Canaries’ Microbiota: The Gut Bacterial Communities along One Female Reproductive Cycle
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Extraction
2.3. 16S rRNA Sequencing
2.4. Data Analysis
3. Results
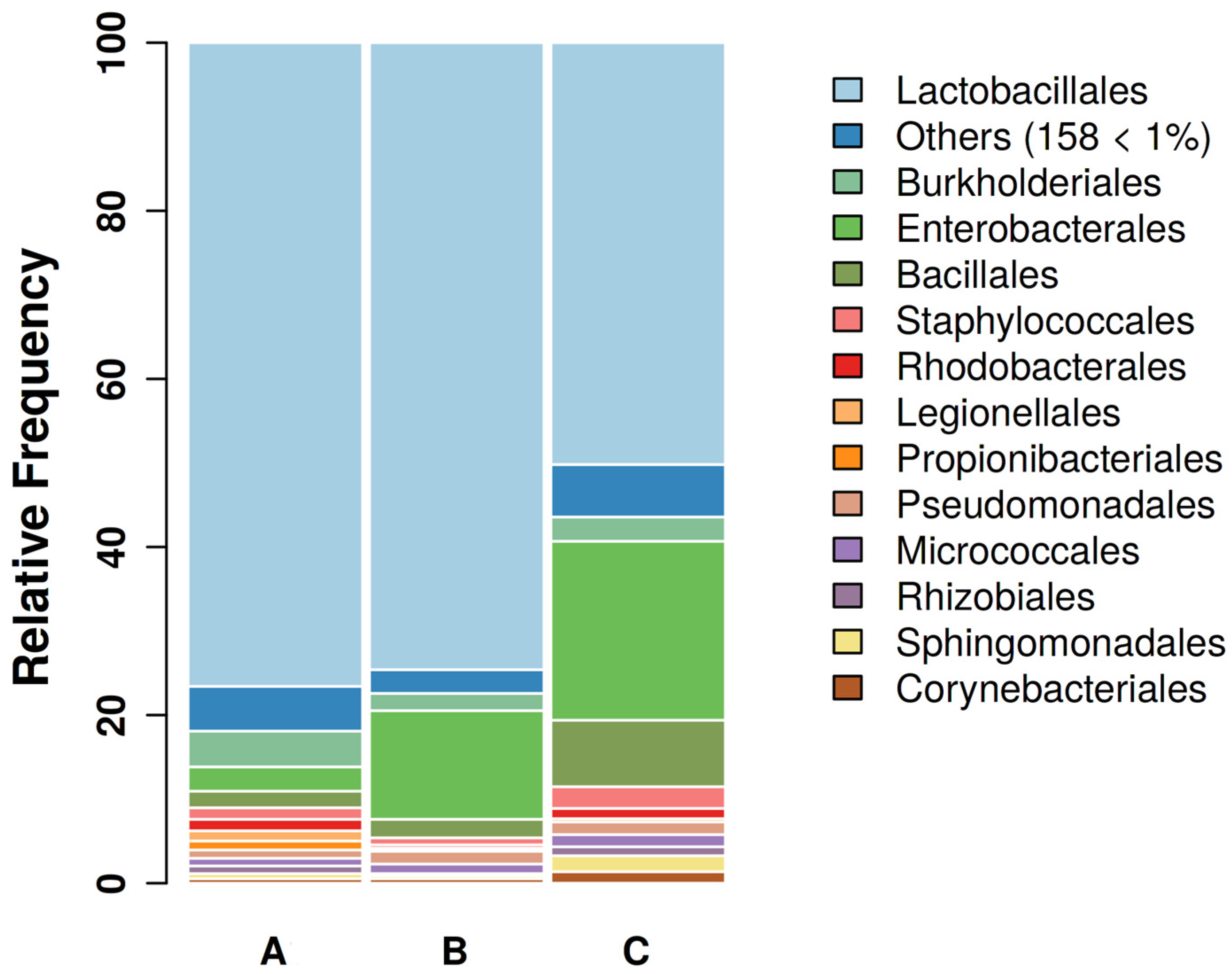
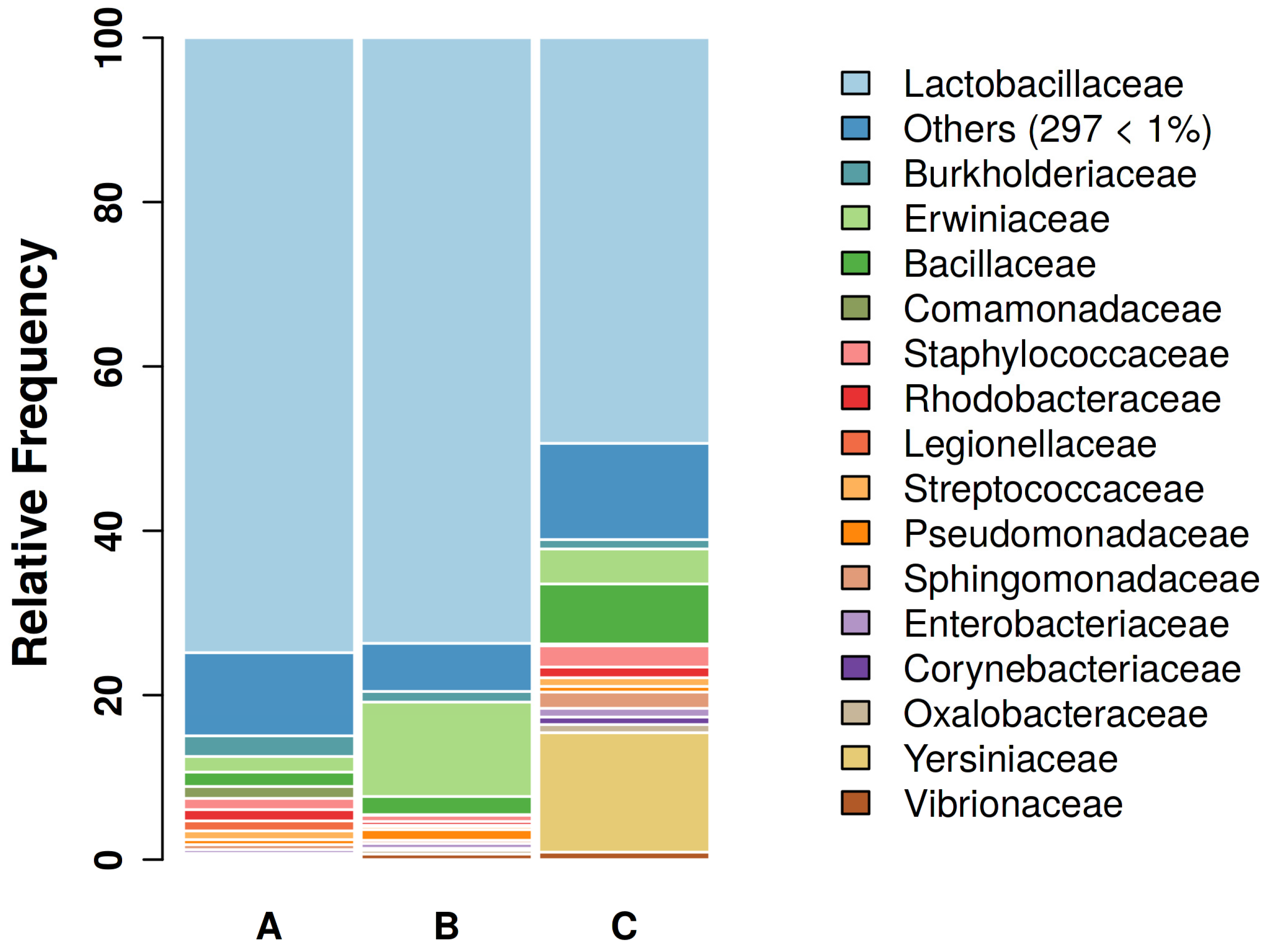
3.1. Sequencing Results and GBC Composition
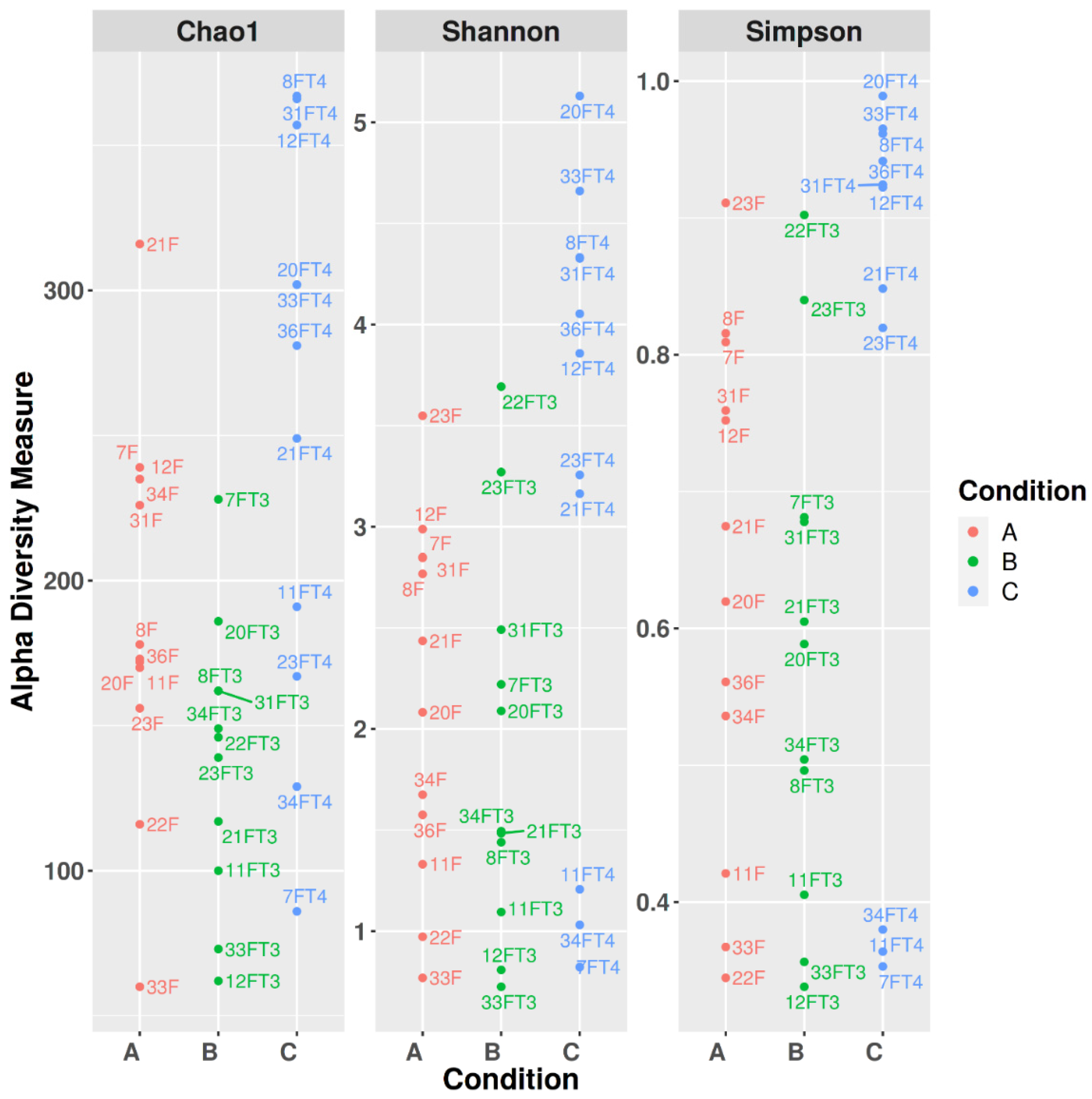
3.2. Alpha Diversity
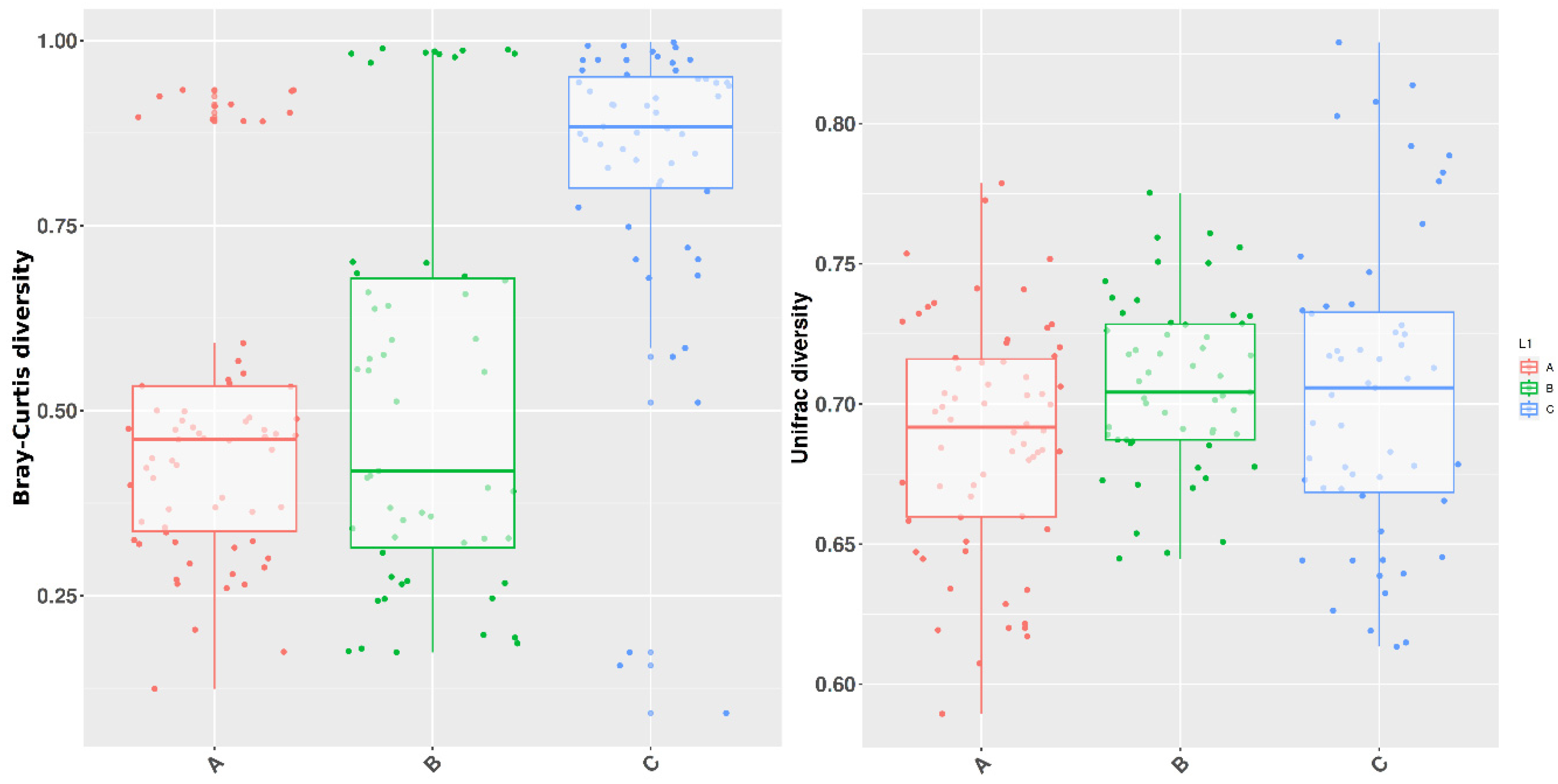
3.3. Beta Diversity
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Scott, R.T. Introduction: Microbiome in human reproduction. Fertil. Steril. 2015, 104, 1341–1343. [Google Scholar] [CrossRef]
- Kwa, W.T.; Sundarajoo, S.; Toh, K.Y.; Lee, J. Application of emerging technologies for gut microbiome research. Singap. Med. J. 2023, 64, 45–52. [Google Scholar] [CrossRef]
- Singer, E.; Bushnell, B.; Coleman-Derr, D.; Bowman, B.; Bowers, R.M.; Levy, A.; Gies, E.A.; Cheng, J.F.; Copeland, A.; Klenk, H.P.; et al. High-resolution phylogenetic microbial community profiling. ISME J. 2016, 10, 2020–2032. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Garcia-Reyero, N.; Martyniuk, C.J.; Tubbs, C.W.; Bisesi, J.H., Jr. Regulation of endocrine systems by the microbiome: Perspectives from comparative animal models. Gen. Comp. Endocrinol. 2020, 292, 113437. [Google Scholar] [CrossRef] [PubMed]
- Góngora, E.; Elliott, K.H.; Whyte, L. Gut microbiome is affected by inter-sexual and inter-seasonal variation in diet for thick-billed murres (Uria lomvia). Sci. Rep. 2021, 11, 1200. [Google Scholar] [CrossRef]
- Sun, F.; Chen, J.; Liu, K.; Tang, M.; Yang, Y. The avian gut microbiota: Diversity, influencing factors, and future directions. Front. Microbiol. 2022, 13, 934272. [Google Scholar] [CrossRef]
- Dai, D.; Qi, G.H.; Wang, J.; Zhang, H.J.; Qiu, K.; Wu, S.G. Intestinal microbiota of layer hens and its association with egg quality and safety. Poult. Sci. 2022, 101, 102008. [Google Scholar] [CrossRef]
- Ran, J.; Wan, Q.H.; Fang, S.G. Gut microbiota of endangered crested ibis: Establishment, diversity, and association with reproductive output. PLoS ONE 2021, 16, e0250075. [Google Scholar] [CrossRef]
- Maraci, Ö.; Antonatou-Papaioannou, A.; Jünemann, S.; Castillo-Gutiérrez, O.; Busche, T.; Kalinowski, J.; Caspers, B.A. The Gut Microbial Composition Is Species-Specific and Individual-Specific in Two Species of Estrildid Finches, the Bengalese Finch and the Zebra Finch. Front. Microbiol. 2021, 12, 619141. [Google Scholar] [CrossRef]
