Yachimec, C.; Doyle, J.; Jewell, L.; De Simone, C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001, 121, 580–591. [Google Scholar]
- Schlee, M.; Wehkamp, J.; Altenhoefer, A.; Oelschlaeger, T.A.; Stange, E.F.; Fellermann, K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect. Immun. 2007, 75, 2399–2407. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- Francisco, D.; Alexis, J.; Rubén, J. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in vivo Models. Front. Microbiol. 2021, 12, 630695. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Reynolds, K.; Martino-Catt, S.; Cui, Y.; Hennighausen, L.; Gonzalez, F.; Rohrer, J.; Benninghoff, A.U.; Hontecillas, R. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 2004, 127, 777–791. [Google Scholar] [CrossRef]
- von Schillde, M.-A.; Hörmannsperger, G.; Weiher, M.; Alpert, C.-A.; Hahne, H.; Bäuerl, C.; van Huynegem, K.; Steidler, L.; Hrncir, T.; Pérez-Martínez, G.; et al. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe 2012, 11, 387–396. [Google Scholar]
- Chinnadurai, K.; Tyagi, A. Conjugated Linoleic Acid: A Milk Fatty Acid with Unique Health Benefit Properties. Edited by Hany El-Shemy, In Soybean and Health; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Kojima, T.; Fujieda, M.; Takahashi, M.; Michibata, T. Influences of probiotic bacteria on organic acid production by pig caecal bacteria in vitro. Proc. Nutr. Soc. 2003, 62, 73–80. [Google Scholar] [CrossRef]
- Feng, C.; Jin, C.; Liu, K.; Yang, Z. Microbiota-derived short chain fatty acids: Their role and mechanisms in viral infections. Biomed. Pharmacother. 2023, 160, 114414. [Google Scholar] [CrossRef]
- Larabi, A.B.; Masson, H.L.P.; Bäumler, A.J. Bile acids as modulators of gut microbiota composition and function. Gut Microbes 2023, 15, 2172671. [Google Scholar] [CrossRef]
- Segawa, S.; Fujiya, M.; Konishi, H.; Ueno, N.; Kobayashi, N.; Shigyo, T.; Kohgo, Y. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS ONE 2011, 6, e23278. [Google Scholar] [CrossRef] [PubMed]
- Selber-Hnatiw, S.; Rukundo, B.; Ahmadi, M.; Akoubi, H.; Al-Bizri, H.; Aliu, A.F.; Ambeaghen, T.U.; Avetisyan, L.; Bahar, I.; Baird, A.; et al. Human gut microbiota: Toward an ecology of disease. Front. Microbiol. 2017, 8, 1265. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Ogura, J.; Sivaprakasam, S.; Ganapathy, V. Gut microbiome and colon cancer: Role of bacterial metabolites and their molecular targets in the host. Curr. Colorectal Cancer Rep. 2017, 13, 111–118. [Google Scholar] [CrossRef]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef]
- Levit, R.; de Giori, G.S.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Evaluation of the effect of soymilk fermented by a ribofla-vin-producing Lactobacillus plantarum strain in a murine model of colitis. Benef. Microbes 2017, 8, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef]
- Huang, C.; Hao, W.; Wang, X.; Zhou, R.; Lin, Q. Probiotics for the treatment of ulcerative colitis: A review of experimental research from 2018 to 2022. Front. Microbiol. 2023, 14, 1211271. [Google Scholar] [CrossRef]
- Pujo, J.; Petitfils, C.; Le Faouder, P.; Eeckhaut, V.; Payros, G.; Maurel, S.; Perez-Berezo, T.; Van Hul, M.; Barreau, F.; Blanpied, C.; et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 2021, 70, 1088–1097. [Google Scholar] [CrossRef]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; de Vos, M.; Boon, N.; de Wiele, T.V. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2016, 52, 1–8. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottiere, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional im-portance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [PubMed]
- Ramírez-Macías, I.; Orenes-Piñero, E.; Camelo-Castillo, A.; Rivera-Caravaca, J.M.; López-García, C.; Marín, F. Novel insights in the relationship of gut microbiota and coronary artery diseases. Crit. Rev. Food Sci. Nutr. 2022, 62, 3738–3750. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef] [PubMed]
- Mischke, M.; Plösch, T. The gut microbiota and their metabolites: Potential implications for the host epigenome. Microb. Hum. Body 2016, 902, 33–44. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.-P.; Zhang, Y.-N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Hendrikx, T.; Schnabl, B. Indoles: Metabolites produced by intestinal bacteria capable of controlling liver disease manifestation. J. Intern. Med. 2019, 286, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, B.; Hu, Y.; Zhao, Y. New Insights Into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases. Front. Pharmacol. 2021, 12, 769501. [Google Scholar] [CrossRef]
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Tofalo, R.; Cocchi, S.; Suzzi, G. Polyamines and gut microbiota. Front. Nutr. 2019, 6, 16. [Google Scholar] [CrossRef]
- Smallwood, T.; Allayee, H.; Bennett, B.J. Choline metabolites: Gene by diet interactions. Curr. Opin. Lipidol. 2016, 27, 33. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Lv, J.; Dong, L.; Zhang, L.; Wang, T. Choline supplementation improves the lipid metabolism of intrauterine-growth-restricted pigs. Asian-Australas. J. Anim. Sci. 2018, 31, 686–695. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Forster, V.J.; McDonnell, A.; Theobald, R.; McKay, J.A. Effect of methotrexate/vitamin B12 on DNA methylation as a potential factor in leukemia treatment-related neurotoxicity. Epigenomics 2017, 9, 1205–1218. [Google Scholar] [CrossRef]
- Afanasiev, I. New nucleophilic mechanisms of ros-dependent epigenetic modifications: Comparison of aging and cancer. Aging Dis. 2014, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. Hydrogen sulfide, reactive sulfur species and coping with reactive oxygen species. Free Radic. Biol. Med. 2019, 140, 74–83. [Google Scholar] [CrossRef]
- Available online: https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional/ (accessed on 6 January 2024).
