Abstract
Hospital-acquired infections caused by P. aeruginosa contribute to global distress because of the elevated rates of microbial antibiotic resistance. Aminoglycosides are antipseudomonal agents that are effectively and frequently utilized to eradicate this infection. This current study is a retrospective study investigating plasmid-mediated aminoglycoside resistance by focusing on the prevalence of the genes encoding aminoglycoside-modifying enzymes (AMEs) and 16S rRNA methylase among P. aeruginosa clinical isolates from Taif, Saudi Arabia. A hundred clinical isolates of P. aeruginosa were collected. The isolates were identified from February 2021 to February 2022. Antibiotic susceptibility testing and MICs were determined using (DD) and (BM-MIC) testing, respectively. AMEs and 16S rRNA methylase variants in bacterial isolates were amplified via PCR for genetic detection. A relatively high multiple antibiotic resistance rate corresponding to 10–32% was reported. Eighteen percent of P. aeruginosa isolates were gentamicin–amikacin–tobramycin resistant according to the MIC levels. The aminoglycoside-resistant strains were additionally identified via GyrA gene sequencing. The phylogenic relatedness dendrogram of the sequenced GyrA genes was performed using a neighbor-joining method via MEGAX software version 10.2.6. The most prevalent AME encoding gene was aac(6′)-Ib, observed in 94.4% of resistant isolates, while a resistance gene cocktail of [aac(6′)-Ib and ant(3″)-I] was a highly frequent combination (27.8%). This study updated the knowledge about aminoglycoside resistance mechanisms in P. aeruginosa, which constitutes an urgent need, especially after the COVID-19 crisis, which was associated with increased antimicrobial use and resistance rates.
1. Introduction
P. aeruginosa is one of the major opportunistic pathogens that cause acute or chronic infections. On the parallel side, illogical and widespread use of antibiotics has led to long-term impact on the antimicrobial resistance (AMR) of such microorganisms [1,2,3].
Aminoglycosides are a large category of antibiotics that bind specifically to 16S rRNA in 30S ribosomal subunits and disturb protein translation. They are extensively used in the treatment of serious bacterial infections, especially aerobic Gram-negative bacteria. The increasing problem of multi-resistance in Gram-negative bacteria, such as P. aeruginosa, warrants new studies focused on understanding aminoglycoside resistance [4]. The widespread occurrence of antibiotic-resistant strains of P. aeruginosa in hospitals is a matter of growing resistance as they cause various types of hospital-acquired human infections, with an expected elevation of resistance rates due to the antibiotic-dependent protocols that had already been utilized during the COVID-19 pandemic [5].
ARGs acquisition represents the major cause of plasmid-mediated aminoglycoside resistance via the encoding of AMEs, namely acetyltransferases (aac), phosphotransferases (aph), and nucleotidyl transferases (ant) [6]. In addition, 16S rRNA methylases (rmts) are another aminoglycoside resistance pathway among clinical P. aeruginosa isolates [7]. The aac(6′)-Ib gene has an obvious relationship to high gentamicin resistance; it is found in the majority of Gram-negative bacteria, including P. aeruginosa [7,8]. Other common AME encoded by ant(3″)-I, aph(3′)-VI, ArmA, aac(3′)-II, and aac(6′)-II was investigated in pan-resistant P. aeruginosa [9,10].
The resistance mechanism complexity was encountered by the co-existence of more than one ARG, where the spread of these genes is particularly based on the type of bacterium causing the infection and misuse of aminoglycosides among different hospitals or geographic regions [11]. This current study is a retrospective, observational analytical study aimed at detecting the antibiotic resistance patterns of P. aeruginosa clinical isolates and focusing on the mechanism of resistance against the frequently used aminoglycosides (gentamicin, amikacin, and tobramycin) and the prevalence of the genes encoding for resistance in P. aeruginosa clinical isolates. Furthermore, it displays a general view of the probability of resistant microbial transmission.
2. Materials and Methods
2.1. Collection of P. aeruginosa Isolates
We recovered 100 clinical isolates as a microbiology laboratory procedure from the microbiology laboratory at King Abdulaziz Specialist Hospital from February 2021 to February 2022, and all isolates were obtained from adult male/female patients (above 18 years old). Children and pregnant women were not included in this study. All strains were initially recovered on MacConkey’s agar (Oxoid, Basingstoke, UK) and then purified on cetrimide agar (Scharlau, Barcelona, Spain). All isolates were primarily identified by the Vitek 2® system (BioMérieux, Craponne, France) and API 20NE® (BioMérieux, Craponne, France). The genus level was also confirmed via the amplification of the algD gene using the primer pairs (Macrogen, Geumcheon-gu, Seoul, Republic of Korea) listed in Table 1. Long-term storage of isolates at −84 °C in glycerol brain/heart infusion was undertaken for further tests. This study was performed with ethical approval No. 42-0107 following the regulations of the ethical committee at Taif University.
