Mediterranean Plants with Antimicrobial Activity against Staphylococcus aureus, a Meta-Analysis for Green Veterinary Pharmacology Applications
Abstract
:1. Introduction
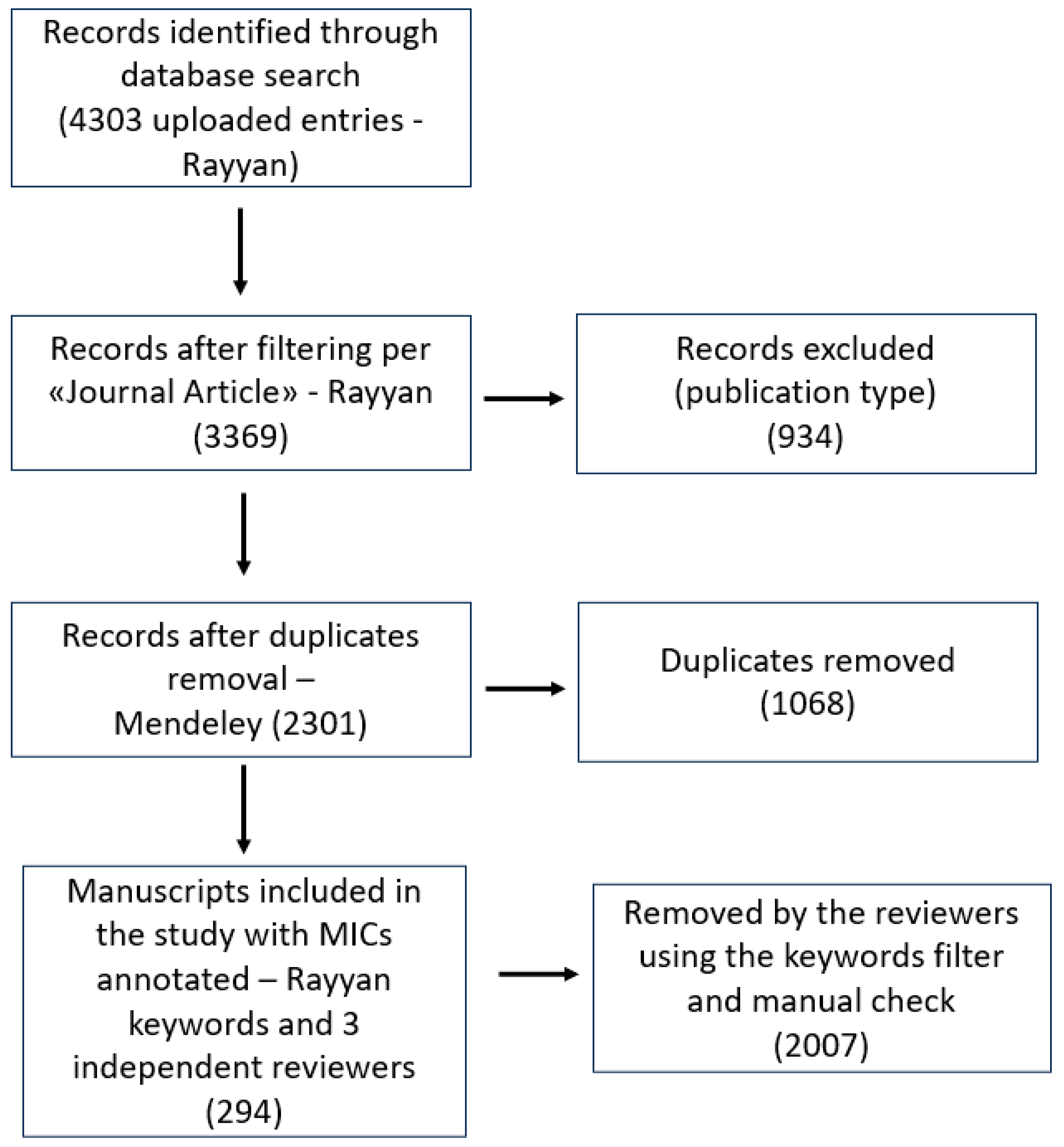
2. Materials and Methods
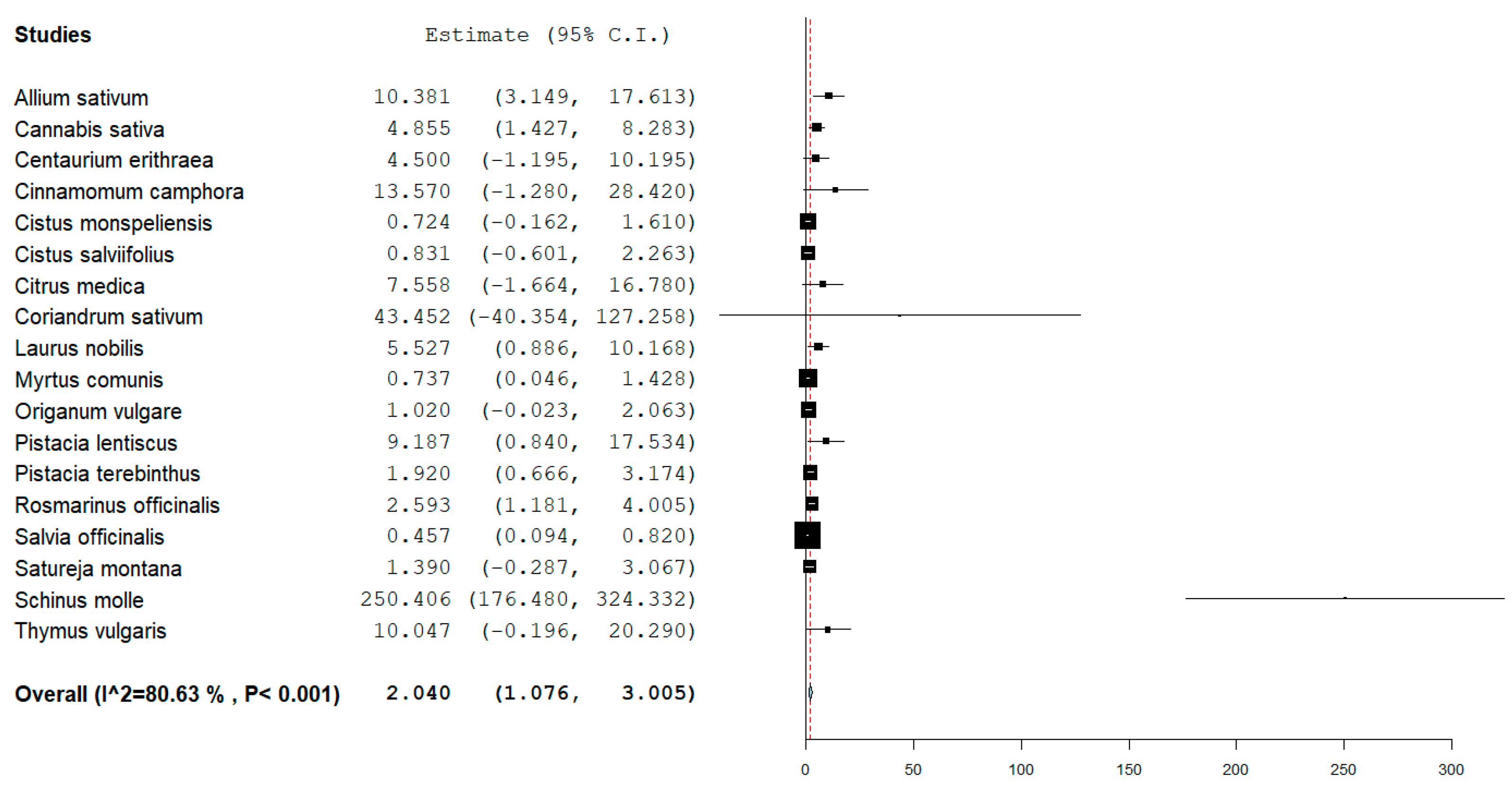
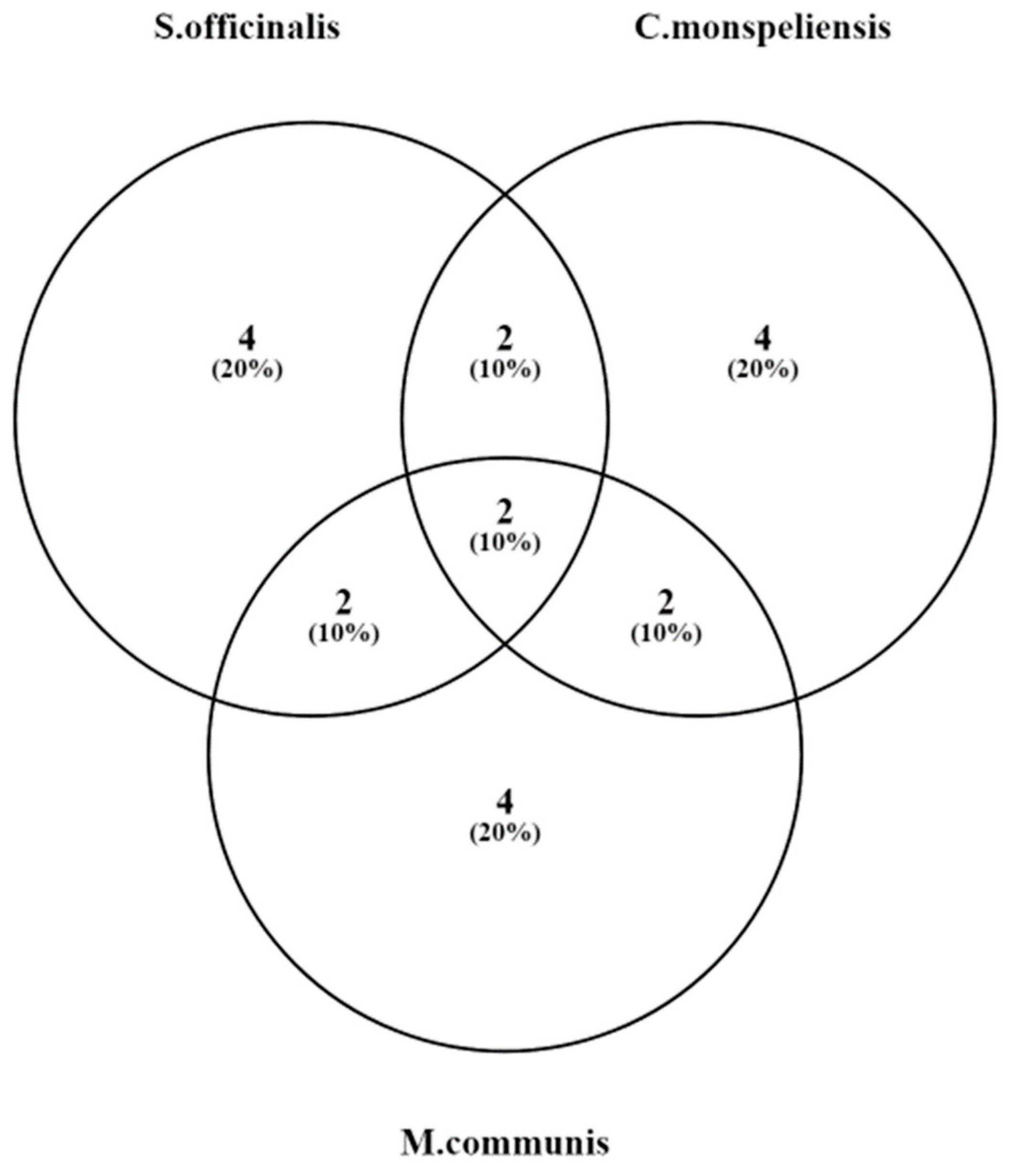
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Piras, C.; Tilocca, B.; Castagna, F.; Roncada, P.; Britti, D.; Palma, E. Plants with Antimicrobial Activity Growing in Italy: A Pathogen-Driven Systematic Review for Green Veterinary Pharmacology Applications. Antibiotics 2022, 11, 919. [Google Scholar] [CrossRef]
- Blasi, C.; Capotorti, G.; Michetti, L.; Rosati, L.; Smiraglia, D. Landscape heterogeneity and vegetation potential in Italy. Bocconea 2009, 23, 2009. [Google Scholar]
- Selvi, F.; Campetella, G.; Canullo, R.; Chelli, S.; Domina, G.; Farris, E.; Gasperini, C.; Rosati, L.; Wellstein, C.; Carrari, E. The Italian endemic forest plants. Plant Ecol. Evol. 2023, 156, 29–45. [Google Scholar] [CrossRef]
- Croce, A.; Stinca, A.; Santangelo, A.; Esposito, A. Exploring vascular flora diversity of two protected sandy coastal areas in southern Italy. Rend. Lincei. Sci. Fis. Nat. 2019, 30, 323–336. [Google Scholar] [CrossRef]
- Fois, M.; Farris, E.; Calvia, G.; Campus, G.; Fenu, G.; Porceddu, M.; Bacchetta, G. The endemic vascular flora of Sardinia: A dynamic checklist with an overview of biogeography and conservation status. Plants 2022, 11, 601. [Google Scholar] [CrossRef]
- Piras, C.; Hale, O.; Reynolds, C.K.; Jones, B.; Taylor, N.; Morris, M.; Cramer, R. LAP-MALDI MS coupled with machine learning: An ambient mass spectrometry approach for high-throughput diagnostics. Chem. Sci. 2022, 13, 1746–1758. [Google Scholar] [CrossRef]
- Piras, C.; Soggiu, A.; Bonizzi, L.; Greco, V.; Ricchi, M.; Arrigoni, N.; Bassols, A.; Urbani, A.; Roncada, P. Identification of immunoreactive proteins of Mycobacterium avium subsp. paratuberculosis. Proteomics 2015, 15, 813–823. [Google Scholar] [CrossRef]
- Greco, V.; Piras, C.; Pieroni, L.; Urbani, A. Direct assessment of plasma/serum sample quality for proteomics biomarker investigation. In Serum/Plasma Proteomics. Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; pp. 3–21. [Google Scholar]
- Piras, C.; Hale, O.J.; Reynolds, C.K.; Jones, A.K.; Taylor, N.; Morris, M.; Cramer, R. Speciation and milk adulteration analysis by rapid ambient liquid MALDI mass spectrometry profiling using machine learning. Sci. Rep. 2021, 11, 3305. [Google Scholar] [CrossRef]
- Castagna, F.; Piras, C.; Palma, E.; Musolino, V.; Lupia, C.; Bosco, A.; Rinaldi, L.; Cringoli, G.; Musella, V.; Britti, D. Green veterinary pharmacology applied to parasite control: Evaluation of punica granatum, artemisia campestris, salix caprea aqueous macerates against gastrointestinal nematodes of sheep. Vet. Sci. 2021, 8, 237. [Google Scholar] [CrossRef]
- Piras, C.; Gugliandolo, E.; Castagna, F.; Palma, E.; Britti, D. Ivermectin (IVM) possible side activities and implications in antimicrobial resistance and animal welfare: The authors’ perspective. Vet. Sci. 2022, 9, 24. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Impieri, M. Ethnobotanical notes about some uses of medicinal plants in Alto Tirreno Cosentino area (Calabria, Southern Italy). J. Ethnobiol. Ethnomed. 2007, 3, 34. [Google Scholar] [CrossRef]
- Passalacqua, N.G.; Guarrera, P.M.; De Fine, G. Contribution to the knowledge of the folk plant medicine in Calabria region (Southern Italy). Fitoterapia 2007, 78, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Dey, A.; Koirala, N.; Shaheen, S.; El Omari, N.; Salehi, B.; Goloshvili, T.; Cirone Silva, N.C.; Bouyahya, A.; Vitalini, S.; et al. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front. Pharmacol. 2021, 12, 600139. [Google Scholar] [CrossRef] [PubMed]
- Bottoni, M.; Milani, F.; Mozzo, M.; Kolloffel, D.A.R.; Papini, A.; Fratini, F.; Maggi, F.; Santagostini, L. Sub-tissue localization of phytochemicals in cinnamomum camphora (L.) j. presl. growing in northern Italy. Plants 2021, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Juliano, C.; Usai, M. Analytical Profiling of Phenolic Compounds in Extracts of Three Cistus Species from Sardinia and Their Potential Antimicrobial and Antioxidant Activity. Chem. Biodivers. 2021, 18, e2100053. [Google Scholar] [CrossRef]
