Genome Analysis for Cholesterol-Lowing Action and Bacteriocin Production of Lactiplantibacillus plantarum WLPL21 and ZDY04 from Traditional Chinese Fermented Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Genome Sequencing and Annotation
2.3. Phylogenetic Analysis
2.4. Identification of Bacteriocin-Encoding Genes
2.5. CRISPR Analysis
2.6. Probiotic Characteristics
2.7. Statistical Analysis
3. Results
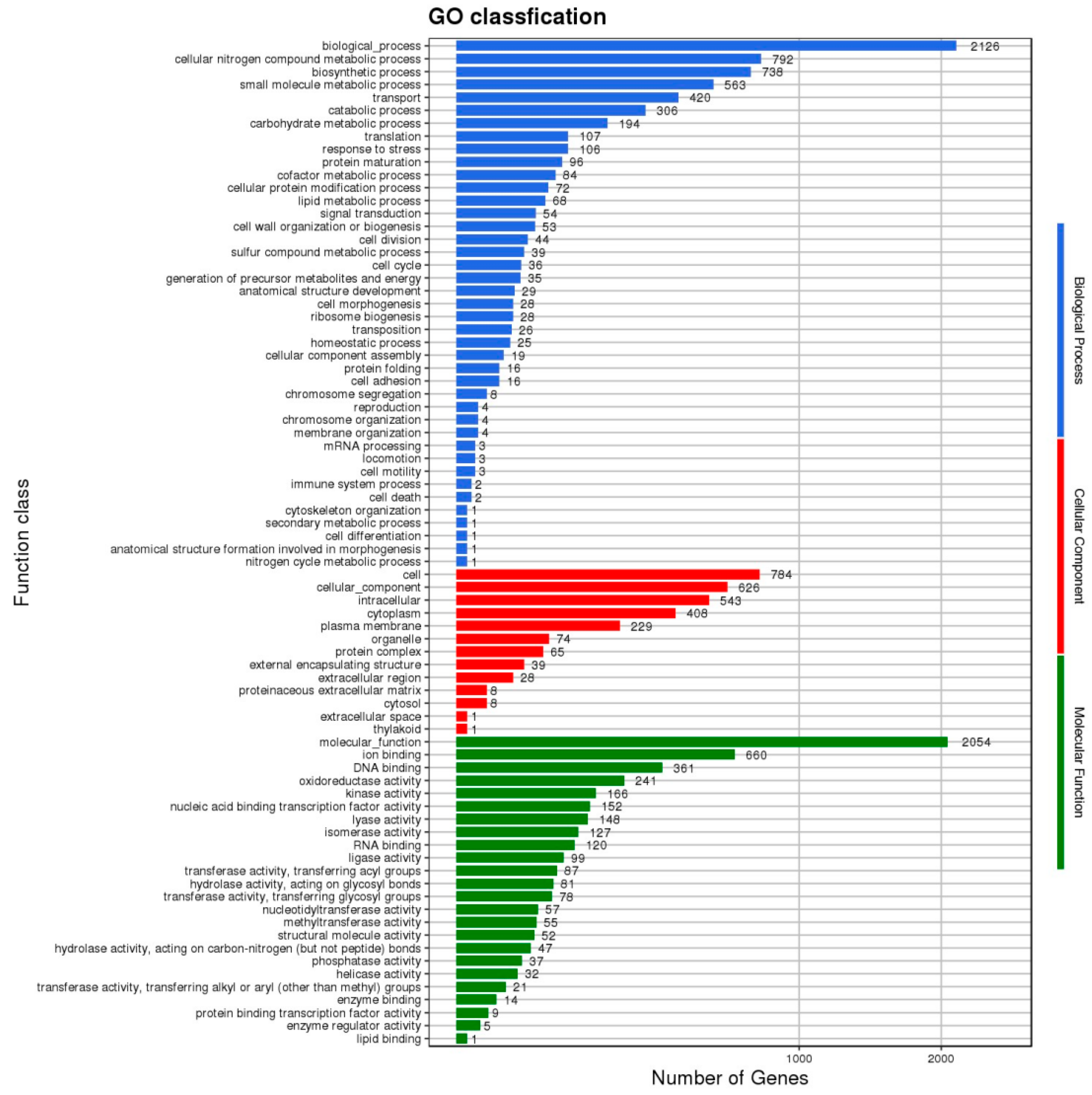
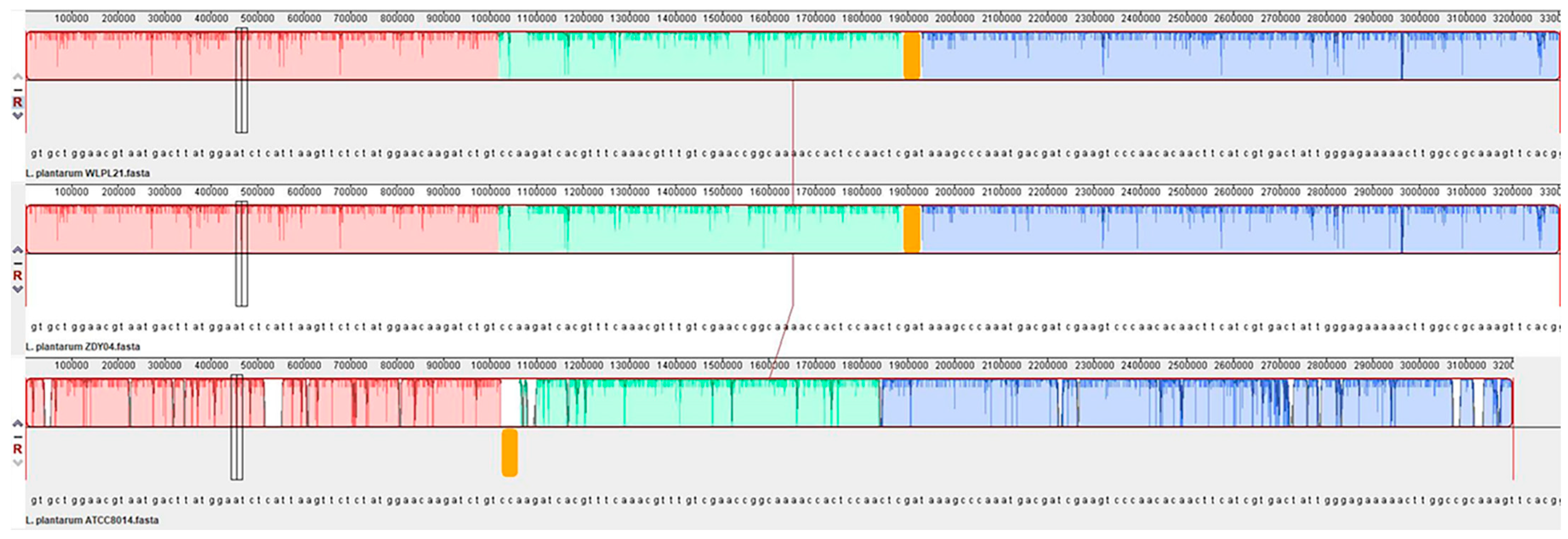
3.1. Genome Analysis of L. plantarum
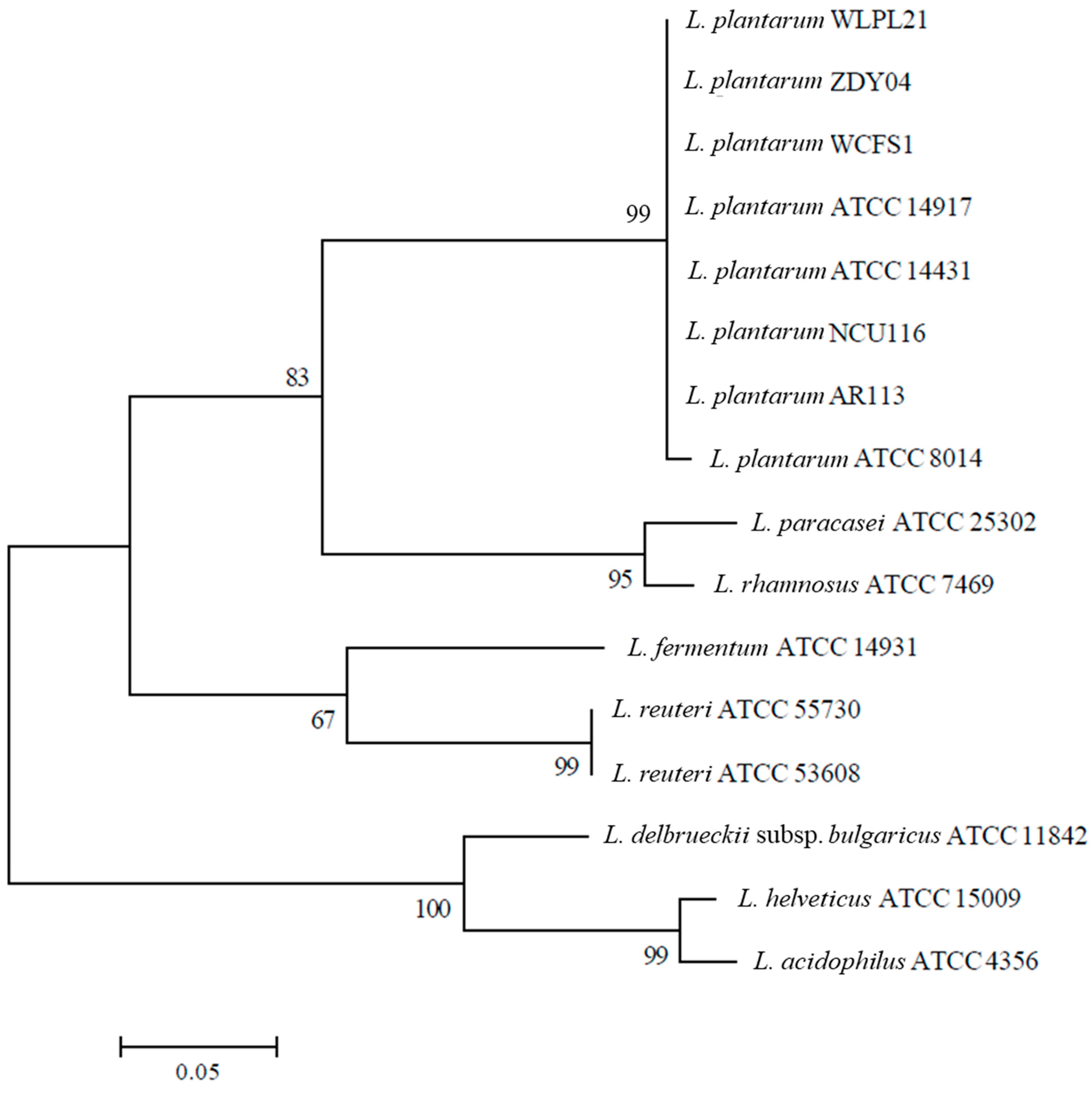
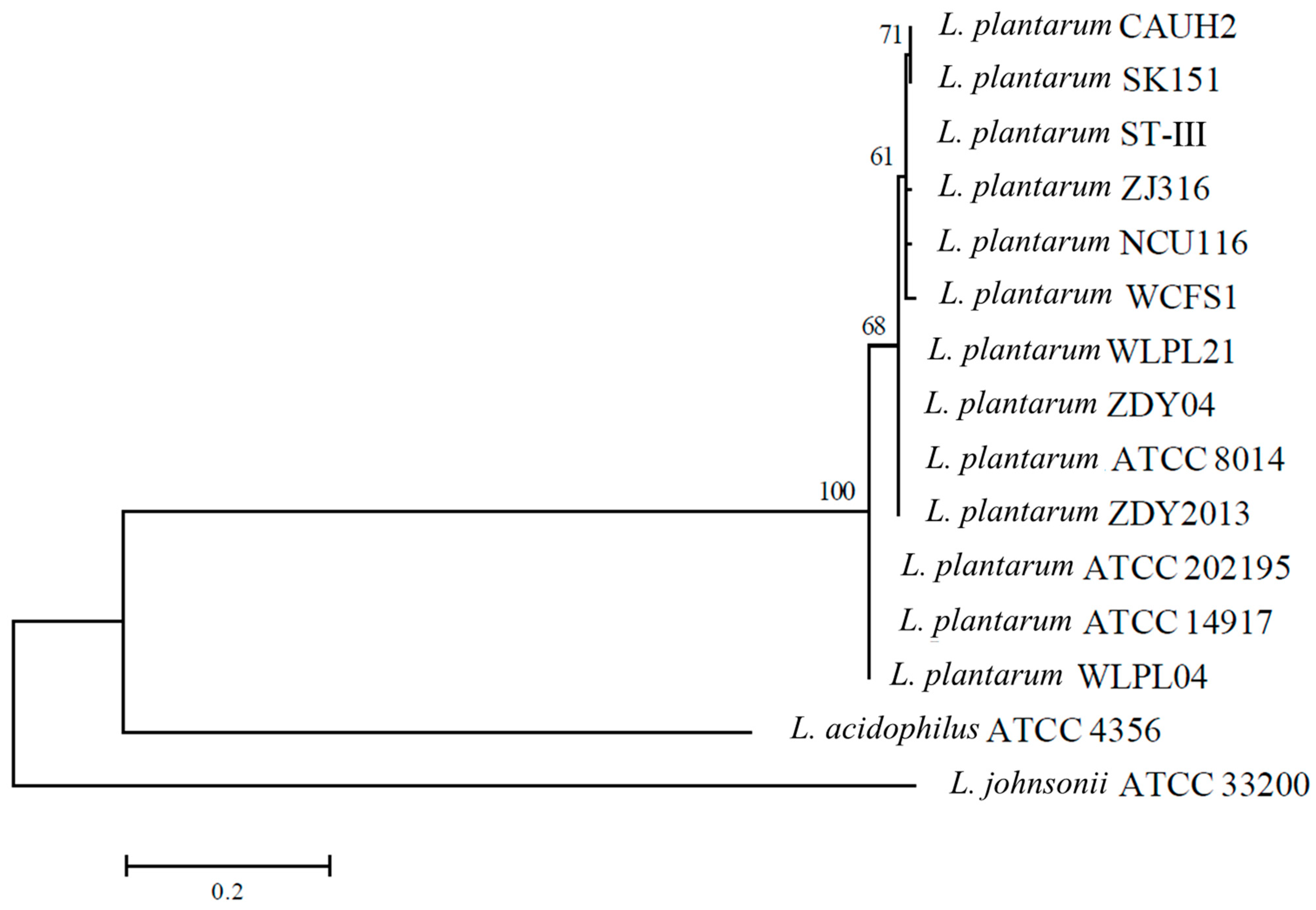
3.2. Phylogenetic Analysis
3.3. Transport System
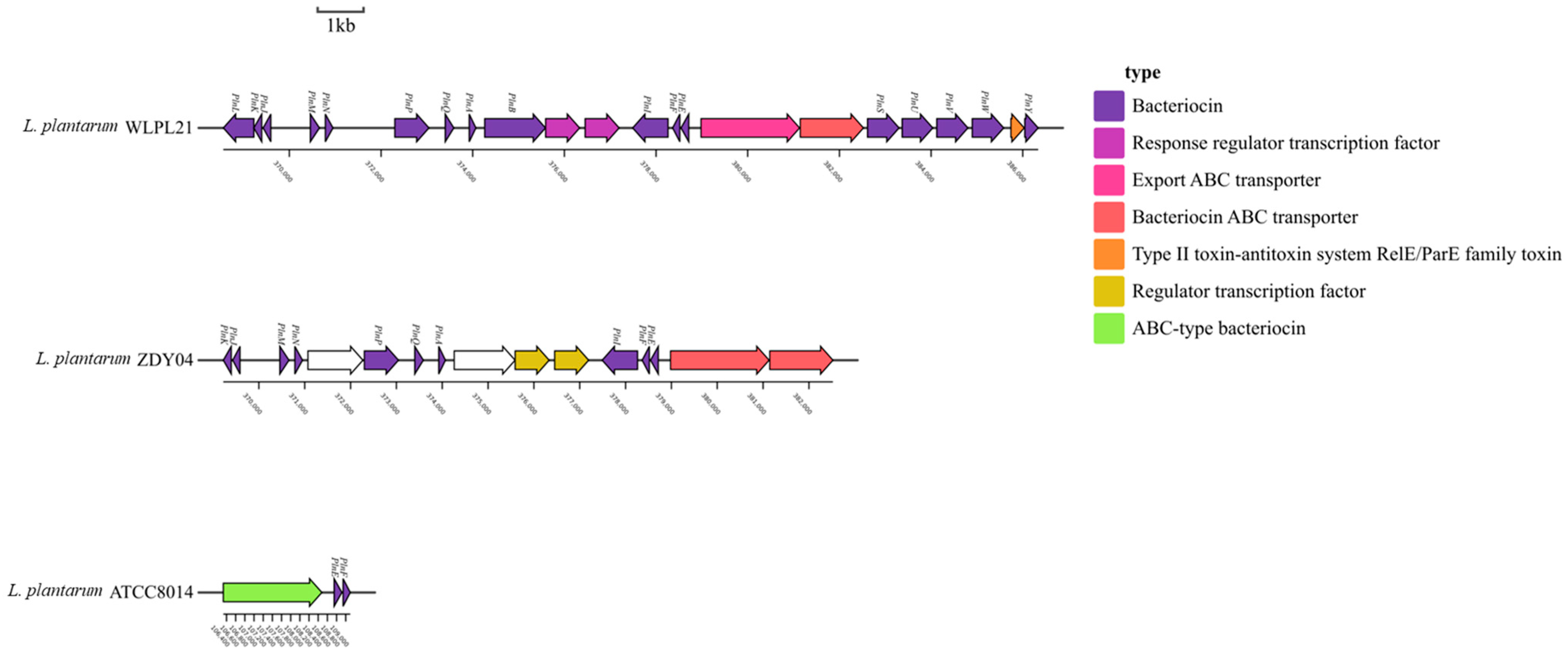
3.4. Bacteriocin Production
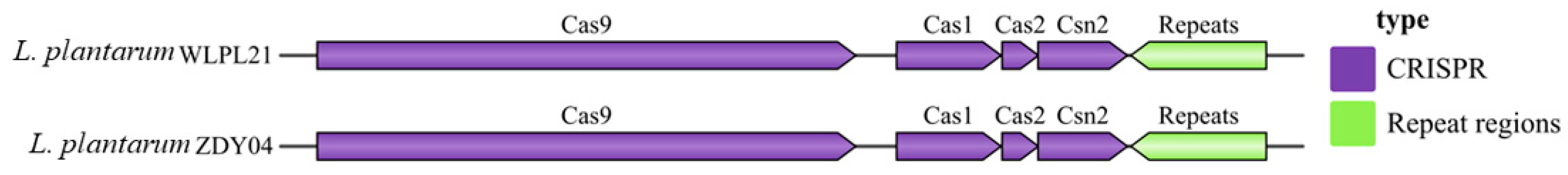
3.5. CRISPR Analysis
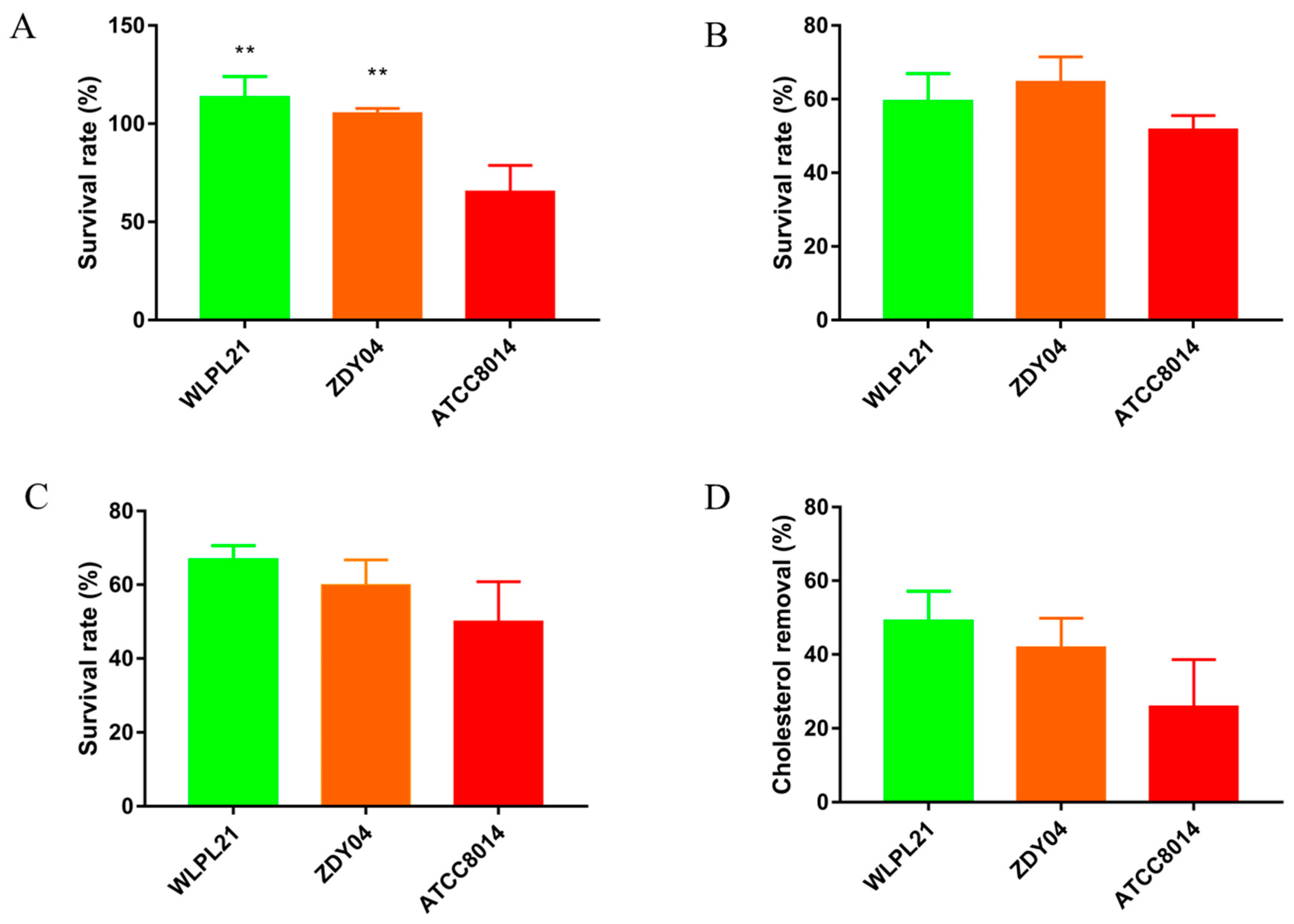
3.6. Probiotic Characteristics In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ryu, J.A.; Kim, E.; Yang, S.M.; Lee, S.; Yoon, S.R.; Jang, K.S.; Kim, H.Y. High-throughput sequencing of the microbial community associated with the physicochemical properties of meju (dried fermented soybean) and doenjang (traditional Korean fermented soybean paste). LWT 2021, 146, 111473. [Google Scholar] [CrossRef]
- Tamang, J.P.; Anupma, A.; Shangpliang, H.N.J. Ethno-microbiology of Tempe, an Indonesian fungal-fermented soybean food and Koji, a Japanese fungal starter culture. Curr. Opin. Food Sci. 2022, 48, 100912. [Google Scholar] [CrossRef]
- Cheng, S.; Li, H.; Huang, Y.; Su, Y.; Li, Y.; Jia, A.; Jiang, Y.; Zhang, Y.; Man, C. Lactobacillus gasseri JM1 isolated from infant feces alleviates colitis in mice via protecting the intestinal barrier. Nutrients 2022, 15, 139. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Feng, N.; Zhang, C.; Liu, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Lactobacillus reuteri A9 and Lactobacillus mucosae A13 isolated from Chinese superlongevity people modulate lipid metabolism in a hypercholesterolemia rat model. FEMS Microbio. Lett. 2019, 366, fnz254. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, L.; Wen, R.; Chen, Q.; Kong, B. Role of lactic acid bacteria in flavor development in traditional Chinese fermented foods: A review. Crit. Rev. Food Sci. 2022, 62, 2741–2755. [Google Scholar] [CrossRef] [PubMed]
- Valledor, S.J.D.; Dioso, C.M.; Bucheli, J.E.V.; Park, Y.J.; Suh, D.H.; Jung, E.S.; Kim, B.; Holzapfel, W.H.; Todorov, S.D. Characterization and safety evaluation of two beneficial, enterocin-producing Enterococcus faecium strains isolated from kimchi, a Korean fermented cabbage. Food Microbiol. 2022, 102, 103886. [Google Scholar] [CrossRef]
- Liu, C.J.; Wang, R.; Gong, F.M.; Liu, X.F.; Zheng, H.J.; Luo, Y.Y.; Li, X.R. Complete genome sequences and comparative genome analysis of Lactobacillus plantarum strain 5-2 isolated from fermented soybean. Genomics 2015, 106, 404–411. [Google Scholar] [CrossRef]
- Somashekaiah, R.; Mottawea, W.; Gunduraj, A.; Joshi, U.; Hammami, R.; Sreenivasa, M.Y. Probiotic and antifungal Attributes of Levilactobacillus brevis MYSN105, isolated from an Indian traditional fermented food Pozha. Front. Microbiol. 2021, 12, 696267. [Google Scholar] [CrossRef]
- Garcia, N.; Bottacini, F.; Van, S.D.; Gahan, C.G.M.; Corsetti, A. Comparative Genomics of Lactiplantibacillus plantarum: Insights into probiotic markers in strains isolated from the human gastrointestinal tract and fermented foods. Front. Microbiol. 2022, 13, 854266. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, Y.; Park, H.; Kang, H.J.; Ji, Y.; Holzapfel, W.H. Lactobacillus johnsonii BFE6154 ameliorates diet-induced hypercholesterolemia. Probiotics Antimicrob. Proteins 2023, 15, 451–459. [Google Scholar] [CrossRef]