- Arnaiz-Villena, A.; Areces, C.; Ruiz-Del-Valle, V. El origen de los canarios. Revta Ornitol. Práct. 2012, 53, 3–11. [Google Scholar]
- Boseret, G.; Losson, B.; Mainil, J.G.; Thiry, E.; Saegerman, C. Zoonoses in pet birds: Review and perspectives. Vet. Res. 2013, 44, 36. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, J.C. Organization of vertebrate annual cycles: Implications for control mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, C.E.; Todisco, G.; Montani, A.; Profeta, F.; Di Provvido, A.; Foschi, G.; Persiani, T.; Marsilio, F. Reproductive disorders in domestic canaries (Serinus canarius domesticus): A retrospective study on bacterial isolates and their antimicrobial resistance in Italy from 2009 to 2012. Vet. Ital. 2018, 54, 169–174. [Google Scholar] [CrossRef]
- Rosen, L.B. Topics in Medicine and Surgery. Avian Reproductive Disorders. J. Exot. Pet. Med. 2012, 21, 124–131. [Google Scholar] [CrossRef]
- Rouse, M.L., Jr.; Ball, G.F. Lesions targeted to the anterior forebrain disrupt vocal variability associated with testosterone-induced sensorimotor song development in adult female canaries, Serinus canaria. Dev. Neurobiol. 2016, 76, 3–18. [Google Scholar] [CrossRef]
- Lalot, M.; Bovet, D. Prosociality and reciprocity according to parental status, communication, and personality in domestic canaries (Serinus canaria). Behav. Process. 2023, 205, 104818. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Castillo-Carranza, S.A.; Guard, B.; Gomez-Vazquez, J.P.; Dowd, S.E.; Brigthsmith, D.J. Comprehensive Molecular Characterization of Bacterial Communities in Feces of Pet Birds Using 16S Marker Sequencing. Microb. Ecol. 2017, 73, 224–235. [Google Scholar] [CrossRef]
- Robino, P.; Ferrocino, I.; Rossi, G.; Dogliero, A.; Alessandria, V.; Grosso, L.; Galosi, L.; Tramuta, C.; Cocolin, L.; Nebbia, P. Changes in gut bacterial communities in canaries infected by Macrorhabdus ornithogaster. Avian Pathol. 2019, 48, 111–120. [Google Scholar] [CrossRef]