- Zucca, P.; Pintus, M.; Manzo, G.; Nieddu, M.; Steri, D.; Rinaldi, A.C. Antimicrobial, antioxidant and anti-tyrosinase properties of extracts of the Mediterranean parasitic plant Cytinus hypocistis. BMC Res. Notes 2015, 8, 562. [Google Scholar] [CrossRef]
- Sanna, C.; Maxia, A.; Fenu, G.; Loi, M.C. So uncommon and so singular, but underexplored: An updated overview on ethnobotanical uses, biological properties and phytoconstituents of sardinian endemic plants. Plants 2020, 9, 958. [Google Scholar] [CrossRef]
- Mir, M.A.; Bashir, N.; Alfaify, A.; Oteef, M.D.Y. GC-MS analysis of Myrtus communis extract and its antibacterial activity against gram-positive bacteria. BMC Complement. Med. Ther. 2020, 20, 86. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Barros, J.C.; de Oliveira, C.E.V.; da Conceição, M.L. Influence of Origanum vulgare L. essential oil on enterotoxin production, membrane permeability and surface characteristics of Staphylococcus aureus. Int. J. Food Microbiol. 2010, 137, 308–311. [Google Scholar] [CrossRef]
- Mezni, F.; Aouadhi, C.; Khouja, M.L.; Khaldi, A.; Maaroufi, A. In vitro antimicrobial activity of Pistacia lentiscus L. edible oil and phenolic extract. Nat. Prod. Res. 2015, 29, 565–570. [Google Scholar] [CrossRef]
- Pulaj, B.; Mustafa, B.; Nelson, K.; Quave, C.L.; Hajdari, A. Chemical composition and in vitro antibacterial activity of Pistacia terebinthus essential oils derived from wild populations in Kosovo. BMC Complement. Altern. Med. 2016, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Hillebrand, G.G.; Nunez, G. Rosmarinus officinalis l. (rosemary) extracts containing carnosic acid and carnosol are potent quorum sensing inhibitors of staphylococcus aureus virulence. Antibiotics 2020, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Farahpour, M.R.; Pirkhezr, E.; Ashrafian, A.; Sonboli, A. Accelerated healing by topical administration of Salvia officinalis essential oil on Pseudomonas aeruginosa and Staphylococcus aureus infected wound model. Biomed. Pharmacother. 2020, 128, 110120. [Google Scholar] [CrossRef]
- Juliano, C.; Mattana, A.; Usai, M. Composition and in vitro antimicrobial activity of the essential oil of thymus herba-barona loisel growing wild in sardinia. J. Essent. Oil Res. 2000, 12, 516–522. [Google Scholar] [CrossRef]
- de Carvalho, R.J.; de Souza, G.T.; Honório, V.G.; de Sousa, J.P.; da Conceição, M.L.; Maganani, M.; de Souza, E.L. Comparative inhibitory effects of Thymus vulgaris L. essential oil against Staphylococcus aureus, Listeria monocytogenes and mesophilic starter co-culture in cheese-mimicking models. Food Microbiol. 2015, 52, 59–65. [Google Scholar] [CrossRef]
- D’Agostino, G.; Badalamenti, N.; Franco, P.; Bruno, M.; Gallo, G. The chemical composition of the flowers essential oil of Inula crithmoides (Asteraceae) growing in aeolian islands, Sicily (Italy) and its biocide properties on microorganisms affecting historical art crafts. Nat. Prod. Res. 2021, 36, 2993–3001. [Google Scholar] [CrossRef]
- Ouassou, H.; Bouhrim, M.; Kharchoufa, L.; Imtara, H.; Elhouda Daoudi, N.; Benoutman, A.; Bencheikh, N.; Ouahhoud, S.; Elbouzidi, A.; Bnouham, M. Caralluma europaea (Guss) N.E.Br.: A review on ethnomedicinal uses, phytochemistry, pharmacological activities, and toxicology. J. Ethnopharmacol. 2021, 273, 113769. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Bezruk, I.; Ivanauskas, L.; Georgiyants, V. Comparative analysis of apocarotenoids and phenolic constituents of Crocus sativus stigmas from 11 countries: Ecological impact. Arch. Pharm. 2022, 355, e2100468. [Google Scholar] [CrossRef]
- Najar, B.; Nardi, V.; Cervelli, C.; Mancianti, F.; Nardoni, S.; Ebani, V.V.; Pistelli, L. Helichrysum araxinum Takht. ex Kirp. grown in Italy: Volatiloma composition and in vitro antimicrobial activity. Zeitschrift Naturforsch.-Sect. C J. Biosci. 2020, 75, 265–270. [Google Scholar] [CrossRef]
- Turchetti, G.; Garzoli, S.; Masci, V.L.; Sabia, C.; Iseppi, R.; Giacomello, P.; Tiezzi, A.; Ovidi, E. Antimicrobial testing of schinus molle (l.) leaf extracts and fractions followed by gc-ms investigation of biological active fractions. Molecules 2020, 25, 1977. [Google Scholar] [CrossRef]
- Zengin, G.; Menghini, L.; Sotto, A.D.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic analyses, in vitro biological activities, and cytotoxicity of cannabis sativa l. Essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [PubMed]
