Concurrent Brain Subregion Microgliosis in an HLA-II Mouse Model of Group A Streptococcal Skin Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. GAS Bacteria and In Vivo Infection
2.3. GAS Burden in the Blood, Brain, Skin, and the Subcutaneous Adipose Tissue
2.4. Assessment of Cytokines in the Skin and the Brain Homogenates
2.5. Western Blot Detection of the Glial Fibrillary Acidic Protein (GFAP) and Ionized Calcium-Binding Adaptor-1 (Iba-1) Protein
2.6. GAS and Iba-1 Immunoreactivity
2.7. Image Acquisition
2.8. Statistical Analysis
3. Results
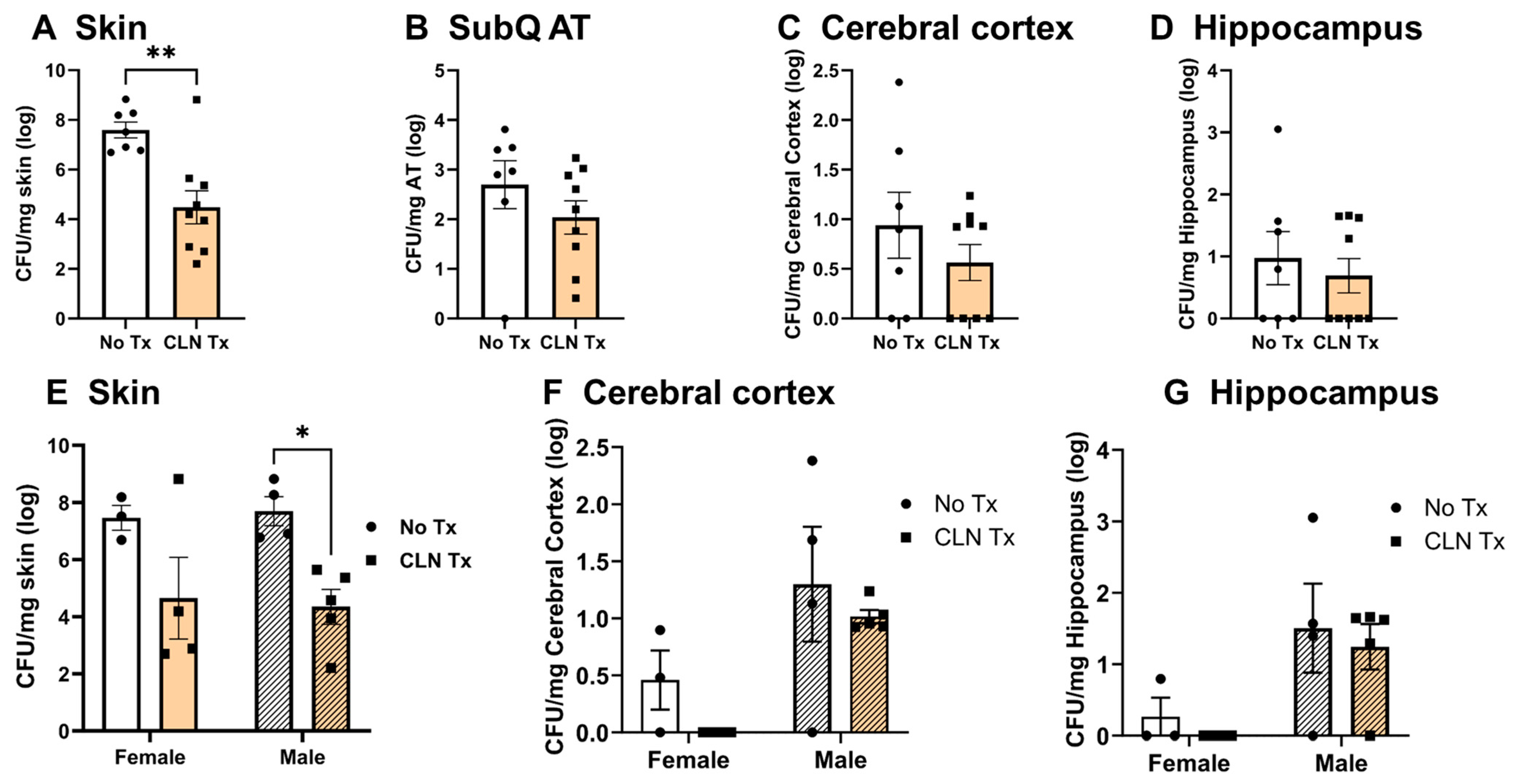
3.1. Clindamycin Treatment Significantly Reduced Weight Loss and Lesion Areas following Subcutaneous GAS Infection
3.2. Heterogeneity in GAS Burden in the Skin, Subcutaneous Adipose Tissue, and the Brain
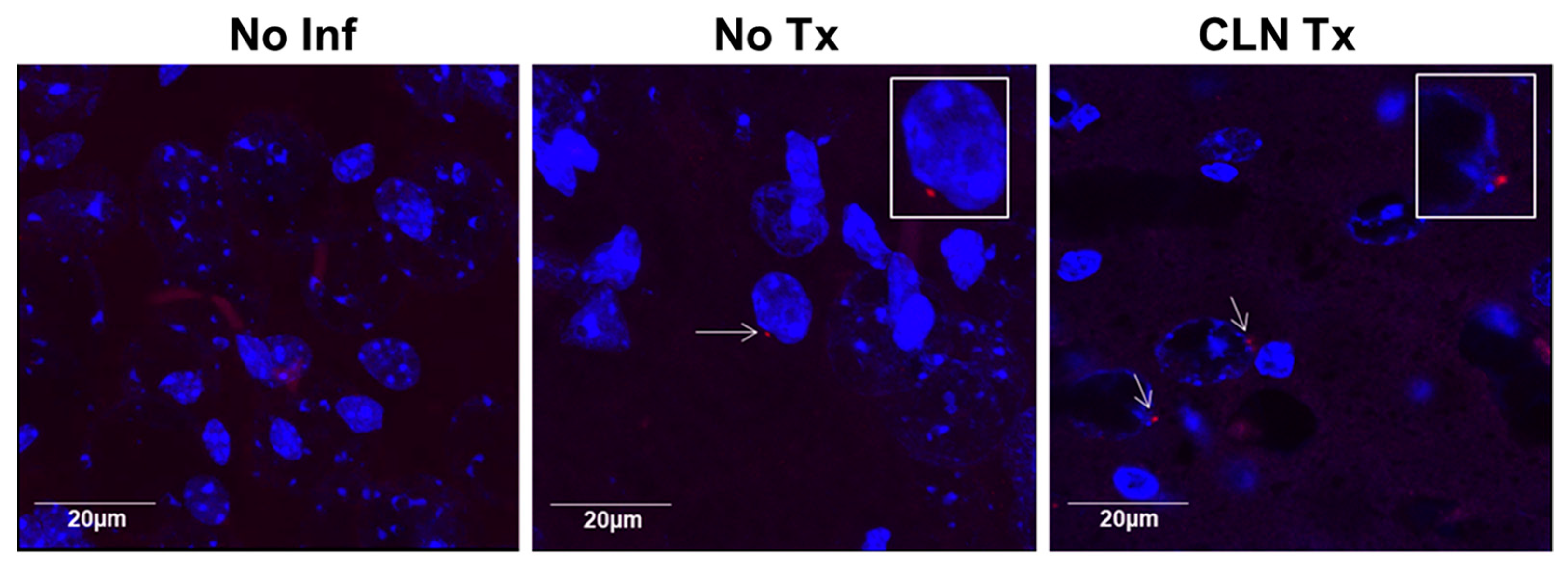
3.3. GAS Bacteria Localize to the Brain
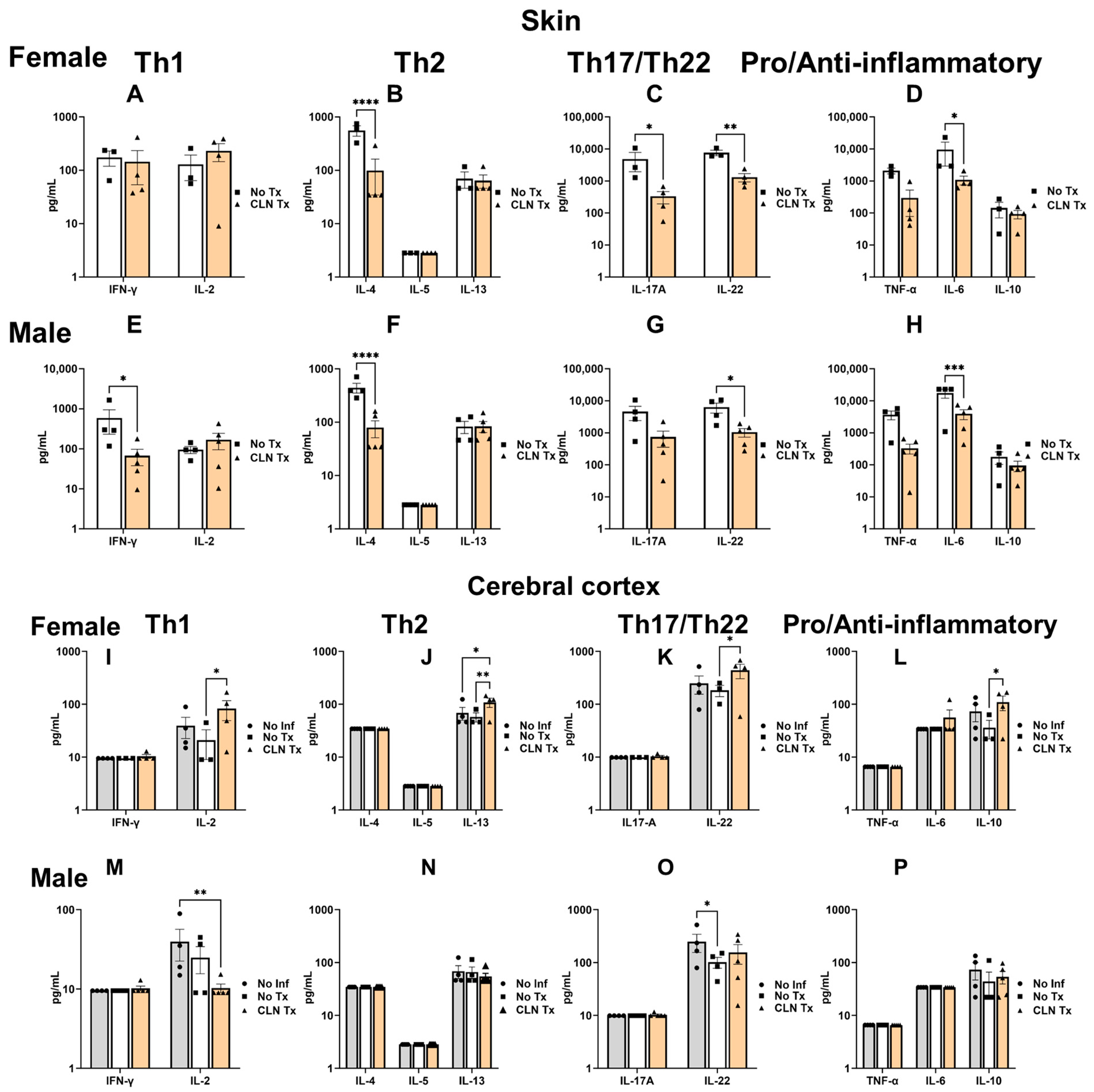
3.4. Clindamycin Treatment Significantly Altered the Cytokine Profile in the Skin and Cerebral Cortex
3.5. Subcutaneous GAS Infection Increased Cortical and Hippocampal Iba-1 Protein Levels
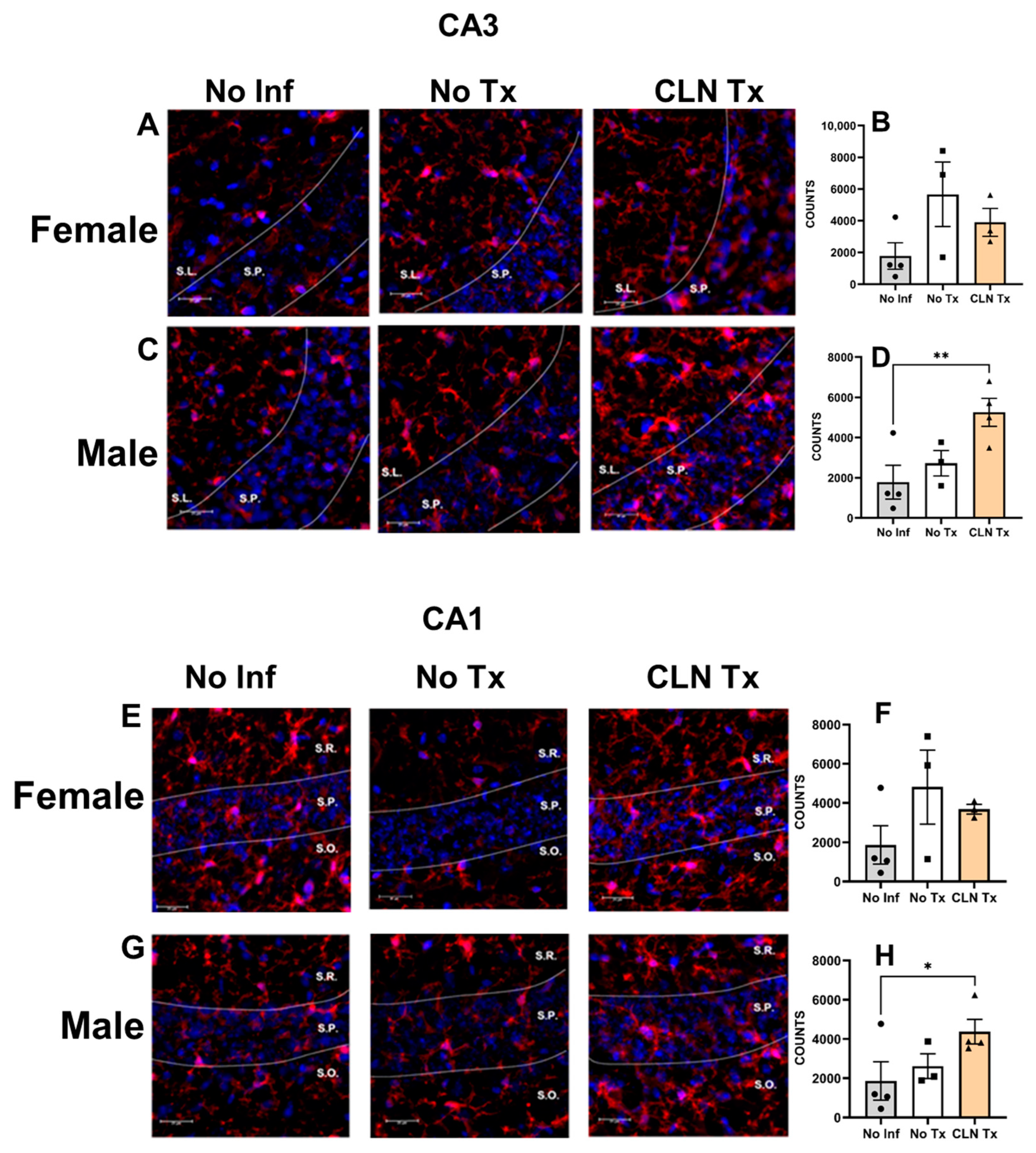
3.6. Subcutaneous GAS Infection Increased Iba-1 Immunoreactivity in Male Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunningham, M.W. Pathogenesis of Group A Streptococcal Infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Kotb, M. Rise and Persistence of Global M1T1 Clone of Streptococcus Pyogenes. Emerg. Infect. Dis. 2008, 14, 1511–1517. [Google Scholar] [CrossRef]
- Henningham, A.; Barnett, T.C.; Maamary, P.G.; Walker, M.J. Pathogenesis of Group A Streptococcal Infections. Discov. Med. 2012, 13, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, R.; Rosenheinrich, M.; Kreikemeyer, B.; Oehmcke-Hecht, S. Streptococcus Pyogenes Triggers Activation of the Human Contact System by Streptokinase. Infect. Immun. 2015, 83, 3035–3042. [Google Scholar] [CrossRef] [PubMed]
- Kotb, M.; Norrby-Teglund, A.; McGeer, A.; El-Sherbini, H.; Dorak, M.T.; Khurshid, A.; Green, K.; Peeples, J.; Wade, J.; Thomson, G.; et al. An Immunogenetic and Molecular Basis for Differences in Outcomes of Invasive Group A Streptococcal Infections. Nat. Med. 2002, 8, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Nooh, M.M.; Nookala, S.; Kansal, R.; Kotb, M. Individual Genetic Variations Directly Effect Polarization of Cytokine Responses to Superantigens Associated with Streptococcal Sepsis: Implications for Customized Patient Care. J. Immunol. 2011, 186, 3156–3163. [Google Scholar] [CrossRef]