- Kumar, L.; Dwivedi, M.; Jain, N.; Shete, P.; Solanki, S.; Gupta, R.; Jain, A. The Female Reproductive Tract Microbiota: Friends and Foe. Life 2023, 13, 1313. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Chiu, C.H. Species Richness: Estimation and Comparison. In Wiley StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2016; pp. 1–26. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Jaccard, P. The Distribution of the Flora in the Alpine Zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A Comprehensive Online Resource for Quality Checked and Aligned Ribosomal RNA Sequence Data Compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 7 August 2023).
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). In Wiley Statsref: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 1–15. [Google Scholar]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Huynh, U.; Zastrow, M.L. Metallobiology of Lactobacillaceae in the gut microbiome. J. Inorg. Biochem. 2023, 238, 112023. [Google Scholar] [CrossRef] [PubMed]
- Mohsin Bukhari, S.; Ahmed Alghamdi, H.; Ur Rehman, K.; Andleeb, S.; Ahmad, S.; Khalid, N. Metagenomics analysis of the fecal microbiota in Ring-necked pheasants (Phasianus colchicus) and Green pheasants (Phasianus versicolor) using next generation sequencing. Saudi J. Biol. Sci. 2022, 29, 1781–1788. [Google Scholar] [CrossRef]
- De Cesare, A.; Sirri, F.; Manfreda, G.; Moniaci, P.; Giardini, A.; Zampiga, M.; Meluzzi, A. Effect of dietary supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) on caecum microbioma and productive performance in broiler chickens. PLoS ONE 2017, 12, e0176309. [Google Scholar] [CrossRef]
- Mattar, A.F.; Teitelbaum, D.H.; Drongowski, R.A.; Yongyi, F.; Harmon, C.M.; Coran, A.G. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr. Surg. Int. 2002, 18, 586–590. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.H.; Whang, K.Y.; Kim, Y.J.; Oh, S. Inhibition of Escherichia coli O157:H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells. J. Microbiol. Biotechnol. 2008, 18, 1278–1285. [Google Scholar]
- Fujisawa, T.; Shirasaka, S.; Watabe, J.; Mitsuoka, T. Lactobacillus aviarius sp. nov.: A new species isolated from the intestine of chickens. Syst. Appl. Microbiol. 1984, 5, 414–420. [Google Scholar] [CrossRef]