- El Menyiy, N.; Guaouguaou, F.E.; El Baaboua, A.; El Omari, N.; Taha, D.; Salhi, N.; Shariati, M.A.; Aanniz, T.; Benali, T.; Zengin, G.; et al. Phytochemical properties, biological activities and medicinal use of Centaurium erythraea Rafn. J. Ethnopharmacol. 2021, 276, 114171. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Cozzolino, A.; de Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò cultivated in southern Italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of essential oil and its biological activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Quave, C.L.; Estévez-Carmona, M.; Compadre, C.M.; Hobby, G.; Hendrickson, H.; Beenken, K.E.; Smeltzer, M.S. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE 2012, 7, e28737. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Coppola, R.; Nazzaro, F. Phenolic composition and antimicrobial and antiquorum sensing activity of an ethanolic extract of peels from the apple cultivar annurca. J. Med. Food 2011, 14, 957–963. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Yang, J.L.; Venditti, A.; Moridi Farimani, M. A review of the phytochemistry, ethnopharmacology and biological activities of Teucrium genus (Germander). Nat. Prod. Res. 2021, 36, 5647–5664. [Google Scholar] [CrossRef]
- Badalamenti, N.; Modica, A.; Ilardi, V.; Bruno, M.; Maresca, V.; Zanfardino, A.; Di Napoli, M.; Castagliuolo, G.; Varcamonti, M.; Basile, A. Daucus carota subsp. maximus (Desf.) Ball from Pantelleria, Sicily (Italy): Isolation of essential oils and evaluation of their bioactivity. Nat. Prod. Res. 2021, 36, 5842–5847. [Google Scholar] [CrossRef]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complement. Altern. Med. 2019, 19, 82. [Google Scholar] [CrossRef]
- Pellegrini, M.; Ricci, A.; Serio, A.; Chaves-López, C.; Mazzarrino, G.; D’Amato, S.; Lo Sterzo, C.; Paparella, A. Characterization of essential oils obtained from Abruzzo autochthonous plants: Antioxidant and antimicrobial activities assessment for food application. Foods 2018, 7, 19. [Google Scholar] [CrossRef]
- Fratianni, F.; Riccardi, R.; Spigno, P.; Ombra, M.N.; Cozzolino, A.; Tremonte, P.; Coppola, R.; Nazzaro, F. Biochemical Characterization and Antimicrobial and Antifungal Activity of Two Endemic Varieties of Garlic (Allium sativum L.) of the Campania Region, Southern Italy. J. Med. Food 2016, 19, 686–691. [Google Scholar] [CrossRef]
- Mancini, E.; Senatore, F.; Del Monte, D.; De Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; De Feo, V. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules 2015, 20, 12016–12028. [Google Scholar] [CrossRef]
- Carlo Tenore, G.; Troisi, J.; Di Fiore, R.; Basile, A.; Novellino, E. Chemical composition, antioxidant and antimicrobial properties of Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) seed meal, a promising protein source of Campania region (southern Italy) horticultural germplasm. J. Sci. Food Agric. 2012, 92, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Loy, G.; Cottiglia, F.; Garau, D.; Deidda, D.; Pompei, R.; Bonsignore, L. Chemical composition and cytotoxic and antimicrobial activity of Calycotome villosa (Poiret) Link leaves. Farmaco 2001, 56, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Barra, A.; Russo, M.T.; Coroneo, V.; Dessí, S.; Cabras, P. Chemical composition of the essential oils of Juniperus from ripe and unripe berries and leaves and their antimicrobial activity. J. Agric. Food Chem. 2003, 51, 3073–3078. [Google Scholar] [CrossRef]
- Mazzanti, G.; Battinelli, L.; Salvatore, G. Antimicrobial properties of the linalol-rich essential oil of Hyssopos officinalis L. var decumbens (Lamiaceae). Flavour Fragr. J. 1998, 13, 289–294. [Google Scholar] [CrossRef]
- Piras, C.; Di Ciccio, P.A.; Soggiu, A.; Greco, V.; Tilocca, B.; Costanzo, N.; Ceniti, C.; Urbani, A.; Bonizzi, L.; Ianieri, A.S. aureus biofilm protein expression linked to antimicrobial resistance: A proteomic study. Animals 2021, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; Fabres-Klein, M.H.; de Oliveira Mendes, T.A.; Fitzgerald, J.R.; de Oliveira Barros Ribon, A. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: Current understanding and future perspectives. BMC Vet. Res. 2022, 18, 115. [Google Scholar] [CrossRef]
- Piras, C.; Greco, V.; Gugliandolo, E.; Soggiu, A.; Tilocca, B.; Bonizzi, L.; Zecconi, A.; Cramer, R.; Britti, D.; Urbani, A.; et al. Raw cow milk bacterial consortium as bioindicator of circulating anti-microbial resistance (Amr). Animals 2020, 10, 2378. [Google Scholar] [CrossRef] [PubMed]