- Kotb, M.; Norrby-Teglund, A.; McGeer, A.; Green, K.; Low, D.E. Association of Human Leukocyte Antigen with Outcomes of Infectious Diseases: The Streptococcal Experience. Scand. J. Infect. Dis. 2003, 35, 665–669. [Google Scholar] [CrossRef]
- Bessen, D.E. Tissue Tropisms in Group A Streptococcus: What Virulence Factors Distinguish Pharyngitis from Impetigo Strains? Curr. Opin. Infect. Dis. 2016, 29, 295–303. [Google Scholar] [CrossRef]
- Nookala, S.; Mukundan, S.; Fife, A.; Alagarsamy, J.; Kotb, M. Heterogeneity in FoxP3- and GARP/LAP-Expressing T Regulatory Cells in an HLA Class II Transgenic Murine Model of Necrotizing Soft Tissue Infections by Group A Streptococcus. Infect. Immun. 2018, 86, 10–1128. [Google Scholar] [CrossRef]
- Cutforth, T.; Demille, M.M.C.; Agalliu, I.; Agalliu, D. CNS Autoimmune Disease after Streptococcus Pyogenes Infections: Animal Models, Cellular Mechanisms and Genetic Factors. Future Neurol. 2016, 11, 63–76. [Google Scholar] [CrossRef]
- Martino, D.; Defazio, G.; Giovannoni, G. The PANDAS Subgroup of Tic Disorders and Childhood-Onset Obsessive-Compulsive Disorder. J. Psychosom. Res. 2009, 67, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.; Chiarotti, F.; Buttiglione, M.; Cardona, F.; Creti, R.; Nardocci, N.; Orefici, G.; Veneselli, E.; Rizzo, R. The Relationship between Group A Streptococcal Infections and Tourette Syndrome: A Study on a Large Service-Based Cohort. Dev. Med. Child Neurol. 2011, 53, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.K.; Petitto, J.M.; Voeller, K.K.; Goodman, W.K. Obsessive Compulsive Disorder: Is There an Association with Childhood Streptococcal Infections and Altered Immune Function? Semin. Clin. Neuropsychiatry 2001, 6, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Fujinami, R.S.; White, H.S.; Preux, P.M.; Blümcke, I.; Sander, J.W.; Löscher, W. Infections, Inflammation and Epilepsy. Acta Neuropathol. 2016, 131, 211–234. [Google Scholar] [CrossRef]
- Nielsen, H.; Storgaard, M.; Helweg-Larsen, J.; Larsen, L.; Jepsen, M.P.G.; Hansen, B.R.; Wiese, L.; Bodilsen, J. Group A Streptococcus Meningitis in Adults, Denmark. Emerg. Infect. Dis. 2023, 29, 1937–1939. [Google Scholar] [CrossRef]
- Dehority, W.; Uchiyama, S.; Khosravi, A.; Nizet, V. Brain Abscess Caused by Streptococcus Pyogenes in a Previously Healthy Child. J. Clin. Microbiol. 2006, 44, 4613–4615. [Google Scholar] [CrossRef]
- Hayashi, A.; Takano, T.; Suzuki, A.; Narumiya, S. Group A Streptococcal Brain Abscess: A Case Report and a Review of the Literature since 1988. Scand. J. Infect. Dis. 2011, 43, 553–555. [Google Scholar] [CrossRef]