- Abdul Hakim, B.N.; Xuan, N.J.; Hazwani Oslan, S.N. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, G.; Ali, S.A. Dairy-Based Probiotic-Fermented Functional Foods: An Update on Their Health-Promoting Properties. Fermentation 2022, 8, 425. [Google Scholar] [CrossRef]
- Voidarou, C.; Antoniadou, Μ.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2020, 10, 69. [Google Scholar] [CrossRef]
- Fatima Sherwani, K.; Ara Abbas Bukhari, D. Probiotics in Processed Dairy Products and Their Role in Gut Microbiota Health. In Effect of Microbiota on Health and Disease; El-Sayed, H., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar]
- Kumar, B.V.; Naga Vijayendra, S.V.; Sarathi Reddy, O.V. Trends in dairy and non-dairy probiotic products—A review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The Functional Roles of Lactobacillus acidophilus in Different Physiological and Pathological Processes. J. Microbiol. Biotechnol. 2022, 32, 1226–1233. [Google Scholar] [CrossRef]
- Ziarno, M.; Cichońska, P. Lactic Acid Bacteria-Fermentable Cereal- and Pseudocereal-Based Beverages. Microorganisms 2021, 9, 2532. [Google Scholar] [CrossRef] [PubMed]
- Asfari, M.M.; Sarmini, M.T.; Kendrick, K.; Hudgi, A.; Uy, P.; Sridhar, S.; Sifuentes, H. Association between Inflammatory Bowel Disease and Lactose Intolerance: Fact or Fiction. Korean J. Gastroenterol. 2020, 76, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, P.; Tomasik, P. Probiotics, non-dairy prebiotics and postbiotics in nutrition. Appl. Sci. 2020, 10, 1470. [Google Scholar]
- Küçükgöz, K.; Trząskowska, M. Nondairy Probiotic Products: Functional Foods That Require More Attention. Nutrients 2022, 14, 753. [Google Scholar] [CrossRef]
- Rasika, D.M.; Vidanarachchi, J.K.; Luiz, S.F.; Azeredo, D.R.; Cruz, A.G.; Ranadheera, C.S. Probiotic Delivery through Non-Dairy Plant-Based Food Matrices. Agriculture 2021, 11, 599. [Google Scholar] [CrossRef]
- Zheng, L.; Wen, L. Gut microbiota and inflammatory bowel disease: The current status and perspectives. World J. Clin. Cases 2020, 9, 321–333. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Tharu, R.; Ahlawat, G.M.; Kushwaha, S.; Khanna, P. Gut microbiota disparities between active Crohn’s disease and healthy controls: A global systematic review. Clin. Epidemiol. Glob. Health 2023, 25, 101497. [Google Scholar] [CrossRef]
- Sanders, D.J.; Inniss, S.; Sebepos-Rogers, G.; Rahman, F.Z.; Smith, A.M. The role of the microbiome in gastrointestinal inflammation. Biosci. Rep. 2021, 41, BSR20203850. [Google Scholar] [CrossRef]
- Guarner, F.; Khan, A.G.; Garisch, J.; Eliakim, R.; Gangl, A.; Thomson, A.; Krabshuis, J.; Lemair, T.; Kaufmann, P.; de Paula, J.A.; et al. World gastroenterology organisation global guidelines: Probiotics and prebiotics october 2011. J. Clin. Gastroenterol. 2012, 46, 468–481. [Google Scholar] [CrossRef]
- Villoria, A.; García, V.; Dosal, A.; Moreno, L.; Montserrat, A.; Figuerola, A.; Horta, D.; Calvet, X.; Ramírez-Lázaro, M.J. Fatigue in out-patients with inflammatory bowel disease: Prevalence and predictive factors. PLoS ONE 2017, 12, e0181435. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, Z.; Cai, J. Fecal microbiota transplantation: Emerging applications in autoimmune diseases. J. Autoimmun. 2023, 141, 103038. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, G.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation and Other Gut Microbiota Manipulation Strategies. Microorganisms 2022, 10, 2424. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Rizzello, F.; Helwig, U.; Poggioli, G.; Schreiber, S.; Talbot, I.C.; Nicholls, R.J.; Gionchetti, P.; Campieri, M.; Kamm, M.A. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 2004, 53, 108–114. [Google Scholar] [CrossRef]
- e Silva, N.O.; de Brito, B.B.; da Silva FA, F.; Santos ML, C.; de Melo, F.F. Probiotics in inflammatory bowel disease: Does it work? World J. Meta-Anal. 2020, 8, 54–66. [Google Scholar]
- Matías-Pérez, D.; Hernández-Bautista, E.; García-Montalvo, I.A. Intermittent fasting may optimize intestinal microbiota, adipocyte status and metabolic health. Asia Pac. J. Clin. Nutr. 2022, 31, 16–23. [Google Scholar]
- Lupu, V.V.; Ghiciuc, C.M.; Stefanescu, G.; Mihai, C.M.; Popp, A.; Sasaran, M.O.; Bozomitu, L.; Starcea, I.M.; Raileanu, A.A.; Lupu, A. Emerging role of the gut microbiome in post-infectious irritable bowel syndrome: A literature review. World J. Gastroenterol. 2023, 29, 3241–3256. [Google Scholar] [CrossRef]