- Hassan, A.; Din, A.U.; Zhu, Y.; Zhang, K.; Li, T.H.; Wang, Y.; Xu, S.C.; Lei, H.K.; Yu, X.; Wang, G.X. Anti-atherosclerotic effects of Lactobacillus plantarum ATCC 14917 in ApoE−/− mice through modulation of proinflammatory cytokines and oxidative stress. Appl. Microbio. Biot. 2020, 104, 6337–6350. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.Y.; Ren, W.B.; Wang, P.P.; Zheng, L. Lactobacillus rhamnosus GG protects against atherosclerosis by improving ketone body synthesis. Appl. Microbio. Biot. 2022, 106, 8233–8243. [Google Scholar] [CrossRef] [PubMed]
- El-Dein, A.N.; El-Deen, A.M.N.; El-Shatoury, E.H.; Awad, G.A.; Ibrahim, M.K.; Awad, H.M.; Farid, M.A. Assessment of exopolysaccharides, bacteriocins and in vitro and in vivo hypocholesterolemic potential of some Egyptian Lactobacillus spp. Int. J. Biol. Macromol. 2021, 173, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Jitpakdee, J.; Kantachote, D.; Kanzaki, H.; Nitoda, T. Potential of lactic acid bacteria to produce functional fermented whey beverage with putative health promoting attributes. LWT 2022, 160, 113269. [Google Scholar] [CrossRef]
- Liu, L.; Li, P.L. Complete genome sequence of Lactobacillus paraplantarum L-ZS9, a probiotic starter producing class II bacteriocins. J. Biotechnol. 2016, 222, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Halami, P.M.; Tamang, J.P. Genome analysis of Lactobacillus plantarum isolated from some indian fermented foods for Bacteriocin production and probiotic marker genes. Front. Microbiol. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Upendra, R.S.; Khandelwal, P.; Ahmed, M.R. Bacteriocin production optimization applying RSM and hybrid (ANN-GA) method for the indigenous culture of Pediococcus pentosaceus Sanna 14. J. Appl. Pharm. Sci. 2021, 11, 050–060. [Google Scholar] [CrossRef]