2.2. Antimicrobial Susceptibility Testing (AST)
Both DD and BM-MIC testing was conducted among all isolates using Muller Hinton agar and broth, respectively [12]. Breakpoints for different antibiotics were interpreted based on the guidelines of CLSI, 2017 [13]. Seven antibiotics that represent different categories of antimicrobial agents were utilized, including three aminoglycosides (gentamicin—10 µg (GM), amikacin—30 µg (AK), and tobramycin—10 µg (TM)) and ceftazidime—30 µg (CTZ), imipenem—10 µg (IMP), piperacillin/tazobactam—100/10 µg (TZP), and ciprofloxacin—5 µg (CIP) (Merseyside, United Kingdom). MICs were determined for the mentioned 7 antibiotics utilizing a wide concentration range from (0.5–1024 µg/mL), as shown in Table 2.

Table 2.
DD and BM-MIC methods (μg/mL) of P. aeruginosa clinical isolates.

Table 1.
Primer sets and PCR cycling conditions were used for genotyping and amplification of aminoglycosides resistance genes.
Table 1.
Primer sets and PCR cycling conditions were used for genotyping and amplification of aminoglycosides resistance genes.
| Primer/Gene | Sequence | PCR Condition | Amplicon Size (pb) | References |
|---|---|---|---|---|
| VIC/algD | F: TTCCCTCGCAGAGAAAACATC R: CCTGGTTGATCAGGTCGATCT | Initial denaturation at 95 °C for 15 min, then 30 cycles of 95 °C for 1 min, 58 °C for 1 min and 72 °C for 5 min and one cycle of final elongation at 72 °C | 520 | [14] |
| GyrA | F: TTATGCCATGAGCGAGCTGGGCAACGACT R: AACCGTTGACCAGCAGGTTGGGAATCTT | Initial denaturation at 95 °C for 15 min, then 35 cycles of 95 °C for 1 min, 57 °C for 1 min, and 72 °C for 5 min, and one cycle of final elongation at 72 °C. | 365 | [2] |
| Aph(3′)-VI | F: ATGGAATTGCCCAATATTATT R: TCAATTCAATTCATCAAGTTT | Initial denaturation at 95 °C for 15 min, then 30 cycles of 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 5 min, and one cycle of final elongation at 72 °C. | 780 | [15] |
| aac(3′)-II/aac(3′)-II | F: ATATCGCGATGCATACGCGG R: GACGGCCTCTAACCGGAAGG | 877 | ||
| aac(6′)-Ib/aac(6′)-Ib | F: TTGCGATGCTCTATGAGTGGCTA R: CTCGAATGCCTGGCGTGTTT | 472 | ||
| aac(6′)-II/aac(6′)-II | F: CGACCATTTCATGTCC R: GAAGGCTTGTCGTGTTT | 542 | ||
| ant(3″)-I/ant(3″)-I/ | F: CATCATGAGGGAAGCGGTG R: GACTACCTTGGTGATCTCG | 787 | ||
| ArmA/ArmA | F: CCGAAATGACAGTTCCTATC R: GAAAATGAGTGCCTTGGAGG | 846 | ||
| rmtB/rmtB | F: ATGAACATCAACGATGCCCTC R: CCTTCTGATTGGCTTATCCA | Initial denaturation at 95 °C for 15 min, then 30 cycles of 95 °C for 1min, 60 °C for 1 min, and 72 °C for 5 min, and one cycle of final elongation at 72 °C. | 769 | [15] |
2.3. Genomic DNA Extraction
Genomic DNA was extracted using Gene JET Genomic DNA Purification Kit (Thermo Fisher Scientific, Waltham, WA, USA) according to the manufacturer’s protocol.
2.4. PCR and Gel Electrophoresis
Genetically identification of Pseudomonal isolates was investigated via algD PCR amplification (Macrogen, Geumcheon-gu, Seoul, Republic of Korea). Furthermore, GyrA Sequencing was performed on aminoglycosides resistant isolates only as an extra confirmatory tool.
ARGs PCRs were carried out on all isolates. Primers, cycling conditions, and amplicon sizes are listed in Table 1 (references in Table 1). Amplification of targeted ARGs include (Aph(3′)-VI, aac(3′)-II, aac(6′)-Ib, aac(6′)-II, ant(3″)-I, Arm, and rmtB). The target genes were amplified in the total PCR reaction mixture of 20 µL containing (4 µL of DNA, 4 µL of 5× master mix (Solis BioDyne, Tartu, Estonia), 0.6 µL for each of forward and reverse primer (10 pmol/μL)), P. aeruginosa ATCC 27853 was utilized as a reference. Visualization PCR fragments (2 µL of RCR product) carried out on 1.5% agarose gels with 0.3 µg/mL ethidium bromide (EtBr) and GeneRuler 100 bp DNA Ladder, using a power supply (Labnet International Inc., Taipei, Taiwan).