- Fakharian, F.; Thirugnanam, S.; Welsh, D.A.; Kim, W.-K.; Rappaport, J.; Bittinger, K.; Rout, N. The Role of Gut Dysbiosis in the Loss of Intestinal Immune Cell Functions and Viral Pathogenesis. Microorganisms 2023, 11, 1849. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017, 12, CD006095. [Google Scholar] [CrossRef] [PubMed]
- Davidovics, Z.H.; Michail, S.; Nicholson, M.R.; Kociolek, L.K.; Pai, N.; Hansen, R.; Schwerd, T.; Maspons, A.; Shamir, R.; Szajewska, H.; et al. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection and Other Conditions in Children: A Joint Position Paper From the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 68, 130. [Google Scholar] [CrossRef]
- Sokol, H.; Galperine, T.; Kapel, N.; Bourlioux, P.; Seksik, P.; Barbut, F.; Scanzi, J.; Chast, F.; Batista, R.; Joly, F.; et al. Fecal Microbiota Transplantation for Treatment of Relapsing Clostridium Difficile Infection: Guidelines for Clinical Practice. Available online: https://www.researchgate.net/publication/281993297_Fecal_microbiota_transplantation_for_treatment_of_relapsing_Clostridium_difficile_infection_Guidelines_for_clinical_practice (accessed on 29 November 2023).
- Bai, M.; Guo, H.; Zheng, Y. Inflammatory bowel disease and Clostridium difficile infection: Clinical presentation, diagnosis, and management. Ther. Adv. Gastroenterol. 2023, 16, 1–14. [Google Scholar] [CrossRef]
- Sangurima, L.; Malik, M.M.; Ganatra, N.; Siby, R.; Kumar, S.; Khan, S.; Cheriachan, D.; Mohammed, L. Clostridioides difficile Infection in Inflammatory Bowel Disease Patients: A Systematic Review of Risk Factors and Approach in Management. Cureus 2023, 15, e43134. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.C.; Paterson, J.L.; Ferguson, S.A.; Stanley, D.; Wright, K.P., Jr.; Dawson, D. The shift work and health research agenda: Considering changes in gut microbiota as a pathway linking shift work, sleep loss and circadian misalignment, and metabolic disease. Sleep Med. Rev. 2017, 34, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Regolisti, G.; Brusasco, I.; Cabassi, A.; Morabito, S.; Fiaccadori, E. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Fallani, M.; Amarri, S.; Uusijarvi, A.; Adam, R.; Khanna, S.; Aguilera, M.; Gil, A.; Vieites, J.M.; Norin, E.; Young, D.; et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 2011, 157 Pt 5, 1385–1392. [Google Scholar] [CrossRef]
- Fallani, M.; Young, D.; Scott, J.; Norin, E.; Amarri, S.; Adam, R.; Aguilera, M.; Khanna, S.; Gil, A.; Edwards, C.A.; et al. Intestinal microbiota of 6-week-old infants across Europe: Geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 77–84. [Google Scholar] [CrossRef]
- Angelis, M.D.; Rizzello, C.G.; Fasano, A.; Clemente, M.G.; Simone, C.D.; Silano, M.; Vincenzi, M.D.; Losito, I.; Gobbetti, M. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for Celiac Sprue probiotics and gluten intolerance. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2005, 1762, 80–93. [Google Scholar] [CrossRef]
- Moraes, S.; Grzeskowiak, L.M.; Teixeira, S.; Peluzio, C.G. Intestinal Microbiota and Probiotics in Celiac Disease. Clin. Microbiol. Rev. 2014, 27, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J. Gastroenterol. 2014, 20, 14105. [Google Scholar] [CrossRef]
- Lebeer, S.; Bron, P.A.; Marco, M.L.; Van Pijkeren, J.P.; O’Connell Motherway, M.; Hill, C.; Van Sinderen, D. Identification of probiotic effector molecules: Present state and future perspectives. Curr. Opin. Biotechnol. 2018, 49, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Fasulo, E.; Ungaro, F.; Massimino, L.; Sinagra, E.; Danese, S.; Mandarino, F.V. Gut Dysbiosis in Irritable Bowel Syndrome: A Narrative Review on Correlation with Disease Subtypes and Novel Therapeutic Implications. Microorganisms 2023, 11, 2369. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.D.; Sun, N.; Canakis, A.; Park, W.Y.; Weber, H.C. Irritable Bowel Syndrome and the Gut Microbiome: A Comprehensive Review. J. Clin. Med. 2023, 12, 2558. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients 2017, 9, 555. [Google Scholar]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- Pandey, H.; Tang, D.W.; Wong, S.H.; Lal, D. Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities. Cancers 2022, 15, 866. [Google Scholar] [CrossRef]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef]
