Abstract
Probiotics for humans and direct-fed microbials for livestock are increasingly popular dietary ingredients for supporting immunity. The aim of this study was to determine the effects of dietary supplementation of Bacillus subtilis MB40 (MB40) on immunity in piglets challenged with the foodborne pathogen Listeria monocytogenes (LM). Three-week-old piglets (n = 32) were randomly assigned to four groups: (1) basal diet, (2) basal diet with LM challenge, (3) MB40-supplemented diet, and (4) MB40-supplemented diet with LM challenge. Experimental diets were provided throughout a 14-day (d) period. On d8, piglets in groups 2 and 4 were intraperitoneally inoculated with LM at 108 CFU/mL per piglet. Blood samples were collected at d1, d8, and d15 for biochemical and immune response profiling. Animals were euthanized and necropsied at d15 for liver and spleen bacterial counts and intestinal morphological analysis. At d15, LM challenge was associated with increased spleen weight (p = 0.017), greater circulating populations of neutrophils (p = 0.001) and monocytes (p = 0.008), and reduced ileal villus height to crypt depth ratio (p = 0.009), compared to non-challenged controls. MB40 supplementation reduced LM bacterial counts in the liver and spleen by 67% (p < 0.001) and 49% (p < 0.001), respectively, following the LM challenge, compared to the basal diet. MB40 supplementation was also associated with decreased circulating concentrations of monocytes (p = 0.007). Altogether, these data suggest that MB40 supplementation is a safe and well-tolerated approach to enhance immunity during systemic Listeria infection.
1. Introduction
Probiotics are live microorganisms that confer a health benefit on a host when administered in adequate amounts [1]. Beyond supporting digestion and immunity in healthy adults [2], probiotics may also lessen the incidence or severity of certain diseases. The American Gastroenterology Association issued guidance that probiotics may be useful for (1) prevention of Clostridioides difficile infection in adults and children taking antibiotics, (2) prevention of necrotizing enterocolitis in preterm, low birthweight infants, and (3) management of pouchitis, a complication of inflammatory bowel disease [3]. Several meta-analyses support the use of probiotics to mitigate antibiotic-associated diarrhea [4,5] and gastrointestinal (GI) infections among infants and children [6,7]. These data highlight the potential role of probiotics in modulating the gut immune response and mitigating the expansion of pathogenic bacteria. In particular, probiotic supplementation may be an effective strategy to lessen the severity and duration of GI symptoms during foodborne bacterial infection.
Foodborne illness is one of the most prevalent GI disorders across the globe. The Centers for Disease Control and Prevention estimates 48 million cases, 128,000 hospitalizations, and 3000 deaths annually due to foodborne illness in the United States [8]. Common foodborne bacterial pathogens include Salmonella, Clostridium perfringens, Campylobacter, certain Escherichia coli strains, and Listeria monocytogenes (LM). LM is the Gram-positive, rod-shaped, non-spore-forming, motile, facultative anaerobe that causes listeriosis [9]. In at-risk individuals, listeriosis can lead to systemic infection, sepsis, encephalitis, meningitis, and death following the translocation of LM from intestinal tissue to mesenteric lymph nodes and visceral organs [10,11]. An estimated 1600 cases of listeriosis occur annually in the US, leading to an estimated 255 deaths and the highest rate of hospitalization among 31 known foodborne pathogens [12]. LM is genetically diverse with 14 serotypes, of which serotypes 1/2a, 1/2b, 1/2c, and 4b comprise most food and clinical isolates, and serotype 4b is most associated with human listeriosis [13,14].
Individuals can become ill with listeriosis after consuming contaminated foods such as pork, chicken, deli meats, dairy products, and vegetables [15,16]. Several post-harvest mitigation strategies are used to reduce the growth and survival of LM in food and food processing facilities (e.g., acid treatment, osmotic stress, desiccation, disinfectants, ionization radiation, and antimicrobial peptides) [17]. However, LM exhibits considerable stress tolerance and adaptability [18]. With regard to meat pre-harvest practices, antibiotics have been administered to livestock since the 1950s to improve growth performance and reduce the burden of subacute bacterial diseases that are caused by pathogens such as LM [19,20]. The emergence of antibiotic-resistant bacteria has led to policies banning the use of antibiotics in animal feed as prophylaxis and growth promoters in the European Union and the United States, respectively [21,22,23].
One alternative approach to antibiotics to improve livestock performance and increase resistance to disease is the use of beneficial, live microorganisms as feed additives, also known as direct-fed microbials (DFMs) [24,25,26]. The term DFM is often used interchangeably with probiotics. Studies dating back to the 1970s have shown a growth performance benefit of DFM supplementation in calves and piglets using strains of bacteria from the Lactobacillaceae family and Bifidobacterium genus [27,28]. Strains from the Bacillaceae family (including the Bacillus genus) have also shown benefits as DFMs [25,29,30,31]. Bacillaceae strains exert probiotic activity, in part, by secreting antimicrobial molecules and digestive enzymes that contribute to disease resistance and nutrient absorption, respectively [32,33,34]. Bacillaceae-based DFMs may also indirectly improve growth performance by promoting the growth of beneficial gut bacteria [35,36] and modulating gut and systemic immune responses [37,38,39]. Bacillaceae strains are particularly suited for animal DFM and human probiotic products because they can be manufactured as thermostable spores that tolerate gastric acidity following oral administration [40,41,42,43,44]. Certain probiotic strains of Bacillus subtilis and Weizmannia coagulans (formerly B. coagulans) have been clinically shown to support digestion and GI health in healthy human participants [45,46,47] and participants with symptoms of inflammatory bowel syndrome or dyspepsia [48,49,50,51,52,53,54,55,56].
The effects of Bacillaceae spore supplementation on growth performance and diarrhea severity have previously been studied in pigs [57,58,59], as well as acute challenge studies with pathogens like C. perfringens and E. coli [60,61]. However, we are not aware of any LM challenge study in pigs. Bacillaceae strains are especially promising antilisterial candidates, given the many reports of live Bacillaceae strains and cell-free supernatants with lytic or competitive growth activity toward cultured LM bacteria [62,63,64,65,66,67,68,69,70]. Therefore, we set out to study the effects of B. subtilis strain MB40 (MB40) as a candidate DFM in a new porcine model of listeriosis. Probiotic MB40 supplementation has previously been shown to be safe and supports GI health in clinical trials of healthy human participants [45,71]. MB40 has yet to be studied in patients with symptomatic disease or foodborne illness, so this preclinical study also served to assess the probiotic potential of MB40 to improve human immunity. We hypothesized that MB40 supplementation would improve the immune response, limit visceral dissemination of LM, and reduce intestinal histopathology following intraperitoneal LM challenge in weaned piglets.
2. Materials and Methods
2.1. Animals and Diets
This study was approved by and conducted under the guidelines of the Virginia Tech Institutional Animal Care and Use Committee (IACUC-18-010-APSC; August 2018). A total of 32 cross-bred, weaned piglets (21 days old) were acquired from the Virginia Tech swine farm and transported to a biosafety level 2 facility. The animals were individually housed and fed ad libitum a standard, non-medicated, pelleted, swine starter commercial diet (Big Spring Mill, Elliston, VA, USA) for 9 days while acclimating to the facility in environmentally controlled rooms maintained at 24 °C with a standard light cycle (12 h light/dark). The diet contained crude protein (18% minimum), crude fat (3% minimum), crude fiber (4% maximum), calcium (1% minimum, 1.5% maximum), and phosphorus (0.5% minimum). The following ingredients were listed on the label: two-grain products, processed grain by-products, plant protein products, animal protein products, forage products, cane molasses, sugar, salt, dicalcium phosphate, monocalcium phosphate, calcium carbonate, potassium chloride, iron sulfate, cobalt sulfate, copper sulfate, manganese sulfate, manganous oxide, zinc sulfate, zinc oxide, red iron oxide, calcium iodate, sodium selenite, vitamin A supplement, vitamin D3 supplement, vitamin E supplement, menadione sodium bisulfite complex (source of vitamin K activity), vitamin B-12 supplement, choline chloride, niacin, calcium pantothenate, riboflavin supplement, and mineral oil. No vaccinations were provided since the animals were in isolation in clean rooms.
Following acclimation, animals were randomly assigned to 4 experimental groups in a 2 × 2 factorial arrangement (2 dietary treatments, with and without LM challenge): (1) basal diet (Basal), (2) basal diet and LM challenge (Basal + LM), (3) MB40-supplemented diet (MB40), and (4) MB40-supplemented diet and LM challenge (MB40 + LM, Figure 1). Each experimental group included 4 gilts and 4 barrows. Bacillus subtilis strain MB40, registered as American Type Culture Collection (ATCC) No. PTA-122264 was derived from the Bacillus subtilis Marburg type strain DSM 10 (ATCC No. 6051) and displayed enhanced spore stability without genetic engineering. MB40 has also demonstrated a robust safety profile and probiotic characteristics in cell culture experiments and clinical trials [45,71]. Additionally, the United States Food and Drug Administration issued a “no objection letter” for the use of MB40 in foods according to a “generally regarded as safe” (GRAS) dossier [72]. MB40 was manufactured and provided by BIO-CAT Microbials, LLC (Shakopee, MN, USA). Briefly, MB40 was grown using aerobic fermentation methods to achieve nearly 100% spores. After fermentation, pure MB40 spore culture was concentrated via centrifugation and then spray-dried using maltodextrin as a carrier. In this animal study, MB40 was supplemented at 7.5 billion colony-forming units (CFU) per day by mixing 1 g MB40 (7.5 billion CFU/g) with the basal diet in pelleted form. Experimental diets were fed to animals for 14 days. All diets were formulated to meet or exceed the 2012 National Research Council nutrient recommendations for weaned piglets [73] and did not contain antibiotics or pharmaceutical levels of copper or zinc.