2.5. DNA Sequencing
The purified PCR products of the GyrA gene were purified, and Sanger sequenced in one direction by capillary electrophoresis sequencing (CES) utilizing the forward primer. Nucleotide sequences were determined at the Macrogen sequencing facility (Macrogen Inc., Seoul, Republic of Korea).
GyrA gene sequences were corrected using the molecular evolutionary genetic analysis MEGAX software version 10.2.6, (Bio Design Institute, Tempe, AZ, USA). Homology searches of nucleotide sequences were performed via FASTA and BLAST screen. Original nucleotide sequences of GyrA were obtained from the GenBank nucleotide sequence database with accession numbers L29147 [2].
Molecular phylogenetic analysis of the sequenced GyrA genes of resistant pseudomonal isolates was performed using the neighbor-joining method via MEGAX version 10.2.6.
3. Results
All the isolates under investigation in this study were unduplicated and obtained from different body parts at both male and female wards at the tertiary health care center (King Abdulaziz Specialist Hospital) in Taif City, Saudi Arabia, including sputum (n = 44), urine (n = 36), blood (n = 7), wound swap (n = 7), bile (n = 2), and (n = 1) for each of eye swab, vaginal swab, peritoneal fluid, and catheter tip, as shown in Table S1.
The resistance profiles of the P. aeruginosa isolates were determined using both DD and BM-MIC methods. Table 2 summarizes all the resulting data obtained from these methods. A paired t-test was conducted for the differences between the two methods in the means of percentage of resistant/susceptible isolates (% R:S) and [total number of resistant and intermediate isolates/susceptible isolates [% (R + I):S]. The statistical analysis and data interpretations revealed a strong correlation between the two methods with a confidence level of 0.95, as presented in Table S2.
According to BM-MIC, the tested isolates showed that 32.0% (32/100) were resistant to one or more antibiotics as 31.0% (31/100) were resistant to gentamicin, 18.0% (18/100) to both amikacin and tobramycin, 10.0% (10/100) to ceftazidime, 32.0% (32/100) to imipenem, 13.0% (13/100) to piperacillin/tazobactam, and 29.0% (29/100) to ciprofloxacin, as presented in Table 2. The corrected GyrA sequences were uploaded to the National Center for Biotechnology Information (NCBI), Accession no. (OR188196 to OR188213). The aminoglycoside-resistant isolates were subjected to homology searches of their GyrA sequences. The results indicated a high identity percentage of 99.32–99.67% compared to the reference nucleotide sequence of GyrA obtained from the GenBank nucleotide sequence database (accession number L29147). This finding confirms the previous genetic identification of the isolates (Figure S1).
Among all isolates, twenty-nine (29%) strains were gentamicin-resistant via DD in contrast to (31%) using the BM-MIC method. However, both methods showed the same amikacin and tobramycin-resistant rate (18%). Generally, eighteen isolates were resistant to all the aminoglycosides assayed (gentamicin, amikacin, and tobramycin). It is important to note that the MIC of piperacillin-tazobactam was determined using a constant concentration of tazobactam (4 µg/mL) [16].
For the same antibiotic, elevated MIC was observed compared to reduced inhibition zone diameter and vice versa due to the concurrent existence of a wide range of resistance rates from high, intermediate, and low-resistant strains [17].
All ARGs focused in this study (aac(6′)-Ib, ant(3″)-I, aph(3′)-VI, armA, aac (3′)-II, aac(6′)-II and rmtB were amplified via PCR according to the conditions and primer sets mentioned previously. The resulting PCR products were (472 bp for aac(6′)-Ib, 780 bp for aph(3′)-VI, 787 bp for ant(3″)-I, 846 pb for armA, 877 bp for aac(3′)-II) while rmtB was completely absent in all isolates under investigation. The PCR fragments were separated on 1.5% agarose gel using a 100-base pair DNA Ladder. The resistance profile of pan aminoglycoside isolates is reported in Table 3.

Table 3.
ARGs profile of pan aminoglycoside isolates.
The most frequent ARGs was aac(6′)-Ib, detected in 94.4% (17/18) of the resistant strains, followed by ant(3″)-I (6/18, 33.3%), then aph(3′)-VI (3/18, 16.7%), while armA, aac(3′)-II and aac(6′)-II were found in (1/18, 5.5%). Conversely, rmtB was negative in all the isolates under investigation (Figure 1).