- Cui, W.; Xu, L.; Huang, L.; Tian, Y.; Yang, Y.; Li, Y.; Yu, Q. Changes of gut microbiota in patients at different phases of stroke. CNS Neurosci. Ther. 2023, 29, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F. Diet and the human gut microbiome: An international review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Guan, X.-X.; Tang, Y.-J.; Sun, J.-F.; Wang, X.-K.; Wang, W.-D.; Fan, J.-M. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef]
- Toh, J.W.T.; Wilson, R.B. Pathways of Gastric Carcinogenesis, Helicobacter pylori Virulence and Interactions with Antioxidant Systems, Vitamin C and Phytochemicals. Int. J. Mol. Sci. 2020, 21, 6451. [Google Scholar] [CrossRef]
- Reznikov, E.A.; Suskind, D.L. Current Nutritional Therapies in Inflammatory Bowel Disease: Improving Clinical Remission Rates and Sustainability of Long-Term Dietary Therapies. Nutrients 2023, 15, 668. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Ilango, S.; Antony, U. Probiotic microorganisms from non-dairy traditional fermented foods. Trends Food Sci. Technol. 2021, 118, 617–638. [Google Scholar] [CrossRef]
- Ganguly, S.; Sabikhi, L.; Singh, A.K. Effect of whey-pearl millet-barley based probiotic beverage on Shigella-induced pathogenicity in murine model. J. Funct. Foods 2019, 54, 498–505. [Google Scholar] [CrossRef]
- Doar, N.W.; Samuthiram, S.D. Qualitative Analysis of the Efficacy of Probiotic Strains in the Prevention of Antibiotic-Associated Diarrhea. Cureus 2023, 15, e40261. [Google Scholar] [CrossRef]
- Szajewska, H.; Kołodziej, M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 2015, 42, 793–801. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, L.; Yan, Z.; Jia, J.; Gao, J.; Wei, Y. The mechanisms and safety of probiotics against toxigenic clostridium difficile. Expert Rev. Anti-Infect. Ther. 2020, 18, 967–975. [Google Scholar] [PubMed]
- Fragkou, I.A.; Gougoulis, D.A.; Billinis, C.; Mavrogianni, V.S.; Bushnell, M.J.; Cripps, P.J.; Tzora, A.; Fthenakis, G.C. Transmission of Mannheimia haemolytica from the tonsils of lambs to the teat of ewes during sucking. Vet. Microbiol. 2011, 148, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Fthenakis, G.C.; Saratsis, P.; Tzora, A.; Linde, K. Naturally occurring subclinical ovine mastitis associated with Listeria monocytogenes. Small Rumin. Res. 1998, 31, 23–27. [Google Scholar] [CrossRef]
- Voidarou, C.; Vassos, D.; Kegos, T.; Koutsotoli, A.; Tsiotsias, A.; Skoufos, J.; Tzora, A.; Maipa, V.; Alexopoulos, A.; Bezirtzoglou, E. Aerobic and anaerobic microbiology of the immersion chilling procedure during poultry processing. Poult. Sci. 2007, 86, 1218–1222. [Google Scholar] [PubMed]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Wang, L.; Du, M.; Wang, K.; Khandpur, N.; Rossato, S.L.; Drouin-Chartier, J.-P.; Steele, E.M.; Giovannucci, E.; Song, M.; Zhang, F.F. Association of ultra-processed food consumption with colorectal cancer risk among men and women: Results from three prospective US cohort studies. BMJ 2022, 378, e068921. [Google Scholar] [CrossRef]
- Requena, T.; Martínez-Cuesta, M.C.; Peláez, C. Diet and microbiota linked in health and disease. Food Funct. 2018, 9, 688–704. [Google Scholar] [CrossRef]
- Healey, G.R.; Celiberto, L.S.; Lee, S.M.; Jacobson, K. Fiber and Prebiotic Interventions in Pediatric Inflammatory Bowel Disease: What Role Does the Gut Microbiome Play? Nutrients 2020, 12, 3204. [Google Scholar] [CrossRef]
- Scarallo, L.; Lionetti, P. Dietary Management in Pediatric Patients with Crohn’s Disease. Nutrients 2021, 13, 1611. [Google Scholar] [CrossRef]
- Jadhav, A.; Jagtap, S.; Vyavahare, S.; Sharbidre, A.; Kunchiraman, B. Reviewing the potential of probiotics, prebiotics and synbiotics: Advancements in treatment of ulcerative colitis. Front. Cell. Infect. Microbiol. 2023, 13, 1268041. [Google Scholar] [CrossRef]
- Kuellmer, A.; Mangold, T.; Bettinger, D.; Maruschke, L.; Wannhoff, A.; Caca, K.; Wedi, E.; Hosseini, A.S.A.; Kleemann, T.; Schulz, T.; et al. Over-the-scope clip versus transcatheter arterial embolization for refractory peptic ulcer bleeding—A propensity score matched analysis. UEG J. 2021, 9, 1048–1056. [Google Scholar]
- Lopes, G.; Campanari, G.S.d.S.; Barbalho, S.M.; Matias, J.N.; Lima, V.M.; Araújo, A.C.; Goulart, R.d.A.; Detregiachi, C.R.P.; Haber, J.F.S.; de Carvalho, A.C.A.; et al. Effects of probiotics on inflammatory bowel disease: A systematic review. Jpn. J. Gastroenterol. Res. 2021, 1, 1009. [Google Scholar]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotics and Probiotic-Derived Functional Factors—Mechanistic Insights Into Applications for Intestinal Homeostasis. Front. Immunol. 2020, 11, 560388. [Google Scholar] [CrossRef]