- Zhao, K.; Qiu, L.; He, Y.; Tao, X.Y.; Zhang, Z.H.; Wei, H. Alleviation syndrome of high-cholesterol-diet-induced hypercholesterolemia in mice by intervention with Lactiplantibacillus plantarum WLPL21 via regulation of cholesterol metabolism and transportation as well as gut microbiota. Nutrients 2023, 15, 2600. [Google Scholar] [CrossRef]
- Surachat, K.; Sangket, U.; Deachamag, P.; Chotigeat, W. In silico analysis of protein toxin and bacteriocins from Lactobacillus paracasei SD1 genome and available online databases. PLoS ONE 2017, 12, e0183548. [Google Scholar] [CrossRef]
- Lindgreen, S. AdapterRemoval: Easy cleaning of next-generation sequencing reads. BMC Res. Notes 2012, 5, 337. [Google Scholar] [CrossRef]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. GigaScience 2012, 1, 18. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Coil, D.; Jospin, G.; Darling, A.E. A5-miseq: An updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics 2014, 31, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.D.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Delcher, A.L.; Harmon, D.; Kasif, S.; White, O.; Salzberg, S.L. Improved microbial gene identification with GLIMMER. Nucleic. Acids Res. 1999, 27, 4636–4641. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic. Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Lagesen, K.; Hallin, P.; Rodland, E.A.; Stærfeldt, H.H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic. Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Burge, S.W.; Daub, J.; Eberhardt, R.; Tate, J.; Barquist, L.; Nawrocki, E.P.; Eddy, S.R.; Gardner, P.P.; Bateman, A. Rfam 11.0: 10 years of RNA families. Nucleic. Acids Res. 2013, 41, D226–D232. [Google Scholar] [CrossRef]