- Sheeler, C.; Rosa, J.G.; Ferro, A.; McAdams, B.; Borgenheimer, E.; Cvetanovic, M. Glia in Neurodegeneration: The Housekeeper, the Defender and the Perpetrator. Int. J. Mol. Sci. 2020, 21, 9188. [Google Scholar] [CrossRef]
- Vainchtein, I.D.; Molofsky, A.V. Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 2020, 43, 144–154. [Google Scholar] [CrossRef]
- Nooh, M.M.; El-Gengehi, N.; Kansal, R.; David, C.S.; Kotb, M. HLA Transgenic Mice Provide Evidence for a Direct and Dominant Role of HLA Class II Variation in Modulating the Severity of Streptococcal Sepsis. J. Immunol. 2007, 178, 3076–3083. [Google Scholar] [CrossRef]
- Ascough, S.; Ingram, R.J.; Chu, K.K.; Reynolds, C.J.; Musson, J.A.; Doganay, M.; Metan, G.; Ozkul, Y.; Baillie, L.; Sriskandan, S.; et al. Anthrax Lethal Factor as an Immune Target in Humans and Transgenic Mice and the Impact of HLA Polymorphism on CD4+ T Cell Immunity. PLoS Pathog. 2014, 10, e1004085. [Google Scholar] [CrossRef] [PubMed]
- Nabozny, G.H.; Baisch, J.M.; Cheng, S.; Cosgrove, D.; Griffiths, M.M.; Luthra, H.S.; David, C.S. HLA-DQ8 Transgenic Mice Are Highly Susceptible to Collagen-Induced Arthritis: A Novel Model for Human Polyarthritis. J. Exp. Med. 1996, 183, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Taneja, V.; David, C.S. Role of HLA Class II Genes in Susceptibility/Resistance to Inflammatory Arthritis: Studies with Humanized Mice. Immunol. Rev. 2010, 233, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Taneja, V.; Taneja, N.; Paisansinsup, T.; Behrens, M.; Griffiths, M.; Luthra, H.; David, C.S. CD4 and CD8 T Cells in Susceptibility/Protection to Collagen-Induced Arthritis in HLA-DQ8-Transgenic Mice: Implications for Rheumatoid Arthritis. J. Immunol. 2002, 168, 5867–5875. [Google Scholar] [CrossRef]
- Germundson, D.L.; Nookala, S.; Smith, N.A.; Warda, Y.; Nagamoto-Combs, K. HLA-II Alleles Influence Physical and Behavioral Responses to a Whey Allergen in a Transgenic Mouse Model of Cow’s Milk Allergy. Front. Allergy 2022, 3, 870513. [Google Scholar] [CrossRef]
- Neeno, T.; Krco, C.J.; Harders, J.; Baisch, J.; Cheng, S.; David, C.S. HLA-DQ8 Transgenic Mice Lacking Endogenous Class II Molecules Respond to House Dust Allergens: Identification of Antigenic Epitopes. J. Immunol. 1996, 156, 3191–3195. [Google Scholar] [CrossRef]
- Pavelko, K.D.; Drescher, K.M.; McGavern, D.B.; David, C.S.; Rodriguez, M. HLA-DQ Polymorphism Influences Progression of Demyelination and Neurologic Deficits in a Viral Model of Multiple Sclerosis. Mol. Cell. Neurosci. 2000, 15, 495–509. [Google Scholar] [CrossRef][Green Version]
