Effect of Dietary Fiber on Reproductive Performance, Intestinal Microorganisms and Immunity of the Sow: A Review
Abstract
1. Introduction
2. Composition and Classification of Dietary Fiber
3. Effects of Dietary Fiber on Sow Reproduction and Related Mechanisms
3.1. Effect of Dietary Fiber on Reproductive Performance of the Sow
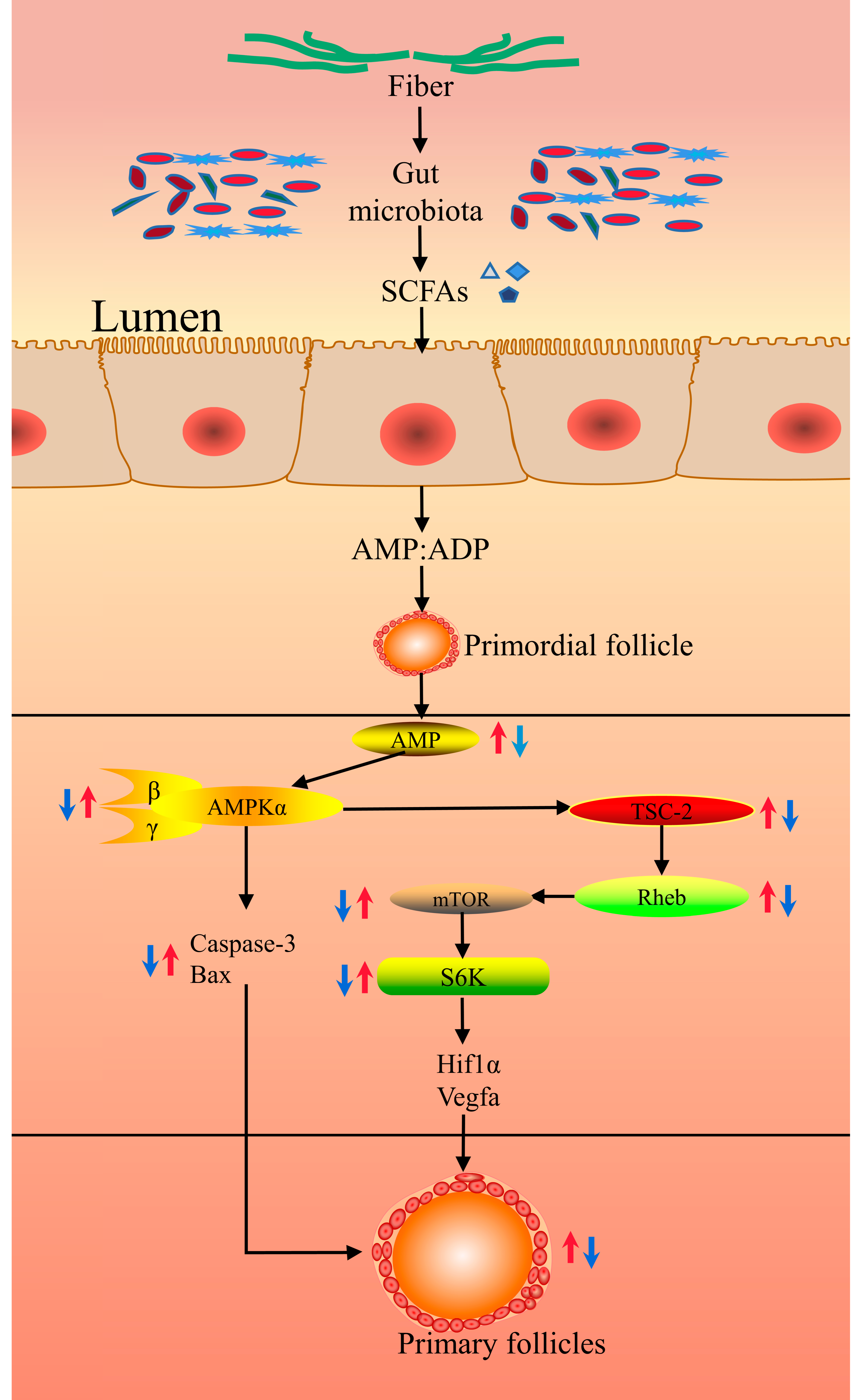
3.2. Mechanism by Which Dietary Fiber Improves Reproductive Performance of the Sow
4. Effects of Dietary Fiber on Intestinal Microflora of the Sow and the Underlying Mechanism
4.1. Effect of Dietary Fiber on the Gut Microbiota of the Sow
4.2. Mechanism by Which Dietary Fiber Influences the Intestinal Microflora in the Sow
5. Effects of Dietary Fiber on Immunity of Pregnant Sow and the Underlying Mechanism
5.1. Effect of Dietary Fiber on Immunity of Pregnant Sow
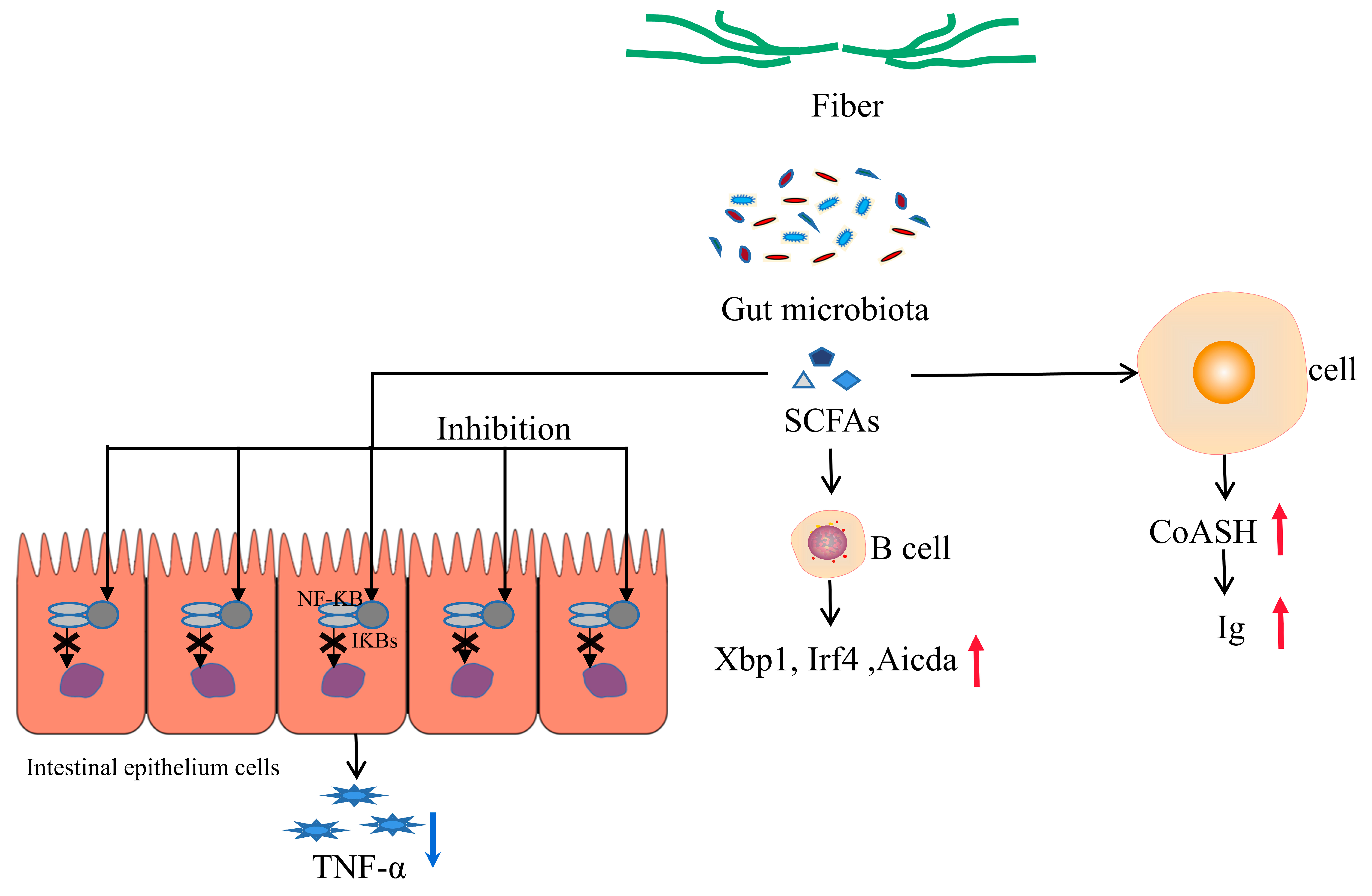
5.2. Mechanisms Underlying the Effect of Dietary Fiber on Immunity in Pregnant Sow
6. Summary
Funding
Conflicts of Interest
References
- Veum, T.L.; Crenshaw, J.D.; Crenshaw, T.D.; Cromwell, G.L.; Easter, R.A.; Ewan, R.C.; Nelssen, J.L.; Miller, E.R.; Pettigrew, J.E.; Ellersieck, M.R.; et al. The addition of ground wheat straw as a fiber source in the gestation diet of sows and the effect on sow and litter performance for three successive parities. J. Anim. Sci. 2009, 87, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Berrocoso, J.D. Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 2015, 9, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wei, J.; Yu, H.; Hao, X.; Zuo, J.; Tan, C.; Deng, J. Effects of Dietary Fiber Sources during Gestation on Stress Status, Abnormal Behaviors and Reproductive Performance of Sows. Animals 2020, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Loisel, F.; Farmer, C.; Ramaekers, P.; Quesnel, H. Effects of high fiber intake during late pregnancy on sow physiology, colostrum production, and piglet performance1. J. Anim. Sci. 2013, 91, 5269–5279. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Feng, D.; Wu, D.; Fang, Z.; Lin, Y.; Yan, T. Effect of Dietary Fibre on Reproductive Performance of Sows During the First Two Parities. Reprod. Domest. Anim. 2011, 46, 1061–1066. [Google Scholar] [CrossRef]
- Cheng, C.; Wei, H.; Xu, C.; Xie, X.; Jiang, S.; Peng, J. Maternal Soluble Fiber Diet during Pregnancy Changes the Intestinal Microbiota, Improves Growth Performance, and Reduces Intestinal Permeability in Piglets. Appl. Environ. Microbiol. 2018, 84, e01047-18. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Du, J.; Anderson, C.T.; Xiao, C. Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nat. Plants 2022, 8, 332–340. [Google Scholar] [CrossRef]