- Bland, C.; Ramsey, T.L.; Sabree, F.; Lowe, M.; Brown, K.; Kyrpides, N.C.; Hugenholtz, P. CRISPR recognition tool (CRT): A tool for automatic detection of clustered regularly interspaced palindromic repeats. Bioinformatics 2007, 8, 1–8. [Google Scholar] [CrossRef]
- Blake, J.D.; Cohen, F.E. Pairwise sequence alignment below the twilight zone. J. Mol. Biol. 2001, 307, 721–735. [Google Scholar] [CrossRef]
- Conesa, A.; Stefan, G. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int. J. Plant Sci. 2008, 2008, 619832. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic. Acids Res. 2007, 35 (Suppl. 2), W182–W185. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.; Forslund, K.; Szklarczyk, D.; Trachana, K.; Roth, A.; Huerta-Cepas, J.; Gabaldón, T.; Rattei, T.; Creevey, C.; Kuhn, M.; et al. eggNOG v4. 0: Nested orthology inference across 3686 organisms. Nucleic. Acids Res. 2014, 42, D231–D239. [Google Scholar] [CrossRef] [PubMed]
- Stothard, P.; Wishart, D.S. Circular genome visualization and exploration using CGView. Bioinformatics 2005, 21, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.F.; Zou, K.X.; Zhan, Y.; Cai, Y.J.; Zhang, Z.H.; Tao, X.Y.; Qiu, L.; Wei, H. Enterococcus faecium GEFA01 alleviates hypercholesterolemia by promoting reverse cholesterol transportation via modulating the gut microbiota-SCFA axis. Front. Nutr. 2022, 9, 1020734. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.G.; Kim, K.W.; Yi, H. Subspecies-level genome comparison of Lactobacillus delbrueckii. Sci. Rep. 2023, 13, 3171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Z.L.; Zhang, S.Q.; Li, P.L. Complete genome sequencing revealed the potential application of a novel Weizmannia coagulans PL-W production with promising bacteriocins in food preservative. Foods 2023, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, S.; Allonsius, C.N.; Wittouck, S.; Thys, S.; Lievens, B.; Weckx, S.; De Vuyst, L.; Sarah, L. Comparative genome analysis of Lactobacillus mudanjiangensis, an understudied member of the Lactobacillus plantarum group. Microb. Genom. 2019, 5, e000286. [Google Scholar] [CrossRef]
- Surachat, K.; Kantachote, D.; Deachamag, P.; Wonglapsuwan, M. Genomic insight into pediococcus acidilactici HN9, a potential probiotic strain isolated from the traditional Thai-style fermented beef Nhang. Microorganisms 2021, 9, 50. [Google Scholar] [CrossRef]
- Yu, X.H.; Zhang, D.W.; Zheng, X.L.; Tang, C.K. Cholesterol transport system: An integrated cholesterol transport model involved in atherosclerosis. Prog. Lipid Res. 2019, 73, 65–91. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Zhang, D.Y.; Liu, H.; Wang, S.X.; Wang, Y.M.; Ji, H.F. Complete genome sequencing and comparative genome characterization of Lactobacillus johnsonii ZLJ010, a potential probiotic with health-promoting properties. Front. Genet. 2019, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.H.; Choi, S.H.; Park, S.W.; Choi, N.J.; Kim, Y.; Kim, S.H. Synbiotic impact of tagatose on viability of Lactobacillus rhamnosus strain GG mediated by the phosphotransferase system (PTS). Food Microbiol. 2013, 36, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Multiple bacteriocin production in lactic acid bacteria. J. Biosci. Bioeng. 2022, 134, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Guo, L.D.; Zhang, X.Y.; Mu, H.Q.; Sha, S.F.; Lin, Y.; Hou, H.W.; Wang, L.P. Whole-genome sequencing combined with mass spectrometry to identify bacteriocin and mine silent genes. LWT 2022, 169, 113975. [Google Scholar] [CrossRef]
