Using Wastewater Surveillance to Monitor Gastrointestinal Pathogen Infections in the State of Oklahoma
Abstract
:1. Introduction
2. Methods
2.1. Wastewater Sampling
2.2. Genetic Material Extraction and Quantification
2.3. Human Cases and Outbreaks
2.4. Statistical Methods
3. Results
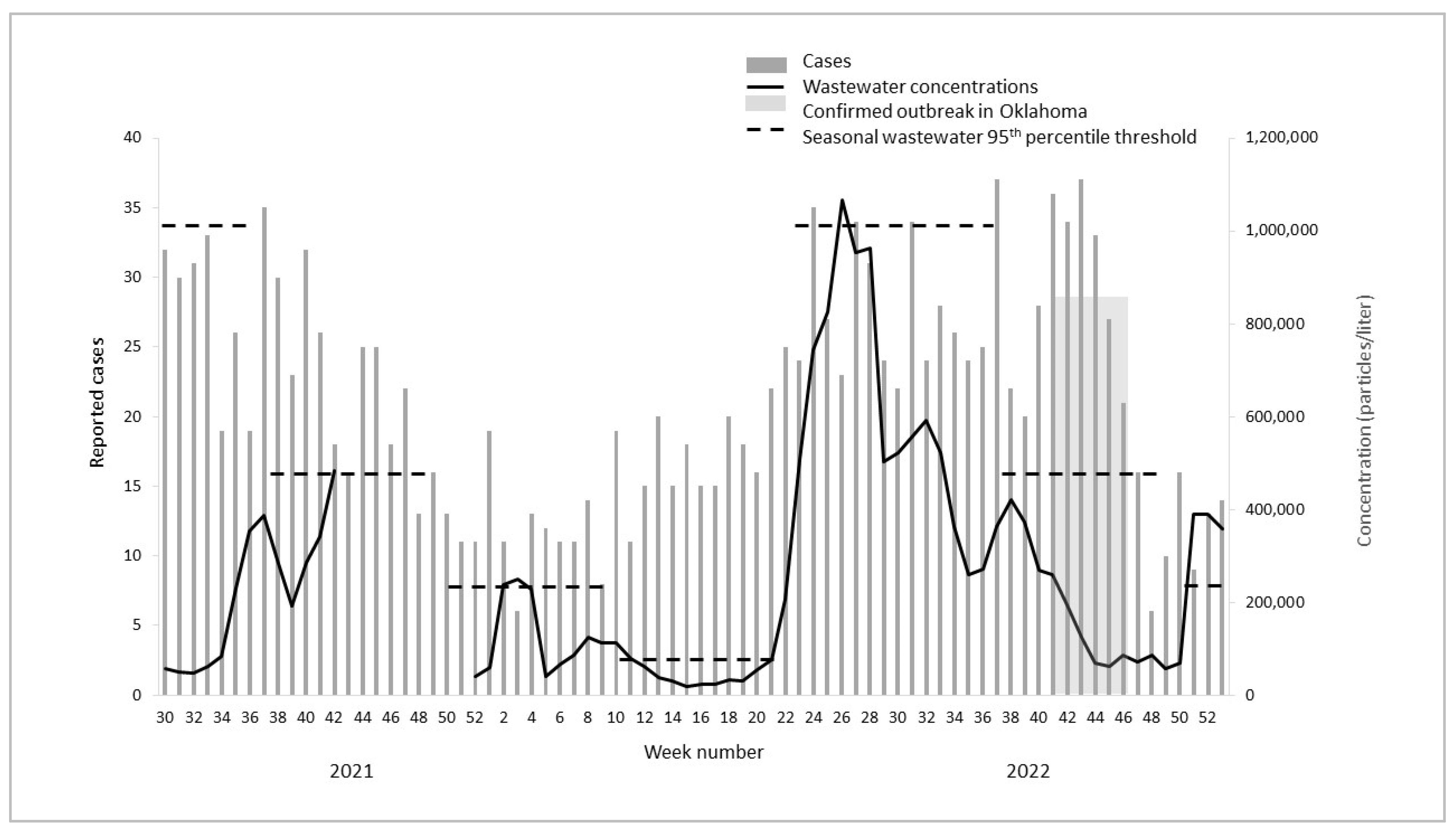
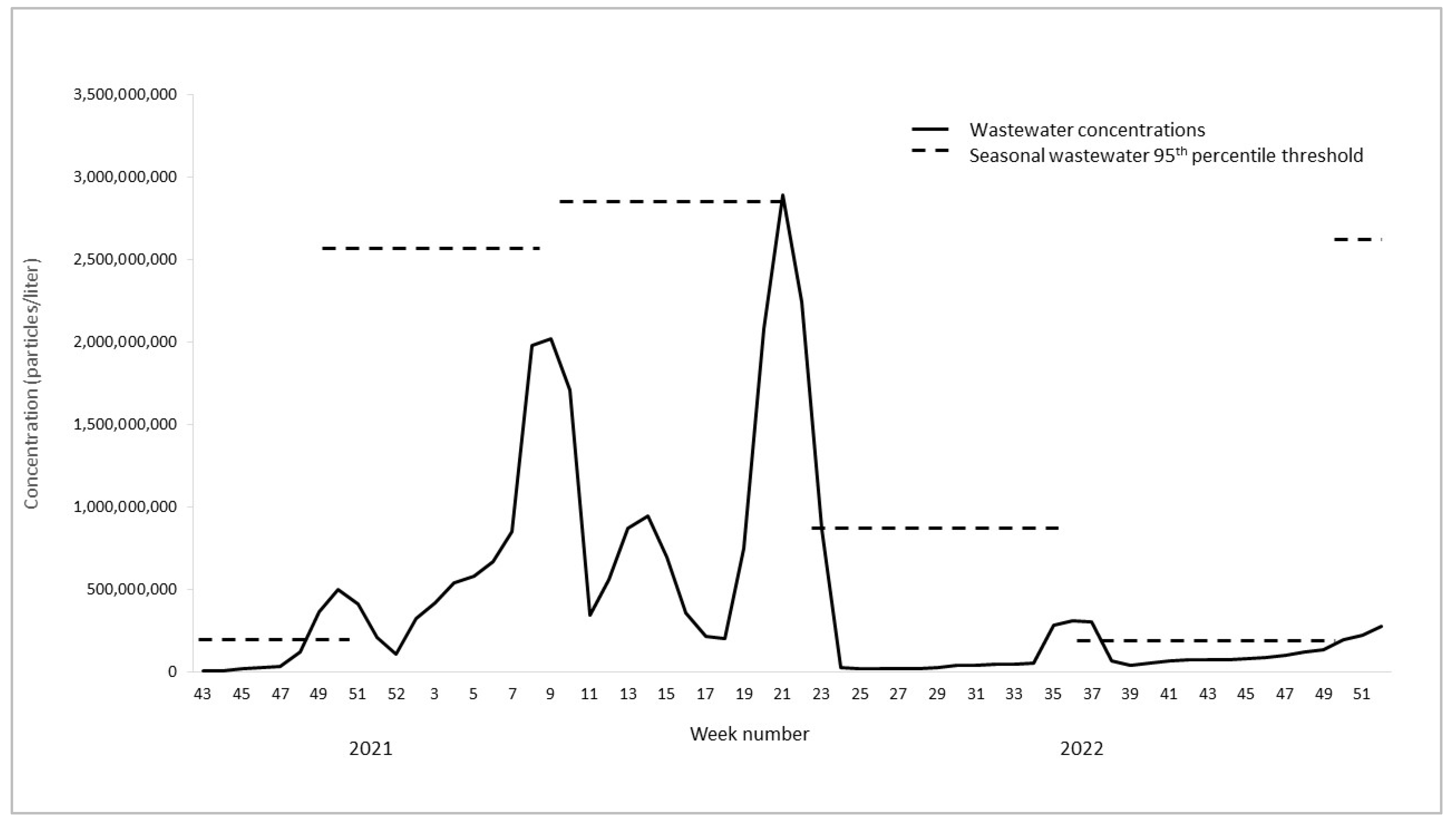
3.1. Wastewater Concentrations and Cases over Time
3.2. Outbreaks
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibbons, C.L.; Mangen, M.-J.J.; Plass, D.; Havelaar, A.H.; Brooke, R.J.; Kramarz, P.; Peterson, K.L.; Stuurman, A.L.; Cassini, A.; Fèvre, E.M.; et al. Measuring Underreporting and Under-Ascertainment in Infectious Disease Datasets: A Comparison of Methods. BMC Public Health 2014, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Haagsma, J.A.; Geenen, P.L.; Ethelberg, S.; Fetsch, A.; Hansdotter, F.; Jansen, A.; Korsgaard, H.; O’Brien, S.J.; Scavia, G.; Spitznagel, H.; et al. Community Incidence of Pathogen-Specific Gastroenteritis: Reconstructing the Surveillance Pyramid for Seven Pathogens in Seven European Union Member States. Epidemiol. Infect. 2013, 141, 1625–1639. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.B.; Keshaviah, A.; Bento, A.I.; Conroy-Ben, O.; Driver, E.M.; Ensor, K.B.; Halden, R.U.; Hopkins, L.P.; Kuhn, K.G.; Moe, C.L.; et al. Wastewater Surveillance of Pathogens Can Inform Public Health Responses. Nat. Med. 2022, 28, 1992–1995. [Google Scholar] [CrossRef] [PubMed]
- McClary-Gutierrez, J.S.; Mattioli, M.C.; Marcenac, P.; Silverman, A.I.; Boehm, A.B.; Bibby, K.; Balliet, M.; de los Reyes, F.L.; Gerrity, D.; Griffith, J.F.; et al. SARS-CoV-2 Wastewater Surveillance for Public Health Action. Emerg. Infect. Dis. 2021, 27, e210753. [Google Scholar] [CrossRef]
