A Fatal A/H5N1 Avian Influenza Virus Infection in a Cat in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Description
2.2. Blood Analysis
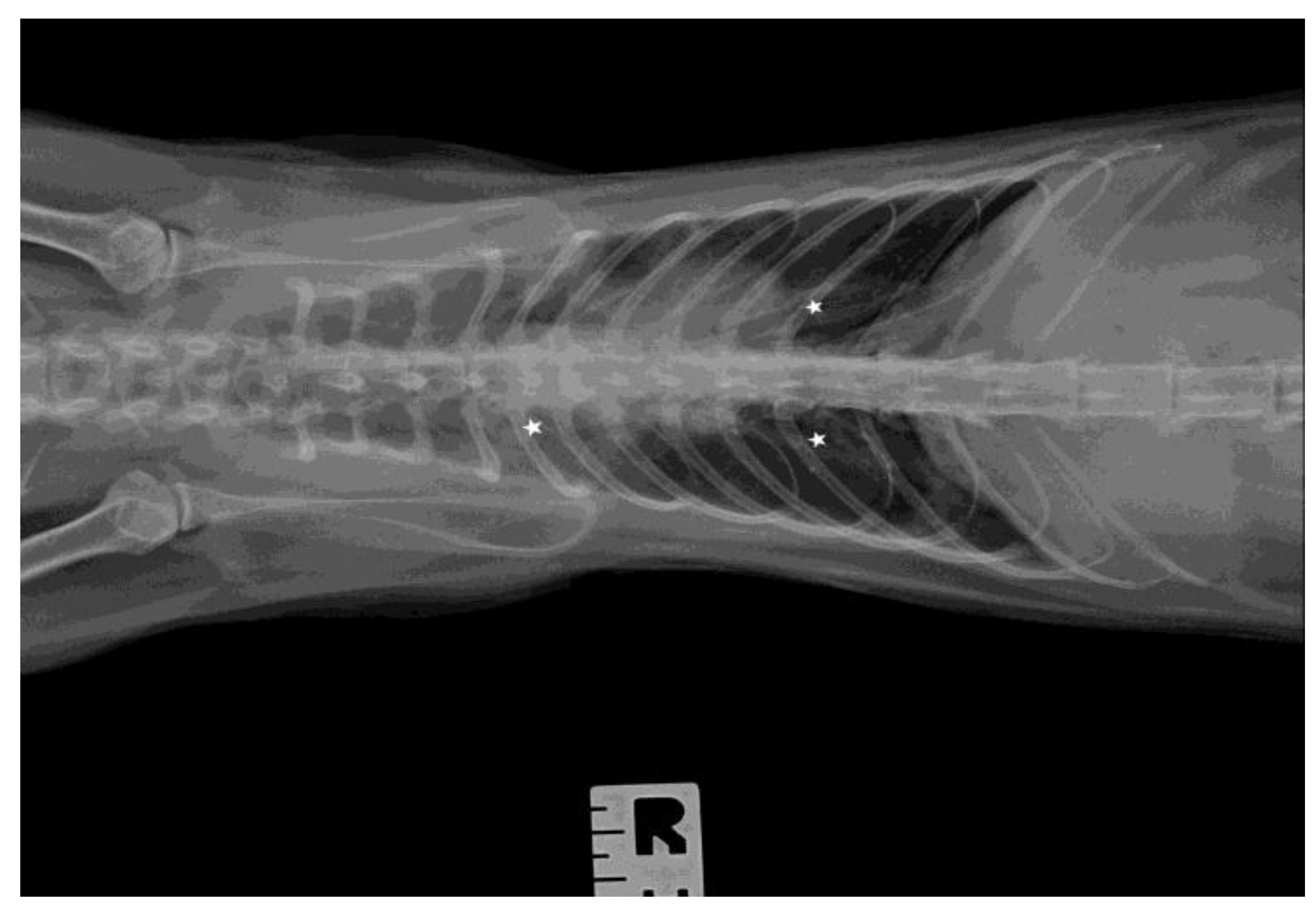
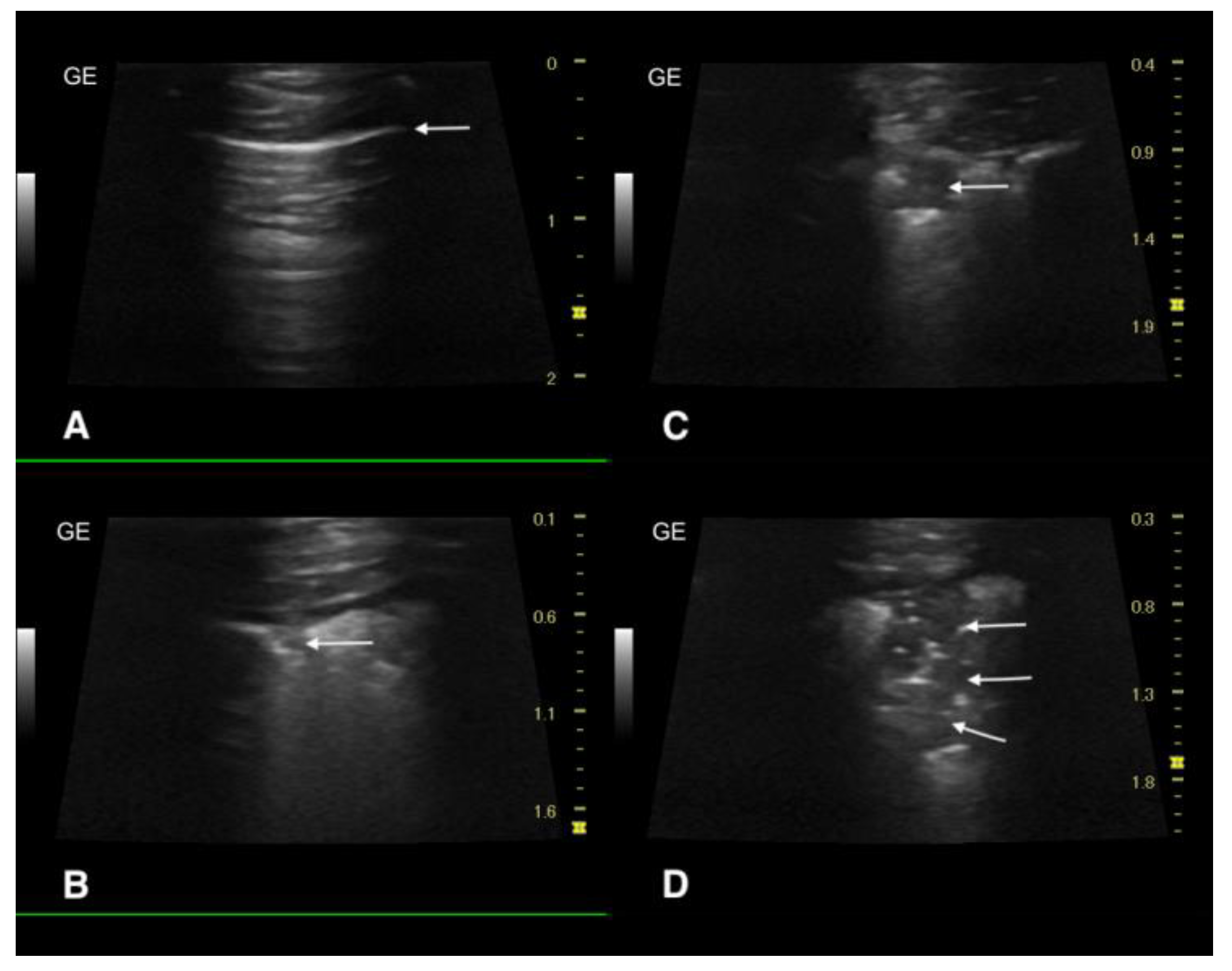