- Gong, J.; Si, W.; Forster, R.J.; Huang, R.; Yu, H.; Yin, Y.; Yang, C.; Han, Y. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: From crops to ceca. FEMS Microbiol. Ecol. 2007, 59, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Z.; Hong, Y.; Jiang, K.; Yu, L.; Xie, X.; Mi, Y.; Zhu, S.J.; Zhang, C.; Li, J. Transplantion of predominant Lactobacilli from native hens to commercial hens could indirectly regulate their ISC activity by improving intestinal microbiota. Microb. Biotechnol. 2022, 15, 1235–1252. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Y.; Zhu, B.; Gao, G.F.; Guo, Y.; Hu, Y. Metagenome-assembled genomes and gene catalog from the chicken gut microbiome aid in deciphering antibiotic resistomes. Commun. Biol. 2021, 4, 1305. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fang, M.; Hu, Y.; Yang, Y.; Yang, M.; Chen, Y. Characterization of the most abundant Lactobacillus species in chicken gastrointestinal tract and potential use as probiotics for genetic engineering. Acta Biochim. Biophys. Sin. (Shanghai) 2014, 46, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Danzeisen, J.L.; Calvert, A.J.; Noll, S.L.; McComb, B.; Sherwood, J.S.; Logue, C.M.; Johnson, T.J. Succession of the turkey gastrointestinal bacterial microbiome related to weight gain. PeerJ 2013, 1, e237. [Google Scholar] [CrossRef]
- West, A.G.; DeLaunay, A.; Marsh, P.; Perry, E.K.; Jolly, M.; Gartrell, B.D.; Pas, A.; Digby, A.; Taylor, M.W. Gut microbiota of the threatened takahē: Biogeographic patterns and conservation implications. Anim. Microbiome 2022, 4, 11. [Google Scholar] [CrossRef]
- Czerwiński, J.; Højberg, O.; Smulikowska, S.; Engberg, R.M.; Mieczkowska, A. Effects of sodium butyrate and salinomycin upon intestinal microbiota, mucosal morphology and performance of broiler chickens. Arch. Anim. Nutr. 2012, 66, 102–116. [Google Scholar] [CrossRef]
- Chang, J.; Wang, T.; Wang, P.; Yin, Q.; Liu, C.; Zhu, Q.; Lu, F.; Gao, T. Compound probiotics alleviating aflatoxin B1 and zearalenone toxic effects on broiler production performance and gut microbiota. Ecotoxicol. Environ. Saf. 2020, 194, 110420. [Google Scholar] [CrossRef]
- Guo, H.; Wang, P.; Liu, C.; Chang, J.; Yin, Q.; Wang, L.; Jin, S.; Zhu, Q.; Lu, F. Compound mycotoxin detoxifier alleviating aflatoxin B1 toxic effects on broiler growth performance, organ damage and gut microbiota. Poult. Sci. 2023, 102, 102434. [Google Scholar] [CrossRef]