- Mangalam, A.K.; Luo, N.; Luckey, D.; Papke, L.; Hubbard, A.; Wussow, A.; Smart, M.; Giri, S.; Rodriguez, M.; David, C. Absence of IFN-γ Increases Brain Pathology in Experimental Autoimmune Encephalomyelitis–Susceptible DRB1*0301.DQ8 HLA Transgenic Mice through Secretion of Proinflammatory Cytokine IL-17 and Induction of Pathogenic Monocytes/Microglia into the Central Nerv. J. Immunol. 2014, 193, 4859–4870. [Google Scholar] [CrossRef]
- Mangalam, A.; Luckey, D.; Basal, E.; Jackson, M.; Smart, M.; Rodriguez, M.; David, C. HLA-DQ8 (DQB1*0302)-Restricted Th17 Cells Exacerbate Experimental Autoimmune Encephalomyelitis in HLA-DR3-Transgenic Mice. J. Immunol. 2009, 182, 5131–5139. [Google Scholar] [CrossRef]
- Ambigapathy, G.; Mukundan, S.; Nagamoto-Combs, K.; Combs, C.K.; Nookala, S. HLA-II-Dependent Neuroimmune Changes in Group A Streptococcal Necrotizing Fasciitis. Pathogens 2023, 12, 1000. [Google Scholar] [CrossRef]
- Knopick, P.; Terman, D.; Riha, N.; Alvine, T.; Larson, R.; Badiou, C.; Lina, G.; Ballantyne, J.; Bradley, D. Endogenous HLA-DQ8αβ Programs Superantigens (SEG/SEI) to Silence Toxicity and Unleash a Tumoricidal Network with Long-Term Melanoma Survival. J. Immunother. Cancer 2020, 8, e001493. [Google Scholar] [CrossRef] [PubMed]
- Chatellier, S.; Ihendyane, N.; Kansal, R.G.; Khambaty, F.; Basma, H.; Norrby-Teglund, A.; Low, D.E.; McGeer, A.; Kotb, M. Genetic Relatedness and Superantigen Expression in Group A Streptococcus Serotype M1 Isolates from Patients with Severe and Nonsevere Invasive Diseases. Infect. Immun. 2000, 68, 3523–3534. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.C.; Mukundan, S.; Alagarsamy, J.; Laturnus, D.; Kotb, M. Host Genetic Variations and Sex Differences Potentiate Predisposition, Severity, and Outcomes of Group A Streptococcus-Mediated Necrotizing Soft Tissue Infections. Infect. Immun. 2016, 84, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Chella Krishnan, K.; Mukundan, S.; Alagarsamy, J.; Hur, J.; Nookala, S.; Siemens, N.; Svensson, M.; Hyldegaard, O.; Norrby-Teglund, A.; Kotb, M. Genetic Architecture of Group A Streptococcal Necrotizing Soft Tissue Infections in the Mouse. PLoS Pathog. 2016, 12, e1005732. [Google Scholar] [CrossRef]