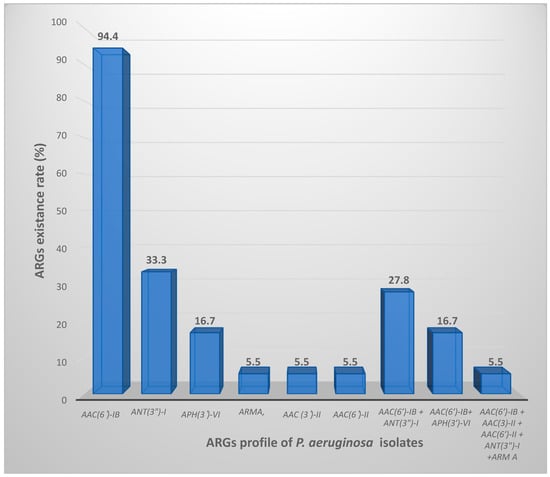
Figure 1.
ARGs distribution profile among the isolated P. aeruginosa resistant strains.
Nine isolates were investigated with more than one ARG. The genetic combination of aac(6′)-Ib and ant(3″)-I was the highly frequent one (5/18, 27.8%) followed by aac(6′)-Ib and aph(3′)-VI (3/18, 16.7%). One isolate harbored five genes: aac(6′)-Ib + aac (3′)-II + aac(6′)-II + ant(3″)-I + arm A. Although resistance phenotypes against gentamicin and/or amikacin were observed, negative tests for ARGs had been evaluated in this study. As expected, all the susceptible isolates were free from ARGs under the test. The findings of this study revealed that the most common ARG was aac(6′)-Ib corresponding to (17/18, 94.4%), followed by ant(3″)-I (6/18, 33.3%), aph(3′)-VI (3/18, 16.7%). Furthermore, the detection rate for each armA, aac(3′)-II, and aac(6′)-II was equal occurring in 1 out of 18 isolates (5.5%). Conversely, rmtB was negative in any of the isolates. Although resistance phenotypes against gentamicin and/or amikacin were observed, negative tests for ARGs had been evaluated in this study. As expected, all the susceptible isolates were free from ARGs under the test. All 18 aminoglycoside-resistant strains included in this study were classified as MDR due to their resistance to at least one antibiotic from three different classes: Aminoglycoside, Carbapenems, and Quinolones. The MIC ranges of resistant Pseudomonal isolates were visually represented in Table 1 using red color.
Molecular phylogenetic analysis of the sequenced GyrA genes using the neighbor-joining method via MEGAX version 10.2.6. revealed four distinct phylogenic clades [clade A, B, C, and D] within two major clusters denoted as Cluster I and Cluster II as depicted in Figure 2. Notably, all the sequences in the clade A [seq14, seq20, seq4, seq2, and seq1] were isolated from sputum specimens in the male ward. Clade B primarily consisted of seq13, seq19, seq3, and seq22 obtained from sputum specimens in the male ward, except for sequence no.19, which was derived from a wound swap in the female ward. In Cluster II, clade C contained a single sequence no. 5 obtained from a wound swap in the female ward. Within clade D, all sequences [seq.6, seq.7, seq.8, seq.10, seq.16, seq. seq.23, seq.11, and seq.17] were from the male ward, except no. 16, which was isolated from a female wound swab.
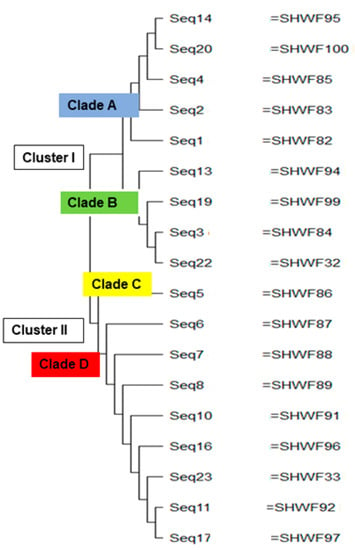
Figure 2.
Phylogenetic relatedness between the sequenced GyrA genes of aminoglycoside-resistant P. aeruginosa using the neighbor-joining method.
4. Discussion
Despite the ongoing usefulness of aminoglycoside as a group of antipseudomonal agents. The issue of resistance remains a growing concern, with consideration given to geographical variations. Like other antibiotics, there are regional disparities in resistance rates that directly reflect a prescription pattern and the efficacy of infection control measures. [18]. This study exhibited a significant prevalence of plasmid-mediated P. aeruginosa resistance with 10–32% of isolates that were resistant among the selected antibiotics [aminoglycosides (gentamicin, amikacin, and tobramycin), piperacillin/Tazobactam, ceftazidime, imipenem, and ciprofloxacin. Although twenty-nine (29%) of isolates were gentamicin-resistant by DD versus (31%) by the BM-MIC method, this result is completely acceptable as there was no statistically significant difference observed between the two methods (DD vs. BM-MIC), Table S2. Our investigation agrees with the study about the evaluation of different gentamicin susceptibility methods among enterobacterial strains. The DD method showed the best compatibility and performance compared to the broth microdilution as a reference method, with a categorical agreement of 98.1% [19].