- Jarrett, S.; Ashworth, C.J. The role of dietary fibre in pig production, with a particular emphasis on reproduction. J. Anim. Sci. Biotechnol. 2018, 9, 59. [Google Scholar] [CrossRef]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. 1. Preparation of fiber residues of low nitrogen content. J. Assoc. Off. Agric. Chem. 1963, 46, 825–829. [Google Scholar]
- Van Soest, P.J. Development of a Comprehensive System of Feed Analyses and its Application to Forages. J. Anim. Sci. 1967, 26, 119–128. [Google Scholar] [CrossRef]
- Esposito, F.; Arlotti, G.; Bonifati, A.M.; Napolitano, A.; Vitale, D.; Fogliano, V. Antioxidant activity and dietary fibre in durum wheat bran by-products. Food Res. Int. 2005, 38, 1167–1173. [Google Scholar] [CrossRef]
- Knudsen, K.B. The nutritional significance of “dietary fibre” analysis. Anim. Feed. Sci. Technol. 2001, 90, 3–20. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zhang, L.; Yang, Y.; Lin, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Feng, B.; Xu, S.; et al. Maternal Dietary Fiber Composition during Gestation Induces Changes in Offspring Antioxidative Capacity, Inflammatory Response, and Gut Microbiota in a Sow Model. Int. J. Mol. Sci. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.P.; Johnston, L.J.; Baidoo, S.K.; Shurson, G.C. Effects of a high-fiber diet and frequent feeding on behavior, reproductive performance, and nutrient digestibility in gestating sows. J. Anim. Sci. 2006, 84, 946–955. [Google Scholar] [CrossRef]
- Zhuo, Y.; Shi, X.; Lv, G.; Hua, L.; Zhou, P.; Che, L.; Fang, Z.; Lin, Y.; Xu, S.; Li, J.; et al. Beneficial effects of dietary soluble fiber supplementation in replacement gilts: Pubertal onset and subsequent performance. Anim. Reprod. Sci. 2017, 186, 11–20. [Google Scholar] [CrossRef]
- Zhuo, Y.; Hua, L.; Che, L.; Fang, Z.; Lin, Y.; Xu, S.; Wang, J.; Li, J.; Feng, B.; Wu, D. Dietary Fiber Supplementation in Replacement Gilts Improves the Reproductive Performance From the Second to Fifth Parities. Front. Vet. Sci. 2022, 9, 839926. [Google Scholar] [CrossRef]
- Feng, D.D.; Wu, D.; Che, L.Q.; Zhou, D.S.; Fang, Z.F.; Lin, Y.; Wang, Y.T. Effects of dietary fiber levels on reproductive performance and hormone levels of gestating sows and organ development of the piglets. Chin. J. Anim. Nutr. 2011, 23, 25–33. [Google Scholar] [CrossRef]