- Tang, G.; Xu, Z.; Goldman, J.E. Synergistic Effects of the SAPK/JNK and the Proteasome Pathway on Glial Fibrillary Acidic Protein (GFAP) Accumulation in Alexander Disease. J. Biol. Chem. 2006, 281, 38634–38643. [Google Scholar] [CrossRef]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.È.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia Are an Essential Component of the Neuroprotective Scar That Forms after Spinal Cord Injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Seung, H.S. Trainable Weka Segmentation: A Machine Learning Tool for Microscopy Pixel Classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef]
- Talukdar, S.N.; Osan, J.; Ryan, K.; Grove, B.; Perley, D.; Kumar, B.D.; Yang, S.; Dallman, S.; Hollingsworth, L.; Bailey, K.L.; et al. RSV-Induced Expanded Ciliated Cells Contribute to Bronchial Wall Thickening. Virus Res. 2023, 327, 199060. [Google Scholar] [CrossRef]
- Seeger, D.R.; Golovko, S.A.; Grove, B.D.; Golovko, M.Y. Cyclooxygenase Inhibition Attenuates Brain Angiogenesis and Independently Decreases Mouse Survival under Hypoxia. J. Neurochem. 2021, 158, 246–261. [Google Scholar] [CrossRef]
- Schwendy, M.; Unger, R.E.; Bonn, M.; Parekh, S.H. Automated Cell Segmentation in FIJI® Using the DRAQ5 Nuclear Dye. BMC Bioinformatics 2019, 20, 39. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, A.B.; Hennessy, E.; Murray, C.L.; Nazmi, A.; Delaney, H.J.; Healy, D.; Fagan, S.G.; Rooney, M.; Stewart, E.; Lewis, A.; et al. Acute Systemic Inflammation Exacerbates Neuroinflammation in Alzheimer’s Disease: IL-1β Drives Amplified Responses in Primed Astrocytes and Neuronal Network Dysfunction. Alzheimer’s Dement. 2021, 17, 1735–1755. [Google Scholar] [CrossRef] [PubMed]
- Geyer, S.; Jacobs, M.; Hsu, N.J. Immunity against Bacterial Infection of the Central Nervous System: An Astrocyte Perspective. Front. Mol. Neurosci. 2019, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Korzhevskii, D.E.; Kirik, O.V. Brain Microglia and Microglial Markers. Neurosci. Behav. Physiol. 2016, 46, 284–290. [Google Scholar] [CrossRef]
- Bhusal, A.; Nam, Y.; Seo, D.; Lee, W.H.; Suk, K. Cathelicidin-Related Antimicrobial Peptide Negatively Regulates Bacterial Endotoxin-Induced Glial Activation. Cells 2022, 11, 3886. [Google Scholar] [CrossRef]
- Singer, B.H.; Dickson, R.P.; Denstaedt, S.J.; Newstead, M.W.; Kim, K.; Falkowski, N.R.; Erb-Downward, J.R.; Schmidt, T.M.; Huffnagl, G.B.; Standiford, T.J. Bacterial Dissemination to the Brain in Sepsis. Am. J. Respir. Crit. Care Med. 2018, 197, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Eiche, T.P.; Mohajeri, M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14, 2661. [Google Scholar] [CrossRef] [PubMed]