- Wiersema, M.L.; Koester, L.R.; Schmitz-Esser, S.; Koltes, D.A. Comparison of intestinal permeability, morphology, and ileal microbial communities of commercial hens housed in conventional cages and cage-free housing systems. Poult. Sci. 2021, 100, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Walter, J. Ecological role of lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.K.; Chen, Y.Y.; Fang, A.; Shaw, G.T.; Hung, C.M.; Wang, D. Maternal gut microbes shape the early-life assembly of gut microbiota in passerine chicks via nests. Microbiome 2020, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Alanis-Lopez, C.; Marroquin-Cardona, A.G.; Kawas, J.R. Composition and Potential Function of Fecal Bacterial Microbiota from Six Bird Species. Birds 2021, 2, 42–59. [Google Scholar] [CrossRef]
- Alcaraz, L.D.; Hernández, A.M.; Peimbert, M. Exploring the cockatiel (Nymphicus hollandicus) fecal microbiome, bacterial inhabitants of a worldwide pet. PeerJ 2016, 4, e2837. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A. Avian Molting. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 907–917. [Google Scholar] [CrossRef]
- Vézina, F.; Gustowska, A.; Jalvingh, K.M.; Chastel, O.; Piersma, T. Hormonal correlates and thermoregulatory consequences of molting on metabolic rate in a northerly wintering shorebird. Physiol. Biochem. Zool. 2009, 82, 129–142. [Google Scholar] [CrossRef]
- Kuenzel, W.J. Neurobiology of molt in avian species. Poult. Sci. 2003, 82, 981–991. [Google Scholar] [CrossRef]
- Smiley, K.O. Prolactin and avian parental care: New insights and unanswered questions. Horm. Behav. 2019, 111, 114–130. [Google Scholar] [CrossRef]
- Murphy, M.E. Energetics and nutrition of molt. In Avian Energetics and Nutritional Ecology; Carey, C., Ed.; Chapman and Hall: London, UK, 1996; pp. 158–198. [Google Scholar]
- Cho, H.; Lee, W.Y. Interspecific comparison of the fecal microbiota structure in three Arctic migratory bird species. Ecol. Evol. 2020, 10, 5582–5594. [Google Scholar] [CrossRef]
- Dewar, M.L.; Arnould, J.P.; Krause, L.; Trathan, P.; Dann, P.; Smith, S.C. Influence of fasting during moult on the faecal microbiota of penguins. PLoS ONE 2014, 9, e99996. [Google Scholar] [CrossRef]
- Yaqoob, M.U.; El-Hack, M.E.A.; Hassan, F.; El-Saadony, M.T.; Khafaga, A.F.; Batiha, G.E.; Yehia, N.; Elnesr, S.S.; Alagawany, M.; El-Tarabily, K.A.; et al. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021, 100, 101143. [Google Scholar] [CrossRef] [PubMed]
- Coutteel, P. Veterinary aspects of breeding management in captive passerines. Semin. Avian Exot. Pet Med. 2003, 12, 3–10. [Google Scholar] [CrossRef]