2.3. Ultrasound Examination and X-ray
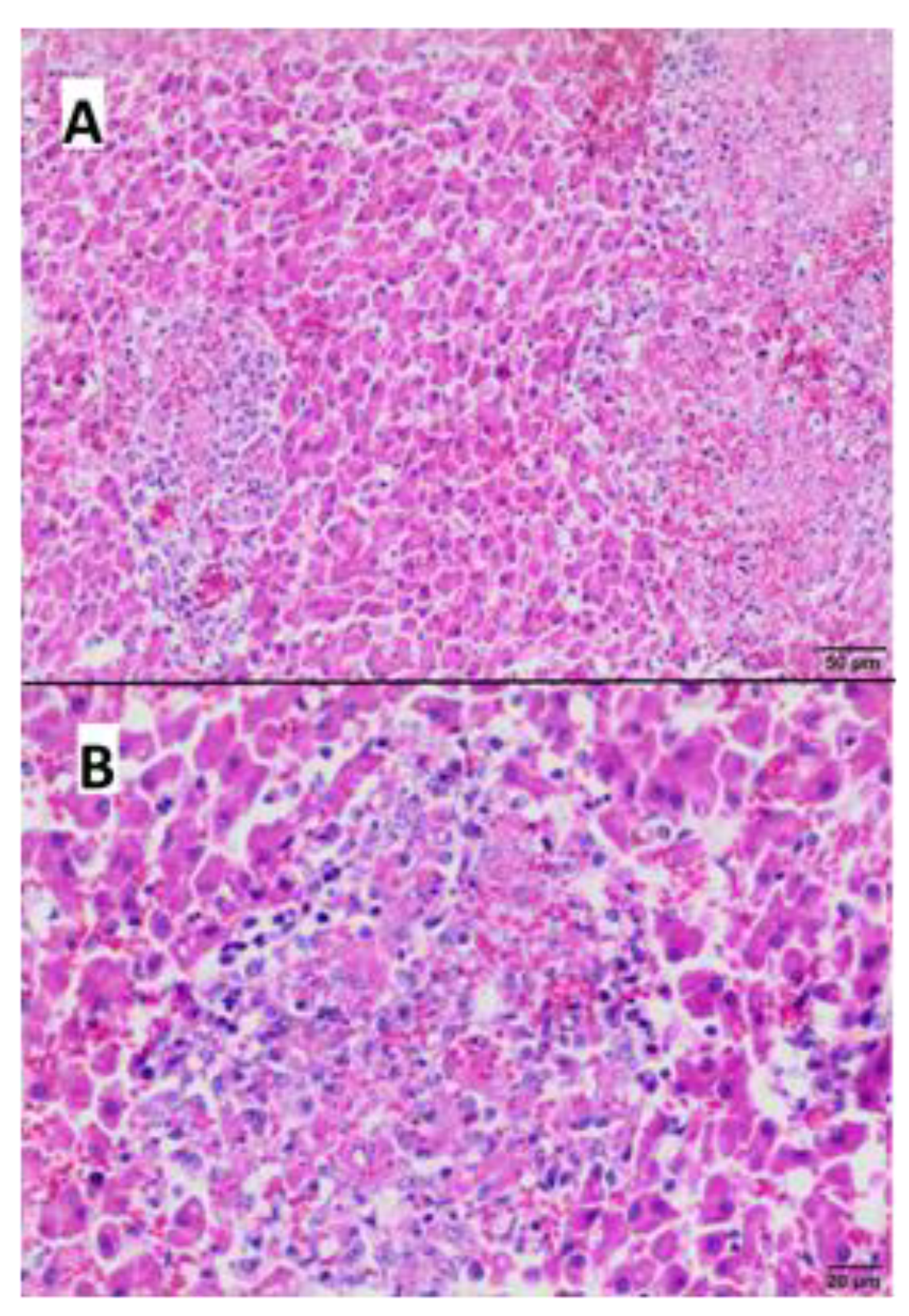
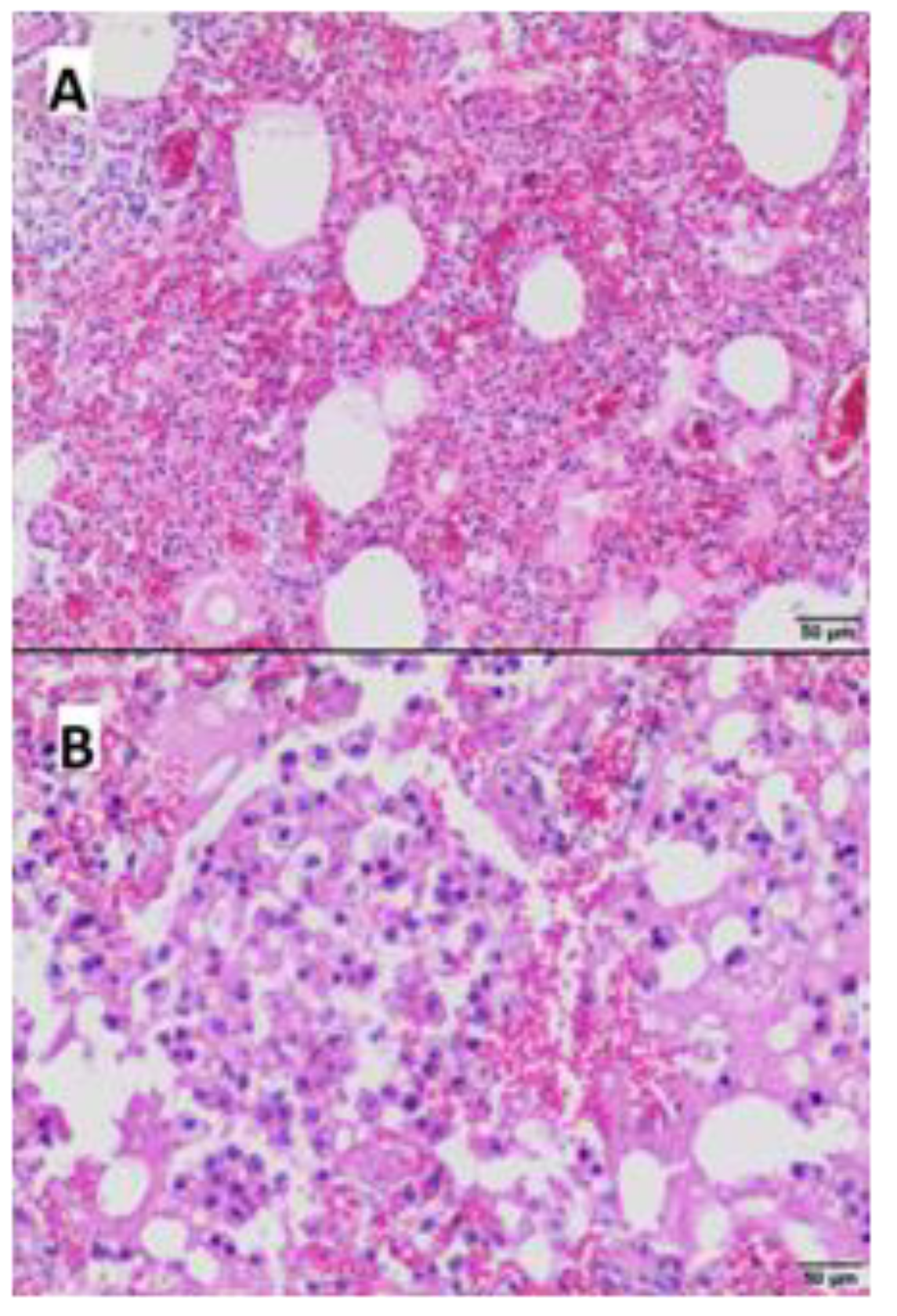
2.4. Pathological Examination
2.5. Bacteriological Tests
2.6. Virological and Serological Tests
- In order to detect antibodies to the feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) antigen serum samples were tested using the FIV Ab/FeLV Ag Combo Test (VetExpert, Łomianki, Poland).