- Thornsberry, C.; Burton, P.J.; Yee, Y.C.; Watts, J.L.; Yancey Jr, R.J. The activity of a combination of penicillin and novobiocin against bovine mastitis pathogens: Development of a disk diffusion test. J. Dairy Sci. 1997, 80, 413–421. [Google Scholar] [CrossRef]
- Silva, D.M.; Costa, P.A.D.A.; Ribon, A.O.B.; Purgato, G.A.; Gaspar, D.-M.; Diaz, M.A.N. Plant Extracts Display Synergism with Different Classes of Antibiotics. An. Acad. Bras. Cienc. 2019, 91, e20180117. [Google Scholar] [CrossRef]
- Radulovic, N.S.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Stojanovic, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [PubMed]
- Riyadi, S.A.; Naini, A.A.; Supratman, U. Sesquiterpenoids from Meliaceae Family and Their Biological Activities. Molecules 2023, 28, 4874. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.V.; Pant, S.; Khan, M.A.H.; Shah, A.A.; Siddiqui, S.; Jeridi, M.; Alhamdi, H.W.S.; Ahmad, S. Phytochemicals as Antimicrobials: Prospecting Himalayan Medicinal Plants as Source of Alternate Medicine to Combat Antimicrobial Resistance. Pharmaceuticals 2023, 16, 881. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Hossain, M.E.; Mithi, F.M.; Ahmed, M.; Saldías, M.; Akkol, E.K.; Sobarzo-Sánchez, E. Multifunctional therapeutic potential of phytocomplexes and natural extracts for antimicrobial properties. Antibiotics 2021, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A promising weapon in the arsenal against antibiotic-resistant bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, B.; Dar, K.K.; Ali, S.; Awan, U.A.; Nayyer, A.Q.; Ghous, T.; Andleeb, S. Short communication: In vitro assessment of antioxidant, antibacterial and phytochemical analysis of peel of Citrus sinensis. Pak. J. Pharm. Sci. 2015, 28, 231–239. [Google Scholar]
- Galgano, M.; Capozza, P.; Pellegrini, F.; Cordisco, M.; Sposato, A.; Sblano, S.; Camero, M.; Lanave, G.; Fracchiolla, G.; Corrente, M.; et al. Antimicrobial Activity of Essential Oils Evaluated In Vitro against Escherichia coli and Staphylococcus aureus. Antibiotics 2022, 11, 979. [Google Scholar] [CrossRef]
- Sehnal, K.; Uhlirova, D.; Stankova, M.; Vsetickova, M.; Tothova, Z.; Hosnedlova, B.; Kepinska, M.; Ruttkaynedecký, B.; Bach, D.N.; Sochor, J.; et al. Green synthesis of silver nanoparticles using sage and their antibacterial effect on gram-positive and gram-negative bacteria. In Proceedings of the NANOCON Conference Proceedings—International Conference on Nanomaterials, Brno, Czech Republic, 21–23 October 2020; pp. 469–473. [Google Scholar]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRIE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef]
- Kot, B.; Wierzchowska, K.; Piechota, M.; Czerniewicz, P.; Chrzanowski, G. Antimicrobial activity of five essential oils from lamiaceae against multidrug-resistant Staphylococcus aureus. Nat. Prod. Res. 2019, 33, 3587–3591. [Google Scholar] [CrossRef]
- Stefanovic, O.D.; Stanojevic, D.D.; Comic, L.R. Synergistic antibacterial activity of salvia officinalis and cichorium intybus extracts and antibiotics. ACTA Pol. Pharm. 2012, 69, 457–463. [Google Scholar] [PubMed]
- Snowden, R.; Harrington, H.; Morrill, K.; Jeane, L.; Garrity, J.; Orian, M.; Lopez, E.; Rezaie, S.; Hassberger, K.; Familoni, D.; et al. A Comparison of the Anti-Staphylococcus aureus Activity of Extracts from Commonly Used Medicinal Plants. J. Altern. Complement. Med. 2014, 20, 375–382. [Google Scholar] [CrossRef]
- Rota, C.; Carramiñana, J.J.; Burillo, J.; Herrera, A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J. Food Prot. 2004, 67, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, T.; Hashemzadeh, M.S.; Nazarizadeh, A.; Neyriz-Naghadehi, M.; Tat, M.; Ghalavand, M.; Dorostkar, R. Chemical composition and antibacterial properties of Ocimum basilicum, Salvia officinalis and Trachyspermum ammi essential oils alone and in combination with nisin. Res. J. Pharmacogn. 2016, 3, 51–58. [Google Scholar]
- Leelapornpisid, P.; Chansakao, S.; Ittiwittayawat, T.; Pruksakorn, S. Antimicrobial activity of herbal extracts on Staphylococcus aureus and Propionibacterium acnes. ISHS Acta Horticulturae 679: III WOCMAP Congress on Medicinal and Aromatic Plants—Volume 5: Quality, Efficacy, Safety, Processing and Trade in Medicinal and Aromatic Plants. 2005, pp. 97–104. Available online: https://www.actahort.org/books/679/679_11.htm (accessed on 6 September 2023).