- Ferguson, E.; Slevin, J.; Edwards, S.; Hunter, M.; Ashworth, C. Effect of alterations in the quantity and composition of the pre-mating diet on embryo survival and foetal growth in the pig. Anim. Reprod. Sci. 2006, 96, 89–103. [Google Scholar] [CrossRef]
- Diao, X.Y.; Yang, X.; Li, F.; Zhang, X.X.; Niu, J.K.; Ma, S.; Wang, Z.C.; Cui, Y.L.; Shi, Y.H. Effects of feeding high fiber diet in gestation on farrowing performance of sows and growth performance of offspring piglets. Chin. J. Anim. Nutr. 2022, 34, 2229–2237. [Google Scholar] [CrossRef]
- Cao, M. Effects of dietary fiber level on follicle development of gilts and the underlying mechanisms. Ph.D. Thesis, Sichuan Agricultural University, Chengdu, China, 2019. [Google Scholar]
- Arias-Álvarez, M.; García-García, R.M.; Rebollar, P.G.; Nicodemus, N.; Millán, P.; Revuelta, L.; Lorenzo, P.L. Follicular, Oocyte and Embryo Features Related to Metabolic Status in Primiparous Lactating does Fed with High-Fibre Rearing Diets. Reprod. Domest. Anim. 2009, 45, e91–e100. [Google Scholar] [CrossRef]
- Cao, M.; Zhuo, Y.; Gong, L.; Tang, L.; Li, Z.; Yang, M.; Xu, S.; Che, L.; Lin, Y.; Feng, B.; et al. Optimal Dietary Fiber Intake to Retain a Greater Ovarian Follicle Reserve for Gilts. Animals 2019, 9, 881. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.M.; Slevin, J.; Hunter, M.G.; Edwards, S.A.; Ashworth, C.J. Beneficial effects of a high fibre diet on oocyte maturity and embryo survival in gilts. Reproduction 2007, 133, 433–439. [Google Scholar] [CrossRef]
- Weaver, A.C.; Kelly, J.M.; Kind, K.L.; Gatford, K.L.; Kennaway, D.J.; Herde, P.J.; van Wettere, W.H.E.J. Oocyte maturation and embryo survival in nulliparous female pigs (gilts) is improved by feeding a lupin-based high-fibre diet. Reprod. Fertil. Dev. 2013, 25, 1216–1223. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K. Cellular and molecular regulation of the activation of mammalian primordial follicles: Somatic cells initiate follicle activation in adulthood. Hum. Reprod. Updat. 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Xu, M.; Che, L.; Zhang, P.; Wu, D.; Xu, S. Regulation of adenosine 5’-monophosphate-activated protein kinase in mammali-an ovary development and mechanisms. Chin. J. Anim. Nutr. 2015, 27, 3006–3011. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, B.; Chen, H.; Chen, D. Carbohydrates effects on nutrition and health functions in pigs. Anim. Sci. J. 2021, 92, e13557. [Google Scholar] [CrossRef]