3. Results
3.1. Clinical Course of the Disease and Treatment
3.2. Blood Analysis
3.3. Ultrasound Examination and X-ray
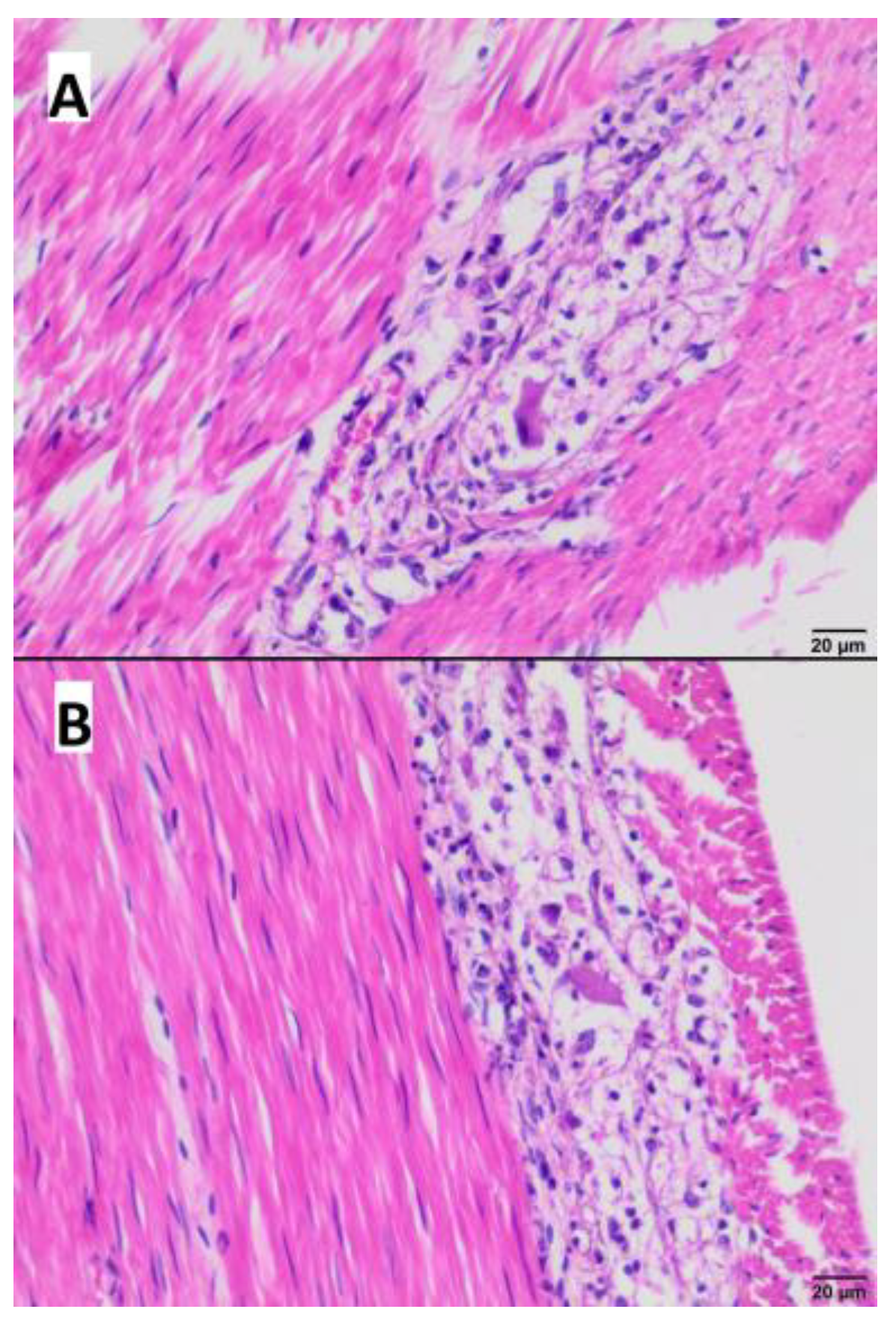
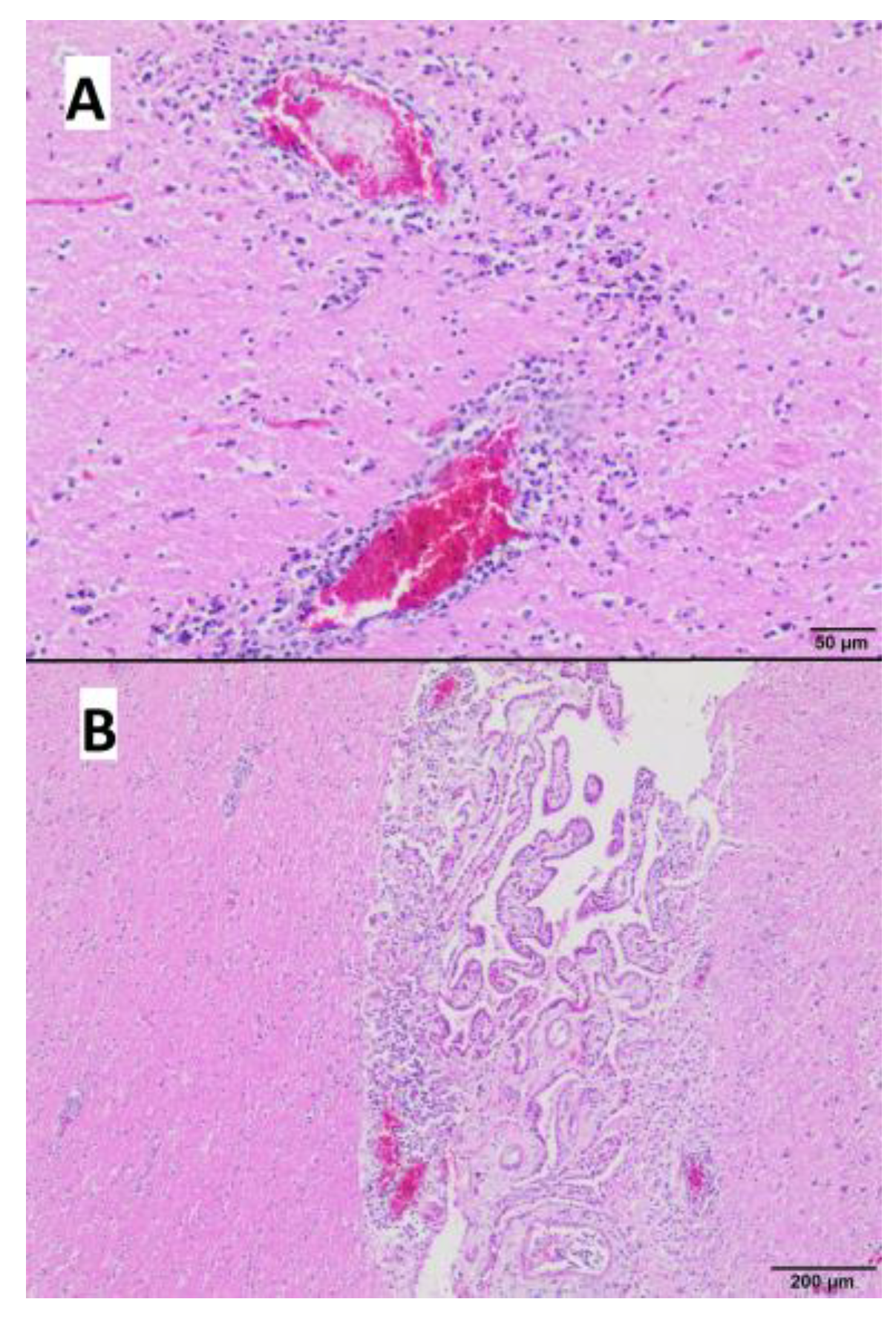
3.4. Gross and Microscopic Pathological Examination
3.5. Bacteriological Examination
3.6. Virological and Serological Tests
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wasik, B.R.; Voorhees, I.E.H.; Parrish, C.R. Canine and Feline Influenza. Cold Spring Harb. Perspect. Med. 2021, 11, a038562. [Google Scholar] [CrossRef] [PubMed]
- Frymus, T.; Belák, S.; Egberink, H.; Hofmann-Lehmann, R.; Marsilio, F.; Addie, D.D.; Boucraut-Baralon, C.; Hartmann, K.; Lloret, A.; Lutz, H.; et al. Influenza Virus Infections in Cats. Viruses 2021, 13, 1435. [Google Scholar] [CrossRef]
- Paniker, C.K.; Nair, C.M. Infection with A2 Hong Kong Influenza Virus in Domestic Cats. Bull. World Health Organ. 1970, 43, 859–862. [Google Scholar]
- Romváry, J.; Rózsa, J.; Farkas, E. Infection of Dogs and Cats with the Hong Kong Influenza A (H3N2) Virus during an Epidemic Period in Hungary. Acta Vet. Acad. Sci. Hung. 1975, 25, 255–259. [Google Scholar] [PubMed]
- Newbury, S.P.; Cigel, F.; Killian, M.L.; Leutenegger, C.M.; Seguin, M.A.; Crossley, B.; Brennen, R.; Suarez, D.L.; Torchetti, M.; Toohey-Kurth, K. First Detection of Avian Lineage H7N2 in Felis Catus. Genome Announc. 2017, 5, e00457-17. [Google Scholar] [CrossRef]
- Jeoung, H.-Y.; Lim, S.-I.; Shin, B.-H.; Lim, J.-A.; Song, J.-Y.; Song, D.-S.; Kang, B.-K.; Moon, H.-J.; An, D.-J. A Novel Canine Influenza H3N2 Virus Isolated from Cats in an Animal Shelter. Vet. Microbiol. 2013, 165, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Song, D.S.; An, D.J.; Moon, H.J.; Yeom, M.J.; Jeong, H.Y.; Jeong, W.S.; Park, S.J.; Kim, H.K.; Han, S.Y.; Oh, J.S.; et al. Interspecies Transmission of the Canine Influenza H3N2 Virus to Domestic Cats in South Korea, 2010. J. Gen. Virol. 2011, 92, 2350–2355. [Google Scholar] [CrossRef]
- Wille, M.; Holmes, E.C. The Ecology and Evolution of Influenza Viruses. Cold Spring Harb. Perspect. Med. 2020, 10, a038489. [Google Scholar] [CrossRef]
- Alexander, D.J.; Brown, I.H. History of Highly Pathogenic Avian Influenza. Rev. Sci. Tech. 2009, 28, 19–38. [Google Scholar] [CrossRef]
- Sonnberg, S.; Webby, R.J.; Webster, R.G. Natural History of Highly Pathogenic Avian Influenza H5N1. Virus Res. 2013, 178, 63–77. [Google Scholar] [CrossRef]