- Isaiah, S.; Loots, D.T.; Solomons, R.; van der Kuip, M.; Tutu Van Furth, A.M.; Mason, S. Overview of Brain-to-Gut Axis Exposed to Chronic CNS Bacterial Infection(s) and a Predictive Urinary Metabolic Profile of a Brain Infected by Mycobacterium Tuberculosis. Front. Neurosci. 2020, 14, 296. [Google Scholar] [CrossRef]
- Bottasso, O.; Bay, M.L.; Besedovsky, H.; del Rey, A. Adverse Neuro-Immune-Endocrine Interactions in Patients with Active Tuberculosis. Mol. Cell. Neurosci. 2013, 53, 77–85. [Google Scholar] [CrossRef]
- Zager, A.; Andersen, M.L.; Lima, M.M.S.; Reksidler, A.B.; Machado, R.B.; Tufik, S. Modulation of Sickness Behavior by Sleep: The Role of Neurochemical and Neuroinflammatory Pathways in Mice. Eur. Neuropsychopharmacol. 2009, 19, 589–602. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wu, P.H.; Kang, C.C.; Tsai, Y.S.; Chou, C.K.; Liang, C.T.; Wu, J.J.; Tsai, P.J. Group A Streptococcus Subcutaneous Infection-Induced Central Nervous System Inflammation Is Attenuated by Blocking Peripheral TNF. Front. Microbiol. 2019, 10, 265. [Google Scholar] [CrossRef]
- Pillai, A.; Thomas, S.; Williams, C. Clindamycin in the Treatment of Group G β-Haemolytic Streptococcal Infections. J. Infect. 2005, 51, e207–e211. [Google Scholar] [CrossRef]
- Andreoni, F.; Zürcher, C.; Tarnutzer, A.; Schilcher, K.; Neff, A.; Keller, N.; Marques Maggio, E.; Poyart, C.; Schuepbach, R.A.; Zinkernagel, A.S.; et al. Clindamycin Affects Group a Streptococcus Virulence Factors and Improves Clinical Outcome. J. Infect. Dis. 2017, 215, 269–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunningham, M.W.; Cox, C.J. Autoimmunity against Dopamine Receptors in Neuropsychiatric and Movement Disorders: A Review of Sydenham Chorea and Beyond. Acta Physiol. 2016, 216, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Hiltensperger, M. Role of IL-6 in the Commitment of T Cell Subsets. Cytokine 2021, 146, 155654. [Google Scholar] [CrossRef] [PubMed]
- Laho, D.; Blumental, S.; Botteaux, A.; Smeesters, P.R. Invasive Group A Streptococcal Infections: Benefit of Clindamycin, Intravenous Immunoglobulins and Secondary Prophylaxis. Front. Pediatr. 2021, 9, 697938. [Google Scholar] [CrossRef]
- Avire, N.J.; Whiley, H.; Ross, K. A Review of Streptococcus Pyogenes: Public Health Risk Factors, Prevention and Control. Pathogens 2021, 10, 248. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Marriott, I. The Interleukin-10 Family of Cytokines and Their Role in the CNS. Front. Cell. Neurosci. 2018, 12, 458. [Google Scholar] [CrossRef]