- Kuhn, K.G.; Jarshaw, J.; Jeffries, E.; Adesigbin, K.; Maytubby, P.; Dundas, N.; Miller, A.C.; Rhodes, E.; Stevenson, B.; Vogel, J.; et al. Predicting COVID-19 Cases in Diverse Population Groups Using SARS-CoV-2 Wastewater Monitoring across Oklahoma City. Sci. Total Environ. 2022, 812, 151431. [Google Scholar] [CrossRef]
- Manor, Y.; Shulman, L.M.; Kaliner, E.; Hindiyeh, M.; Ram, D.; Sofer, D.; Moran-Gilad, J.; Lev, B.; Grotto, I.; Gamzu, R.; et al. Intensified Environmental Surveillance Supporting the Response to Wild Poliovirus Type 1 Silent Circulation in Israel, 2013. Eurosurveillance 2014, 19, 20708. [Google Scholar] [CrossRef]
- Battistone, A.; Buttinelli, G.; Bonomo, P.; Fiore, S.; Amato, C.; Mercurio, P.; Cicala, A.; Simeoni, J.; Foppa, A.; Triassi, M.; et al. Detection of Enteroviruses in Influent and Effluent Flow Samples from Wastewater Treatment Plants in Italy. Food Environ. Virol. 2014, 6, 13–22. [Google Scholar] [CrossRef]
- Chacón, L.; Morales, E.; Valiente, C.; Reyes, L.; Barrantes, K. Wastewater-Based Epidemiology of Enteric Viruses and Surveillance of Acute Gastrointestinal Illness Outbreaks in a Resource-Limited Region. Am. J. Trop. Med. Hyg. 2021, 105, 1004–1012. [Google Scholar] [CrossRef]
- Diemert, S.; Yan, T. Municipal Wastewater Surveillance Revealed a High Community Disease Burden of a Rarely Reported and Possibly Subclinical Salmonella Enterica Serovar Derby Strain. Appl. Environ. Microbiol. 2020, 86, e00814-20. [Google Scholar] [CrossRef]
- Haramoto, E.; Katayama, H.; Oguma, K.; Yamashita, H.; Tajima, A.; Nakajima, H.; Ohgaki, S. Seasonal Profiles of Human Noroviruses and Indicator Bacteria in a Wastewater Treatment Plant in Tokyo, Japan. Water Sci. Technol. 2006, 54, 301–308. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Munk, P.; Njage, P.; van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global Monitoring of Antimicrobial Resistance Based on Metagenomics Analyses of Urban Sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, P.M.C.; Larsson, D.G.J.; Flach, C.-F. Surveillance of Antibiotic Resistant Escherichia Coli in Human Populations through Urban Wastewater in Ten European Countries. Environ. Pollut. 2020, 261, 114200. [Google Scholar] [CrossRef] [PubMed]
- John, P.; Varga, C.; Cooke, M.; Majowicz, S.E. Incidence, Demographic, and Seasonal Risk Factors of Infections Caused by Five Major Enteric Pathogens, Ontario, Canada, 2010–2017. Foodborne Pathog. Dis. 2022, 19, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.B.; Zhou, B.; Naumova, E.N. Seasonal Synchronization of Foodborne Outbreaks in the United States, 1996–2017. Sci. Rep. 2020, 10, 17500. [Google Scholar] [CrossRef]
- Hall, A.J.; Wikswo, M.E.; Manikonda, K.; Roberts, V.A.; Yoder, J.S.; Gould, L.H. Acute Gastroenteritis Surveillance through the National Outbreak Reporting System, United States. Emerg. Infect. Dis. 2013, 19, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, A.K.; Calderwood, L.; Wikswo, M.E.; Barclay, L.; Mattison, C.P.; Balachandran, N.; Vinjé, J.; Hall, A.J.; Mirza, S.A. Spatiotemporal Trends in Norovirus Outbreaks in the United States, 2009–2019. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 76, 667–673. [Google Scholar] [CrossRef]
- Ørmen, Ø.; Madslien, E.H. Molecular Monitoring of Enteropathogens in Sewage During NATO Exercise Trident Juncture 2018: Potential Tool in Early Outbreak Warning? Mil. Med. 2021, 186, e661–e666. [Google Scholar] [CrossRef] [PubMed]
- Hellmér, M.; Paxéus, N.; Magnius, L.; Enache, L.; Arnholm, B.; Johansson, A.; Bergström, T.; Norder, H. Detection of Pathogenic Viruses in Sewage Provided Early Warnings of Hepatitis A Virus and Norovirus Outbreaks. Appl. Environ. Microbiol. 2014, 80, 6771–6781. [Google Scholar] [CrossRef]