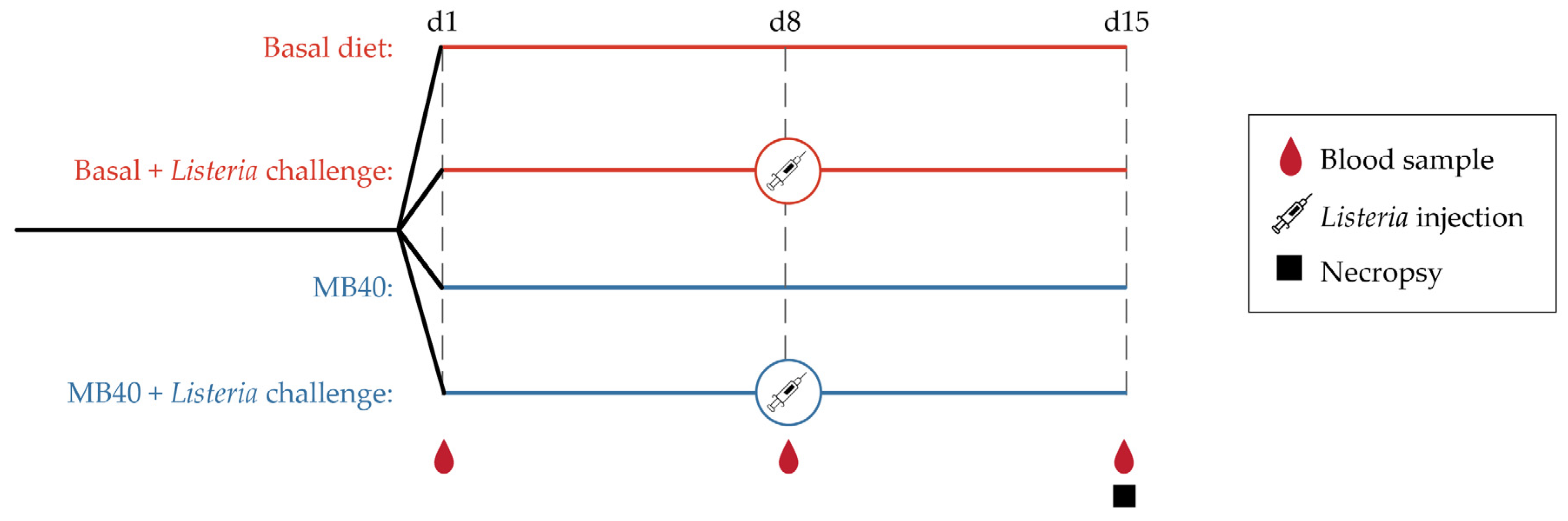
Figure 1.
Experimental design.
2.2. Bacterial Challenge
L. monocytogenes (4b strain) was sub-cultured from a frozen stock on blood agar plates and enriched overnight in brain heart infusion (BHI) broth. Log dilutions of BHI broth were plated on blood agar in order to determine CFU/mL. After counting and diluting to the appropriate dose, the culture was centrifuged to remove the broth, and the bacterial pellet was resuspended in phosphate-buffered saline (PBS) at 108 CFU/mL before a single intraperitoneal injection at 1 mL/animal in groups 2 and 4 at d8. The non-challenged groups (1 and 3) received a similar 1 mL injection of sterile PBS. Intraperitoneal injection models the visceral spread of LM and avoids confounding variables associated with oral dosing (e.g., gastric pH, gastric volume of chyme, intestinal pH, intestinal volume of digesta). The intraperitoneal LM dose was chosen to cause systemic infection and mild GI distress without mortality. Animals were euthanized at d15, 7 days after the single dose of LM. It has previously been recognized that systemic inflammation and visceral infection can be sustained for up to 10 days following intraperitoneal bacterial pathogen challenge with LM in mice [74,75].
2.3. Necropsy and Sample Collection
Animals were individually weighed, and feed refusals were measured daily to determine daily feed intake throughout this study to calculate average daily weight gain (ADG), average daily feed intake (ADFI), and feed conversion ratio. Animals did not fast before sample collection. At d1, d8, and d15, blood samples were collected for standard serum chemistry and hematological profiling. Blood samples were collected in EDTA or lithium heparin BD Vacutainer® blood collection tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and stored on ice. Blood samples collected in EDTA-coated tubes were transported on ice for hematologic analysis. Plasma was separated from blood collected in lithium heparin-coated tubes at 4 °C for 10 min at 2500× g (Eppendorf, Hamburg, Germany), and aliquots were stored at −80 °C until further analysis. Fresh plasma samples were sent to the Virginia-Maryland College of Veterinary Medicine (Blacksburg, VA, USA) for blood chemistry analysis using the AU480 Chemistry Analyzer with ion-selective electrode (Beckman Coulter, Inc., Brea, CA, USA). At the end of this study (d15), animals were euthanized with an overdose (1 mL/5 kg body weight) of pentobarbital sodium (390 mg/mL) and phenytoin sodium (50 mg/mL) and exsanguinated. Abdomens were opened, and livers, kidneys, and spleens were aseptically collected and weighed. Intestinal samples were flushed with ice-cold sterile saline to remove digesta prior to weighing. Samples from the liver, spleen, and small intestine were snap-frozen in liquid nitrogen and stored at −80 °C. Fresh liver and spleen samples from animals challenged with LM were cultured for CFU enumeration.
2.4. Plasma Cytokine Analysis
Plasma tumor necrosis factor-alpha (TNF-α), interleukin (IL)-10, and IL-6 concentrations were determined using porcine-specific, enzyme-linked immunosorbent assay kits (Catalog Nos. EPT0015, EPI0030, and RK09048, ABclonal, Inc., Woburn, MA, USA) according to manufacturer’s instructions. Samples and standards were assayed in duplicate using a plate reader (BioTek Instruments, Inc., Winooski, VT, USA). The assays and analyses were performed according to the manufacturer’s protocol for each kit.
2.5. Intestinal Morphology
Following euthanasia, intestinal segments were collected, flushed with cold PBS, and fixed in 10% neutral buffered formalin. Intestinal segments were removed from the fixative and sliced into five 10 mm sections, and placed into a tissue cassette. Tissue samples were dehydrated through a graded alcohol series, cleared with xylene, and embedded in paraffin wax. Tissue samples were then sliced into 5 µm sections using a microtome, mounted onto slides (5 sections per slide), and stained with hematoxylin and eosin stain for determination of gross morphology of intestinal villus height (VH) and crypt depth (CD). Images of intestinal sections were taken using an OLYMPUS® BX50 microscope (Evident Corporation, Tokyo, Japan) equipped with a Nikon Digital Sight camera. Five cross-sections per slide per animal were viewed, and a total of 12 VHs and 12 CDs were photographed for each animal, and analyzed using NIS-Elements AR 3.10 software (Nikon Instruments Inc., Melville, NY, USA).
2.6. Statistical Analysis
To investigate temporal effects, data were considered in repeated measures analyses. Log10 transformation was applied to the quantification of bacterial groups, and, if necessary, to some dependent variables to improve model assumptions of normality. All other data were analyzed by analysis of variance (ANOVA) using GraphPad Prism version 9.2.0 for Windows (GraphPad Software, Inc., San Diego, CA, USA). The experimental arrangement was a 3-way factorial design with two between-factors (diet and LM challenge) and one within-factor (time). The full model for the ANOVA analysis was:
where:
Ynijk = µ + αi + βj + γk + (αβ)ij + (αγ)ik + (βγ)jk + (αβγ)ijk + εnijk
Ynijk: nth observation at level i of level α, level j of level β, level k of level γ;
µ: overall data mean;
αi: ith level of the factor diet;
βj: jth level of the factor LM challenge;
γk: kth level of the factor time;
(αβ)ij: interaction term between the diet and LM challenge factors;
(αγ)ik: interaction term between the diet and time factors;
(βγ)jk: interaction term between the LM challenge and time factors;
(αβγ)ijk: interaction term between the diet, LM challenge, and time factors;
εnijk: error term.
A simplified model without time as a factor was used to evaluate differences between factors across each timepoint:
Ynij = µ + αi + βj + (αβ)ij + εnij
Differences were considered significant if p < 0.05. When a significant effect was determined by ANOVA, means were compared using Fisher’s least significant differences post hoc test. p values for the full and simplified models for growth parameters, organ weights, hematology, plasma cytokines, intestinal morphology, and plasma biochemistry are provided as Supplementary Tables (Tables S2–S4).
Bacterial counts in the liver and spleen of LM-challenged animals were analyzed by unpaired t-test, and correlation analysis was used to assess the relationship between organ bacterial count and blood monocyte concentration.
3. Results
3.1. Growth Parameters
Growth performance and feed conversion ratio (FCR) were evaluated during dietary MB40 supplementation (d1–d15) and following LM challenge at d8 (see Figure 1 for schematic of experimental design). The average body weight of all animals at d1 was 7.12 ± 1.70 kg. Baseline body weights (d1) did not differ between diets (Table 1). There was a main effect of time on body weight, weight gain, average daily weight gain (ADG), and average daily food intake (ADFI), whereby animals consumed more feed and gained more weight throughout the 14 days of this study (Table 1). Neither diet (p = 0.672) nor LM challenge (p = 0.739) had significant effects on body weight. Neither feed intake nor FCR was significantly different across diets (Table 1). There was a main effect of LM challenge on FCR, whereby feed conversion was less efficient in the LM-challenged animals from d1–d15 (p = 0.031); however, this effect was not significant following LM challenge from d8–d15 (p = 0.539, Table 1). No overt symptomology of gastrointestinal illness, including diarrhea, was observed in any animals.

Table 1.
Effects of B. subtilis MB40 supplementation and Listeria challenge on growth parameters 1.
3.2. Organ Weights and Bacterial Counts
Average weights of small intestine, liver, kidney, and spleen generally increased in LM-challenged groups compared with non-challenged controls (Table 2). A main effect of diet was observed for small intestine weight, in which the MB40-supplemented animals had lower weights (p = 0.036); however, this effect was no longer observed following normalization to body weight (Table 2). Normalized spleen weights were significantly higher after the LM challenge (p = 0.017), and normalized kidney weights were slightly higher after the LM challenge (p = 0.052).

Table 2.
Effects of B. subtilis MB40 supplementation and Listeria challenge on organ weights 1.