- Ottolenghi, A.; Bolel, P.; Sarkar, R.; Greenshpan, Y.; Iraqi, M.; Ghosh, S.; Bhattacharya, B.; Taylor, Z.V.; Kundu, K.; Radinsky, O.; et al. Life-Extended Glycosylated IL-2 Promotes Treg Induction and Suppression of Autoimmunity. Sci. Rep. 2021, 11, 7676. [Google Scholar] [CrossRef]
- Harris, F.; Berdugo, Y.A.; Tree, T. IL-2-Based Approaches to Treg Enhancement. Clin. Exp. Immunol. 2023, 211, 149–163. [Google Scholar] [CrossRef]
- Ciccia, F.; Guggino, G.; Rizzo, A.; Saieva, L.; Peralta, S.; Giardina, A.; Cannizzaro, A.; Sireci, G.; De Leo, G.; Alessandro, R.; et al. Type 3 Innate Lymphoid Cells Producing IL-17 and IL-22 Are Expanded in the Gut, in the Peripheral Blood, Synovial Fluid and Bone Marrow of Patients with Ankylosing Spondylitis. Ann. Rheum. Dis. 2015, 74, 1739–1747. [Google Scholar] [CrossRef]
- Liu, C.; Yang, J. Enteric Glial Cells in Immunological Disorders of the Gut. Front. Cell. Neurosci. 2022, 16, 895871. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Zhang, Y.; Zuloaga, K.; Yang, Q. The Role of Innate Lymphocytes in Regulating Brain and Cognitive Function. Neurobiol. Dis. 2023, 179, 106061. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhan, H.; Liang, Y.; He, Q.; Cui, D. Role of Th22 Cells in Human Viral Diseases. Front. Med. 2021, 8, 708140. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, L.; Zhu, C.; Zhang, M.; Liang, C. Current Knowledge of Th22 Cell and IL-22 Functions in Infectious Diseases. Pathogens 2023, 12, 176. [Google Scholar] [CrossRef]
- Liang, S.C.; Tan, X.Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 Are Coexpressed by Th17 Cells and Cooperatively Enhance Expression of Antimicrobial Peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Thorsdottir, S.; Henriques-Normark, B.; Iovino, F. The Role of Microglia in Bacterial Meningitis: Inflammatory Response, Experimental Models and New Neuroprotective Therapeutic Strategies. Front. Microbiol. 2019, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Afridi, R.; Kim, J.-H.; Rahman, M.H.; Suk, K. Metabolic Regulation of Glial Phenotypes: Implications in Neuron–Glia Interactions and Neurological Disorders. Front. Cell. Neurosci. 2020, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Joh, T.H. Microglia, Major Player in the Brain Inflammation: Their Roles in the Pathogenesis of Parkinson’s Disease. Exp. Mol. Med. 2006, 38, 333–347. [Google Scholar] [CrossRef]
- Cenker, J.J.; Stultz, R.D.; McDonald, D. Brain Microglial Cells Are Highly Susceptible to HIV-1 Infection and Spread. AIDS Res. Hum. Retroviruses 2017, 33, 1155–1165. [Google Scholar] [CrossRef]