- Vincent, V.; Scott, H.M.; Harvey, R.B.; Alali, W.Q.; Hume, M.E. Novel Surveillance of Salmonella enterica Serotype Heidelberg Epidemics in a Closed Community. Foodborne Pathog. Dis. 2007, 4, 375–385. [Google Scholar] [CrossRef]
- Myers, D.N.; Stoeckel, D.M.; Bushon, R.N.; Francy, D.S.; Brady, A.M.G. Fecal Indicator Bacteria: U.S. Geological Survey Techniques of Water-Resources Investigations. In National Field Manual for the Collection of Water-Quality Data; Book 9; USGS: Reston, VA, USA, 2014; Chapter A7.1. [Google Scholar] [CrossRef]
- Oberacker, P.; Stepper, P.; Bond, D.M.; Höhn, S.; Focken, J.; Meyer, V.; Schelle, L.; Sugrue, V.J.; Jeunen, G.-J.; Moser, T.; et al. Bio-On-Magnetic-Beads (BOMB): Open Platform for High-Throughput Nucleic Acid Extraction and Manipulation. PLoS Biol. 2019, 17, e3000107. [Google Scholar] [CrossRef]
- Vondrakova, L.; Pazlarova, J.; Demnerova, K. Detection, Identification and Quantification of Campylobacter Jejuni, Coli and Lari in Food Matrices All at Once Using Multiplex QPCR. Gut Pathog. 2014, 6, 12. [Google Scholar] [CrossRef]
- Miura, T.; Parnaudeau, S.; Grodzki, M.; Okabe, S.; Atmar, R.L.; Le Guyader, F.S. Environmental Detection of Genogroup I, II and IV Norovirus Using a Generic Real-Time Reverse Transcription-PCR Assay. Appl. Environ. Microbiol. 2013, 79, 6585–6592. [Google Scholar] [CrossRef]
- Shannon, K.E.; Lee, D.-Y.; Trevors, J.T.; Beaudette, L.A. Application of Real-Time Quantitative PCR for the Detection of Selected Bacterial Pathogens during Municipal Wastewater Treatment. Sci. Total Environ. 2007, 382, 121–129. [Google Scholar] [CrossRef]
- List of Multistate Foodborne Outbreak Notices|CDC. Available online: https://www.cdc.gov/foodsafety/outbreaks/lists/outbreaks-list.html (accessed on 25 January 2023).