- Larsen, O.F.A.; Claassen, E. The mechanistic link between health and gut microbiota diversity. Sci. Rep. 2018, 8, 2183. [Google Scholar] [CrossRef]
- Offret, C.; Paulino, S.; Gauthier, O.; Château, K.; Bidault, A.; Corporeau, C.; Miner, P.; Petton, B.; Pernet, F.; Fabioux, C.; et al. The marine intertidal zone shapes oyster and clam digestive bacterial microbiota. FEMS Microbiol. Ecol. 2020, 96, fiaa078. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Gao, H.; Yang, Y.; Liu, H.; Yu, H.; Chen, Z.; Dong, B. Seasonal dynamics and starvation impact on the gut microbiome of urochordate ascidian Halocynthia roretzi. Anim. Microbiome 2020, 2, 30. [Google Scholar] [CrossRef]
- Graells, T.; Ishak, H.; Larsson, M.; Guy, L. The all-intracellular order Legionellales is unexpectedly diverse, globally distributed and lowly abundant. FEMS Microbiol. Ecol. 2018, 94, fiy185. [Google Scholar] [CrossRef]
- Lan, X.; Peng, X.; Du, T.; Xia, Z.; Gao, Q.; Tang, Q.; Yi, S.; Yang, G. Alterations of the Gut Microbiota and Metabolomics Associated with the Different Growth Performances of Macrobrachium rosenbergii Families. Animals 2023, 13, 1539. [Google Scholar] [CrossRef]
- Assis, B.A.; Bell, T.H.; Engler, H.I.; King, W.L. Shared and unique responses in the microbiome of allopatric lizards reared in a standardized environment. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2023, 339, 5–12. [Google Scholar] [CrossRef]
- Cunha, M.P.V.; Guimarães, M.B.; Davies, Y.M.; Milanelo, L.; Knöbl, T. Bactérias gram-negativas em cardeais (Paroaria coronata e Paroaria dominicana) apreendidos do tráfico de animais silvestres. Braz. J. Vet. Res. Anim. Sci. 2016, 53, 107–111. [Google Scholar] [CrossRef][Green Version]
- Davies, Y.M.; Guimarães, M.B.; Milanelo, L.; de Oliveira, M.G.X.; de Moura Gomes, V.T.; Azevedo, N.P.; Cunha, M.P.V.; Moreno, L.Z.; Romero, D.C.; Christ, A.P.G.; et al. A survey on gram-negative bacteria in saffron finches (Sicalis flaveola) from illegal wildlife trade in Brazil. Braz. J. Vet. Res. Anim. Sci. 2016, 53, 286–294. [Google Scholar] [CrossRef][Green Version]
- Davies, Y.M.; Franco, L.S.; Barbosa, F.B.; Vanin, C.L.; Gomes, V.T.M.; Moreno, L.Z.; Barbosa, M.R.F.; Sato, M.I.Z.; Moreno, A.M.; Knöbl, T. Use of MALDI-TOF for identification and surveillance of gram-negative bacteria in captive wild psittacines. Braz. J. Biol. 2021, 82, e233523. [Google Scholar] [CrossRef] [PubMed]