Our investigation revealed that the rate of aminoglycoside resistance was (18%). This finding in Taif City agrees with another Saudi finding in Hail province, which demonstrated a 16% aminoglycoside resistance rate of P. aeruginosa isolates [20,21]. Comparable estimated resistance rate of MDR- P. aeruginosa had been reported by NHSN in patients with pneumonia, bacteremia, and UT infections [22]. Recently, highly resistant isolates to antipseudomonal agents have been reported in Qatar [23]. In Egypt, phenotypically and genotypically detection of MDR Gram-negative clinical isolates were reported with rates of gentamicin and amikacin resistance corresponding to (11/26, 42.3%, and 10/26, 38.4%), respectively [24]. Other studies in Kosovo showed an elevated P. aeruginosa resistance rate over consecutive 2 years from 2013 to 2015, against each of gentamicin, carbapenems, and ciprofloxacin. Thus, there is a global warrant to emphasize uncontrolled antibiotic use and perfectly adhere to the conception of “reserve drugs” [25].
On the other hand, the findings of a Turkish study reported relatively high gentamicin–mikacin resistance rates of 70.7% and 42.2%, respectively [5]. About twenty years ago, a Saudi study conducted in the Al-Dharan region reported a declining level of MDR—P. aeruginosa corresponding to 1–2% regarding the inpatient isolates [26]. Compared to our findings, a warning alarm for a future outbreak of antibiotic resistance should be considered, and regulatory issues and antibiotics stewardship protocols are urgently needed to restrict microbial resistance distribution as soon as possible. Furthermore, there is extreme importance to update our knowledge about the P. aeruginosa resistance profiles according to time and geographic regional variations, which ideally give back the diversity of aminoglycoside utilization as antipseudomonal agents, application of the infection quality control and the health care facilities all over the world [27].
In the current work, the highly frequent ARG among resistant isolates was aac(6′)-Ib corresponding to (17/18, 94.4%). This observation was in agreement with an Iranian study that reported 71.2% of the resistant P. aeruginosa isolates were aac(6′)-Ib positive [28]. Furthermore, the study investigated the existence of aph(3′)-VI among three P. aeruginosa isolates (3/18, 16.7%); these three isolates were found to be highly amikacin resistant with MIC levels between (256–1024 ug/mL), (Table 2). Our finding agreed with Torres et al., who reported the contribution of phosphotransferase in amikacin-resistant P. aeruginosa strains [29]. Another study in Venezuela investigated that ARGs (aac(6′)-Ib, aphA1, and aad B) were highly distributed among the examined clinical isolates [30].
In an Iranian study, a mixture of ARGs was demonstrated among tested pseudomonal strains, including [aac(6′)-II, aad(2″), and aph(3′)-VI], that emphasize the concurrently multiple existences of ARGs in the same isolate. In addition, the work investigated one isolate from 18 that was phenotypically resistant to both gentamicin and amikacin, while it showed a negative genotyping test for ARGs evaluated in this study, this may be revealed to the implication of other variants that were not covered here, as reported elsewhere [31]. In the same manner, a negative test for ARG genotyping was reported for all the susceptible isolates, which agrees with other studies conducted worldwide [32]. An extremely high aminoglycoside resistance was observed previously, combined with a wide range existence of ARGs including different variants of aad gene (A1, B, A2), ant(2″)-Ia, aph(3′)-IIb, aac(3′)-Ia, and aac(6′)-Iia [33]. The interplay between efflux pumps MexXY, MexZ, and the level of mexXY expression plays an essential role in aminoglycoside resistance in clinical isolates of P. aeruginosa, but the magnitude of the contribution of this efflux pump to resistance is isolate-specific [34].
The molecular phylogenetic analysis of GyrA nucleotide sequences reported that almost all the nucleotide sequences isolated from the same hospital ward (the male or female ward) belonged to the same phylogenic group, which indicated a high probability of microbial transmission from colonized or infected patients to other patients or to health care workers and visitors who may subsequently transmit them to others. Meoli et al. reported the importance of preventive measures to reduce the prevalence of microbial transmission, especially in the case of surgical site infections [35].
This study highlighted the effect of plasmid-mediated resistance among P. aeruginosa as one of the common infection causative agents. Other resistance mechanisms might contribute an axial role in P. aeruginosa resistance. Therefore, additional genetic information is required for the implementation of new therapeutic strategies along with infection prevention and quality control policies.
In parallel, this work confirmed the global awareness against microbial multiple drug resistance, including (1) how one microbe harbored more than one resistance mechanism and (2) the phylogenic relatedness analysis reported high expectations of microbial transmission that accelerates the resistance incidence rate So, the current type of studies are obligatory for continuous updating of clinical practice knowledge, to hinder antibiotic resistance, support regulatory aspects and antibiotics stewardship’s programs among aminoglycoside as anti-pseudomonal agents along with other antibiotics.