- Dhopatkar, N.; Keeler, J.L.; Mutwalli, H.; Whelan, K.; Treasure, J.; Himmerich, H. Gastrointestinal symptoms, gut microbiome, probiotics and prebiotics in anorexia nervosa: A review of mechanistic rationale and clinical evidence. Psychoneuroendocrinology 2022, 147, 105959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, W.; Feng, C.; Kwok, L.-Y.; He, Q.; Sun, Z. Stronger gut microbiome modulatory effects by postbiotics than probiotics in a mouse colitis model. npj Sci. Food 2022, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, D.; Yang, M.; Hao, Y.; Zhu, Y.; Chen, Z.; Aziz, T.; Sarwar, A.; Yang, Z. Screening of folate-producing lactic acid bacteria and modulatory effects of folate-biofortified yogurt on gut dysbacteriosis of folate-deficient rats. Food Funct. 2020, 11, 6308–6318. [Google Scholar] [CrossRef]
- Gomez Quintero, D.F.; Kok, C.R.; Hutkins, R. The Future of Synbiotics: Rational Formulation and Design. Front. Microbiol. 2022, 13, 919725. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Meneghin, F.; Fabiano, V.; Mameli, C.; Zuccotti, G.V. Probiotics and Atopic Dermatitis in Children. Pharmaceuticals 2012, 5, 727–744. [Google Scholar] [CrossRef]
- Rafique, N.; Jan, S.Y.; Dar, A.H.; Dash, K.K.; Sarkar, A.; Shams, R.; Pandey, V.K.; Khan, S.A.; Amin, Q.A.; Hussain, S.Z. Promising bioactivities of postbiotics: A comprehensive review. J. Agric. Food Res. 2023, 14, 100708. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Roshan, H.; Ghaedi, E.; Rahmani, J.; Barati, M.; Najafi, M.; Karimzedeh, M.; Nikpayam, O. Effects of probiotics and synbiotic supplementation on antioxidant status: A meta-analysis of randomized clinical trials. Clin. Nutr. ESPEN 2019, 30, 81–88. [Google Scholar] [CrossRef]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota–gut–brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef]
- Nambiar, R.B.; Perumal, A.B.; Shittu, T.; Sadiku, E.R.; Sellamuthu, P.S. Probiotics, prebiotics, synbiotics, post-biotics, & paraprobiotics-New perspective for functional foods and nutraceuticals. Front. Nutr. 2023, 10, 1164676. [Google Scholar] [PubMed]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic delivery through fermentation: Dairy vs. non-dairy beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Witkowski, M.; Witkowski, M.; Friebel, J.; Buffa, J.A.; Li, X.S.; Wang, Z.; Sangwan, N.; Li, L.; DiDonato, J.A.; Tizian, C.; et al. Vascular endothelial Tissue Factor contributes to trimethylamine N-oxide enhanced arterial thrombosis. Cardiovasc. Res. 2022, 118, 2367–2384. [Google Scholar] [CrossRef]
- Ahlawat, S.; Sharma, K.K. Gut–organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-biotics, and Post-biotics. Front. Nutr. 2022, 8, 634897. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2022, 12, 184. [Google Scholar] [CrossRef]
- Yeşilyurt, N.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Involvement of Probiotics and Postbiotics in the Immune System Modulation. Biologics 2021, 1, 89–110. [Google Scholar] [CrossRef]
- Isolauri, E.; Sütas, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73, 444s–450s. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Ali, S.A. Probiotics and gut microbiota: Mechanistic insights into gut immune homeostasis through TLR pathway regulation. Food Funct. 2022, 13, 7423–7447. [Google Scholar]
- Boricha, A.A.; Shekh, S.L.; Pithva, S.P.; Ambalam, P.S.; Manuel Vyas, B.R. In vitro evaluation of probiotic properties of Lactobacillus species of food and human origin. LWT 2019, 106, 201–208. [Google Scholar] [CrossRef]
- Asgari, B.; Kermanian, F.; Yaghoobi, M.H.; Vaezi, A.; Soleimanifar, F.; Yaslianifard, S. The Anti-Helicobacter pylori Effects of Lactobacillus acidophilus, L. plantarum, and L. rhamnosus in Stomach Tissue of C57BL/6 Mice. Visc. Med. 2020, 36, 137–143. [Google Scholar] [CrossRef]
- Parmjit, S.P.; Anal, A.K.; Kaur, R. Probiotics, prebiotics and synbiotics: Opportunities, health benefits and industrial challenges. In Probiotics, Prebiotics and Synbiotics: Technological Advancements Towards Safety and Industrial Applications; Panesar, P.S., Anal, A.K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 1–13. [Google Scholar]