- Hong-Brown, L.Q.; Brown, C.R.; Navaratnarajah, M.; Lang, C.H. Activation of AMPK/TSC2/PLD by Alcohol Regulates mTORC1 and mTORC2 Assembly in C2C12 Myocytes. Alcohol. Clin. Exp. Res. 2013, 37, 1849–1861. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Xu, S.; Wu, X.; Dong, Y.; Xu, M.; Li, Z.; Chen, S.; Zhuo, Y.; Lin, Y.; Che, L.; Fang, Z.; et al. Glucose activates the primordial follicle through the AMPK/mTOR signaling pathway. Clin. Transl. Med. 2020, 10, e122. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Li, J.; Zheng, N.; Xu, X.; Yang, J.; Xia, G.; Zhang, M. MAPK3/1 participates in the activation of primordial follicles through mTORC1-KITL signaling. J. Cell. Physiol. 2018, 233, 226–237. [Google Scholar] [CrossRef]
- Lindberg, J.E. Fiber effects in nutrition and gut health in pigs. J. Anim. Sci. Biotechnol. 2014, 5, 15. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Cui, Y.; Wang, W.; Liu, H.; Li, Z.; Guo, Z.; Ma, S.; Li, D.; Wang, C.; et al. Consumption of Dietary Fiber from Different Sources during Pregnancy Alters Sow Gut Microbiota and Improves Performance and Reduces Inflammation in Sows and Piglets. mSystems 2021, 6, e00591-20. [Google Scholar] [CrossRef] [PubMed]
- Heinritz, S.N.; Weiss, E.; Eklund, M.; Aumiller, T.; Heyer, C.M.; Messner, S.; Rings, A.; Louis, S.; Bischoff, S.C.; Mosenthin, R. Impact of a High-Fat or High-Fiber Diet on Intestinal Microbiota and Metabolic Markers in a Pig Model. Nutrients 2016, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, J.; Tan, B.; Chen, J.; Zhang, H.; Li, Z.; Ma, X. Physiological function and application of dietary fiber in pig nutrition: A review. Anim. Nutr. 2021, 7, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gao, T.; Liu, Z.; Diao, X. Effects of Dietary Supplementation with High Fiber (Stevia Residue) on the Fecal Flora of Pregnant Sows. Animals 2020, 10, 2247. [Google Scholar] [CrossRef]
- Li, S.; Zheng, J.; He, J.; Liu, H.; Huang, Y.; Huang, L.; Wang, K.; Zhao, X.; Feng, B.; Che, L.; et al. Dietary fiber during gestation improves lactational feed intake of sows by modulating gut microbiota. J. Anim. Sci. Biotechnol. 2023, 14, 65. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, H.; Liu, S.; He, T.; Piao, X. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets1. J. Anim. Sci. 2019, 97, 4922–4933. [Google Scholar] [CrossRef]