The limitations of this current study include (1) the local level of data about aminoglycoside resistance mechanisms, (2) limited numbers of ARGs and 16S RNA methylases were searched, especially rmtA and rmtD, and (3) sequencing of the amplified genes would have been important to distinguish aac(6′)-Ib from aac(6′)-Ib-cr. Hence, we suggest conducting parallel studies across different populations to expand the scope of research by investigating more aminoglycoside resistance mechanisms utilizing a larger number of pseudomonal isolates to improve global therapeutic outcomes. Furthermore, a future study is recommended to evaluate whether the isolates are XDR or DTR-P. aeruginosa.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11092293/s1, Table S1: Correlation of each isolate with the clinical origin (different body parts at both male and female wards, including sputum (n = 44), urine (n = 36), blood (n = 7), wound swap (n = 7), bile (n = 2), and (n = 1) for each of eye swab, vaginal swab, peritoneal fluid, and catheter tip), Table S2: Paired t-test statistical analysis correlation among both the DD and the BM-MIC methods in means of (A) Hypothesis Test for the difference of two means: dependent sample (Paired t-test) − % (R/S) [percentage of resistant/susceptible isolates], and (B) Hypothesis Test for the difference of two means: dependent sample (Paired t-test) − % (R + I)/S [total number of resistant and intermediate isolates/susceptible isolates]. Figure S1: GyrA PCR amplification and sequence alignment, (A) GyrA PCR amplification of samples (1G–14G), 3 µL per Lane showed the band at Mwt of 365 bp, utilizing 100 bp ladder, (B) GyrA nucleotide sequence alignment versus the reference sequence from the GenBank nucleotide sequence database with accession numbers L29147.
Author Contributions
Conceptualization, S.W.E.-F.; methodology, S.W.E.-F.; software, S.W.E.-F.; validation, S.W.E.-F. and M.W.A.; formal analysis, S.W.E.-F. and M.W.A.; investigation, S.W.E.-F. and M.W.A.; resources, S.W.E.-F.; statistics and data curation, S.W.E.-F. and M.W.A.; writing—original draft preparation, S.W.E.-F.; writing—review and editing, S.W.E.-F. and M.W.A.; visualization, S.W.E.-F. and M.W.A.; supervision, M.W.A.; funding acquisition, S.W.E.-F. All authors have read and agreed to the published version of the manuscript.
Funding
The researchers would like to acknowledge the Deanship of Scientific Research at Taif University for funding this work.
Institutional Review Board Statement
This study was performed after the ethical approval No. 42-0107 following the regulations of the ethical committee at Taif University.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Acknowledgments
The researchers would like to acknowledge the microbiology laboratory at King Abdulaziz Specialist Hospital and the microbiology laboratory at the College of Pharmacy, Taif University.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Aminoglycoside modifying enzymes (AMEs), disk diffusion method (DD), broth-microdilution MIC testing (BM-MIC), antimicrobial resistance (AMR), aminoglycosides resistant genes (ARGs), multiple drug resistance (MDR), the Clinical and Laboratory Standards Institute (CLSI), National Health-care Safety Network (NHSN), Extensively-Drug Resistance (XDR) and Difficult-to-Treat Resistance (DTR).
References
- Rawson, T.M.; Ming, D.; Ahmad, R.; Moore, L.S.P.; Holmes, A.H. Antimicrobial use, drug-resistant infections and COVID-19. Nat. Rev. Microbiol. 2020, 18, 409–410. [Google Scholar] [CrossRef]
- Nguyen, K.V.; Nguyen, T.V.; Nguyen, H.T.T.; Le, D.V. Mutations in the gyrA, parC, and mexR genes provide functional insights into the fluoroquinolone-resistant Pseudomonas aeruginosa isolated in Vietnam. Infect Drug Resist. 2018, 28, 275–282. [Google Scholar] [CrossRef]
- Atassi, G.; Medernach, R.; Scheetz, M.; Nozick, S.; Rhodes, N.J.; Murphy-Belcaster, M.; Murphy, K.R.; Alisoltani, A.; Ozer, E.A.; Hauser, A.R. Genomics of Aminoglycoside Resistance in Pseudomonas aeruginosa Bloodstream Infections at a United States Academic Hospital. Microbiol. Spectr. 2023, 11, e0508722. [Google Scholar] [CrossRef]