- Zalegh, I.; Bourhia, M.; Zerouali, K.; Katfy, K.; Nayme, K.; Khallouki, F.; Benzaarate, I.; Mohammad Salamatullah, A.; Alzahrani, A.; Nafidi, H.-A.; et al. Molecular Characterization of Gene-Mediated Resistance and Susceptibility of ESKAPE Clinical Isolates to Cistus monspeliensis L. and Cistus salviifolius L. Extracts. Evid.-Based Complement. Altern. Med. 2022, 2022, 7467279. [Google Scholar] [CrossRef] [PubMed]
- Hickl, J.; Argyropoulou, A.; Sakavitsi, M.E.; Halabalaki, M.; Al-Ahmad, A.; Hellwig, E.; Aligiannis, N.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; et al. Mediterranean herb extracts inhibit microbial growth of representative oral microorganisms and biofilm formation of Streptococcus mutans. PLoS ONE 2018, 13, e0207574. [Google Scholar] [CrossRef] [PubMed]
- Rebaya, A.; Belghith, S.I.; Hammrouni, S.; Maaroufi, A.; Ayadi, M.T.; Chérif, J.K. Antibacterial and antifungal activities of ethanol extracts of Halimium halimifolium, Cistus salviifolius and Cistus monspeliensis. Int. J. Pharm. Clin. Res. 2016, 8, 243–247. [Google Scholar]
- Nefzi, K.; Charfi, K.; Maaroufi, A.; Hosni, K.; Msaada, K.; Baraket, M.; Nasr, Z. Biological activities and determination of the mode of action of Tunisian Globularia alypum and Cistus monspeliensis ethanolic extracts. Int. J. Environ. Health Res. 2022. [Google Scholar] [CrossRef]
- Liu, M.; Luo, F.; Qing, Z.; Yang, H.; Liu, X.; Yang, Z.; Zeng, J. Chemical composition and bioactivity of essential oil of ten labiatae species. Molecules 2020, 25, 4862. [Google Scholar] [CrossRef]
- Rodrigues, J.B.D.; de Carvalho, R.J.; de Souza, N.T.; Oliveira, K.D.; Franco, O.L.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Control 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Prazina, N.; Bacic, A.; Hadzic-Hasanovic, V.; Mahmutovic, O. The antibacterial activity of essential oil of oregano (origanum vulgare l.) from bosnia and herzegovina against selected atcc strains. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 140–144. [Google Scholar]
- Stojkovic, D.; Glamoclija, J.; Ciric, A.; Nikolic, M.; Ristic, M.; Siljegovic, J.; Sokovic, M. Investigation on antibacterial synergism of origanum vulgare and thymus vulgaris essential oils. Arch. Biol. Sci. 2013, 65, 639–643. [Google Scholar] [CrossRef]
- Zitek, T.; Borjan, D.; Golle, A.; Knez, Z.; Knez, M.; Žitek, T.; Borjan, D.; Golle, A.; Knez, Ž.; Knez, M. Optimization of extraction of phenolic compounds with antimicrobial properties from origanum vulgare. Processes 2021, 9, 1032. [Google Scholar] [CrossRef]
- de Barros, J.C.; da Conceicao, M.L.; Neto, N.J.G.; da Costa, A.C.V.; de Souza, E.L. Combination of Origanum vulgare L. essential oil and lactic acid to inhibit staphylococcus aureus in meat broth and meat model. Braz. J. Microbiol. 2012, 43, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Bogavac, M.; Radovanović, B.; Sudji, J.; Tešanović, K.; Janjušević, L. Origanum vulgare essential oil affects pathogens causing vaginal infections. J. Appl. Microbiol. 2017, 122, 1177–1185. [Google Scholar] [CrossRef]
- Neto, L.T.; Monteiro, M.L.G.; Machado, M.A.M.; Galvan, D.; Conte, C.A. An Optimization of Oregano, Thyme, and Lemongrass Essential Oil Blend to Simultaneous Inactivation of Relevant Foodborne Pathogens by Simplex-Centroid Mixture Design. Antibiotics 2022, 11, 1572. [Google Scholar] [CrossRef]
- Licina, B.Z.; Stefanovic, O.D.; Vasic, S.M.; Radojevic, I.D.; Dekic, M.S.; Comic, L.R.; Ličina, B.Z.; Stefanović, O.D.; Vasić, S.M.; Radojević, I.D.; et al. Biological activities of the extracts from wild growing Origanum vulgare L. Food Control 2013, 33, 498–504. [Google Scholar] [CrossRef]
- Hulankova, R.; Borilova, G.; Hulánková, R.; Bořilová, G. In vitro combined effect of oregano essential oil and caprylic acid against salmonella serovars, escherichia coli O157:H7, staphylococcus aureus and listeria monocytogenes. Acta Vet. Brno 2011, 80, 343–348. [Google Scholar] [CrossRef]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control 2009, 20, 157–160. [Google Scholar] [CrossRef]
- de Souza, G.T.; de Carvalho, R.J.; de Sousa, J.P.; Tavares, J.F.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of the Essential Oil from Origanum vulgare L. on Survival of Pathogenic Bacteria and Starter Lactic Acid Bacteria in Semihard Cheese Broth and Slurry. J. Food Prot. 2016, 79, 246–252. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Barros, J.C.; da Conceicao, M.L.; Neto, N.J.G.; da Costa, A.C.V. Combined application of Origanum vulgare L. essential oil and acetic acid for controlling the growth of staphylococcus aureus in foods. Braz. J. Microbiol. 2009, 40, 387–393. [Google Scholar] [CrossRef]
- Heni, S.; Boughendjioua, H.; Saida, M.; Bennadja, S.; Djahoudi, A. Use of Origanum vulgare Essential Oil as an Antibacterial Additive in the Preservation of Minced Meat. J. Pharm. Res. Int. 2020, 32, 1–9. [Google Scholar] [CrossRef]
- de Oliveira, J.; Diniz, M.D.M.; Lima, E.D.; de Souza, E.L.; Trajano, V.N.; Santos, B.H.C. Effectiveness of Origanum vulgare L. and Origanum majorana L. Essential oils in Inhibiting the Growth of Bacterial Strains Isolated from the Patients with Conjunctivitis. Braz. Arch. Biol. Technol. 2009, 52, 45–50. [Google Scholar] [CrossRef]
- de Souza, E.L.; Stamford, T.L.M.; Lima, E.D.O.; Barbosa, J.M.; Marques, M.O.M. Interference of heating on the antimicrobial activity and chemical composition of Origanum vulgare L. (Lamiaceae) essential oil. Cienc. E Tecnol. Aliment. 2008, 28, 418–422. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Consoli, G.M.L.; Cafiso, V.; Stefani, S.; Geraci, C. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef]
- Karaboduk, K.; Karabacak, O.; Karaboduk, H.; Tekinay, T. Chemical analysis and antimicrobial activities of the Origanum vulgare subsp. Hirtum. J. Environ. Prot. Ecol. 2014, 15, 1283–1292. [Google Scholar]
- Béjaoui, A.; Chaabane, H.; Jemli, M.; Boulila, A.; Boussaid, M. Essential oil composition and antibacterial activity of Origanum vulgare subsp. glandulosum Desf. at different phenological stages. J. Med. Food 2013, 16, 1115–1120. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Saikia, D.; Chauhan, A.; Krishna, V. Antibacterial Activity of Origanum vulgare L. Popul. Indian Orig. J. Biol. Act. Prod. from Nat. 2012, 2, 353–359. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Rasheed, S.; Nigam, P.S.; Janneh, O.; Sarker, S.D. Composition, antioxidant and chemotherapeutic properties of the essential oils from two Origanum species growing in Pakistan. Rev. Bras. Farmacogn. 2011, 21, 943–952. [Google Scholar] [CrossRef]
- Da Costa, A.C.; Dos Santos, B.H.C.; Filho, L.S.; Lima, E.D.O. Antibacterial activity of the essential oil of Origanum vulgare L. (Lamiaceae) against bacterial multiresistant strains isolated from nosocomial patients. Rev. Bras. Farmacogn. 2009, 19, 236–241. [Google Scholar] [CrossRef]