- Xu, X.; Subbarao, K.; Cox, N.J.; Guo, Y. Genetic Characterization of the Pathogenic Influenza A/Goose/Guangdong/1/96 (H5N1) Virus: Similarity of Its Hemagglutinin Gene to Those of H5N1 Viruses from the 1997 Outbreaks in Hong Kong. Virology 1999, 261, 15–19. [Google Scholar] [CrossRef]
- Wille, M.; Barr, I.G. Resurgence of Avian Influenza Virus. Science 2022, 376, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Antigua, K.J.C.; Choi, W.-S.; Baek, Y.H.; Song, M.-S. The Emergence and Decennary Distribution of Clade 2.3.4.4 HPAI H5Nx. Microorganisms 2019, 7, 156. [Google Scholar] [CrossRef]
- Cui, P.; Shi, J.; Wang, C.; Zhang, Y.; Xing, X.; Kong, H.; Yan, C.; Zeng, X.; Liu, L.; Tian, G.; et al. Global Dissemination of H5N1 Influenza Viruses Bearing the Clade 2.3.4.4b HA Gene and Biologic Analysis of the Ones Detected in China. Emerg. Microbes Infect. 2022, 11, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; et al. Avian Influenza Overview September–December 2022. EFSA J. 2023, 21, e07786. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Mirinaviciute, G.; Niqueux, É.; et al. Avian Influenza Overview December 2022–March 2023. EFSA J. 2023, 21, e07917. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinaviciute, G.; Niqueux, É.; Stahl, K.; et al. Avian Influenza Overview March–April 2023. EFSA J. 2023, 21, e08039. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Melidou, A.; Mirinavičiūtė, G.; Niqueux, É.; et al. Avian Influenza Overview April–June 2023. EFSA J. 2023, 21, e08191. [Google Scholar] [CrossRef]
- Kuiken, T.; Rimmelzwaan, G.; van Riel, D.; van Amerongen, G.; Baars, M.; Fouchier, R.; Osterhaus, A. Avian H5N1 Influenza in Cats. Science 2004, 306, 241. [Google Scholar] [CrossRef] [PubMed]
- Keawcharoen, J.; Oraveerakul, K.; Kuiken, T.; Fouchier, R.A.M.; Amonsin, A.; Payungporn, S.; Noppornpanth, S.; Wattanodorn, S.; Theambooniers, A.; Tantilertcharoen, R.; et al. Avian Influenza H5N1 in Tigers and Leopards. Emerg. Infect. Dis. 2004, 10, 2189–2191. [Google Scholar] [CrossRef]
- Rimmelzwaan, G.F.; van Riel, D.; Baars, M.; Bestebroer, T.M.; van Amerongen, G.; Fouchier, R.A.M.; Osterhaus, A.D.M.E.; Kuiken, T. Influenza A Virus (H5N1) Infection in Cats Causes Systemic Disease with Potential Novel Routes of Virus Spread within and between Hosts. Am. J. Pathol. 2006, 168, 176–183. [Google Scholar] [CrossRef]