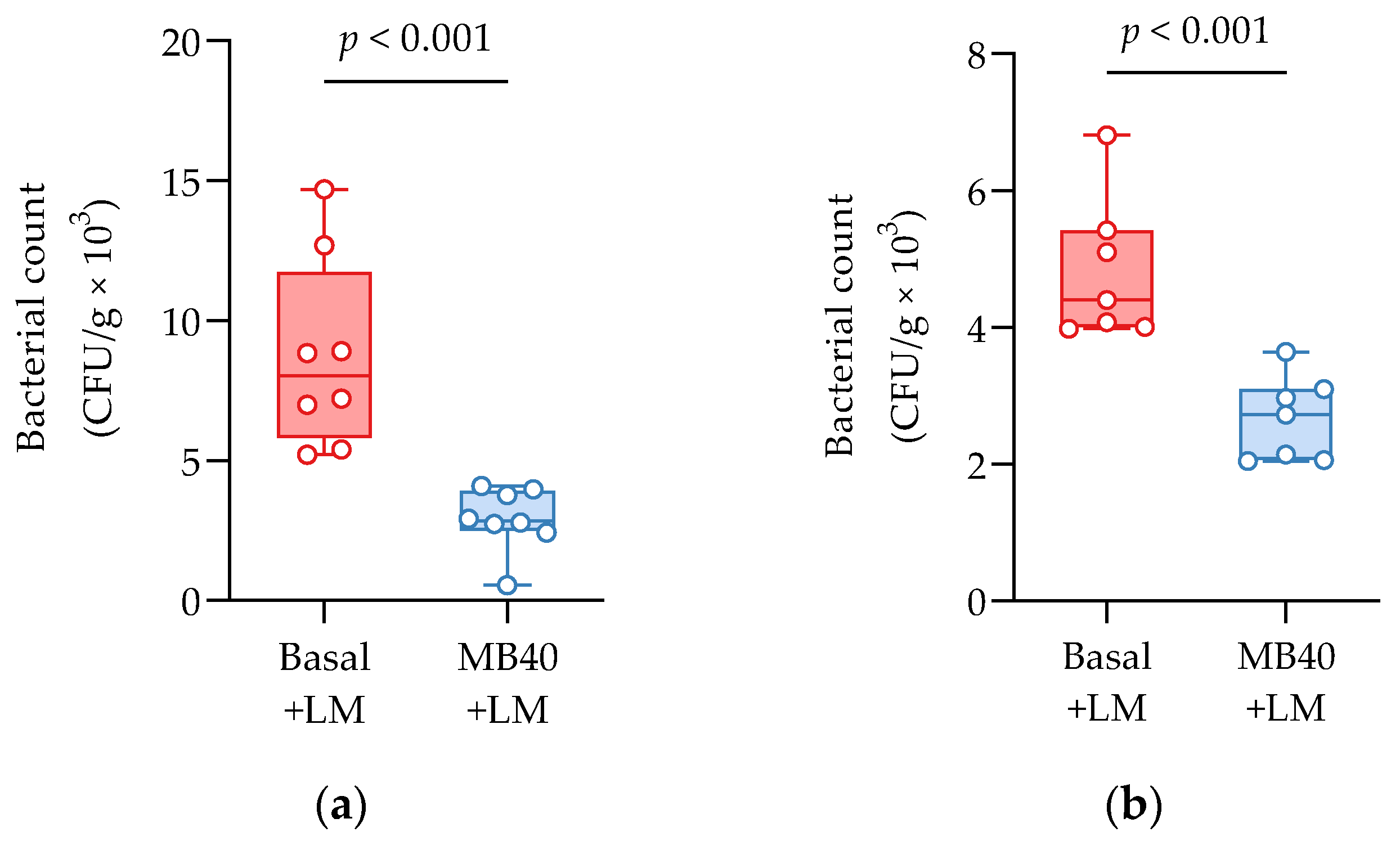
Bacterial invasion of the liver and spleen in challenged animals was investigated by microbial enumeration. Samples from non-challenged piglets were negative for LM. MB40-supplemented animals showed a 67% reduction in liver bacterial counts compared to challenged animals fed basal diet without MB40 (2.91 ± 1.14 vs. 8.75 ± 3.38 CFU × g−1 × 103, p < 0.001, Figure 2a). MB40 supplementation was also associated with a 49% reduction of bacterial counts in the spleen following LM challenge, compared to basal diet (2.46 ± 0.81 vs. 4.84 ± 0.96 CFU × g−1 × 10, p < 0.001, Figure 2b). The presence of bacteria in the liver and spleen, as well as the increased spleen and kidney organ weights, suggest that the dose of LM in this intraperitoneal injection model of listeriosis was sufficient to evoke hepatic and splenic infection and inflammation. Moreover, MB40 supplementation may have mitigated the visceral dissemination of LM from the peritoneal cavity to the liver and spleen.
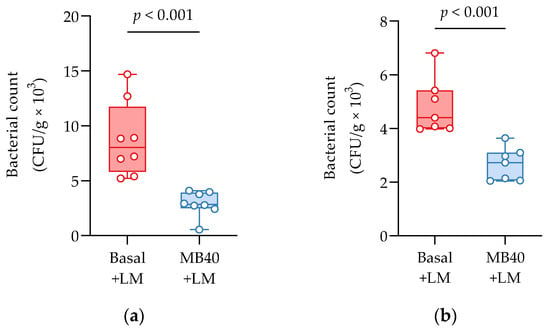
Figure 2.
(a) Liver and (b) spleen bacterial counts 7 days following intraperitoneal Listeria challenge (LM) in animals fed basal diet (Basal) or diet supplemented with B. subtilis MB40 spores (MB40). The box and whisker plots show the median, interquartile range, and minimum and maximum values (n = 8 per group). Statistical differences were determined by unpaired t-tests.
3.3. Hematology and Plasma Cytokines
Whole blood and plasma were collected for hematology and cytokine analysis, respectively, from animals at baseline (d1), before LM challenge (d8), and at the end of study (d15). There was a main effect of time on erythrocyte concentration (p = 0.039), hematocrit (p < 0.001), hemoglobin concentration (p < 0.001), packed cell volume (p < 0.001), and segmented neutrophil concentration (p = 0.020), with values lower at d15 compared to d1 (Table 3). Lymphocytes tended to increase by d8 and d15 compared to d1 (p = 0.087), with no effects of diet or LM challenge (Table 3). A main effect of LM challenge was significant for increased leukocyte concentrations (p = 0.027), including neutrophils (p = 0.004), and monocytes (p < 0.021) in LM-challenged animals at d15 compared to non-challenged animals (Table 3). Despite the inflammatory response to LM challenge and increased hepatic and splenic bacterial load, the concomitantly increased concentrations of neutrophils and monocytes fall within standard reference ranges for 6-week-old pigs [76]. A time × diet effect was significant for eosinophil concentrations (p = 0.032); however, elevated baseline concentrations in the animals prior to MB40 supplementation are confounding (Table 3). More telling for the impact of MB40 on potentially reducing inflammation, a main effect of diet on decreasing monocyte concentrations was observed at d15 (p = 0.007), whereby monocytes in MB40-supplemented animals decreased after LM challenge (d8–d15) and increased in LM-challenged animals fed the basal diet without MB40 (Figure 3).

Table 3.
Effects of B. subtilis MB40 supplementation and Listeria challenge on hematology and plasma cytokines 1.
Table 3.
Effects of B. subtilis MB40 supplementation and Listeria challenge on hematology and plasma cytokines 1.
| Condition | p Value | ||||||
|---|---|---|---|---|---|---|---|
| Parameters (Units) | Basal | Basal + LM | MB40 | MB40 + LM | Diet | Challenge | D × C |
| Erythrocytes (cells/µL) * | |||||||
| d1 | 6.7 ± 0.62 | 6.6 ± 0.69 | 6.5 ± 0.42 | 6.8 ± 0.39 | 0.926 | 0.593 | 0.331 |
| d8 | 6.3 ± 0.66 | 6.2 ± 0.88 | 6.0 ± 0.78 | 6.5 ± 0.37 | 0.880 | 0.502 | 0.334 |
| d15 | 6.5 ± 0.52 | 6.3 ± 0.68 | 6.3 ± 0.53 | 6.5 ± 0.59 | 0.796 | 0.964 | 0.429 |
| Hemoglobin (g/dL) * | |||||||
| d1 | 12.0 ± 0.80 | 11.9 ± 0.91 | 11.9 ± 0.91 | 12.6 ± 0.66 | 0.358 | 0.437 | 0.207 |
| d8 | 10.9 ± 0.50 | 11.1 ± 1.50 | 10.6 ± 1.39 | 11.5 ± 0.38 | 0.847 | 0.191 | 0.415 |
| d15 | 11.4 ± 0.59 | 11.3 ± 0.56 | 11.2 ± 0.92 | 11.3 ± 0.92 | 0.674 | 0.971 | 0.605 |
| Hematocrit (%) * | |||||||
| d1 | 39.1 ± 2.90 | 38.3 ± 2.56 | 38.9 ± 2.91 | 40.7 ± 2.38 | 0.289 | 0.610 | 0.205 |
| d8 | 35.6 ± 2.11 | 36.0 ± 4.89 | 34.5 ± 4.06 | 37.0 ± 1.22 | 0.989 | 0.275 | 0.444 |
| d15 | 37.2 ± 1.73 | 35.8 ± 3.28 | 35.4 ± 2.81 | 36.9 ± 2.62 | 0.721 | 0.985 | 0.176 |
| Packed cell volume (%) * | |||||||
| d1 | 37.1 ± 1.66 | 36.7 ± 2.81 | 37.8 ± 3.17 | 39.0 ± 2.22 | 0.132 | 0.658 | 0.376 |
| d8 | 35.1 ± 3.40 | 35.3 ± 4.41 | 34.3 ± 5.16 | 36.0 ± 1.78 | 0.974 | 0.547 | 0.650 |
| d15 | 36.4 ± 1.74 | 33.8 ± 2.93 | 36.0 ± 3.50 | 35.6 ± 2.39 | 0.508 | 0.146 | 0.297 |
| Leukocytes (cells/µL) ‡ | |||||||
| d1 ‡ | 15.0 ± 1.98 A | 15.5 ± 3.10 B | 12.4 ± 2.29 A | 16.8 ± 3.95 B | 0.564 | 0.047 | 0.104 |
| d8 | 18.4 ± 5.13 | 17.7 ± 6.39 | 15.1 ± 3.79 | 16.6 ± 2.68 | 0.233 | 0.835 | 0.561 |
| d15 ‡ | 13.6 ± 3.35 A | 16.9 ± 3.03 B | 12.0 ± 3.22 A | 17.2 ± 4.93 B | 0.647 | 0.005 | 0.480 |
| Segmented neutrophils (cells/µL) *,‡ | |||||||
| d1 | 7.3 ± 2.32 | 9.1 ± 2.54 | 6.6 ± 2.58 | 8.9 ± 3.45 | 0.689 | 0.062 | 0.806 |
| d8 | 9.4 ± 4.20 | 10.4 ± 4.92 | 7.2 ± 3.39 | 9.1 ± 1.35 | 0.242 | 0.313 | 0.773 |
| d15 ‡ | 4.6 ± 1.70 A | 8.2 ± 2.33 B | 5.5 ± 1.03 A | 7.5 ± 2.45 B | 0.921 | 0.001 | 0.285 |
| Lymphocytes (cells/µL) | |||||||
| d1 | 6.5 ± 1.83 | 5.1 ± 1.00 | 4.9 ± 1.35 | 5.0 ± 1.46 | 0.159 | 0.267 | 0.191 |
| d8 | 7.4 ± 1.20 | 6.1 ± 2.46 | 6.3 ± 1.70 | 6.0 ± 1.67 | 0.417 | 0.255 | 0.468 |
| d15 | 7.8 ± 1.93 | 6.7 ± 2.08 | 5.8 ± 3.17 | 7.0 ± 2.07 | 0.363 | 0.999 | 0.190 |
| Eosinophils (cells/µL) ** | |||||||
| d1 | 0.17 ± 0.23 | 0.04 ± 0.07 | 0.20 ± 0.16 | 0.26 ± 0.20 | 0.072 | 0.645 | 0.152 |
| d8 † | 0.21 ± 0.28 a | 0.07 ± 0.08 a | 0.17 ± 0.19 b | 0.25 ± 0.17 b | 0.040 | 0.690 | 0.369 |
| d15 | 0.16 ± 0.09 | 0.29 ± 0.26 | 0.11 ± 0.16 | 0.21 ± 0.18 | 0.350 | 0.130 | 0.825 |
| Monocytes (cells/µL) ‡,** | |||||||
| d1 ‡ | 1.0 ± 0.43 A | 1.2 ± 0.65 B | 0.75 ± 0.29 A | 1.5 ± 0.72 B | 0.870 | 0.047 | 0.234 |
| d8 | 0.91 ± 0.46 | 1.2 ± 0.53 | 1.5 ± 0.88 | 1.2 ± 0.86 | 0.316 | 0.928 | 0.377 |
| d15 †,‡ | 0.89 ± 0.59 a,A | 1.6 ± 0.68 b,A | 0.44 ± 0.17 a,B | 0.97 ± 0.73 b,B | 0.007 | 0.021 | 0.801 |
| Interleukin-10 (pg/mL) * | |||||||
| d1 | 326 ± 104.4 | 401 ± 153.0 | 354 ± 148.1 | 361 ± 154.5 | 0.903 | 0.425 | 0.500 |
| d8 | 285 ± 73.6 | 343 ± 106.2 | 360 ± 66.5 | 317 ± 87.1 | 0.430 | 0.803 | 0.103 |
| d15 | 283 ± 70.1 | 235 ± 54.4 | 287 ± 62.0 | 276 ± 54.1 | 0.299 | 0.190 | 0.399 |
| TNF-α (pg/mL) * | |||||||
| d1 | 512 ± 156.1 | 595 ± 243.0 | 435 ± 102.0 | 452 ± 163.5 | 0.092 | 0.434 | 0.605 |
| d8 | 433 ± 220.8 | 410 ± 151.1 | 458 ± 177.5 | 330 ± 123.8 | 0.659 | 0.228 | 0.398 |
| d15 | 275 ± 123.5 | 337 ± 147.0 | 407 ± 194.0 | 403 ± 207.8 | 0.123 | 0.646 | 0.599 |
| Interleukin-6 (pg/mL) * | |||||||
| d1 | 736 ± 152.8 | 854 ± 152.6 | 756 ± 300.7 | 775 ± 281.6 | 0.726 | 0.409 | 0.551 |
| d8 | 336 ± 91.3 | 427 ± 171.7 | 439 ± 102.0 | 421 ± 137.7 | 0.299 | 0.435 | 0.242 |
| d15 | 505 ± 70.0 | 474 ± 141.2 | 504 ± 134.5 | 492 ± 106.3 | 0.837 | 0.608 | 0.818 |
1 Data are means ± standard deviation (n = 6–8 per group); * main effect of time; ** time × diet interaction; † main effect of diet; ‡ main effect of LM challenge.; significant differences between groups (p < 0.05, ANOVA) are denoted by unshared letters (diet factor: a, b; challenge factor: A, B); significant p values are italicized and bold (p values for three-way repeated measures ANOVA are shown in Table S2). Abbreviations: Basal, basal diet; d, day; D × C, diet × challenge interaction; LM, Listeria monocytogenes challenge at d8; MB40, MB40-supplemented diet; TNF-α, tumor necrosis factor-alpha.