- Enayatifard, R.; Akbari, J.; Babaei, A.; Rostamkalaei, S.S.; Hashemi, S.M.H.; Habibi, E. Anti-microbial potential of nano-emulsion form of essential oil obtained from aerial parts of Origanum vulgare L. as food additive. Adv. Pharm. Bull. 2021, 11, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Turgis, M.; Vu, K.D.; Dupont, C.; Lacroix, M. Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Food Res. Int. 2012, 48, 696–702. [Google Scholar] [CrossRef]
- Koca, T.; Koca, O.; Korcum, A.F. Antimicrobial activities of essential oils on microorganisms isolated from radiation dermatitis. J. Clin. Anal. Med. 2019, 10, 307–310. [Google Scholar] [CrossRef]
- Kovačević, Z.; Kladar, N.; Čabarkapa, I.; Radinović, M.; Maletić, M.; Erdeljan, M.; Božin, B. New Perspective of Origanum vulgare L. and Satureja montana L. Essential Oils as Bovine Mastitis Treatment Alternatives. Antibiotics 2021, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, A.; Plessas, S.; Kimbaris, A.; Varvatou, M.; Mantzourani, I.; Fournomiti, M.; Tzouti, V.; Nerantzaki, A.; Bezirtzoglou, E. Mode of antimicrobial action of Origanum vulgare essential oil against clinical pathogens. Curr. Res. Nutr. Food Sci. 2017, 5, 109–115. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Kimbaris, A.C.; Plessas, S.; Mantzourani, I.; Theodoridou, I.; Stavropoulou, E.; Polissiou, M.G.; Bezirtzoglou, E. Antibacterial activities of essential oils from eight Greek aromatic plants against clinical isolates of Staphylococcus aureus. Anaerobe 2011, 17, 399–402. [Google Scholar] [CrossRef]
- Tanasescu, S.; Nitu, R.; Dahma, G.; Pilut, C.; Diaconu, M.; Neagoe, O.; Muntean, D.; Horhat, I.D.; Dragomir, A.; Lighezan, D.; et al. Chemical Composition and Antimicrobial Activity of Essential Oil of Romanian Origanum vulgare. Rev. Chim. 2019, 70, 1744–1745. [Google Scholar] [CrossRef]
- Pesavento, G.; Maggini, V.; Maida, I.; Lo Nostro, A.; Calonico, C.; Sassoli, C.; Perrin, E.; Fondi, M.; Mengoni, A.; Chiellini, C.; et al. Essential Oil from Origanum vulgare Completely Inhibits the Growth of Multidrug-Resistant Cystic Fibrosis Pathogens. Nat. Prod. Commun. 2016, 11, 861–864. [Google Scholar] [CrossRef]
- Fikry, S.; Khalil, N.; Salama, O. Chemical profiling, biostatic and biocidal dynamics of Origanum vulgare L. essential oil. AMB Express 2019, 9, 41. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Nikmaram, N.; Esteghlal, S.; Mousavi Khaneghah, A.; Niakousari, M.; Barba, F.J.; Roohinejad, S.; Koubaa, M.; Khaneghah, A.M.; Niakousari, M.; et al. Efficiency of Ohmic assisted hydrodistillation for the extraction of essential oil from oregano (Origanum vulgare subsp viride) spices. Innov. FOOD Sci. Emerg. Technol. 2017, 41, 172–178. [Google Scholar] [CrossRef]
- Helal, I.M.; El-Bessoumy, A.; Al-Bataineh, E.; Joseph, M.R.P.; Rajagopalan, P.; Chandramoorthy, H.C.; Ben Hadj Ahmed, S.; Ahmed, S.B.; Ben Hadj Ahmed, S. Antimicrobial efficiency of essential oils from traditional medicinal plants of asir region, Saudi Arabia, over drug resistant isolates. Biomed Res. Int. 2019, 2019, 8928306. [Google Scholar] [CrossRef]
- Lopez, P.; Sanchez, C.; Batlle, R.; Nerin, C.; López, P.; Sanchez, C.; Batlle, R.; Nerín, C. Vapor-phase activities of cinnamon, thyme, and oregano essential oils and key constituents against foodborne microorganisms. J. Agric. Food Chem. 2007, 55, 4348–4356. [Google Scholar] [CrossRef]
- Hao, Y.P.; Li, J.Y.; Shi, L. A Carvacrol-Rich Essential Oil Extracted From Oregano (Origanum vulgare “Hot & Spicy”) Exerts Potent Antibacterial Effects Against Staphylococcus aureus. Front. Microbiol. 2021, 12, 741861. [Google Scholar] [CrossRef]
- Bonjar, G.H.S.; Shahidi Bonjar, G.H. Antibacterial screening of plants used in Iranian folkloric medicine. Fitoterapia 2004, 75, 231–235. [Google Scholar] [CrossRef]
- Erdogan Eliuz, E.A.; Ayas, D.; Goksen, G. In Vitro Phototoxicity and Antimicrobial Activity of Volatile Oil Obtained from Some Aromatic Plants. J. Essent. Oil-Bearing Plants 2017, 20, 758–768. [Google Scholar] [CrossRef]
- Kaya, D.A.; Duran, N. The antimicrobial activities of myrtus communis AND Micromeria fruticosa essential oils. In International Conference on Advanced Materials and Systems (ICAMS); The National Research & Development Institute for Textiles and Leather-INCDTP: Bucharest, Romania, 2018; pp. 249–254. [Google Scholar]
- Nabavizadeh, M.; Abbaszadegan, A.; Gholami, A.; Sheikhiani, R.; Shokouhi, M.; Shams, M.S.; Ghasemi, Y. Chemical constituent and antimicrobial effect of essential oil from Myrtus communis leaves on microorganisms involved in persistent endodontic infection compared to two common endodontic irrigants: An in vitro study. J. Conserv. Dent. 2014, 17, 449–453. [Google Scholar] [CrossRef]
- Caputo, L.; Capozzolo, F.; Amato, G.; De Feo, V.; Fratianni, F.; Vivenzio, G.; Nazzaro, F. Chemical composition, antibiofilm, cytotoxic, and anti-acetylcholinesterase activities of Myrtus communis L. leaves essential oil. BMC Complement. Med. Ther. 2022, 22, 142. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Yanguela, J.; Amrouche, T.; Boubrit, S.; Boussad, N.; Roncales, P. Chemical composition and antimicrobial effects of essential oils of Eucalyptus globulus, Myrtus communis and Satureja hortensis against Escherichia coli O157:H7 and Staphylococcus aureus in minced beef. Food Sci. Technol. Int. 2011, 17, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Sisay, M.; Bussa, N.; Gashaw, T.; Mengistu, G. Investigating In Vitro Antibacterial Activities of Medicinal Plants Having Folkloric Repute in Ethiopian Traditional Medicine. J. evidence-based Integr. Med. 2019, 24, 2515690X19886276. [Google Scholar] [CrossRef] [PubMed]
- Raeiszadeh, M.; Pardakhty, A.; Sharififar, F.; Mehrabani, M.; Nejat-Mehrab-Kermani, H.; Mehrabani, M. Phytoniosome: A Novel Drug Delivery for Myrtle Extract. Iran. J. Pharm. Res. IJPR 2018, 17, 804–817. [Google Scholar]
- Mansouri, S.; Foroumadi, A.; Ghaneie, T.; Najar, A.G. Antibacterial activity of the crude extracts and fractionated constituents of Myrtus communis. Pharm. Biol. 2001, 39, 399–401. [Google Scholar] [CrossRef]
- Benhadid, R.; Hadef, Y.; Djahoudi, A.; Hosni, K. Chemical Characterization, Evaluation of the Antibacterial and Antioxidant Activities of the Essential Oil of Algerian (Myrtus communis L). Prog. Nutr. 2022, 24, e2022029. [Google Scholar] [CrossRef]
- Karaçam, M.; Kaya, D.A. Effect of essential oils on some pathogens that cause eczema. In Proceedings of the ICAMS Proceedings of the International Conference on Advanced Materials and Systems, Langkawi, Turkey, 26–27 November 2020; pp. 183–188. [Google Scholar]
- El Hartiti, H.; El Mostaphi, A.; Barrahi, M.; Ali, A.B.; Chahboun, N.; Amiyare, R.; Zarrouk, A.; Bourkhiss, B.; Ouhssine, M. Chemical composition and antibacterial activity of the essential oil of Myrtus communis leaves. Karbala Int. J. Mod. Sci. 2020, 6, 251–258. [Google Scholar] [CrossRef]
- Najar, A.G.; Mansouri, S.; Rahighi, S. Effects of sub-inhibitory concentrations of Myrtus communis leave extracts on the induction of free radicals in Staphylococcus aureus; a possible mechanism for the antibacterial action. Asian J. Plant Sci. 2009, 8, 551–556. [Google Scholar]