- Shabbir, I.; Al-Asmari, F.; Saima, H.; Nadeem, M.T.; Ambreen, S.; Kasankala, L.M.; Khalid, M.Z.; Rahim, M.A.; Özogul, F.; Bartkiene, E.; et al. The Biochemical, Microbiological, Antioxidant and Sensory Characterization of Fermented Skimmed Milk Drinks Supplemented with Probiotics Lacticaseibacillus casei and Lacticaseibacillus rhamnosus. Microorganisms 2023, 11, 2523. [Google Scholar] [CrossRef]
- Nami, Y.; Bakhshayesh, R.V.; Manafi, M.; Hejazi, M.A. Hypocholesterolaemic activity of a novel autochthonous potential probiotic Lactobacillus plantarum YS5 isolated from yogurt. LWT 2019, 111, 876–882. [Google Scholar] [CrossRef]
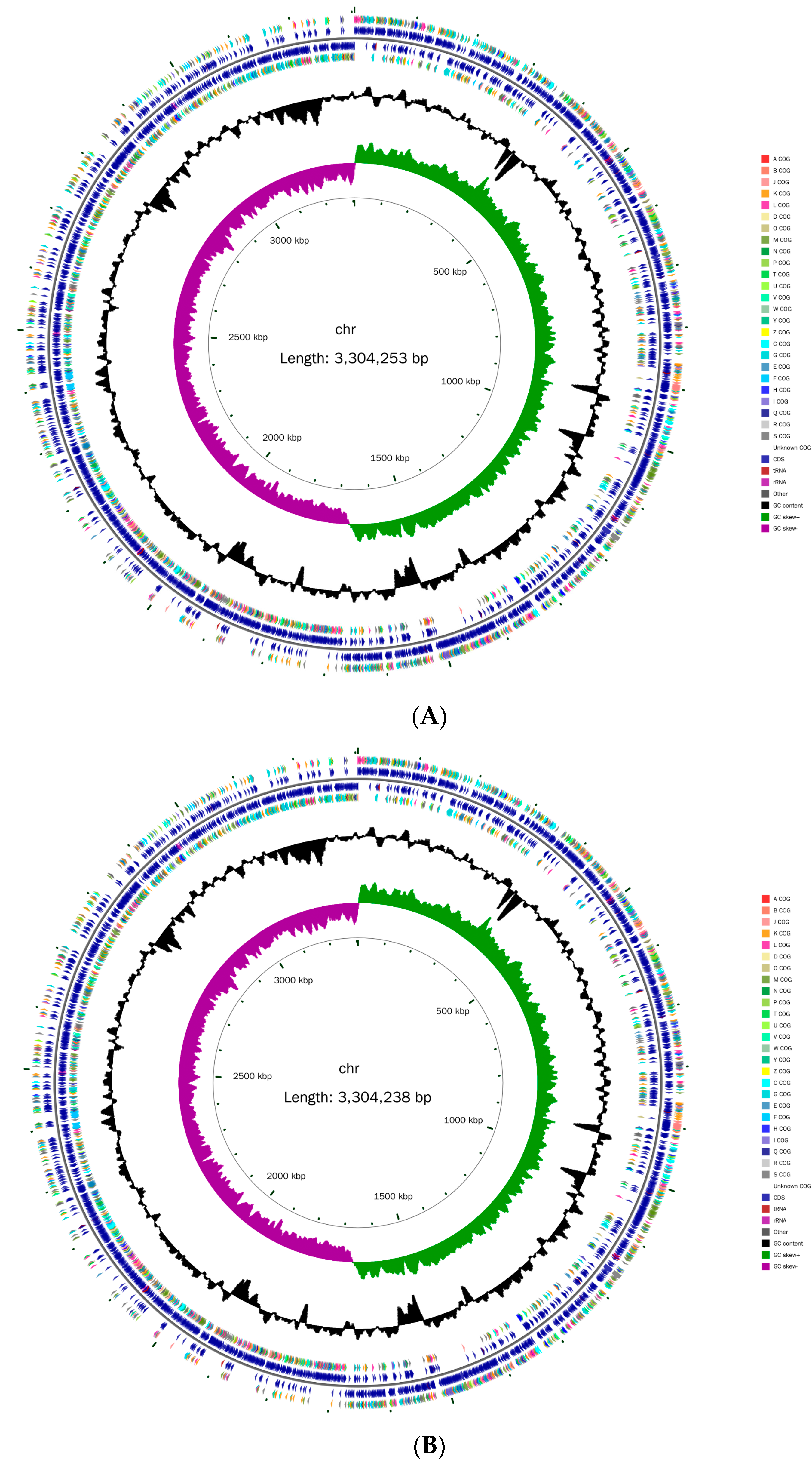
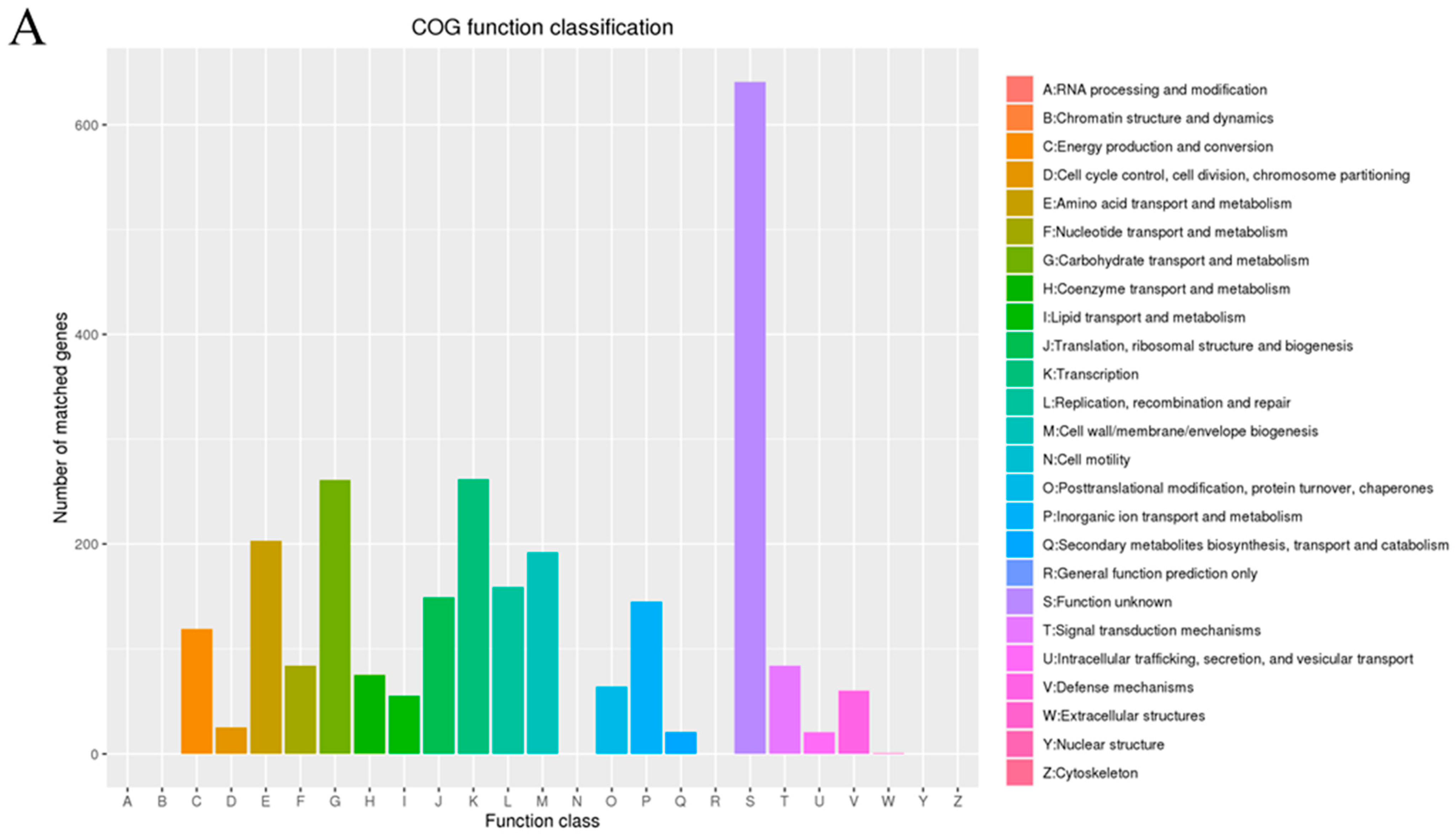
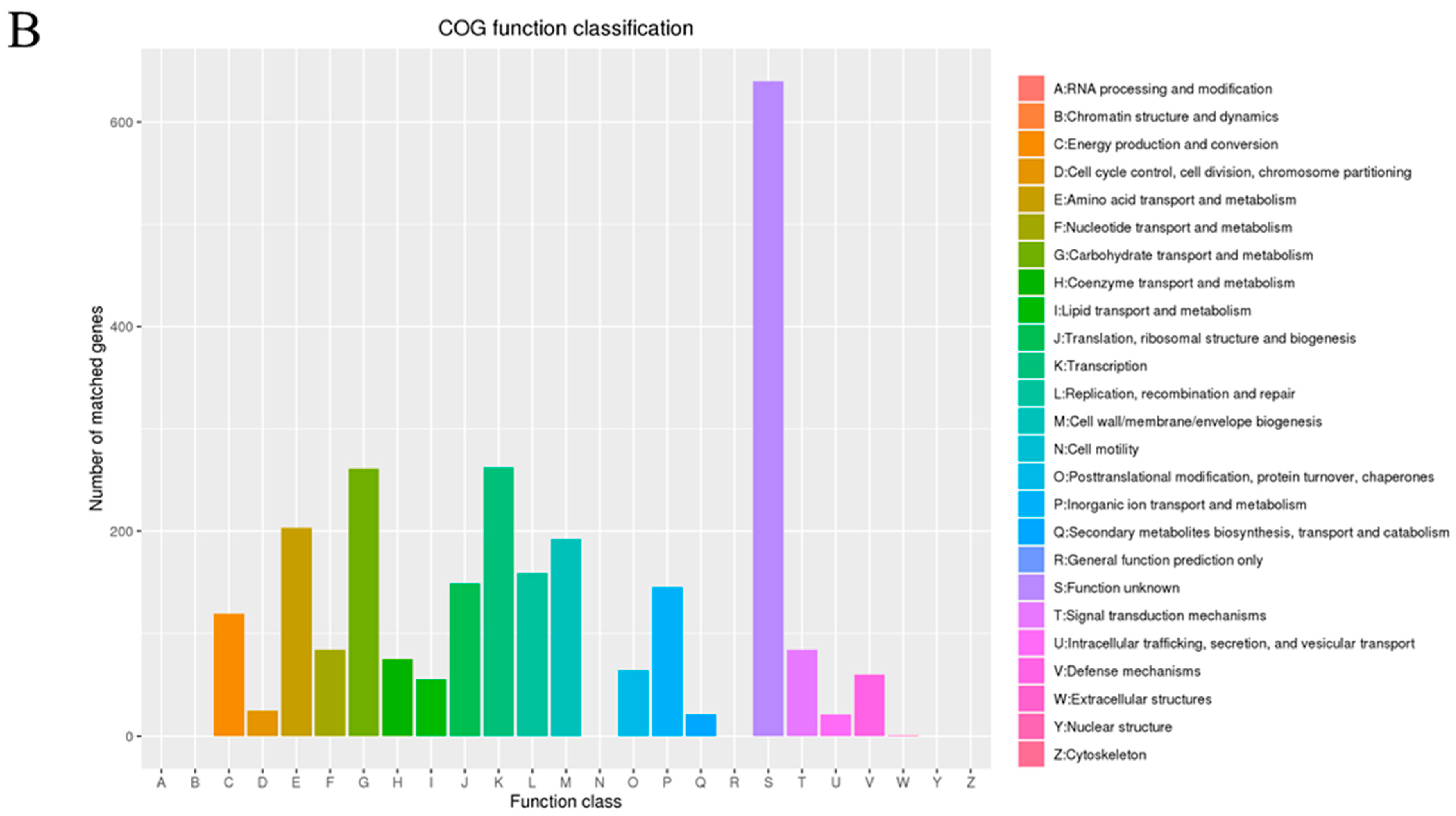
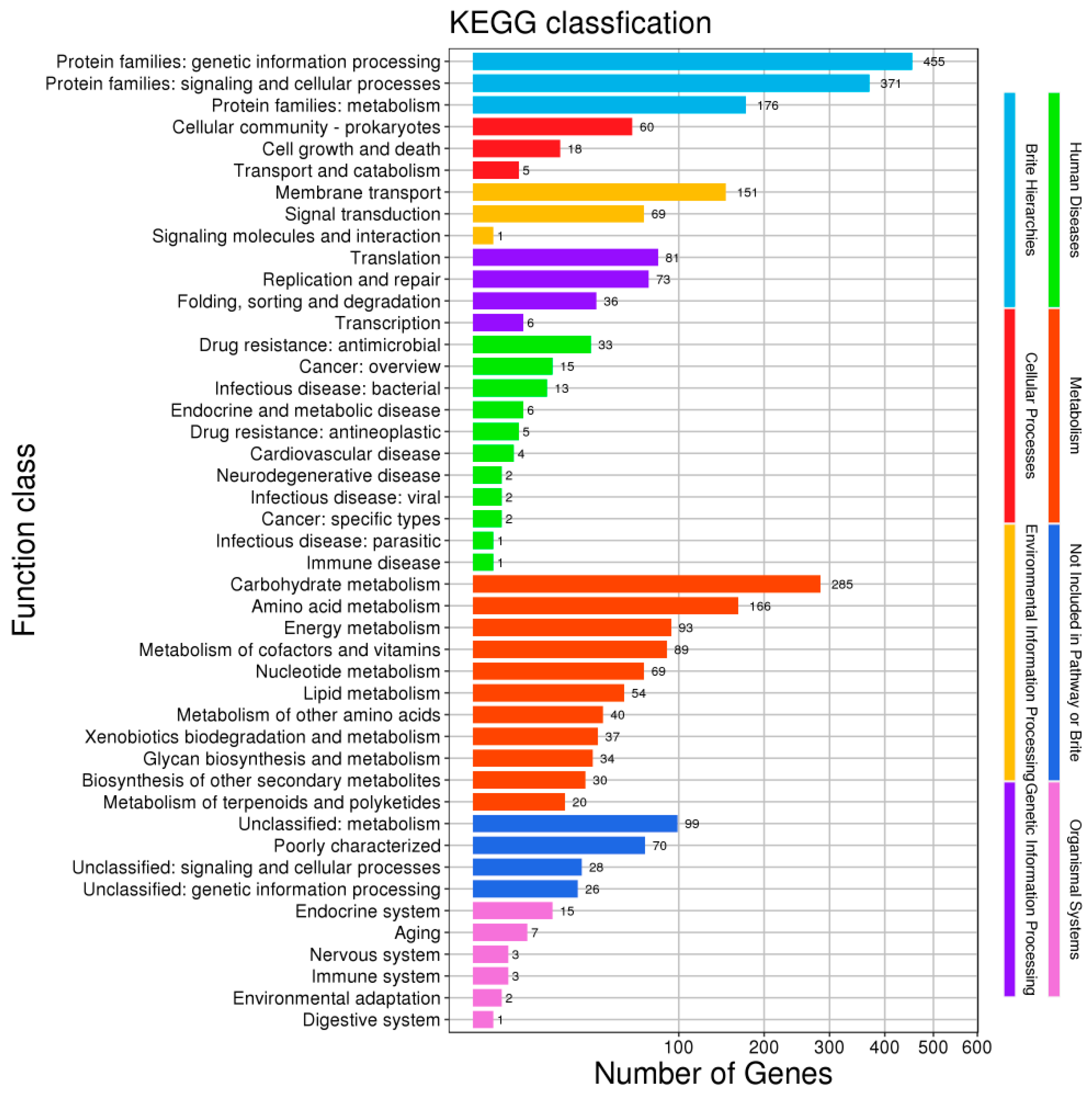
| Strain | Lactiplantibacillus plantarum | ||
| WLPL21 | ZDY04 | ATCC8014 | |
| Genomic size | 3,304,253 | 3,304,238 | 3,202,808 |
| G + C content | 44.56% | 44.56% | 44.43% |
| Number of ORFs | 3072 | 3075 | 3029 |
| Number of tRNA | 65 | 65 | 66 |
| Number of rRNA | 16 | 16 | 13 |
| Number of nc RNA | 61 | 61 | 4 |
| Number of repeat regions | 3 | 3 | 0 |
| Strain | Lactiplantibacillus plantarum | |
|---|---|---|
| WLPL21/ZDY04 | ATCC8014 | |
| Transport system genes | 386 | 232 |
| ABC transport system genes | 191 | 137 |
| Betaine/proline/choline family ABC transporter ATP-binding protein gene | 1 | 1 |
| Peptide ABC transport genes | 19 | 7 |
| Energy-coupling factor ABC transporter genes | 6 | 4 |
| Sugar ABC transporter genes | 9 | 4 |
| ABC transporter ATP-binding protein genes | 71 | 49 |