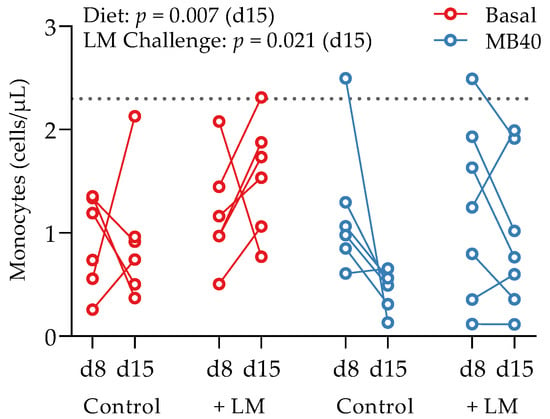
Figure 3.
Effects of B. subtilis MB40 supplementation and Listeria challenge on blood monocyte concentrations. The line connecting data from individual animals (circles) shows the change from d8 to d15. The dotted line shows the upper reference limit (2.3 cells/µL) for pigs up to 6 weeks of age [76]. Basal, basal diet; Control, no LM challenge; d, day; LM, Listeria monocytogenes challenge at d8; MB40, MB40-supplemented diet.
Figure 3.
Effects of B. subtilis MB40 supplementation and Listeria challenge on blood monocyte concentrations. The line connecting data from individual animals (circles) shows the change from d8 to d15. The dotted line shows the upper reference limit (2.3 cells/µL) for pigs up to 6 weeks of age [76]. Basal, basal diet; Control, no LM challenge; d, day; LM, Listeria monocytogenes challenge at d8; MB40, MB40-supplemented diet.
There was a main effect of time on plasma cytokine concentrations (p < 0.05, Table 3). Whereas IL-10 concentration was highest in animals at d1 and lowest at the end of study, TNF-α and IL-6 were greatest at d1, generally decreased by d8, but increased at d15 (Table 3). There were no differences in plasma cytokine concentrations across diet or LM challenge. The significant changes in hematology and plasma cytokine concentrations over time likely represent typical immune maturation in piglets.
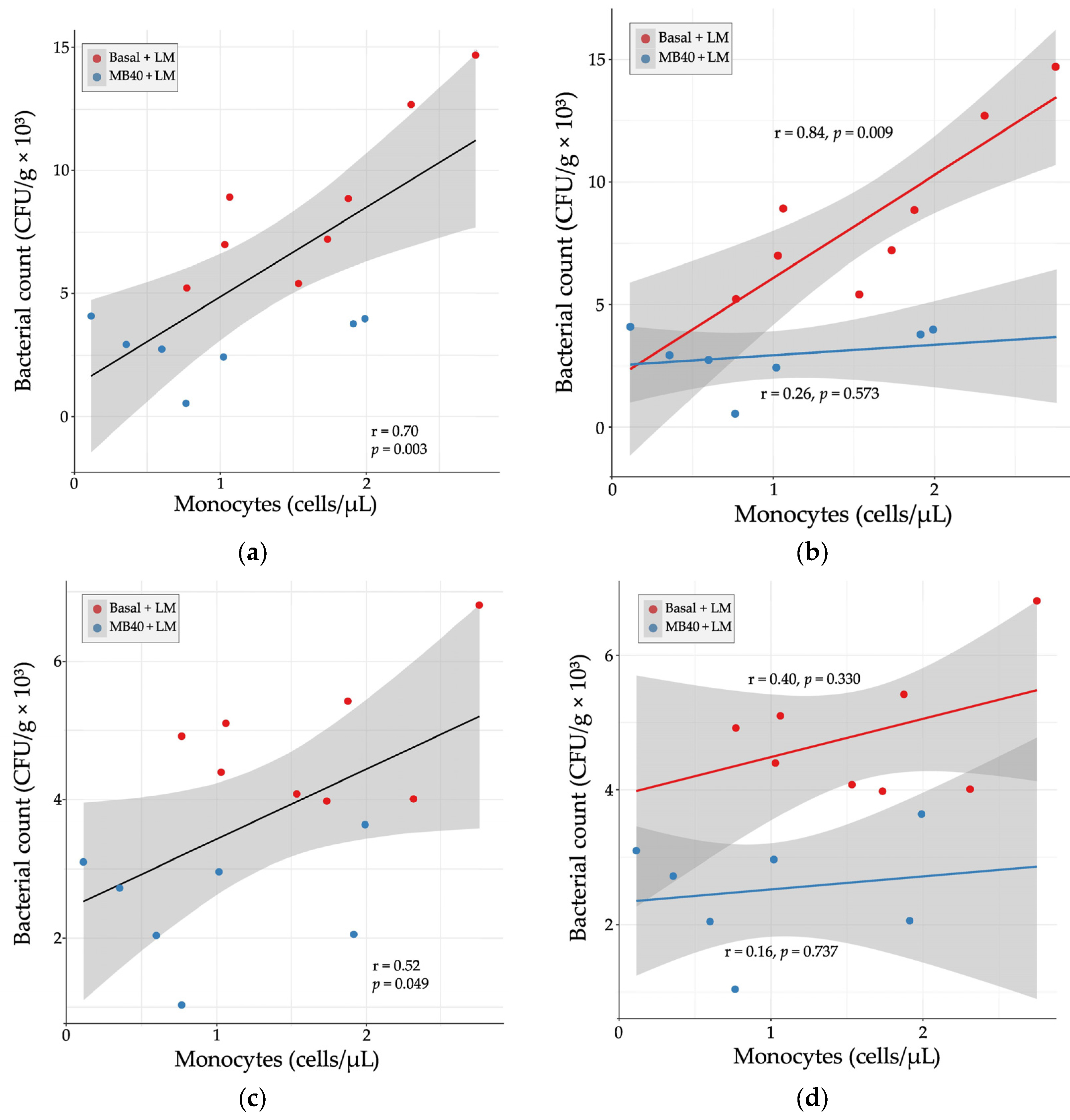
Hematology and plasma cytokine data were also tested for correlation with organ bacterial counts in LM-challenged animals. There was a strong, positive correlation between liver bacterial count and blood monocyte concentration (r = 0.70, p = 0.003, Figure 4a). When separated by diet, the correlation was stronger with basal diet (r = 0.84, p = 0.009) and weakly correlated with MB40 supplementation (r = 0.26, p = 0.573, Figure 4b). In the spleen, there was a moderate, positive correlation between bacterial count and blood monocyte concentration (r = 0.52, p = 0.049, Figure 4c). When separated by diet, the correlations were weaker (basal diet, r = 0.40; MB40, r = 0.16) and not significant (Figure 4d).
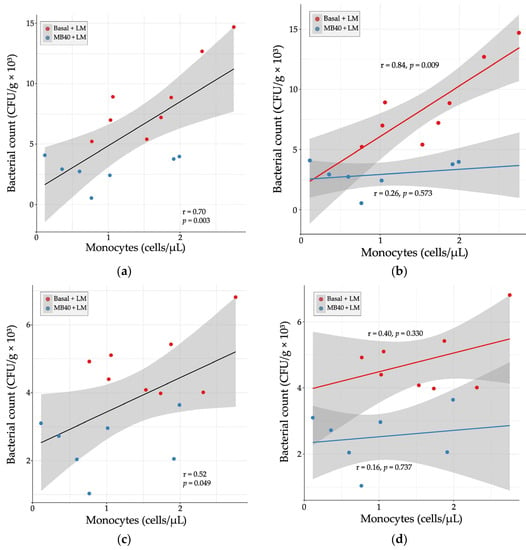
Figure 4.