- Tay, E.L.; Grant, K.; Kirk, M.; Mounts, A.; Kelly, H. Exploring a Proposed WHO Method to Determine Thresholds for Seasonal Influenza Surveillance. PLoS ONE 2013, 8, e77244. [Google Scholar] [CrossRef]
- Outbreak Toolkit. Available online: https://www.who.int/emergencies/outbreak-toolkit (accessed on 13 February 2023).
- Statistical Software for Data Science|Stata. Available online: https://www.stata.com/ (accessed on 13 February 2023).
- Arshad, M.M.; Wilkins, M.J.; Downes, F.P.; Rahbar, M.H.; Erskine, R.J.; Boulton, M.L.; Saeed, A.M. A Registry-Based Study on the Association between Human Salmonellosis and Routinely Collected Parameters in Michigan, 1995–2001. Foodborne Pathog. Dis. 2007, 4, 16–25. [Google Scholar] [CrossRef]
- Bisseux, M.; Colombet, J.; Mirand, A.; Roque-Afonso, A.-M.; Abravanel, F.; Izopet, J.; Archimbaud, C.; Peigue-Lafeuille, H.; Debroas, D.; Bailly, J.-L.; et al. Monitoring Human Enteric Viruses in Wastewater and Relevance to Infections Encountered in the Clinical Setting: A One-Year Experiment in Central France, 2014 to 2015. Eurosurveillance 2018, 23, 17. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, N.; Zhang, S.; Yi, Y.; Han, Y.; Liu, M.; Han, Y.; Shi, N.; Yang, L.; Wang, Q.; et al. Norovirus Detection in Wastewater and Its Correlation with Human Gastroenteritis: A Systematic Review and Meta-Analysis. Environ. Sci. Pollut. Res. Int. 2022, 29, 22829–22842. [Google Scholar] [CrossRef]
- Ahmed, W.; Tscharke, B.; Bertsch, P.M.; Bibby, K.; Bivins, A.; Choi, P.; Clarke, L.; Dwyer, J.; Edson, J.; Nguyen, T.M.H.; et al. SARS-CoV-2 RNA Monitoring in Wastewater as a Potential Early Warning System for COVID-19 Transmission in the Community: A Temporal Case Study. Sci. Total Environ. 2021, 761, 144216. [Google Scholar] [CrossRef]
- Simonsen, J.; Strid, M.A.; Mølbak, K.; Krogfelt, K.A.; Linneberg, A.; Teunis, P. Sero-Epidemiology as a Tool to Study the Incidence of Salmonella Infections in Humans. Epidemiol. Infect. 2008, 136, 895–902. [Google Scholar] [CrossRef]
- Melvin, R.G.; Hendrickson, E.N.; Chaudhry, N.; Georgewill, O.; Freese, R.; Schacker, T.W.; Simmons, G.E. A Novel Wastewater-Based Epidemiology Indexing Method Predicts SARS-CoV-2 Disease Prevalence across Treatment Facilities in Metropolitan and Regional Populations. Sci. Rep. 2021, 11, 21368. [Google Scholar] [CrossRef]
- Markt, R.; Stillebacher, F.; Nägele, F.; Kammerer, A.; Peer, N.; Payr, M.; Scheffknecht, C.; Dria, S.; Draxl-Weiskopf, S.; Mayr, M.; et al. Expanding the Pathogen Panel in Wastewater Epidemiology to Influenza and Norovirus. Viruses 2023, 15, 263. [Google Scholar] [CrossRef]

| Measure | Campylobacter | Salmonella | Norovirus |