- Garmasheva, I.; Kovalenko, N.; Voychuk, S.; Ostapchuk, A.; Livins’ka, O.; Oleschenko, L. Lactobacillus species mediated synthesis of silver nanoparticles and their antibacterial activity against opportunistic pathogens in vitro. Bioimpacts 2016, 6, 219–223. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut–Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Watson, K.S.; Boukhloufi, I.; Bowerman, M.; Parson, S.H. The relationship between body composition, fatty acid metabolism and diet in spinal muscular atrophy. Brain Sci. 2021, 11, 131. [Google Scholar]
- Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L. Nutrition, Physical Activity and Supplementation in Irritable Bowel Syndrome. Nutrients 2023, 15, 3662. [Google Scholar] [CrossRef]
- Kopacz, K.; Phadtare, S. Probiotics for the Prevention of Antibiotic-Associated Diarrhea. Healthcare 2022, 10, 1450. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Probiotics, prebiotics, synbiotics, and fermented foods as potential biotics in nutrition improving health via microbiome-gut-brain axis. Fermentation 2022, 8, 303. [Google Scholar] [CrossRef]
- Massuger, W.; Moore GT, C.; Andrews, J.M.; Kilkenny, M.F.; Reyneke, M.; Knowles, S.; Purcell, L.; Alex, G.; Buckton, S.; Page, A.T. Crohn’s & Colitis Australia inflammatory bowel disease audit: Measuring the quality of care in Australia. Intern. Med. J. 2019, 49, 859–866. [Google Scholar]
- Mills, S.; Rea, M.C.; Lavelle, A.; Ghosh, S.; Hill, C.; Ross, R.P. Interplay between inflammatory bowel disease therapeutics and the gut microbiome reveals opportunities for novel treatment approaches. Microbiome Res. Rep. 2023, 2, 35. [Google Scholar] [CrossRef]
- Ng, S.C.; Kamm, M.A.; Yeoh, Y.K.; Chan, P.K.S.; Zuo, T.; Tang, W.; Sood, A.; Andoh, A.; Ohmiya, N.; Zhou, Y.; et al. Original article: Scientific frontiers in faecal microbiota transplantation: Joint document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific Society for Digestive Endoscopy (APSDE). Gut 2019, 69, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thalmensi, J.; Timperi, E.; Gueguen, P.; Névo, N.; Grisard, E.; Sirven, P.; Cocozza, F.; Gouronnec, A.; Martin-Jaular, L.; et al. Extracellular vesicles from triple negative breast cancer promote pro-inflammatory macrophages associated with better clinical outcome. Proc. Natl. Acad. Sci. USA 2022, 119, e2107394119. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.; Kurek, D.; Ng, C.P.; Queiroz, K. Gut-on-a-Chip Models: Current and Future Perspectives for Host–Microbial Interactions Research. Biomedicines 2023, 11, 619. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, W.; Lan, P.; Mou, X. The microbiome in inflammatory bowel diseases: From pathogenesis to therapy. Protein Cell 2021, 12, 331–345. [Google Scholar]
- Yu, Y.; Wen, H.; Li, S.; Cao, H.; Li, X.; Ma, Z.; She, X.; Zhou, L.; Huang, S. Emerging microfluidic technologies for microbiome research. Front. Microbiol. 2022, 13, 906979. [Google Scholar] [CrossRef]
- Duncanson, W.J.; Lin, T.; Abate, A.R.; Seiffert, S.; Shah, R.K.; Weitz, D.A. Microfluidic Synthesis of Advanced Microparticles for Encapsulation and Controlled Release. Lab Chip 2012, 12, 2134–2135. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A. Probiotic Safety-No Guarantees. JAMA Intern Med. 2018, 178, 1577–1578. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef] [PubMed]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Poindexter, B.; COMMITTEE ON FETUS AND NEWBORN; Cummings, J.; Hand, I.; Adams-Chapman, I.; Aucott, S.W.; Puopolo, K.M.; Goldsmith, J.P.; Kaufman, D.; Martin, C.; et al. Use of Probiotics in Preterm Infants. Pediatrics 2021, 147, e2021051485, Erratum in Pediatrics 2021, 148, e2021054370. [Google Scholar] [CrossRef] [PubMed]
- Bongaerts, G.P.; Severijnen, R.S. A reassessment of the PROPATRIA study and its implications for probiotic therapy. Nat. Bi-otechnol. 2016, 34, 55–63. [Google Scholar] [CrossRef]
- Rozos, G.; Voidarou, C.; Stavropoulou, E.; Skoufos, I.; Tzora, A.; Alexopoulos, A.; Bezirtzoglou, E. Biodiversity and Microbial Resistance of Lactobacilli Isolated From the Traditional Greek Cheese Kopanisti. Front. Microbiol. 2018, 9, 517. [Google Scholar] [CrossRef]
- Theophilus, R.J.; Taft, D.H. Antimicrobial Resistance Genes (ARGs), the Gut Microbiome, and Infant Nutrition. Nutrients 2023, 15, 3177. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Hallowell, H.A.; Koutouvalis, K.; Suez, J. Good microbes, bad genes? The dissemination of antimicrobial resistance in the human microbiome. Gut Microbes 2022, 14, 2055944. [Google Scholar] [CrossRef]