- Tokach, M.D.; Menegat, M.B.; Gourley, K.M.; Goodband, R.D. Review: Nutrient requirements of the modern high-producing lactating sow, with an emphasis on amino acid requirements. Animal 2019, 13, 2967–2977. [Google Scholar] [CrossRef]
- Guan, G.; Ding, S.; Yin, Y.; Duraipandiyan, V.; Al-Dhabi, N.A.; Liu, G. Macleaya cordata extract alleviated oxidative stress and altered innate immune response in mice challenged with enterotoxigenic Escherichia coli. Sci. China Life Sci. 2019, 62, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiong, Y.; Zhong, M.; Li, Y.; Wan, H.; Wu, D.; Liu, Q. Effects of purified fibre-mixture supplementation of gestation diet on gut microbiota, immunity and reproductive performance of sows. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cheng, C.; Zhang, X.; Peng, J. Inclusion of Soluble Fiber in the Gestation Diet Changes the Gut Microbiota, Affects Plasma Propionate and Odd-Chain Fatty Acids Levels, and Improves Insulin Sensitivity in Sows. Int. J. Mol. Sci. 2020, 21, 635. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Pluske, J.R.; Hampson, D.J. A review of interactions between dietary fiber and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed. Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Tokunaga, Y.; Ikeda, A.; Ohkura, K.-I.; Kaku-Ohkura, S.; Mamiya, S.; Lim, B.O.; Tachibana, H. Effect of Dietary Fiber on the Lipid Metabolism and Immune Function of Aged Sprague-Dawley Rats. Biosci. Biotechnol. Biochem. 2003, 67, 429–433. [Google Scholar] [CrossRef][Green Version]
- Vogt, M.C.; Brüning, J.C. CNS insulin signaling in the control of energy homeostasis and glucose metabolism—From embryo to old age. Trends Endocrinol. Metab. 2013, 24, 76–84. [Google Scholar] [CrossRef]
- Yui, J.; Garcia-Lloret, M.; Wegmann, T.; Guilbert, L. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta 1994, 15, 819–835. [Google Scholar] [CrossRef]
- Fung, K.Y.C.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef]
- Grilli, E.; Tugnoli, B.; Foerster, C.; Piva, A. Butyrate modulates inflammatory cytokines and tight junctions components along the gut of weaned pigs. J. Anim. Sci. 2016, 94, 433–436. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.P.; Quigley, E.M.M.; Brint, E. Microbiota-host interactions in irritable bowel syndrome: Epithelial barrier, immune regulation and brain-gut interactions. World J. Gastroenterol. 2014, 20, 8859–8866. [Google Scholar] [CrossRef]