- Thanawongnuwech, R.; Amonsin, A.; Tantilertcharoen, R.; Damrongwatanapokin, S.; Theamboonlers, A.; Payungporn, S.; Nanthapornphiphat, K.; Ratanamungklanon, S.; Tunak, E.; Songserm, T.; et al. Probable Tiger-to-Tiger Transmission of Avian Influenza H5N1. Emerg. Infect. Dis. 2005, 11, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Songserm, T.; Amonsin, A.; Jam-on, R.; Sae-Heng, N.; Meemak, N.; Pariyothorn, N.; Payungporn, S.; Theamboonlers, A.; Poovorawan, Y. Avian Influenza H5N1 in Naturally Infected Domestic Cat. Emerg. Infect. Dis. 2006, 12, 681–683. [Google Scholar] [CrossRef]
- Yingst, S.L.; Saad, M.D.; Felt, S.A. Qinghai-like H5N1 from Domestic Cats, Northern Iraq. Emerg. Infect. Dis. 2006, 12, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Wolf, P.U.; Uhl, W.; Gerst, S.; Harder, T.; Starick, E.; Vahlenkamp, T.W.; Mettenleiter, T.C.; Teifke, J.P. Distribution of Lesions and Antigen of Highly Pathogenic Avian Influenza Virus A/Swan/Germany/R65/06 (H5N1) in Domestic Cats after Presumptive Infection by Wild Birds. Vet. Pathol. 2007, 44, 261–268. [Google Scholar] [CrossRef]
- Briand, F.-X.; Souchaud, F.; Pierre, I.; Beven, V.; Hirchaud, E.; Hérault, F.; Planel, R.; Rigaudeau, A.; Bernard-Stoecklin, S.; Van der Werf, S.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Domestic Cat, France, 2022. Emerg. Infect. Dis 2023, 29, 1696. [Google Scholar] [CrossRef]
- Leschnik, M.; Weikel, J.; Möstl, K.; Revilla-Fernández, S.; Wodak, E.; Bagó, Z.; Vanek, E.; Benetka, V.; Hess, M.; Thalhammer, J.G. Subclinical Infection with Avian Influenza A (H5N1) Virus in Cats. Emerg. Infect. Dis. 2007, 13, 243–247. [Google Scholar] [CrossRef]
- Floyd, T.; Banyard, A.C.; Lean, F.Z.X.; Byrne, A.M.P.; Fullick, E.; Whittard, E.; Mollett, B.C.; Bexton, S.; Swinson, V.; Macrelli, M.; et al. Encephalitis and Death in Wild Mammals at a Rehabilitation Center after Infection with Highly Pathogenic Avian Influenza A(H5N8) Virus, United Kingdom. Emerg. Infect. Dis. 2021, 27, 2856–2863. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, X.; Wang, T.; Li, Y.; Li, Y.; Xu, Y.; Chu, D.; Sun, H.; Wu, C.; Li, S.; et al. Fatal H5N6 Avian Influenza Virus Infection in a Domestic Cat and Wild Birds in China. Sci. Rep. 2015, 5, 10704. [Google Scholar] [CrossRef]
- Lee, K.; Lee, E.-K.; Lee, H.; Heo, G.-B.; Lee, Y.-N.; Jung, J.-Y.; Bae, Y.-C.; So, B.; Lee, Y.-J.; Choi, E.-J. Highly Pathogenic Avian Influenza A(H5N6) in Domestic Cats, South Korea. Emerg. Infect. Dis. 2018, 24, 2343–2347. [Google Scholar] [CrossRef]
- Rabalski, L.; Milewska, A.; Pohlmann, A.; Gackowska, K.; Lepionka, T.; Szczepaniak, K.; Swiatalska, A.; Sieminska, I.; Arent, Z.; Beer, M.; et al. Emergence and Potential Transmission Route of Avian Influenza A (H5N1) Virus in Domestic Cats in Poland, June 2023. Eurosurveillance 2023, 28, 2300390. [Google Scholar] [CrossRef] [PubMed]