Relationship between organ bacterial counts and blood monocyte concentrations in (a) liver, (b) liver × diet, (c) spleen, and (d) spleen × diet 7 days following Listeria challenge in animals fed basal diet (Basal) or diet supplemented with B. subtilis MB40 spores (MB40). Statistical differences were determined by Pearson correlation. The areas shaded in gray indicate the 95% confidence interval.
3.4. Plasma Biochemistry
There was a main effect of time on several plasma biochemical parameters across this 14 d study: phosphorous (p < 0.001), calcium (p = 0.001), globulin (p = 0.008), alanine transaminase (p < 0.001), alkaline phosphatase (p < 0.001 sodium (p = 0.014), potassium (p < 0.001), and chloride (p = 0.039) (Tables S1 and S4). Despite small inter-group differences of little physiological relevance, there were significant time × LM challenge interactions for creatinine (p = 0.022), total protein (p = 0.026), albumin (p = 0.011), γ-glutamyltransferase (p = 0.001), and triglyceride (p = 0.001) concentrations (Table S1). A main effect of the LM challenge on increased plasma creatine kinase concentrations was observed at d15 (p = 0.017); however, the differences in values between experimental groups at d8, and over time to d15, suggest that LM challenge does not meaningfully impact creatine kinase levels (Table S1). Altogether, these data suggest that MB40 is safe and well-tolerated in piglets, and that the extent of LM challenge per intraperitoneal dosing of 108 CFU resulted in no marked effects on liver metabolism and kidney function.
3.5. Intestinal Morphology
Sections of the jejunum and ileum from the small intestine and sections of the large intestine were examined for changes in epithelial morphology. There were no effects of MB40 supplementation or intraperitoneal LM challenge on jejunal VH, CD, or VH:CD ratio (Table 4). Ileal VH was comparable across both dietary treatments with and without LM challenge; however, CD was greater in LM-challenged animals irrespective of dietary treatment (p = 0.002, Table 4). Due to this increase in CD, VH:CD ratio was lower in LM-challenged animals (p = 0.009). There were no effects of dietary treatment or LM challenge on large intestinal CD (Table 4).

Table 4.
Effect of MB40 supplementation and Listeria challenge on intestinal morphology 1.
4. Discussion
This study assessed the effect of oral Bacillus subtilis MB40 spore supplementation on the growth and health of piglets for 7 days before and 7 days after Listeria monocytogenes (LM) challenge by single intraperitoneal dose. Probiotic supplementation studies with pathogen challenge in swine have been described, but to our knowledge, this is the first study to specifically investigate Listeria challenge. In this new porcine listeriosis model, a single dose of LM (108 CFU) was administered by intraperitoneal injection. Intraperitoneal injection has the potential to model visceral dissemination of LM between organs and particularly from the mesenteric lymph nodes to the liver and spleen, which can occur following LM invasion of the intestinal epithelium [11,77]. One week following LM dosing, animals showed increased spleen weight and higher bacterial counts in the liver and spleen, without any overt symptomology such as diarrhea or substantial weight loss. Hematological analysis confirmed that the LM dose was sufficient to affect systemic inflammation 7 days post-infection, evidenced by an increase in leukocyte, neutrophil, and monocyte concentrations in the bloodstream compared to non-challenged controls. Surprisingly, visceral LM infection did impact some intestinal morphology, as ileal crypt depth and villus height to crypt depth ratio increased and decreased, respectively. In addition to ileal villus height, all parameters of intestinal morphology in the jejunum and large intestine remained similar between LM-challenged animals and non-challenged controls, suggesting that the ileum is more sensitive to LM-related systemic inflammation. LM could also more directly compromise the intestinal epithelium by direct invasion and colonization, similar to the colonization of Shigella flexneri in the colonic lamina propria following intraperitoneal challenge in mice [78]. However, further studies are needed to better assess mesenteric lymph node infection, visceral spread, and intestinal colonization in our LM challenge model. Altogether, a single 108 CFU intraperitoneal dose of LM in piglets recapitulated many aspects of systemic LM infection, without confounding effects of oral dosing.
We also tested the hypothesis that probiotic supplementation improves immunity in this new model of porcine listeriosis. The results show that 7 days of Bacillus subtilis MB40 supplementation before the LM challenge, and 7 days thereafter, reduced bacterial counts in the liver and spleen, compared to challenged animals provided basal diet without MB40. The lowering of bacterial infection in the liver and spleen with MB40 may have contributed to decreased systemic inflammation, given the lower plasma concentrations of monocytes compared to challenged animals consuming a basal diet. These data demonstrate the efficacy of administering MB40 for as little as 1 week in significantly reducing visceral LM dissemination and invasion of visceral organs. Our results align with observations from other oral pathogen challenge studies with supplementation of Bacillus strains in swine. In one study, weaned piglets were administered a mixture of B. subtilis and B. amyloliquefaciens for 7 days before a single oral gavage of enterotoxigenic E. coli F18 (ETEC F18) [60]. The probiotic group showed reduced fecal shedding of ETEC F18 7 days after infection compared to challenged piglets on a control diet, suggesting Bacillus strains reduced survival, growth, or intestinal attachment of ETEC F18. In a second study, weaned piglets were fed one of two Bacillus strains at 21 days of life onward and orally infected 7 days thereafter, for 3 continuous days, with ETEC F18 [61]. B. subtilis DSM 32540 or B. pumilus DSM 32539 supplementation led to almost 50% and 75% reductions, respectively, in mesenteric lymph node bacterial counts 21 days after infection [61]. The mechanism of Bacillaceae strain antibacterial action involves, in part, a suite of secreted antimicrobial compounds such as fengycins, bacteriocins, surfactins, and macrolides [32,33,79,80]. It is reasonable to project that such well-documented antimicrobial metabolites and peptides may mitigate intestinal survival, epithelial invasion, and visceral spread of pathogens between the intestine, lymph nodes, bloodstream, and organs.
Specific to Listeria, several antimicrobial molecules secreted by Bacillaceae strains have been shown to mitigate LM growth in culture [62,64,69,81,82,83,84,85,86,87,88]. In particular, a subtilin-type bacteriocin peptide from Bacillus subtilis JS-4 was shown by confocal laser-scanning microscopy and electron microscopy to increase LM cell membrane permeability, trigger potassium ion leakage and pore formation, and damage cell membrane integrity [89]. Following oral LM challenge in mice and rats, administration of probiotic strains from the genera Lactobacillus, Lacticaseibacillus, Ligilactobacillus, and Lactococcus was associated with increased visceral organ bacterial clearance and reduced severity of infection [90,91,92,93,94]. In one of these studies, the antilisterial effect of Ligilactobacillus salivarius was attributed to the production and release of a bacteriocin [91]. In the study reported here, though, LM was administered by intraperitoneal injection to model visceral LM dissemination, thus limiting this study of any direct interaction in the GI tract between LM and orally administered MB40 and any of its secreted antimicrobials. It is plausible that a stable antilisterial molecule, such as a subtilin peptide secreted by vegetative MB40 cells in the intestinal lumen, was absorbed into the circulation, leading to direct contact with and killing of LM and mitigation of visceral spread. Future studies are needed to determine whether the administration of MB40 cell-free supernatants, heat-killed postbiotic lysates, and purified bacteriocins are involved in the protective effect of MB40 in this model.
As expected, intraperitoneal LM challenge, according to our protocol, did not remarkably perturb intestinal histology. Despite the reduced reproducibility of symptoms and pathology in oral pathogen challenge experiments, as compared to intraperitoneal challenge, MB40’s role in countering LM intestinal invasion will need to be addressed in a future oral LM challenge study. The oral LM challenge will also be helpful for understanding any contributions of MB40 to gut barrier integrity during infection, which has previously been demonstrated for other Bacillus strains in pigs, chickens, and mice [95,96,97]. As a Gram-positive bacterium, LM does not produce endotoxin; however, it does produce other toxins, such as listeriolysin O which has been shown to disrupt the barrier integrity of human intestinal epithelial cells in cell culture [98,99,100]. It remains to be understood whether oral MB40 supplementation modulated intestinal epithelial cell function, barrier integrity, and gut immune signaling that could indirectly limit the visceral spread of LM.
Beyond gut immunity and secretion of antimicrobial molecules, MB40 could also limit the visceral spread of LM by improving systemic innate immunity, for which immunoglobulin A (IgA) is a positively correlated marker [101]. Other Bacillaceae strains have been shown to impact IgA levels. Dietary supplementation of B. subtilis DSM29784 in broiler chickens increased serum levels of IgA and IgG [97]. Lymphocytes collected from human subjects administered Alkalihalobacillus clausii (formerly B. clausii) showed a greater abundance of membrane-bound IgA and greater secretion of IgA, compared to lymphocytes from subjects administered placebo [102]. A similar probiotic preparation reduced the duration of respiratory infections in children aged 3 to 6 years of age, compared to children not receiving probiotics [103]. In a clinical study of B. subtilis CU1 supplementation in older adults, fecal and salivary IgA levels were increased compared to subjects receiving a placebo [104]. In addition, serum levels of the antiviral factor interferon-gamma were significantly increased by 40% after 10 days of CU1 supplementation, compared to baseline within the supplementation group, and decreased risk of respiratory infection was observed in a post hoc analysis, compared to placebo [104]. One possible explanation for this immunomodulatory activity is the direct interaction between extracellular receptors of Bacillaceae strains, or metabolites like short-chain fatty acids, and Toll-like receptors expressed on the surface of certain gut immune cells that lead to increased IgA production by B lymphocytes [105]. Whereas MB40 had no effects on the cytokines IL-10, TNF-α, and IL-6 in our animal study, future studies will direct attention to IgA and interferon-gamma.
Future research will also direct investigation of the effects of MB40 on the intestinal microbiome. It is of note that dietary supplementation of B. subtilis PB6 in sows was associated with a reduction in the fecal abundance of pathogenic species from the Streptococcus genus [96]. In piglets, 42 days of post-weaning supplementation with three different strains of B. subtilis each improved body weight, increased ileal villus height, decreased the incidence of diarrhea, and decreased fecal abundance of bacteria from the genus Clostridium, compared to pigs fed a control diet [106]. In a recently published, placebo-controlled clinical trial of 115 human participants, daily MB40 supplementation for 30 days remarkably promoted intestinal and nasal decolonization of Staphylococcus aureus [107]. Previous work in mice showed that intestinal decolonization of S. aureus is dependent on the production and secretion of fengycin from B. subtilis [79]. Given this pathogenic species-specific effect, future microbiome analysis will comprise metagenomic sequencing to more comprehensively understand the strain-specific effects of MB40 on the mammalian gut microbiome.