|---|---|---|---|
| Total cases reported | 1459 | 1157 | n.a |
| Average weekly cases reported | 22 | 17 | n.a |
| Average weekly wastewater concentration * | 180,000 | 127,000 | 390 million |
| Peak cases reported (date) | 37 | 64 | n.a |
| (10 September 2022) | (4 September 2021) | ||
| Peak wastewater concentration * (date) | 1.7 million | 4.2 million | 4.5 billion |
| (9 July 2022) | (22 January 2022) | (27 February 2022) |
| Pathogen | Number of Weeks with Outbreaks Reported | Number of Weeks Where Wastewater Concentrations Exceeded 95th Seasonal Threshold | Number of Weeks Where Exceeded Wastewater Thresholds Corresponded with Timing of Reported Outbreaks * | Number of Weeks Where Exceeded Wastewater Thresholds Corresponded with Timing of Outbreaks with Unknown Etiology * (N = 22) |
|---|---|---|---|---|
| Campylobacter | 6 | 10 | 0 | 2 |
| Salmonella | 47 | 11 | 9 | 1 |
| Norovirus | n.a | 7 | n.a | 5 |
| Total (%) | 53 | 28 | 9 (32) | 9 (41) |
| Pathogen | Multistate Outbreak, Week Number/Year Reported * | Oklahoma Wastewater Outbreak Threshold Exceeded, Week Number/Year |
|---|---|---|
| Campylobacter | None reported | n.a |
| Salmonella | Salmonella Senftenberg, 12–21/2022 | 19–20/2022 |
| Norovirus | 14–27/2022 | 21–23/2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhn, K.G.; Shukla, R.; Mannell, M.; Graves, G.M.; Miller, A.C.; Vogel, J.; Malloy, K.; Deshpande, G.; Florea, G.; Shelton, K.; et al. Using Wastewater Surveillance to Monitor Gastrointestinal Pathogen Infections in the State of Oklahoma. Microorganisms 2023, 11, 2193. https://doi.org/10.3390/microorganisms11092193
Kuhn KG, Shukla R, Mannell M, Graves GM, Miller AC, Vogel J, Malloy K, Deshpande G, Florea G, Shelton K, et al. Using Wastewater Surveillance to Monitor Gastrointestinal Pathogen Infections in the State of Oklahoma. Microorganisms. 2023; 11(9):2193. https://doi.org/10.3390/microorganisms11092193
Chicago/Turabian StyleKuhn, Katrin Gaardbo, Rishabh Shukla, Mike Mannell, Grant M. Graves, A. Caitlin Miller, Jason Vogel, Kimberly Malloy, Gargi Deshpande, Gabriel Florea, Kristen Shelton, and et al. 2023. "Using Wastewater Surveillance to Monitor Gastrointestinal Pathogen Infections in the State of Oklahoma" Microorganisms 11, no. 9: 2193. https://doi.org/10.3390/microorganisms11092193
APA StyleKuhn, K. G., Shukla, R., Mannell, M., Graves, G. M., Miller, A. C., Vogel, J., Malloy, K., Deshpande, G., Florea, G., Shelton, K., Jeffries, E., De León, K. B., & Stevenson, B. (2023). Using Wastewater Surveillance to Monitor Gastrointestinal Pathogen Infections in the State of Oklahoma. Microorganisms, 11(9), 2193. https://doi.org/10.3390/microorganisms11092193