- Domańska-Blicharz, K.; Świętoń, E.; Świątalska, A.; Monne, I.; Fusaro, A.; Tarasiuk, K.; Wyrostek, K.; Styś-Fijoł, N.; Giza, A.; Pietruk, M.; et al. Outbreak of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Cats, Poland, June to July 2023. Eurosurveillance 2023, 28, 2300366. [Google Scholar] [CrossRef]
- Giese, M.; Harder, T.C.; Teifke, J.P.; Klopfleisch, R.; Breithaupt, A.; Mettenleiter, T.C.; Vahlenkamp, T.W. Experimental Infection and Natural Contact Exposure of Dogs with Avian Influenza Virus (H5N1). Emerg. Infect. Dis. 2008, 14, 308–310. [Google Scholar] [CrossRef]
- Abdel-Moein, K.A.; El-Hariri, M.D.; Wasfy, M.O.; Samir, A. Occurrence of Ampicillin-Resistant Enterococcus Faecium Carrying Esp Gene in Pet Animals: An Upcoming Threat for Pet Lovers. J. Glob. Antimicrob. Resist. 2017, 9, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Tumpa, A.; Štritof, Z.; Pintarić, S. Prevalence and Antimicrobial Susceptibility of Enterococcus Spp. from Urine of Dogs and Cats in Northwestern Croatia. Res. Vet. Sci. 2022, 151, 42–46. [Google Scholar] [CrossRef]
- Yuan, T.; Liang, B.; Jiang, B.; Sun, S.; Zhou, Y.; Zhu, L.; Liu, J.; Guo, X.; Ji, X.; Sun, Y. Virulence Genes and Antimicrobial Resistance in Enterococcus Strains Isolated from Dogs and Cats in Northeast China. J. Vet. Med. Sci. 2023, 85, 371–378. [Google Scholar] [CrossRef]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The Many Faces of Enterococcus Spp.—Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Golden, N.J.; Schlosser, W.D.; Ebel, E.D. Risk Assessment to Estimate the Probability of a Chicken Flock Infected with H5N1 Highly Pathogenic Avian Influenza Virus Reaching Slaughter Undetected. Foodborne Pathog. Dis. 2009, 6, 827–835. [Google Scholar] [CrossRef]
- Greiner, M.; Müller-Graf, C.; Hiller, P.; Schrader, C.; Gervelmeyer, A.; Ellerbroek, L.; Appel, B. Expert Opinion Based Modelling of the Risk of Human Infection with H5N1 through the Consumption of Poultry Meat in Germany. Berl. Munch. Tierarztl. Wochenschr. 2007, 120, 98–107. [Google Scholar] [PubMed]
- Harder, T.C.; Teuffert, J.; Starick, E.; Gethmann, J.; Grund, C.; Fereidouni, S.; Durban, M.; Bogner, K.H.; Neubauer-Juric, A.; Repper, R.; et al. Highly Pathogenic Avian Influenza Virus (H5N1) in Frozen Duck Carcasses, Germany, 2007. Emerg. Infect. Dis. 2009, 15, 272–279. [Google Scholar] [CrossRef]