5. Conclusions
The data presented in this study indicate that the inclusion of the probiotic Bacillus subtilis MB40 spores in the diet of nursery piglets helps improve innate immunity and disease resistance in an animal model of listeriosis. In this new porcine model, a single intraperitoneal dose of Listeria monocytogenes led to the dissemination of bacteria to the liver and spleen, increased spleen weight, and higher concentrations of circulating leukocytes, neutrophils, and monocytes, compared to non-challenged controls. Remarkably, animals supplemented with B. subtilis MB40 showed reduced bacterial counts in the liver and spleen and lower plasma monocyte concentrations following this Listeria challenge. Because the pathogen challenge was via intraperitoneal injection, it is likely that orally administered B. subtilis MB40 spores germinate in the intestine and secrete an antilisterial molecule that is absorbed and mitigates visceral dissemination of Listeria. Further studies are needed to elucidate MB40’s mechanism of action and investigate MB40’s utility in helping manage foodborne illness and promoting gut microbiota balance in humans. Altogether, B. subtilis MB40 is a promising probiotic in humans and animals for preventing, mitigating, and alleviating symptoms of listeriosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11082110/s1, Table S1: Effects of B. subtilis MB40 supplementation and Listeria challenge on plasma biochemistry; Table S2: p values for effects of B. subtilis MB40 supplementation and Listeria challenge on growth parameters; Table S3: p values for the effects of B. subtilis MB40 supplementation and Listeria challenge on hematology and plasma cytokines; Table S4: p values for the effects of B. subtilis MB40 supplementation and Listeria challenge on plasma biochemistry.
Author Contributions
Conceptualization, S.W.E.-K., R.A.D., C.P. and R.P.R.; methodology, R.A.D., N.K.E. and S.W.E.-K.; formal analysis, J.L.G. and S.W.E.-K.; investigation, N.K.E. and N.S.; resources, J.L.S.; writing—original draft preparation, S.M.G.; writing—review and editing, S.W.E.-K., R.A.D., J.L.G., N.K.E., C.P. and J.L.S.; visualization, J.L.G. and S.M.G.; supervision, S.W.E.-K., R.A.D. and R.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by BIO-CAT, Inc. This work was also supported in part by the USDA National Institute of Food and Agriculture (NIFA) Hatch funds to the Virginia Agricultural Experiment Station.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Virginia Tech (IACUC-18-010-APSC; August 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data not presented within the article or Supplementary Materials are available upon reasonable request from the corresponding authors.
Acknowledgments
The authors thank Dan Little for assistance with project management and Carolyn Anderson and Trish Vandagriff for assistance with the visualization of data.
Conflicts of Interest
S.M.G., J.L.G. and C.P. (retired) are employees of BIO-CAT, Inc., which provided funding for this study. J.L.S. is an employee of BIO-CAT Microbials, LLC, which provided the Bacillus subtilis MB40 spores for this study. BIO-CAT, Inc. is the assignee of a related patent describing methods to reduce the growth of pathogenic bacteria (U.S. Patent No. 10,787,716). The funders were involved in the design of this study, in the analysis of the data, in the writing of the manuscript, and in the decision to publish the results. The authors declare no other conflict of interest.
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L.; Ko, C.W.; Bercik, P.; Falck-Ytter, Y.; Sultan, S.; Weizman, A.V.; Morgan, R.L. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020, 159, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.; Keating, G.; Georgousopoulou, E.; Hespe, C.; Levett, K. Probiotics for the Prevention of Antibiotic-Associated Diarrhoea: A Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e043054. [Google Scholar] [CrossRef]
- Liao, W.; Chen, C.; Wen, T.; Zhao, Q. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Adults: A Meta-Analysis of Randomized Placebo-Controlled Trials. J. Clin. Gastroenterol. 2021, 55, 469–480. [Google Scholar] [CrossRef]
- Pastor-Villaescusa, B.; Blanco-Rojo, R.; Olivares, M. Evaluation of the Effect of Limosilactobacillus fermentum CECT5716 on Gastrointestinal Infections in Infants: A Systematic Review and Meta-Analysis. Microorganisms 2021, 9, 1412. [Google Scholar] [CrossRef]
- Ahmad, H.H.; Peck, B.; Terry, D. The Influence of Probiotics on Gastrointestinal Tract Infections Among Children Attending Childcare: A Systematic Review and Meta-Analysis. J. Appl. Microbiol. 2022, 132, 1636–1651. [Google Scholar] [CrossRef]
- CDC, Centers for Disease Control and Prevention. Estimates of Foodborne Illness in the United States. Available online: https://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html (accessed on 4 August 2023).
- Farber, J.M.; Peterkin, P.I. Listeria monocytogenes, a Food-Borne Pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef]
- Swaminathan, B.; Gerner-Smidt, P. The Epidemiology of Human Listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef]
- Ogawa, M.; Yoshikawa, Y.; Mimuro, H.; Hain, T.; Chakraborty, T.; Sasakawa, C. Autophagy Targeting of Listeria monocytogenes and the Bacterial Countermeasure. Autophagy 2011, 7, 310–314. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Ducey, T.F.; Usgaard, T.; Dunn, K.A.; Bielawski, J.P. Multilocus Genotyping Assays for Single Nucleotide Polymorphism-Based Subtyping of Listeria monocytogenes Isolates. Appl. Environ. Microbiol. 2008, 74, 7629–7642. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.H.; den Bakker, H.C.; Wiedmann, M. Listeria monocytogenes Lineages: Genomics, Evolution, Ecology, and Phenotypic Characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated with Fresh Produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- Cherifi, T.; Arsenault, J.; Pagotto, F.; Quessy, S.; Côté, J.C.; Neira, K.; Fournaise, S.; Bekal, S.; Fravalo, P. Distribution, Diversity and Persistence of Listeria monocytogenes in Swine Slaughterhouses and Their Association with Food and Human Listeriosis Strains. PLoS ONE 2020, 15, e0236807:1–e0236807:19. [Google Scholar] [CrossRef]
- Wiktorczyk-Kapischke, N.; Skowron, K.; Grudlewska-Buda, K.; Wałecka-Zacharska, E.; Korkus, J.; Gospodarek-Komkowska, E. Adaptive Response of Listeria monocytogenes to the Stress Factors in the Food Processing Environment. Front. Microbiol. 2021, 12, 710085. [Google Scholar] [CrossRef]
- Port, G.C.; Miner, M.D.; Freitag, N.E. Listeria monocytogenes–From Saprophyte to Intracellular Pathogen. Nat. Rev. Microbiol. 2009, 7, 623–628. [Google Scholar] [CrossRef]
- Jones, F.T.; Ricke, S.C. Observations on the History of the Development of Antimicrobials and Their Use in Poultry Feeds. Poult. Sci. 2003, 82, 613–617. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic Growth Promoters in Agriculture: History and Mode of Action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef]
- Castanon, J.I. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Foodborne Antimicrobial Resistance as a Biological Hazard–Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2008, 765, 1–87. [Google Scholar] [CrossRef]
- FDA, Food and Drug Administration. Guide for Industry (GFI) #213. New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food-Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI #209. 2013. Available online: https://www.fda.gov/media/83488/download (accessed on 4 August 2023).
- Grant, A.; Gay, C.G.; Lillehoj, H.S. Bacillus spp. as Direct-Fed Microbial Antibiotic Alternatives to Enhance Growth, Immunity, and Gut Health in Poultry. Avian Pathol. 2018, 47, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.; Jaworski, N.W.; et al. Non-Antibiotic Feed Additives in Diets for Pigs: A Review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. The Role of Probiotics, Prebiotics and Synbiotics in Animal Nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, D.S.; Danielson, D.M.; Peo, E.R., Jr. Effects of Microbial Feed Additives on Performance of Starter and Growing-Finishing Pigs. J. Anim. Sci. 1980, 51, 577–581. [Google Scholar] [CrossRef]
- Abe, F.; Ishibashi, N.; Shimamura, S. Effect of Administration of Bifidobacteria and Lactic Acid Bacteria to Newborn Calves and Piglets. J. Dairy Sci. 1995, 78, 2838–2846. [Google Scholar] [CrossRef]
- Alexopoulos, C.; Georgoulakis, I.E.; Tzivara, A.; Kyriakis, C.S.; Govaris, A.; Kyriakis, S.C. Field Evaluation of the Effect of a Probiotic-Containing Bacillus licheniformis and Bacillus subtilis Spores on the Health Status, Performance, and Carcass Quality of Grower and Finisher Pigs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004, 51, 306–312. [Google Scholar] [CrossRef]
- Bahaddad, S.A.; Almalki, M.; Alghamdi, O.A.; Sohrab, S.S.; Yasir, M.; Azhar, E.I.; Chouayekh, H. Bacillus Species as Direct-Fed Microbial Antibiotic Alternatives for Monogastric Production. Probiotics Antimicrob. Proteins 2023, 15, 1–16. [Google Scholar] [CrossRef]
- Luise, D.; Bosi, P.; Raff, L.; Amatucci, L.; Virdis, S.; Trevisi, P. Bacillus spp. Probiotic Strains as a Potential Tool for Limiting the Use of Antibiotics, and Improving the Growth and Health of Pigs and Chickens. Front. Microbiol. 2022, 13, 801827. [Google Scholar] [CrossRef]
- Ilinskaya, O.N.; Ulyanova, V.V.; Yarullina, D.R.; Gataullin, I.G. Secretome of Intestinal Bacilli: A Natural Guard Against Pathologies. Front. Microbiol. 2018, 8, 1666. [Google Scholar] [CrossRef]