| Amino acid ABC transporter genes | 24 | 13 |
| Phosphotransferase system (PTS) genes | 65 | 17 |
| pln Genes | Lactiplantibacillus plantarum | ||
|---|---|---|---|
| WLPL21 | ZDY04 | ATCC8014 | |
| plnA | + | + | - |
| plnB | + | - | - |
| plnE | + | + | + |
| plnF | + | + | + |
| plnI | + | + | - |
| plnJ | + | + | - |
| plnK | - | + | - |
| plnM | + | + | - |
| plnN | + | + | - |
| plnP | + | + | - |
| plnQ | + | + | - |
| plnS | + | - | - |
| plnU | + | - | - |
| plnV | + | - | - |
| plnW | + | - | - |
| plnY | + | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Qiu, L.; Tao, X.; Zhang, Z.; Wei, H. Genome Analysis for Cholesterol-Lowing Action and Bacteriocin Production of Lactiplantibacillus plantarum WLPL21 and ZDY04 from Traditional Chinese Fermented Foods. Microorganisms 2024, 12, 181. https://doi.org/10.3390/microorganisms12010181
Zhao K, Qiu L, Tao X, Zhang Z, Wei H. Genome Analysis for Cholesterol-Lowing Action and Bacteriocin Production of Lactiplantibacillus plantarum WLPL21 and ZDY04 from Traditional Chinese Fermented Foods. Microorganisms. 2024; 12(1):181. https://doi.org/10.3390/microorganisms12010181
Chicago/Turabian StyleZhao, Kui, Liang Qiu, Xueying Tao, Zhihong Zhang, and Hua Wei. 2024. "Genome Analysis for Cholesterol-Lowing Action and Bacteriocin Production of Lactiplantibacillus plantarum WLPL21 and ZDY04 from Traditional Chinese Fermented Foods" Microorganisms 12, no. 1: 181. https://doi.org/10.3390/microorganisms12010181
APA StyleZhao, K., Qiu, L., Tao, X., Zhang, Z., & Wei, H. (2024). Genome Analysis for Cholesterol-Lowing Action and Bacteriocin Production of Lactiplantibacillus plantarum WLPL21 and ZDY04 from Traditional Chinese Fermented Foods. Microorganisms, 12(1), 181. https://doi.org/10.3390/microorganisms12010181