- Yang, W.; Hu, F. Research Updates of Plasmid-Mediated Aminoglycoside Resistance 16S rRNA Methyltransferase. Antibiotics 2022, 11, 906. [Google Scholar] [CrossRef]
- Savaş, L.; Duran, N.; Savaş, N.; Önlen YOcak, S. The prevalence and resistance patterns of Pseudomonas aeruginosa in intensive care units in a university hospital. Turk. J. Med Sci. 2005, 35, 317–322. [Google Scholar]
- Asghar, A.H.; Ahmed, O.B. Prevalence of aminoglycoside resistance genes in Pseudomonas aeruginosa isolated from a tertiary care hospital in Makkah, KSA. Clin. Pract. 2018, 15, 438–441. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Nikolaidis, N.; Tolmasky, M.E. Rise and dissemination of aminoglycoside resistance: The aac(6′)-Ib paradigm. Front. Microbiol. 2013, 4, 121. [Google Scholar] [CrossRef]
- Dubois, V.; Poirel, L.; Marie, C.; Arpin, C.; Nordmann, P.; Quentin, C. Molecular characterization of a novel class 1 integron containing blaGES-1 and a fused product of aac(3)-Ib/aac(6″)-Ib″ gene cassettes in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2002, 46, 638–645. [Google Scholar] [CrossRef]
- Yamane, K.; Doi, Y.; Yokoyama, K.; Yagi, T.; Kurokawa, H.; Shibata, N.; Shibayama, K.; Kato, H.; Arakawa, Y. Genetic Environments of the rmtA Gene in Pseudomonas aeruginosa Clinical Isolates. Antimicrob. Agents Chemother. 2004, 48, 2069–2074. [Google Scholar] [CrossRef]
- Zuhuang, M.; Qin, L. Genes of 16S rRNA Methylase and Aminoglycoside-modifying Enzymes in Pan-drug Resistant Pseudomonas aeruginosa. Chin. J. Nosocomiol. 2006; in press. [Google Scholar]
- Cho, Y.J.; Moon, D.C.; Jin, J.S.; Choi, C.H.; Lee, Y.C.; Lee, J.C. Genetic basis of resistance to aminoglycosides in Acinetobacter spp. and spread of armA in Acinetobacter baumannii sequence group 1 in Korean hospitals. Diagn. Microbiol. Infect. Dis. 2009, 64, 185–190. [Google Scholar] [CrossRef]
- El-Badawy, M.F.; El-Far, S.W.; Althobaiti, S.S.; Abou-Elazm, F.I.A.; Shohayeb, M.M. The First Egyptian Report Showing the Co-Existence of blaNDM-25, blaOXA-23, blaOXA-181, and blaGES-1 Among Carbapenem-Resistant K. pneumoniae Clinical Isolates Genotyped by BOX-PCR. Infect. Drug Resist. 2020, 13, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P. CLSI Performance Standards for Antimicrobial Susceptibility Testing; CLSI Document Clinical Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2017. [Google Scholar]
- El-Badawy, M.F.; Alrobaian, M.M.; Shohayeb, M.M.; Abdelwahab, S.F. Investigation of six plasmid-mediated quinolone resistance genes among clinical isolates of pseudomonas: A genotypic study in Saudi Arabia. Infect. Drug Resist. 2019, 12, 915–923. [Google Scholar] [CrossRef] [PubMed]
- El-Badawy, M.F.; Tawakol, W.M.; El-Far, S.W.; Maghrabi, I.A.; Al-Ghamdi, S.A.; Mansy, M.S.; Ashour, M.S.; Shohayeb, M.M. Molecular Identification of Aminoglycoside-Modifying Enzymes and Plasmid-Mediated Quinolone Resistance Genes among Klebsiella pneumoniae Clinical Isolates Recovered from Egyptian Patients. Int. J. Microbiol. 2017, 2017, 8050432. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A.; Sanders, C.C. Use of a predictor panel to evaluate susceptibility test methods proposed for piperacillin-tazobactam. Antimicrob. Agents Chemother. 1993, 37, 2578–2583. [Google Scholar] [CrossRef]
- Mayrhofer, S.; Domig, K.J.; Mair, C.; Zitz, U.; Huys, G.; Kneifel, W.J. Comparison of broth microdilution, Etest, and agar disk diffusion methods for antimicrobial susceptibility testing of Lactobacillus acidophilus group members. Appl. Environ. Microbiol. 2008, 74, 3745–3748. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Panetta, V.; Della Rocca, M.T.; Durante, A.; Di Caprio, G.; Maggi, P. Profile of Co-Infection Prevalence and Antibiotics Use among COVID-19 Patients. Pathogens 2022, 11, 1250. [Google Scholar] [CrossRef]