- Penner, J.L. Genus XXIX. Proteus. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Garrity, G.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; Volume 2, pp. 745–753. [Google Scholar]
- Wang, W.; Wang, A.; Yang, Y.; Wang, F.; Liu, Y.; Zhang, Y.; Sharshov, K.; Gui, L. Composition, diversity and function of gastrointestinal microbiota in wild red-billed choughs (Pyrrhocorax pyrrhocorax). Int. Microbiol. 2019, 22, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Escallón, C.; Belden, L.K.; Moore, I.T. The Cloacal Microbiome Changes with the Breeding Season in a Wild Bird. Integr. Org. Biol. 2019, 1, oby009. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.; Hucul, C.; Reasor, E.; Smith, T.; McGlothlin, J.W.; Haak, D.C.; Belden, L.K.; Moore, I.T. Assessing age, breeding stage, and mating activity as drivers of variation in the reproductive microbiome of female tree swallows. Ecol. Evol. 2021, 11, 11398–11413. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef]
- Geier, M.S.; Torok, V.A.; Allison, G.E.; Ophel-Keller, K.; Gibson, R.A.; Munday, C.; Hughes, R.J. Dietary omega-3 polyunsaturated fatty acid does not influence the intestinal microbial communities of broiler chickens. Poult. Sci. 2009, 88, 2399–2405. [Google Scholar] [CrossRef]
- Fouhy, F.; Deane, J.; Rea, M.C.; O’Sullivan, Ó.; Ross, R.P.; O’Callaghan, G.; Plant, B.J.; Stanton, C. The effects of freezing on faecal microbiota as determined using MiSeq sequencing and culture-based investigations. PLoS ONE 2015, 10, e0119355. [Google Scholar] [CrossRef]
- Tang, Q.; Jin, G.; Wang, G.; Liu, T.; Liu, X.; Wang, B.; Cao, H. Current Sampling Methods for Gut Microbiota: A Call for More Precise Devices. Front. Cell. Infect. Microbiol. 2020, 10, 151. [Google Scholar] [CrossRef]
- Hadley, T.L. Disorders of the psittacine gastrointestinal tract. Vet. Clin. N. Am. Exot. Anim. Pract. 2005, 8, 329–349. [Google Scholar] [CrossRef]
| Groups | A | B | C |
|---|---|---|---|
| Reproductive phase | Parental care | Molting | Rest |
| N. of samples | 12 | 12 | 11 |
| A vs. B | A vs. C | B vs. C | |
|---|---|---|---|
| Pielou’s Evenness | 0.622461 | 0.048900 | 0.122800 |
| Faith phylogenetic diversity | 0.026716 | 0.218355 | 0.009493 |