- Impey, S.G.; Jevons, E.; Mees, G.; Cocks, M.; Strauss, J.; Chester, N.; Laurie, I.; Target, D.; Hodgson, A.; Shepherd, S.O.; et al. Glycogen Utilization during Running: Intensity, Sex, and Muscle-Specific Responses. Med. Sci. Sports Exerc. 2020, 52, 1966–1975. [Google Scholar] [CrossRef]
- Stefańska, B.; Sroka, J.; Katzer, F.; Goliński, P.; Nowak, W. The effect of probiotics, phytobiotics and their combination as feed additives in the diet of dairy calves on performance, rumen fermentation and blood metabolites during the preweaning period. Anim. Feed. Sci. Technol. 2021, 272, 114738. [Google Scholar] [CrossRef]
- Pariza, M.W.; Gillies, K.O.; Kraak-Ripple, S.F.; Leyer, G.; Smith, A.B. Determining the safety of microbial cultures for consumption by humans and animals. Regul. Toxicol. Pharmacol. 2015, 73, 164–171. [Google Scholar] [CrossRef] [PubMed]
- D’Agostin, M.; Squillaci, D.; Lazzerini, M.; Barbi, E.; Wijers, L.; Da Lozzo, P. Invasive Infections Associated with the Use of Pro-biotics in Children: A Systematic Review. Children 2021, 8, 924. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Dashnyam, P.; Mudududdla, R.; Hsieh, T.J.; Lin, T.C.; Lin, H.Y.; Chen, P.Y.; Hsu, C.Y.; Lin, C.H. β-Glucuronidases of opportunistic bacteria are the major contributors to xenobiotic-induced toxicity in the gut. Sci. Rep. 2018, 8, 16372. [Google Scholar] [CrossRef]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Miftahussurur, M.; Alshawsh, M.A. Pharmacomicrobiomics: Influence of gut micro-biota on drug and xenobiotic metabolism. FASEB J. 2022, 36, e22350. [Google Scholar] [CrossRef] [PubMed]
- Fendt, R.; Hofmann, U.; Schneider AR, P.; Schaeffeler, E.; Burghaus, R.; Yilmaz, A.; Blank, L.M.; Kerb, R.; Lippert, J.; Schlender, J.; et al. Data-driven personalization of a physiologically based pharmacokinetic model for caffeine: A systematic assessment. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 782–793. [Google Scholar] [CrossRef]
- Candeliere, F.; Raimondi, S.; Ranieri, R.; Musmeci, E.; Zambon, A.; Amaretti, A.; Rossi, M. β-Glucuronidase Pattern Predicted From Gut Metagenomes Indicates Potentially Diversified Pharmacomicrobiomics. Front. Microbiol. 2022, 13, 826994. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Campaniello, D.; Bevilacqua, A.; Speranza, B.; Racioppo, A.; Sinigaglia, M.; Corbo, M.R. A narrative review on the use of probiotics in several diseases. Evidence and perspectives. Front. Nutr. 2023, 10, 1209238. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, A.A.; Da Paixão, G.A.; Santos, D.D.; De Morais, M.A.; De Souza, R.B. Journey of the Probiotic Bacteria: Survival of the Fittest. Microorganisms 2022, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Tagliapietra, B.L.; Flores, A.; Richards, S. In vitro test to evaluate survival in the gastrointestinal tract of commercial probiotics. Curr. Res. Food Sci. 2021, 4, 320–325. [Google Scholar] [CrossRef]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Salazar, N.; Arboleya, S.; Valdés, L.; Stanton, C.; Ross, P.; Ruiz, L.; Gueimonde, M.; de Los Reyes-Gavilán, C.G. The human intestinal microbiome at extreme ages of life. Dietary intervention as a way to counteract alterations. Front. Genet. 2014, 5, 406. [Google Scholar] [CrossRef]
- Takagi, T.; Kunihiro, T.; Takahashi, S.; Hisada, T.; Nagashima, K.; Mochizuki, J.; Mizushima, K.; Naito, Y. A newly developed solution for the preservation of short-chain fatty acids, bile acids, and microbiota in fecal specimens. J. Clin. Biochem. Nutr. 2023, 72, 263–269. [Google Scholar] [CrossRef]

| Term | Description |
|---|---|
| Probiotics | Microorganisms, when ingested in sufficient quantities, offer beneficial effects on host health. |
| Pre-biotics | Undegradable food ingredients that stimulate the growth of intestinal normal microbiota. |
| Synbiotics | A combination of probiotics and prebiotics that work synergistically for a healthy gut. |
| Genus | Species | Reference |
|---|---|---|
| Lactobacillus | Lacticaseibacillus casei (previously named Lactobacillus casei), Lcb. rhamnosus, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, Levilactobacillus brevis (previously named L. brevis), Lactobacillus delbrueckii subsp. lactis (previously named L. lactis), Lactiplantibacillus plantarum subsp. plantarum (previously named L. plantarum), Limosilactobacillus fermentum (previously named L. fermentum) | [46,57,58] |
| Bifidobacterium | B. bifidum, Bifidobacterium lactis, Bifidobacterium adolescentis, B. longum, B. breve, Bifidobacterium animalis | [55,56,57,59] |