- Rahimvand, L.; Niakan, M.; Naderi, N.J. The antibacterial effect of aquatic and methanolic extract of Myrtus communis on Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia. Iran. J. Microbiol. 2018, 10, 254. [Google Scholar]
- Dib, K.; Cherrah, Y.; Rida, S.; Filali-Maltouf, A.; Ennibi, O. In vitro antibacterial activity of Myrtus communis L. and Marrubium vulgare L. leaves against Aggregatibacter actinomycetemcomitans and Eikenella corrodens. Evid.-Based Complement. Altern. Med. 2021, 2021, 8351332. [Google Scholar] [CrossRef]
- Gorjian, H.; Khaligh, N.G. Myrtle: A versatile medicinal plant. Nutrire 2023, 48, 10. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Hamdi, N.; Miladi, R.; Abdelkafi, S. Myrtus communis essential oil: Chemical composition and antimicrobial activities against food spoilage pathogens. Chem. Biodivers. 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Usai, M.; Marchetti, M.; Culeddu, N.; Mulas, M. Chemical composition of myrtle (Myrtus communis L.) berries essential oils as observed in a collection of genotypes. Molecules 2018, 23, 2502. [Google Scholar] [CrossRef]
- Savikin-Fodulovic, K.P.; Bulatovic, V.M.; Menkovic, N.R.; Grubisic, D. V Comparison between the essential oil of Myrtus communis L. obtained from naturally grown and in vitro plants. J. Essent. Oil Res. 2000, 12, 75–78. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of eucalyptus oil and 1, 8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Zomorodian, K.; Moein, M.; Pakshir, K.; Karami, F.; Sabahi, Z. Chemical composition and antimicrobial activities of the essential oil from Salvia mirzayanii leaves. J. Evid. Based. Complement. Altern. Med. 2017, 22, 770–776. [Google Scholar] [CrossRef]
- Longaray Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Bouamama, H.; Noël, T.; Villard, J.; Benharref, A.; Jana, M. Antimicrobial activities of the leaf extracts of two Moroccan cistus L. species. J. Ethnopharmacol. 2006, 104, 104–107. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Borrás-Rocher, F.; Micol, V.; Barrajón-Catalán, E. Artificial intelligence applied to improve scientific reviews: The antibacterial activity of Cistus plants as proof of concept. Antibiotics 2023, 12, 327. [Google Scholar] [CrossRef] [PubMed]
- Haida, S.; Bakkouche, K.; Kribii, A.R.; Kribii, A. Chemical Composition of Essential Oil, Phenolic Compounds Content, and Antioxidant Activity of Cistus monspeliensis from Northern Morocco. Biochem. Res. Int. 2021, 2021, 6669877. [Google Scholar] [CrossRef] [PubMed]
- Moghrovyan, A.; Parseghyan, L.; Sevoyan, G.; Darbinyan, A.; Sahakyan, N.; Gaboyan, M.; Karabekian, Z.; Voskanyan, A. Antinociceptive, anti-inflammatory, and cytotoxic properties of Origanum vulgare essential oil, rich with β-caryophyllene and β-caryophyllene oxide. Korean J. Pain 2022, 35, 140–151. [Google Scholar] [CrossRef]
- Ghitea, T.C.; El-Kharoubi, A.; Ganea, M.; Bimbo-Szuhai, E.; Nemeth, T.S.; Ciavoi, G.; Foghis, M.; Dobjanschi, L.; Pallag, A.; Micle, O. The Antimicrobial Activity of Origanum vulgare L. Correlated with the Gastrointestinal Perturbation in Patients with Metabolic Syndrome. Molecules 2021, 26, 283. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Alves, F.C.B.; Andrade, B.F.M.T.; Albano, M.; Rall, V.L.M.; Fernandes, A.A.H.; Buzalaf, M.A.R.; de Lima Leite, A.; de Pontes, L.G.; Dos Santos, L.D. Proteomic analysis and antibacterial resistance mechanisms of Salmonella Enteritidis submitted to the inhibitory effect of Origanum vulgare essential oil, thymol and carvacrol. J. Proteomics 2020, 214, 103625. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hao, Y.; Zhang, W.; Xia, F.; Bai, H.; Li, H.; Shi, L. Comparison of Origanum Essential Oil Chemical Compounds and Their Antibacterial Activity against Cronobacter sakazakii. Molecules 2022, 27, 6702. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Selim, S. Antimicrobial activity of essential oils against vancomycin-resistant enterococci (VRE) and escherichia coli O157: H7 in feta soft cheese and minced beef meat. Braz. J. Microbiol. 2011, 42, 187–196. [Google Scholar] [CrossRef]
- Kolypetri, S.; Kostoglou, D.; Nikolaou, A.; Kourkoutas, Y.; Giaouris, E. Chemical Composition, Antibacterial and Antibiofilm Actions of Oregano (Origanum vulgare subsp. hirtum) Essential Oil against Salmonella Typhimurium and Listeria monocytogenes. Foods 2023, 12, 2893. [Google Scholar] [CrossRef]
- Faleiro, L.; Miguel, G.; Gomes, S.; Costa, L.; Venâncio, F.; Teixeira, A.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antibacterial and antioxidant activities of essential oils isolated from Thymbra capitata L. (Cav.) and Origanum vulgare L. J. Agric. Food Chem. 2005, 53, 8162–8168. [Google Scholar] [CrossRef]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A review on Cistus sp.: Phytochemical and antimicrobial activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Bouabidi, M.; Salamone, F.L.; Gadhi, C.; Bouamama, H.; Speciale, A.; Ginestra, G.; Pulvirenti, L.; Siracusa, L.; Nostro, A.; Cristani, M. Efficacy of Two Moroccan Cistus Species Extracts against Acne Vulgaris: Phytochemical Profile, Antioxidant, Anti-Inflammatory and Antimicrobial Activities. Molecules 2023, 28, 2797. [Google Scholar] [CrossRef]
- Garcia, M.O.; Allend, S.O.; Cunha, K.F.d.; Hartwig, D.D. Myrtaceae family: An update on plant-derived bioactive compounds against bacteria that affect the respiratory system. Rodriguésia 2023, 74, e00822022. [Google Scholar] [CrossRef]
- El Euch, S.K.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Luczkiewicz, M. Celery (Apium graveolens var. dulce (Mill.) Pers.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: London, UK, 2016; pp. 325–338. [Google Scholar]
- Al-Maharik, N.; Jaradat, N.; Al-Hajj, N.; Jaber, S. Myrtus communis L.: Essential oil chemical composition, total phenols and flavonoids contents, antimicrobial, antioxidant, anticancer, and α-amylase inhibitory activity. Chem. Biol. Technol. Agric. 2023, 10, 41. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial susceptibility and antibacterial mechanism of limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef] [PubMed]
- Dias, K.J.S.D.O.; Miranda, G.M.; Bessa, J.R.; Araújo, A.C.J.D.; Freitas, P.R.; Almeida, R.S.D.; Paulo, C.L.R.; Neto, J.B.D.A.; Coutinho, H.D.M.; Ribeiro-Filho, J. Terpenes as bacterial efflux pump inhibitors: A systematic review. Front. Pharmacol. 2022, 13, 953982. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Strategic approach of multifaceted antibacterial mechanism of limonene traced in Escherichia coli. Sci. Rep. 2021, 11, 13816. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial activity and mechanism of limonene against Staphylococcus aureus. J. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Hodaj-Çeliku, E.; Tsiftsoglou, O.; Shuka, L.; Abazi, S.; Hadjipavlou-Litina, D.; Lazari, D. Antioxidant activity and chemical composition of essential oils of some aromatic and medicinal plants from Albania. Nat. Prod. Commun. 2017, 12, 1934578X1701200525. [Google Scholar] [CrossRef]