- Kaspar, F.; Neubauer, P.; Gimpel, M. Bioactive Secondary Metabolites from Bacillus subtilis: A Comprehensive Review. J. Nat. Prod. 2019, 82, 2038–2053. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Cock, I.E.; Chen, X.; Feng, Y. Antimicrobial Bacillus: Metabolites and Their Mode of Action. Antibiotics 2022, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Qian, Y.; Yu, B.; Zhang, T.; Gao, J.; He, J.; Huang, Z.; Zheng, P.; Mao, X.; Luo, J.; et al. Effects of Bacillus subtilis DSM32315 Supplementation and Dietary Crude Protein Level on Performance, Gut Barrier Function and Microbiota Profile in Weaned Piglets. J. Anim. Sci. 2019, 97, 2125–2138. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on Growth Performance, Immunity, Short Chain Fatty Acid Production, Antioxidant Capacity, and Cecal Microflora in Broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef]
- Huang, J.M.; La Ragione, R.M.; Nunez, A.; Cutting, S.M. Immunostimulatory Activity of Bacillus Spores. FEMS Immunol. Med. Microbiol. 2008, 53, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, Y.; Zhang, J.; Yang, Q. Co-administration of Bacillus subtilis RJGP16 and Lactobacillus salivarius B1 Strongly Enhances the Intestinal Mucosal Immunity of Piglets. Res. Vet. Sci. 2013, 94, 62–68. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, L.; Zhang, E.; Yuan, C.; Yang, Q. Oral Administration of Bacillus subtilis Promotes Homing of CD3+ T Cells and IgA-Secreting Cells to the Respiratory Tract in Piglets. Res. Vet. Sci. 2021, 136, 310–317. [Google Scholar] [CrossRef]
- Hyronimus, B.; Le Marrec, C.; Sassi, A.H.; Deschamps, A. Acid and Bile Tolerance of Spore-Forming Lactic Acid Bacteria. Int. J. Food Microbiol. 2000, 61, 193–197. [Google Scholar] [CrossRef]
- Hong, H.A.; Duc, l.; Cutting, S.M. The Use of Bacterial Spore Formers as Probiotics. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef]
- Leser, T.D.; Knarreborg, A.; Worm, J. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J. Appl. Microbiol. 2008, 104, 1025–1033. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus Probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Colom, J.; Freitas, D.; Simon, A.; Brodkorb, A.; Buckley, M.; Deaton, J.; Winger, A.M. Presence and Germination of the Probiotic Bacillus subtilis DE111® in the Human Small Intestinal Tract: A Randomized, Crossover, Double-Blind, and Placebo-Controlled Study. Front. Microbiol. 2021, 12, 715863. [Google Scholar] [CrossRef] [PubMed]
- Penet, C.; Kramer, R.; Little, R.; Spears, J.L.; Parker, J.; Iyer, J.K.; Guthrie, N.; Evans, M. A Randomized, Double-Blind, Placebo-Controlled, Parallel Study Evaluating the Efficacy of Bacillus subtilis MB40 to Reduce Abdominal Discomfort, Gas, and Bloating. Altern. Ther. Health Med. 2021, 27, 146–157. [Google Scholar] [PubMed]
- Garvey, S.M.; Mah, E.; Blonquist, T.M.; Kaden, V.N.; Spears, J.L. The Probiotic Bacillus subtilis BS50 Decreases Gastrointestinal Symptoms in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Gut Microbes 2022, 14, 2122668. [Google Scholar] [CrossRef] [PubMed]
- Walden, K.E.; Hagele, A.M.; Orr, L.S.; Gross, K.N.; Krieger, J.M.; Jäger, R.; Kerksick, C.M. Probiotic BC30 Improves Amino Acid Absorption from Plant Protein Concentrate in Older Women. Probiotics Antimicrob. Proteins 2022, 1–13. [Google Scholar] [CrossRef]
- Dolin, B.J. Effects of a Proprietary Bacillus coagulans Preparation on Symptoms of Diarrhea-Predominant Irritable Bowel Syndrome. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 655–659. [Google Scholar] [CrossRef]
- Hun, L. Bacillus coagulans Significantly Improved Abdominal Pain and Bloating in Patients with IBS. Postgrad. Med. 2009, 121, 119–124. [Google Scholar] [CrossRef]
- Kalman, D.S.; Schwartz, H.I.; Alvarez, P.; Feldman, S.; Pezzullo, J.C.; Krieger, D.R. A Prospective, Randomized, Double-Blind, Placebo-Controlled Parallel-Group Dual Site Trial to Evaluate the Effects of a Bacillus coagulans-Based Product on Functional Intestinal Gas Symptoms. BMC Gastroenterol. 2009, 9, 85. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ali, F.; Pande, A.; Majeed, S.; Karri, S.K. Bacillus coagulans MTCC 5856 Supplementation in the Management of Diarrhea Predominant Irritable Bowel Syndrome: A Double Blind Randomized Placebo Controlled Pilot Clinical Study. Nutr. J. 2016, 15, 21. [Google Scholar] [CrossRef]
- Hatanaka, M.; Yamamoto, K.; Suzuki, N.; Iio, S.; Takara, T.; Morita, H.; Takimoto, T.; Nakamura, T. Effect of Bacillus subtilis C-3102 on Loose Stools in Healthy Volunteers. Benef. Microbes 2018, 9, 357–365. [Google Scholar] [CrossRef]
- Madempudi, R.S.; Ahire, J.J.; Neelamraju, J.; Tripathi, A.; Nanal, S. Randomized Clinical Trial: The Effect of Probiotic Bacillus coagulans Unique IS2 vs. Placebo on the Symptoms Management of Irritable Bowel Syndrome in Adults. Sci. Rep. 2019, 9, 12210. [Google Scholar] [CrossRef] [PubMed]
- Maity, C.; Gupta, A.K. A Prospective, Interventional, Randomized, Double-Blind, Placebo-Controlled Clinical Study to Evaluate the Efficacy and Safety of Bacillus coagulans LBSC in the Treatment of Acute Diarrhea with Abdominal Discomfort. Eur. J. Clin. Pharmacol. 2019, 75, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Maity, C. Efficacy and Safety of Bacillus coagulans LBSC in Irritable Bowel Syndrome: A Prospective, Interventional, Randomized, Double-Blind, Placebo-Controlled Clinical Study [CONSORT Compliant]. Medicine 2021, 100, e23641. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Slaets, H.; De Paepe, K.; Ceulemans, M.; Wetzels, S.; Geboers, K.; Toth, J.; Thys, W.; Dybajlo, R.; Walgraeve, D.; et al. Efficacy and Safety of Spore-Forming Probiotics in the Treatment of Functional Dyspepsia: A Pilot Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Gastroenterol. Hepatol. 2021, 6, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, S.C.; Tsiloyiannis, V.K.; Vlemmas, J.; Sarris, K.; Tsinas, A.C.; Alexopoulos, C.; Jansegers, L. The Effect of Probiotic LSP 122 on the Control of Post-Weaning Diarrhoea Syndrome of Piglets. Res. Vet. Sci. 1999, 67, 223–228. [Google Scholar] [CrossRef]
- Kantas, D.; Papatsiros, V.G.; Tassis, P.D.; Giavasis, I.; Bouki, P.; Tzika, E.D. A Feed Additive Containing Bacillus toyonensis (Toyocerin®) Protects Against Enteric Pathogens in Postweaning Piglets. J. Appl. Microbiol. 2015, 118, 727–738. [Google Scholar] [CrossRef]
- Amalaradjou, M.A.; Bhunia, A.K. Modern Approaches in Probiotics Research to Control Foodborne Pathogens. Adv. Food Nutr. Res. 2012, 67, 185–239. [Google Scholar] [CrossRef]
- Becker, S.L.; Li, Q.; Burrough, E.R.; Kenne, D.; Sahin, O.; Gould, S.A.; Patience, J.F. Effects of an F18 Enterotoxigenic Escherichia coli Challenge on Growth Performance, Immunological Status, and Gastrointestinal Structure of Weaned Pigs and the Potential Protective Effect of Direct-Fed Microbial Blends. J. Anim. Sci. 2020, 98, skaa113. [Google Scholar] [CrossRef]
- He, Y.; Jinno, C.; Kim, K.; Wu, Z.; Tan, B.; Li, X.; Whelan, R.; Liu, Y. Dietary Bacillus spp. Enhanced Growth and Disease Resistance of Weaned Pigs by Modulating Intestinal Microbiota and Systemic Immunity. J. Anim. Sci. Biotechnol. 2020, 11, 101. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Audisio, M.C. Inhibitory Activity of Surfactin, Produced by Different Bacillus subtilis subsp. subtilis Strains, Against Listeria monocytogenes Sensitive and Bacteriocin-Resistant Strains. Microbiol. Res. 2013, 168, 125–129. [Google Scholar] [CrossRef]
- Das, G.; Park, S.; Baek, K.H. Diversity of Endophytic Bacteria in a Fern Species Dryopteris uniformis (Makino) Makino and Evaluation of Their Antibacterial Potential Against Five Foodborne Pathogenic Bacteria. Foodborne Pathog. Dis. 2017, 14, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Zheng, Q.W.; Wei, T.; Zhang, Z.Q.; Zhao, C.F.; Zhong, H.; Xu, Q.Y.; Lin, J.F.; Guo, L.Q. Isolation and Characterization of Fengycins Produced by Bacillus amyloliquefaciens JFL21 and Its Broad-Spectrum Antimicrobial Potential Against Multidrug-Resistant Foodborne Pathogens. Front. Microbiol. 2020, 11, 579621. [Google Scholar] [CrossRef] [PubMed]
- Zilelidou, E.A.; Milina, V.; Paramithiotis, S.; Zoumpopoulou, G.; Poimenidou, S.V.; Mavrogonatou, E.; Kletsas, D.; Papadimitriou, K.; Tsakalidou, E.; Skandamis, P.N. Differential Modulation of Listeria monocytogenes Fitness, In Vitro Virulence, and Transcription of Virulence-Associated Genes in Response to the Presence of Different Microorganisms. Appl. Environ. Microbiol. 2020, 86, e01165-20. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.K.; Orellana, L.; Bryan, D.W.; Moore, A.; Munafo, J.P.; den Bakker, H.C.; Denes, T.G. Phylogeny of the Bacillus altitudinis Complex and Characterization of a Newly Isolated Strain with Antilisterial Activity. J. Food Prot. 2021, 84, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Woo, C.; Fugaban, J.; Vazquez Bucheli, J.E.; Holzapfel, W.H.; Todorov, S.D. Bacteriocinogenic Potential of Bacillus amyloliquefaciens Isolated from Kimchi, a Traditional Korean Fermented Cabbage. Probiotics Antimicrob. Proteins 2021, 13, 1195–1212. [Google Scholar] [CrossRef]
- Johny, L.C.; Suresh, P.V. Complete Genome Sequencing and Strain Characterization of a Novel Marine Bacillus velezensis FTL7 with a Potential Broad Inhibitory Spectrum Against Foodborne Pathogens. World J. Microbiol. Biotechnol. 2022, 38, 164. [Google Scholar] [CrossRef]
- Saggese, A.; De Luca, Y.; Baccigalupi, L.; Ricca, E. An Antimicrobial Peptide Specifically Active Against Listeria monocytogenes is Secreted by Bacillus pumilus SF214. BMC Microbiol. 2022, 22, 3. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X. Isolation and Characterization of Competitive Exclusion Microorganisms from Animal Wastes-Based Composts Against Listeria monocytogenes. J. Appl. Microbiol. 2022, 132, 4531–4543. [Google Scholar] [CrossRef]
- Spears, J.L.; Kramer, R.; Nikiforov, A.I.; Rihner, M.O.; Lambert, E.A. Safety Assessment of Bacillus subtilis MB40 for Use in Foods and Dietary Supplements. Nutrients 2021, 13, 733. [Google Scholar] [CrossRef]
- FDA. GRN No. 955. Bacillus subtilis Strain BS-MB40 PTA-122264 Spore Preparation. 2021. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=955 (accessed on 4 August 2023).