| Observed Features | 0.022741 | 0.056219 | 0.009453 |
| Shannon | 0.423656 | 0.042254 | 0.045201 |
| A vs. B | A vs. C | B vs. C | |
|---|---|---|---|
| Bray-Curtis dissimilarity | 0.863137 | 0.002997 | 0.011988 |
| Unweighted Unifrac | 0.001998 | 0.005994 | 0.003996 |
| Species | Prevalent Microbiota Components | Reference | |
|---|---|---|---|
| Passerines | Canary (Serinus canaria) | Lactobacillus, Clostridium | [19] |
| Lactobacillaceae | [18] | ||
| Bengalese finch (Lonchura striata domestica) | Lactobacillaceae, Campylobacteraceae | [10] | |
| Lactobacillaceae | [56] | ||
| Zebra finch (Taeniopygia guttata) | Lactobacillaceae Campylobacteraceae | [10] | |
| Campylobacteraceae | [56] | ||
| Lactobacillaceae, Campylobacteriaceae, Bifidobacteriaceae | [57] | ||
| Psittacines | Cockatiel (Nymphicus hollandicus) | Erysipelotrichaceae, Lachnospiraceae, Clostridiaceae | [18] |
| Erysipelotrichaceae, Lachnospiraceae, Mycoplasmataceae | [57] | ||
| Erysipelotrichaceae, Lactobacillaceae | [58] | ||
| Budgerigar (Melopsittacus undulatus) | Lactobacillaceae | [18] | |
| Lovebird (Agapornis spp.) | Lactobacillaceae | [57] | |
| Rose-ringed Parakeet (Psittacula krameri) | Lactobacillaceae, Leuconostocaceae | [57] | |
| Red-rumped parrot (Psephotus haematonotus) | Lactobacillaceae | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattab, J.; Marruchella, G.; Sibra, A.; Tiscar, P.G.; Todisco, G. Canaries’ Microbiota: The Gut Bacterial Communities along One Female Reproductive Cycle. Microorganisms 2023, 11, 2289. https://doi.org/10.3390/microorganisms11092289
Hattab J, Marruchella G, Sibra A, Tiscar PG, Todisco G. Canaries’ Microbiota: The Gut Bacterial Communities along One Female Reproductive Cycle. Microorganisms. 2023; 11(9):2289. https://doi.org/10.3390/microorganisms11092289
Chicago/Turabian StyleHattab, Jasmine, Giuseppe Marruchella, Alessandra Sibra, Pietro Giorgio Tiscar, and Gianluca Todisco. 2023. "Canaries’ Microbiota: The Gut Bacterial Communities along One Female Reproductive Cycle" Microorganisms 11, no. 9: 2289. https://doi.org/10.3390/microorganisms11092289
APA StyleHattab, J., Marruchella, G., Sibra, A., Tiscar, P. G., & Todisco, G. (2023). Canaries’ Microbiota: The Gut Bacterial Communities along One Female Reproductive Cycle. Microorganisms, 11(9), 2289. https://doi.org/10.3390/microorganisms11092289