| Saccharomyces | Saccharomyces boulardii, Saccharomyces cerevisiae M41, Saccharomyces cerevisiae B-18 | [48,60] |
| Streptococcus | Streptococcus thermophilus | [53,61,62] |
| Escherichia | Escherichia coli Nissle 1917 | [63,64] |
| Bacillus | Bacillus subtilis | [65,66] |
| Enterococcus | Enterococcus faecalis Enterococcus faecium | [67,68] |
| Positive Implications of Microbiota | Adverse Effects of Microbiota |
|---|---|
| Bacterial competition | Transformation of dietary procarcinogens into carcinogens |
| Enhancement of mucosal immunity and preservation of mucosal integrity | Intestinal dysbiosis disorders |
| Sustaining peristalsis and metabolism of dietary carcinogens | Opportunistic infection and gut-derived translocation |
| Production of vitamin K and B complex | |
| Metabolism of prodrugs |
| Metabolites | Functions | References |
|---|---|---|
| Bile acid metabolites | Glucose, lipid and energy metabolism, antimicrobial effects, signal transduction pathways. | [102,115] |
| Phenolic derivatives | Maintenance of intestinal health and protection against oxidative stress. | [83,116,117] |
| Branched-chain fatty acids (BCFA) | Increased histone acetylation. | [118,119] |
| Indole derivatives | Powerful antioxidant; regulation of intestinal barrier function. | [120,121] |
| Ethanol | Protein fermentation metabolite. | [122,123] |
| Polyamines | Intestinal barrier integrity and enhancement of specific immune system. | [14,124] |
| Choline metabolites | Regulation of lipid metabolism and glucose synthesis. | [125,126] |
| Vitamin K and B complex | Erythrocyte formation, DNA replication/repair, enzymatic co-factor. | [127,128] |
| Hydrogen Sulfide (H2S) | Neutralization of singlet reactive oxygen species. | [118,129,130] |
| Parameters | Characteristics | Targeted Ways to Assess |
|---|---|---|
| Safety | Source of Virulence and Pathogenicity. Antibiotic resistance, toxicity, and metabolic activity are all variables in viral pathogenesis | Evaluation of the source or origin is important; for maximum effectiveness in the target species, it is preferable for the agent to have been isolated from within that species. For human consumption, probiotics derived from humans may be preferable. Constant monitoring both before and after release to the public |
| Technological Acceptance | Carrier foods have a high viability retention rate throughout the production and storage Organoleptic qualities that are of acceptable capacity for mass production Containing no phages | Research in vitro and the creation of new foods Model for sensory evaluation, finished goods, and consumer research on product development |
| Functionality | Ability to withstand acidic conditions and enzymes found in gastric juices Acceptance of bile Mucosal adherence and colonization consequences on health that have been shown and demonstrated | Effects on the stomach and bile have been studied using a variety of animal, in vitro, and human models Research on intestine segments, mucus, cell cultures, and animals/humans in vivo Clinical studies verify beneficial effects for health |
| Desirable physiological criteria | Immunomodulation Effects that are hostile to gastrointestinal pathogens Cancer-preventive and mutation-blocking qualities | Research on animals and people in labs and in the wild Pathogen adhesion and competitive exclusion in culture and animal models |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoufou, M.; Tsigalou, C.; Vradelis, S.; Bezirtzoglou, E. The Networked Interaction between Probiotics and Intestine in Health and Disease: A Promising Success Story. Microorganisms 2024, 12, 194. https://doi.org/10.3390/microorganisms12010194
Skoufou M, Tsigalou C, Vradelis S, Bezirtzoglou E. The Networked Interaction between Probiotics and Intestine in Health and Disease: A Promising Success Story. Microorganisms. 2024; 12(1):194. https://doi.org/10.3390/microorganisms12010194
Chicago/Turabian StyleSkoufou, Maria, Christina Tsigalou, Stergios Vradelis, and Eugenia Bezirtzoglou. 2024. "The Networked Interaction between Probiotics and Intestine in Health and Disease: A Promising Success Story" Microorganisms 12, no. 1: 194. https://doi.org/10.3390/microorganisms12010194
APA StyleSkoufou, M., Tsigalou, C., Vradelis, S., & Bezirtzoglou, E. (2024). The Networked Interaction between Probiotics and Intestine in Health and Disease: A Promising Success Story. Microorganisms, 12(1), 194. https://doi.org/10.3390/microorganisms12010194