- National Research Council. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Hof, H.; Hefner, P. Pathogenicity of Listeria monocytogenes in Comparison to Other Listeria Species. Infection 1988, 16, S141–S144. [Google Scholar] [CrossRef]
- Pine, L.; Malcolm, G.B.; Plikaytis, B.D. Listeria monocytogenes Intragastric and Intraperitoneal Approximate 50% Lethal Doses for Mice are Comparable, but Death Occurs Earlier by Intragastric Feeding. Infect. Immun. 1990, 58, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- Reference Intervals from the Clinical Pathology Laboratory, Department of Veterinary Pathology, College of Veterinary Medicine, Iowa State University. Available online: https://vetmed.iastate.edu/vpath/services/diagnostic-services/clinical-pathology/testing-and-fees/reference-intervals (accessed on 4 August 2023).
- Vázquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Domínguez-Bernal, G.; Goebel, W.; González-Zorn, B.; Wehland, J.; Kreft, J. Listeria Pathogenesis and Molecular Virulence Determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Lee, S.N.; Chang, S.Y.; Ko, H.J.; Ryu, S.; Kweon, M.N. A Mouse Model of Shigellosis by Intraperitoneal Infection. J. Infect. Dis. 2014, 209, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen Elimination by Probiotic Bacillus via Signalling Interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef]
- Mercado, V.; Olmos, J. Bacteriocin Production by Bacillus Species: Isolation, Characterization, and Application. Probiotics Antimicrob. Proteins 2022, 14, 1151–1169. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Liu, S.; Song, B.; Liu, H.; Li, F.; Deng, S.; Wang, G.; Zeng, H.; Zeng, X.; et al. Toyoncin, a Novel Leaderless Bacteriocin That Is Produced by Bacillus toyonensis XIN-YC13 and Specifically Targets B. cereus and Listeria monocytogenes. Appl. Environ. Microbiol. 2021, 87, e00185-21. [Google Scholar] [CrossRef]
- Xin, B.; Xu, H.; Liu, H.; Liu, S.; Wang, J.; Xue, J.; Zhang, F.; Deng, S.; Zeng, H.; Zeng, X.; et al. Identification and Characterization of a Novel Circular Bacteriocin, Bacicyclicin XIN-1, from Bacillus sp. Xin1. Food Control 2021, 121, 107696. [Google Scholar] [CrossRef]
- Cruz Mendoza, I.; Villavicencio-Vasquez, M.; Aguayo, P.; Coello Montoya, D.; Plaza, L.; Romero-Peña, M.; Marqués, A.M.; Coronel-León, J. Biosurfactant from Bacillus subtilis DS03: Properties and Application in Cleaning Out Place System in a Pilot Sausages Processing. Microorganisms 2022, 10, 1518. [Google Scholar] [CrossRef]
- Deng, S.; Liu, S.; Li, X.; Liu, H.; Li, F.; Liu, K.; Zeng, H.; Zeng, X.; Xin, B. Thuricins: Novel Leaderless Bacteriocins with Potent Antimicrobial Activity Against Gram-Positive Foodborne Pathogens. J. Agric. Food Chem. 2022, 70, 9990–9999. [Google Scholar] [CrossRef]
- Hong, S.W.; Kim, J.H.; Cha, H.A.; Chung, K.S.; Bae, H.J.; Park, W.S.; Ham, J.S.; Park, B.Y.; Oh, M.H. Identification and Characterization of a Bacteriocin from the Newly Isolated Bacillus subtilis HD15 with Inhibitory Effects against Bacillus cereus. J. Microbiol. Biotechnol. 2022, 32, 1462–1470. [Google Scholar] [CrossRef]
- Stincone, P.; Fonseca Veras, F.; Micalizzi, G.; Donnarumma, D.; Vitale Celano, G.; Petras, D.; de Angelis, M.; Mondello, L.; Brandelli, A. Listeria monocytogenes Exposed to Antimicrobial Peptides Displays Differential Regulation of Lipids and Proteins Associated to Stress Response. Cell. Mol. Life Sci. 2022, 79, 263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xin, N.; Zhu, Z.; Li, X.; Dai, D.; Pan, C.; Peng, D.; Sun, M. Three Novel Leaderless Bacteriocins Have Antimicrobial Activity Against Gram-Positive Bacteria to Serve as Promising Food Biopreservative. Microb. Cell Fact. 2022, 21, 194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Z.; Zhang, S.; Li, P. Complete Genome Sequencing Revealed the Potential Application of a Novel Weizmannia coagulans PL-W Production with Promising Bacteriocins in Food Preservative. Foods 2023, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Sha, C.; Zhang, L.; Ge, D.; Wang, Y.; Xia, X.; Liu, X.; Zhou, J. A Novel Subtilin-Like Lantibiotics Subtilin JS-4 Produced by Bacillus subtilis JS-4, and its Antibacterial Mechanism Against Listeria monocytogenes. LWT 2021, 142, 110993. [Google Scholar] [CrossRef]
- de Waard, R.; Garssen, J.; Bokken, G.C.; Vos, J.G. Antagonistic Activity of Lactobacillus casei Strain Shirota Against Gastrointestinal Listeria monocytogenes Infection in Rats. Int. J. Food Microbiol. 2002, 73, 93–100. [Google Scholar] [CrossRef]
- Corr, S.C.; Li, Y.; Riedel, C.U.; O’Toole, P.W.; Hill, C.; Gahan, C.G. Bacteriocin Production as a Mechanism for the Antiinfective Activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 2007, 104, 7617–7621. [Google Scholar] [CrossRef]
- dos Santos, L.M.; Santos, M.M.; de Souza Silva, H.P.; Arantes, R.M.; Nicoli, J.R.; Vieira, L.Q. Monoassociation with Probiotic Lactobacillus delbrueckii UFV-H2b20 Stimulates the Immune System and Protects Germfree Mice Against Listeria monocytogenes Infection. Med. Microbiol. Immunol. 2011, 200, 29–38. [Google Scholar] [CrossRef]
- Archambaud, C.; Nahori, M.A.; Soubigou, G.; Bécavin, C.; Laval, L.; Lechat, P.; Smokvina, T.; Langella, P.; Lecuit, M.; Cossart, P. Impact of Lactobacilli on Orally Acquired Listeriosis. Proc. Natl. Acad. Sci. USA 2012, 109, 16684–16689. [Google Scholar] [CrossRef]
- Lukic, J.; Jancic, I.; Mirkovic, N.; Bufan, B.; Djokic, J.; Milenkovic, M.; Begovic, J.; Strahinic, I.; Lozo, J. Lactococcus lactis and Lactobacillus salivarius Differently Modulate Early Immunological Response of Wistar Rats Co-Administered with Listeria monocytogenes. Benef. Microbes 2017, 8, 809–822. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Zhou, J.; Hou, B.; Su, X.; Liu, Z.; Yuan, J.; Li, M. Bacillus licheniformis Zhengchangsheng® Attenuates DSS-Induced Colitis and Modulates the Gut Microbiota in Mice. Benef. Microbes 2019, 10, 543–553. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Cao, M.; Li, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Xu, S.; Feng, B.; Lin, Y.; et al. Dietary Supplementation of Bacillus subtilis PB6 Improves Sow Reproductive Performance and Reduces Piglet Birth Intervals. Anim. Nutr. 2020, 6, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Heng, C.; Zhou, X.; Cao, G.; Jiang, L.; Wang, J.; Li, K.; Wang, D.; Zhan, X. Supplemental Bacillus subtilis DSM 29784 and Enzymes, Alone or in Combination, as Alternatives for Antibiotics to Improve Growth Performance, Digestive Enzyme Activity, Anti-Oxidative Status, Immune Response and the Intestinal Barrier of Broiler Chickens. Br. J. Nutr. 2021, 125, 494–507. [Google Scholar] [CrossRef]
- Richter, J.F.; Gitter, A.H.; Günzel, D.; Weiss, S.; Mohamed, W.; Chakraborty, T.; Fromm, M.; Schulzke, J.D. Listeriolysin O Affects Barrier Function and Induces Chloride Secretion in HT-29/B6 Colon Epithelial Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1350–G1359. [Google Scholar] [CrossRef] [PubMed]
- Cajnko, M.M.; Marušić, M.; Kisovec, M.; Rojko, N.; Benčina, M.; Caserman, S.; Anderluh, G. Listeriolysin O Affects the Permeability of Caco-2 Monolayer in a Pore-Dependent and Ca2+-Independent Manner. PLoS ONE 2015, 10, e0130471. [Google Scholar] [CrossRef] [PubMed]
- Tingting, W.; Tianqi, F.; Xinyu, W.; Can, Z.; Xue, S.; Xuming, D.; Jianfeng, W. Amentoflavone Attenuates Listeria monocytogenes Pathogenicity Through an LLO-Dependent Mechanism. Br. J. Pharmacol. 2022, 179, 3839–3858. [Google Scholar] [CrossRef]
- Albers, R.; Antoine, J.M.; Bourdet-Sicard, R.; Calder, P.C.; Gleeson, M.; Lesourd, B.; Samartín, S.; Sanderson, I.R.; Van Loo, J.; Vas Dias, F.W.; et al. Markers to Measure Immunomodulation in Human Nutrition Intervention Studies. Br. J. Nutr. 2005, 94, 452–481. [Google Scholar] [CrossRef]
- Fiorini, G.; Cimminiello, C.; Chianese, R.; Visconti, G.P.; Cova, D.; Uberti, T.; Gibelli, A. Bacillus subtilis Selectively Stimulates the Synthesis of Membrane Bound and Secreted IgA. Chemioterapia 1985, 4, 310–312. [Google Scholar]
- Marseglia, G.L.; Tosca, M.; Cirillo, I.; Licari, A.; Leone, M.; Marseglia, A.; Castellazzi, A.M.; Ciprandi, G. Efficacy of Bacillus clausii Spores in the Prevention of Recurrent Respiratory Infections in Children: A Pilot Study. Ther. Clin. Risk Manag. 2007, 3, 13–17. [Google Scholar] [CrossRef]
- Lefevre, M.; Racedo, S.M.; Ripert, G.; Housez, B.; Cazaubiel, M.; Maudet, C.; Jüsten, P.; Marteau, P.; Urdaci, M.C. Probiotic Strain Bacillus subtilis CU1 Stimulates Immune System of Elderly During Common Infectious Disease Period: A Randomized, Double-Blind Placebo-Controlled Study. Immun. Ageing 2015, 12, 24. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like Receptors and Their Role in Gut Homeostasis and Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, X.; Duan, Y.; Zhao, Y.; Zhang, W.; Azad, M.; Wang, Z.; Blachier, F.; Kong, X. Dietary Supplementation with Bacillus subtilis Promotes Growth and Gut Health of Weaned Piglets. Front. Vet. Sci. 2021, 7, 600772. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Khongthong, S.; Roekngam, N.; Theapparat, Y.; Sunpaweravong, S.; Faroongsarng, D.; Otto, M. Probiotic for Pathogen-Specific Staphylococcus aureus Decolonisation in Thailand: A Phase 2, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Gastroenterol. Hepatol. 2023, 4, e75–e83. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).