- Cayci, Y.T.; Ulker, K.H.; Birinci, A. Evaluation of three different methods for susceptibility testing of gentamicin in carbapenem-resistant Enterobacterales. Infez. Med. 2021, 29, 568–573. [Google Scholar] [CrossRef]
- Batool, S.; Almaghaslah, D.; Alqahtani, A.; Almanasef, M.; Alasmari, M.; Vasudevan, R.; Attique, S.; Riaz, F. Aetiology and antimicrobial susceptibility pattern of bacterial isolates in community acquired pneumonia patients at Asir region, Saudi Arabia. Int. J. Clin. Pract. 2020, 75, e13667. [Google Scholar] [CrossRef]
- Yezli, S.; Shibl, A.M.; Livermore, D.M.; Memish, Z.A. Prevalence and antimicrobial resistance among Gram-negative pathogens in Saudi Arabia. J. Chemother. 2014, 26, 257–272. [Google Scholar] [CrossRef]
- O’Donnell, J.N.; Bidell, M.R.; Lodise, T.P. Approach to the Treatment of Patients with Serious Multidrug-Resistant Pseudomonas aeruginosa Infections. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 952–969. [Google Scholar] [CrossRef]
- Ahmed, M.A.S.; Khan, F.A.; Sultan, A.A.; Söderquist, B.; Ibrahim, E.B.; Jass, J.; Omrani, A.S. β-lactamase-mediated resistance in MDR-Pseudomonas aeruginosa from Qatar. Antimicrob. Resist. Infect. Control. 2020, 9, 170. [Google Scholar] [CrossRef]
- Helmy, O.M.; Kashef, M.T. Different phenotypic and molecular mechanisms associated with multidrug resistance in Gram-negative clinical isolates from Egypt. Infect. Drug Resist. 2017, 10, 479–498. [Google Scholar] [CrossRef]
- Lila, G.; Mulliqi-Osmani, G.; Bajrami, R.; Kurti, A.; Azizi, E.; Raka, L. The prevalence and resistance patterns of Pseudomonas aeruginosa in a tertiary care hospital in Kosovo. Infez. Med. 2017, 25, 21–26. [Google Scholar]
- Al-Tawfiq, J.A. Occurrence and antimicrobial resistance pattern of inpatient and outpatient isolates of Pseudomonas aeruginosa in a Saudi Arabian hospital: 1998–2003. Int. J. Infect. Dis. 2007, 11, 109–114. [Google Scholar] [CrossRef]
- Poole, K. Aminoglycoside Resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 479–487. [Google Scholar] [CrossRef]
- Panahi, T.; Asadpour, L.; Ranji, N. Distribution of aminoglycoside resistance genes in clinical isolates of Pseudomonas aeruginosa in north of Iran. Gene Rep. 2020, 21, 100929. [Google Scholar] [CrossRef]
- Torres, C.; Perlin, M.H.; Baquero, F.; Lerner, D.L.; Lerner, S.A. High-level amikacin resistance in Pseudomonas aeruginosa associated with a 3′-phosphotransferase with high affinity for amikacin. Int. J. Antimicrob. Agents 2000, 15, 257–263. [Google Scholar] [CrossRef]
- Teixeira, B.; Rodulfo, H.; Carreño, N.; Guzmán, M.; Salazar, E.; De Donato, M. Aminoglycoside resistance genes in Pseudomonas aeruginosa isolates from Cumana. Rev. Inst. Med. Trop. São Paulo 2016, 58, 13. [Google Scholar] [CrossRef]
- Vaziri, F.; Peerayeh, S.N.; Nejad, Q.B.; Farhadian, A. The prevalence of aminoglycoside-modifying enzyme genes (aac(6′)-I, aac(6′)-II, ant(2″)-I, aph(3′)-VI) in Pseudomonas aeruginosa. Clinics 2011, 66, 1519–1522. [Google Scholar]
- Miller, G.H.; Sabatelli, F.J.; Hare, R.S.; Glupczynski, Y.; Mackey, P.; Shlaes, D.; Shimizu, K.; Shaw, K.J.; Aminoglycoside Resistance Study Groups. The most frequent aminoglycoside resistance mechanisms—Changes with time and geographic area: A reflection of aminoglycoside usage patterns? Clin. Infect. Dis. 1997, 24, S46–S62. [Google Scholar] [CrossRef]
- Poonsuk, K.; Tribuddharat, C.; Chuanchuen, R. Aminoglycoside resistance mechanisms in Pseudomonas aeruginosa isolates from non-cystic fibrosis patients in Thailand. Can. J. Microbiol. 2013, 59, 51–56. [Google Scholar] [CrossRef]
- Thacharodi, A.; Lamont, I.L. Aminoglycoside resistance in Pseudomonas aeruginosa: The contribution of the MexXY-OprM efflux pump varies between isolates. J. Med. Microbiol. 2022, 71, 001551. [Google Scholar] [CrossRef]
- Meoli, A.; Ciavola, L.; Rahman, S.; Masetti, M.; Toschetti, T.; Morini, R.; Canto, G.D.; Auriti, C.; Caminiti, C.; Castagnola, E.; et al. Prevention of Surgical Site Infections in Neonates and Children: Non-Pharmacological Measures of Prevention. Antibiotics 2022, 11, 863. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).