- Velasquez-Manoff, M. Gut microbiome: The Peacekeepers. Nature 2015, 518, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Lührs, H.; Gerke, T.; Schauber, J.; Dusel, G.; Melcher, R.; Scheppach, W.; Menzel, T. Cytokine-activated degradation of inhibitory κB protein α is inhibited by the short-chain fatty acid butyrate. Int. J. Color. Dis. 2001, 16, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.; Thangaraju, M.; Mellinger, J.D.; Liu, K.; Ganapathy, V. Colonic Gene Expression in Conventional and Germ-Free Mice with a Focus on the Butyrate Receptor GPR109A and the Butyrate Transporter SLC5A8. J. Gastrointest. Surg. 2010, 14, 449–461. [Google Scholar] [CrossRef]
- Luu, M.; Monning, H.; Visekruna, A. Exploring the Molecular Mechanisms Underlying the Protective Effects of Microbial SCFAs on Intestinal Tolerance and Food Allergy. Front. Immunol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.l.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; Thompson, C.B. ATP-Citrate Lyase Links Cellular Metabolism to Histone Acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef]
- Xuan, Y. Effects of Dietary Fiber Levels on Reproductive Performance and Immunity of Sows during Gestation. Master’s Thesis, Sichuan Agricultural University, Yaan, China, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, F.; Wei, W.; Gao, J.; Jiang, X.; Che, L.; Fang, Z.; Lin, Y.; Feng, B.; Zhuo, Y.; Hua, L.; et al. Effect of Dietary Fiber on Reproductive Performance, Intestinal Microorganisms and Immunity of the Sow: A Review. Microorganisms 2023, 11, 2292. https://doi.org/10.3390/microorganisms11092292
Qin F, Wei W, Gao J, Jiang X, Che L, Fang Z, Lin Y, Feng B, Zhuo Y, Hua L, et al. Effect of Dietary Fiber on Reproductive Performance, Intestinal Microorganisms and Immunity of the Sow: A Review. Microorganisms. 2023; 11(9):2292. https://doi.org/10.3390/microorganisms11092292
Chicago/Turabian StyleQin, Feng, Wenyan Wei, Junjie Gao, Xuemei Jiang, Lianqiang Che, Zhengfeng Fang, Yan Lin, Bin Feng, Yong Zhuo, Lun Hua, and et al. 2023. "Effect of Dietary Fiber on Reproductive Performance, Intestinal Microorganisms and Immunity of the Sow: A Review" Microorganisms 11, no. 9: 2292. https://doi.org/10.3390/microorganisms11092292
APA StyleQin, F., Wei, W., Gao, J., Jiang, X., Che, L., Fang, Z., Lin, Y., Feng, B., Zhuo, Y., Hua, L., Wang, J., Sun, M., Wu, D., & Xu, S. (2023). Effect of Dietary Fiber on Reproductive Performance, Intestinal Microorganisms and Immunity of the Sow: A Review. Microorganisms, 11(9), 2292. https://doi.org/10.3390/microorganisms11092292