- Szczepanik, M.; Walczak, M.; Zawitowska, B.; Michalska-Sionkowska, M.; Szumny, A.; Wawrzeńczyk, C.; Brzezinska, M.S. Chemical composition, antimicromicrobial activity and insecticidal activity against the lesser mealworm Alphitobius diaperinus (Panzer)(Coleoptera: Tenebrionidae) of Origanum vulgare L. ssp. hirtum (Link) and Artemisia dracunculus L. essential oils. J. Sci. Food Agric. 2018, 98, 767–774. [Google Scholar] [CrossRef]
- Bouzouita, N.; Kachouri, F.; Hamdi, M.; Chaabouni, M.M. Antimicrobial activity of essential oils from Tunisian aromatic plants. Flavour Fragr. J. 2003, 18, 380–383. [Google Scholar] [CrossRef]
- Mot, M.-D.; Gavrilaș, S.; Lupitu, A.I.; Moisa, C.; Chambre, D.; Tit, D.M.; Bogdan, M.A.; Bodescu, A.-M.; Copolovici, L.; Copolovici, D.M. Salvia officinalis L. essential oil: Characterization, antioxidant properties, and the effects of aromatherapy in adult patients. Antioxidants 2022, 11, 808. [Google Scholar] [CrossRef]
- Zinno, P.; Guantario, B.; Lombardi, G.; Ranaldi, G.; Finamore, A.; Allegra, S.; Mammano, M.M.; Fascella, G.; Raffo, A.; Roselli, M. Chemical Composition and Biological Activities of Essential Oils from Origanum vulgare Genotypes Belonging to the Carvacrol and Thymol Chemotypes. Plants 2023, 12, 1344. [Google Scholar] [CrossRef]
- Elwira, S.; Marta, S.O.; Rafat, S.; Krystyna, S.W.; Grazyna, G. Natural terpenes influence the activity of antibiotics against isolated mycobacterium tuberculosis. Med. Princ. Pract. 2017, 26, 108–112. [Google Scholar]
- Freitas, P.R.; de Araújo, A.C.J.; Barbosa, C.R.; Muniz, D.F.; Tintino, S.R.; Ribeiro-Filho, J.; Siqueira Júnior, J.P.; de Sousa, G.R.; Coutinho, H.D.M. Inhibition of efflux pumps by monoterpene (α-pinene) and impact on Staphylococcus aureus resistance to tetracycline and erythromycin. Curr. Drug Metab. 2021, 22, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Buriani, A.; Fortinguerra, S.; Sorrenti, V.; Caudullo, G.; Carrara, M. Essential oil phytocomplex activity, a review with a focus on multivariate analysis for a network pharmacology-informed phytogenomic approach. Molecules 2020, 25, 1833. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.; Alves, L. Synergy in plant medicines. Curr. Med. Chem. 2003, 10, 13–20. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simoes, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Abreu, A.C.; Coqueiro, A.; Sultan, A.R.; Lemmens, N.; Kim, H.K.; Verpoorte, R.; Van Wamel, W.J.B.; Simões, M.; Choi, Y.H. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci. Rep. 2017, 7, 3777. [Google Scholar] [CrossRef] [PubMed]
- Dettweiler, M.; Melander, R.J.; Porras, G.; Risener, C.; Marquez, L.; Samarakoon, T.; Melander, C.; Quave, C.L. A clerodane diterpene from Callicarpa americana resensitizes methicillin-resistant Staphylococcus aureus to β-lactam antibiotics. ACS Infect. Dis. 2020, 6, 1667–1673. [Google Scholar] [CrossRef]
- Russell, A.D. Bacterial resistance to disinfectants: Present knowledge and future problems. J. Hosp. Infect. 1999, 43, S57–S68. [Google Scholar] [CrossRef]

| Pathogen | Number of Active Plants | Plant Names and Reference |
|---|---|---|
| Staphylococcus aureus | 39 | Cinnamomum [14]; Cinnamomum camphora (L.) [15]; Cistus monspeliensis L. [16]; Cistus salviifolius L. [16]; Cytinus hypocistis (L.) L. [17]; Limonium morisianum Arrigoni [18]; Myrtus communis L. [19]; Origanum vulgare L. [20]; Pistacia lentiscus L. [21]; Pistacia terebinthus L. [22]; Rosmarinus officinalis L. [23]; Salvia officinalis L. [24]; Thymus herba-barona Loise L. [25]; Thymus vulgaris L. [26]; Inula crithmoides [27]; Caralluma europaea [28]; Crocus sativus [29]; Helichrysum araxinum [30]; Schinus molle (L.) [31]; Cannabis sativa [32]; Centaurium erythraea [33]; Citrus medica L., Citrus bergamia, and Citrus medica [34]; Laurus nobilis [35]; Rubus ulmifolius [36]; Malus domestica var. Annurca [37]; Teucrium genus (Germander) [38]; Daucus carota subsp. maximus (Desf.) [39]; Cytinus [40]; T. vulgaris, Satureja montana and Coriandrum sativum [41]; Garlic (Allium sativum L.) [42]; Thymus vulgaris L. [43]; Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) [44]; Calycotome villosa (Poiret) [45]; Juniperus spp. [46]; Hyssopus officinalis [47] |
| Plant | Total Records for Each Plant | Records Included |
|---|---|---|
| Cinnamomum camphora | 23 | 8 |
| Cistus monspeliensis | 7 | 4 |
| Cistus salviifolius | 6 | 2 |
| Cytinus hypocistis | 3 | 1 |
| Myrtus communis | 69 | 14 |
| Origanum vulgare | 159 | 38 |
| Pistacia lentiscus | 32 | 4 |
| Pistacia terebinthus | 7 | 2 |
| Rosmarinus officinalis | 220 | 75 |
| Salvia officinalis | 102 | 9 |
| Laurus nobilis | 60 | 13 |
| Satureja montana | 15 | 8 |
| Coriandrum sativum | 11 | 7 |
| Garlic (Allium sativum L.) | 263 | 21 |
| Thymus vulgaris L. | 196 | 48 |
| Crocus sativus | 26 | 7 |
| Schinus molle | 28 | 8 |
| Cannabis sativa | 53 | 14 |
| Centaurium erythraea | 8 | 5 |
| Citrus medica L. | 12 | 5 |
| Citrus bergamia | 13 | 1 |
| Salvia officinalis | Cistus monspeliensis | Myrtus communis (Myrtle) |
|---|---|---|
| 1,8-Cineole | Camphene | 1,8-Cineole |
| Borneol | Eugenol | Geraniol |
| Camphor | Geraniol | Limonene |
| Limonene | Limonene | Linalool |
| Linalool | Nerol | Methyl eugenol |
| α-Humulene | Sabinene | Myrtenol |
| α-Pinene | α-Pinene | Myrtenyl acetate |
| α-Thujone | α-Thujone | Nerol |
| β-Pinene | β-Pinene | α-Pinene |
| β-Thujone | δ-3-Carene | α-Terpineol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oppedisano, F.; De Fazio, R.; Gugliandolo, E.; Crupi, R.; Palma, E.; Abbas Raza, S.H.; Tilocca, B.; Merola, C.; Piras, C.; Britti, D. Mediterranean Plants with Antimicrobial Activity against Staphylococcus aureus, a Meta-Analysis for Green Veterinary Pharmacology Applications. Microorganisms 2023, 11, 2264. https://doi.org/10.3390/microorganisms11092264
Oppedisano F, De Fazio R, Gugliandolo E, Crupi R, Palma E, Abbas Raza SH, Tilocca B, Merola C, Piras C, Britti D. Mediterranean Plants with Antimicrobial Activity against Staphylococcus aureus, a Meta-Analysis for Green Veterinary Pharmacology Applications. Microorganisms. 2023; 11(9):2264. https://doi.org/10.3390/microorganisms11092264
Chicago/Turabian StyleOppedisano, Francesca, Rosario De Fazio, Enrico Gugliandolo, Rosalia Crupi, Ernesto Palma, Sayed Haidar Abbas Raza, Bruno Tilocca, Carmine Merola, Cristian Piras, and Domenico Britti. 2023. "Mediterranean Plants with Antimicrobial Activity against Staphylococcus aureus, a Meta-Analysis for Green Veterinary Pharmacology Applications" Microorganisms 11, no. 9: 2264. https://doi.org/10.3390/microorganisms11092264
APA StyleOppedisano, F., De Fazio, R., Gugliandolo, E., Crupi, R., Palma, E., Abbas Raza, S. H., Tilocca, B., Merola, C., Piras, C., & Britti, D. (2023). Mediterranean Plants with Antimicrobial Activity against Staphylococcus aureus, a Meta-Analysis for Green Veterinary Pharmacology Applications. Microorganisms, 11(9), 2264. https://doi.org/10.3390/microorganisms11092264