- Garden, G.A. Microglia in Human Immunodeficiency Virus-Associated Neurodegeneration. Glia 2002, 40, 240–251. [Google Scholar] [CrossRef]
- Wang, T.; Gong, N.; Liu, J.; Kadiu, I.; Kraft-Terry, S.D.; Mosley, R.L.; Volsky, D.J.; Ciborowski, P.; Gendelman, H.E. Proteomic Modeling for HIV-1 Infected Microglia-Astrocyte Crosstalk. PLoS ONE 2008, 3, e2507. [Google Scholar] [CrossRef] [PubMed]
- Ton, H.; Xiong, H. Astrocyte Dysfunctions and HIV-1 Neurotoxicity. J. AIDS Clin. Res. 2013, 4, 255. [Google Scholar] [PubMed]
- Hamadi, N.; Sheikh, A.; Madjid, N.; Lubbad, L.; Amir, N.; Shehab, S.A.D.S.; Khelifi-Touhami, F.; Adem, A. Increased Pro-Inflammatory Cytokines, Glial Activation and Oxidative Stress in the Hippocampus after Short-Term Bilateral Adrenalectomy. BMC Neurosci. 2016, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.A.; Lynch, M.A. Evidence That Increased Hippocampal Expression of the Cytokine Interleukin-1β Is a Common Trigger for Age- and Stress-Induced Impairments in Long-Term Potentiation. J. Neurosci. 1998, 18, 2974–2981. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Wilk, E.; Michaelsen-Preusse, K.; Gerhauser, I.; Baumgärtner, W.; Geffers, R.; Schughart, K.; Korte, M. Long-Term Neuroinflammation Induced by Influenza a Virus Infection and the Impact on Hippocampal Neuron Morphology and Function. J. Neurosci. 2018, 38, 3060–3080. [Google Scholar] [CrossRef]
- Fu, H.; Hardy, J.; Duff, K.E. Selective Vulnerability in Neurodegenerative Diseases. Nat. Neurosci. 2018, 21, 1350–1358. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nookala, S.; Mukundan, S.; Grove, B.; Combs, C. Concurrent Brain Subregion Microgliosis in an HLA-II Mouse Model of Group A Streptococcal Skin Infection. Microorganisms 2023, 11, 2356. https://doi.org/10.3390/microorganisms11092356
Nookala S, Mukundan S, Grove B, Combs C. Concurrent Brain Subregion Microgliosis in an HLA-II Mouse Model of Group A Streptococcal Skin Infection. Microorganisms. 2023; 11(9):2356. https://doi.org/10.3390/microorganisms11092356
Chicago/Turabian StyleNookala, Suba, Santhosh Mukundan, Bryon Grove, and Colin Combs. 2023. "Concurrent Brain Subregion Microgliosis in an HLA-II Mouse Model of Group A Streptococcal Skin Infection" Microorganisms 11, no. 9: 2356. https://doi.org/10.3390/microorganisms11092356
APA StyleNookala, S., Mukundan, S., Grove, B., & Combs, C. (2023). Concurrent Brain Subregion Microgliosis in an HLA-II Mouse Model of Group A Streptococcal Skin Infection. Microorganisms, 11(9), 2356. https://doi.org/10.3390/microorganisms11092356