- Vahlenkamp, T.W.; Harder, T.C.; Giese, M.; Lin, F.; Teifke, J.P.; Klopfleisch, R.; Hoffmann, R.; Tarpey, I.; Beer, M.; Mettenleiter, T.C. Protection of Cats against Lethal Influenza H5N1 Challenge Infection. J. Gen. Virol. 2008, 89, 968–974. [Google Scholar] [CrossRef] [PubMed]
- El-Shesheny, R.; Moatasim, Y.; Mahmoud, S.H.; Song, Y.; El Taweel, A.; Gomaa, M.; Kamel, M.N.; Sayes, M.E.; Kandeil, A.; Lam, T.T.Y.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Clade 2.3.4.4b in Wild Birds and Live Bird Markets, Egypt. Pathogens 2022, 12, 36. [Google Scholar] [CrossRef]
- Wang, H.; Wu, X.; Cheng, Y.; An, Y.; Ning, Z. Tissue Distribution of Human and Avian Type Sialic Acid Influenza Virus Receptors in Domestic Cat. Acta Vet. Hung. 2013, 61, 537–546. [Google Scholar] [CrossRef]

| Clinical Sample | Result | Cq for H5 Gene | Cq for N1 Gene |
|---|---|---|---|
| spleen | (+) | 31.58 | 31.33 |
| intestine | (+) | 32.14 | 31.84 |
| liver | (+) | 33.21 | 33.07 |
| lung | (+) | 34.02 | 33.56 |
| mesenteric lymph node | (+) | 34.71 | 33.92 |
| pancreas | (−) | - | - |
| heart | (−) | - | - |
| kidney | (−) | - | - |
| brain | (−) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaluś-Jordanow, O.; Golke, A.; Dzieciątkowski, T.; Chrobak-Chmiel, D.; Rzewuska, M.; Czopowicz, M.; Sapierzyński, R.; Kardas, M.; Biernacka, K.; Mickiewicz, M.; et al. A Fatal A/H5N1 Avian Influenza Virus Infection in a Cat in Poland. Microorganisms 2023, 11, 2263. https://doi.org/10.3390/microorganisms11092263
Szaluś-Jordanow O, Golke A, Dzieciątkowski T, Chrobak-Chmiel D, Rzewuska M, Czopowicz M, Sapierzyński R, Kardas M, Biernacka K, Mickiewicz M, et al. A Fatal A/H5N1 Avian Influenza Virus Infection in a Cat in Poland. Microorganisms. 2023; 11(9):2263. https://doi.org/10.3390/microorganisms11092263
Chicago/Turabian StyleSzaluś-Jordanow, Olga, Anna Golke, Tomasz Dzieciątkowski, Dorota Chrobak-Chmiel, Magdalena Rzewuska, Michał Czopowicz, Rafał Sapierzyński, Michał Kardas, Kinga Biernacka, Marcin Mickiewicz, and et al. 2023. "A Fatal A/H5N1 Avian Influenza Virus Infection in a Cat in Poland" Microorganisms 11, no. 9: 2263. https://doi.org/10.3390/microorganisms11092263
APA StyleSzaluś-Jordanow, O., Golke, A., Dzieciątkowski, T., Chrobak-Chmiel, D., Rzewuska, M., Czopowicz, M., Sapierzyński, R., Kardas, M., Biernacka, K., Mickiewicz, M., Moroz-Fik, A., Łobaczewski, A., Stefańska, I., Kwiecień, E., Markowska-Daniel, I., & Frymus, T. (2023). A Fatal A/H5N1 Avian Influenza Virus Infection in a Cat in Poland. Microorganisms, 11(9), 2263. https://doi.org/10.3390/microorganisms11092263







