Infant Saliva Microbiome Activity Modulates Nutritional Impacts on Neurodevelopment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Saliva Microbiome Analysis
2.4. Statistical Analysis
3. Results
3.1. Participants
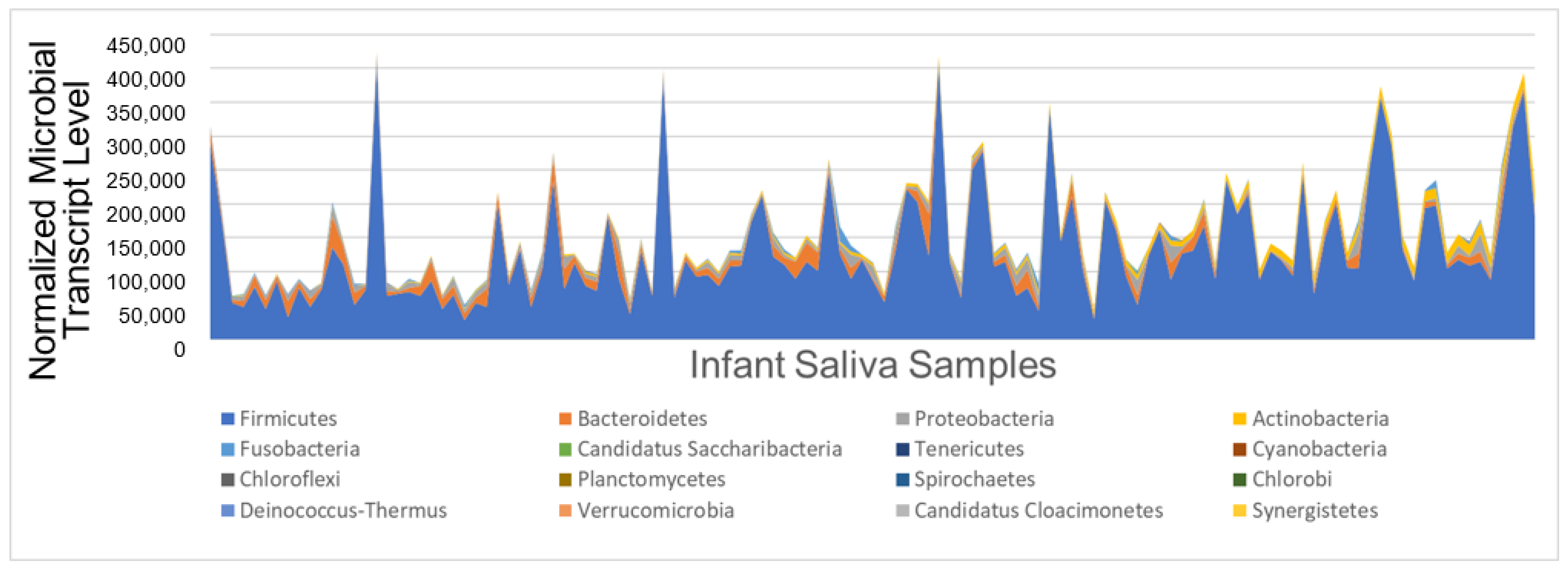
3.2. Oral Microbial Activity
3.3. Nutrition
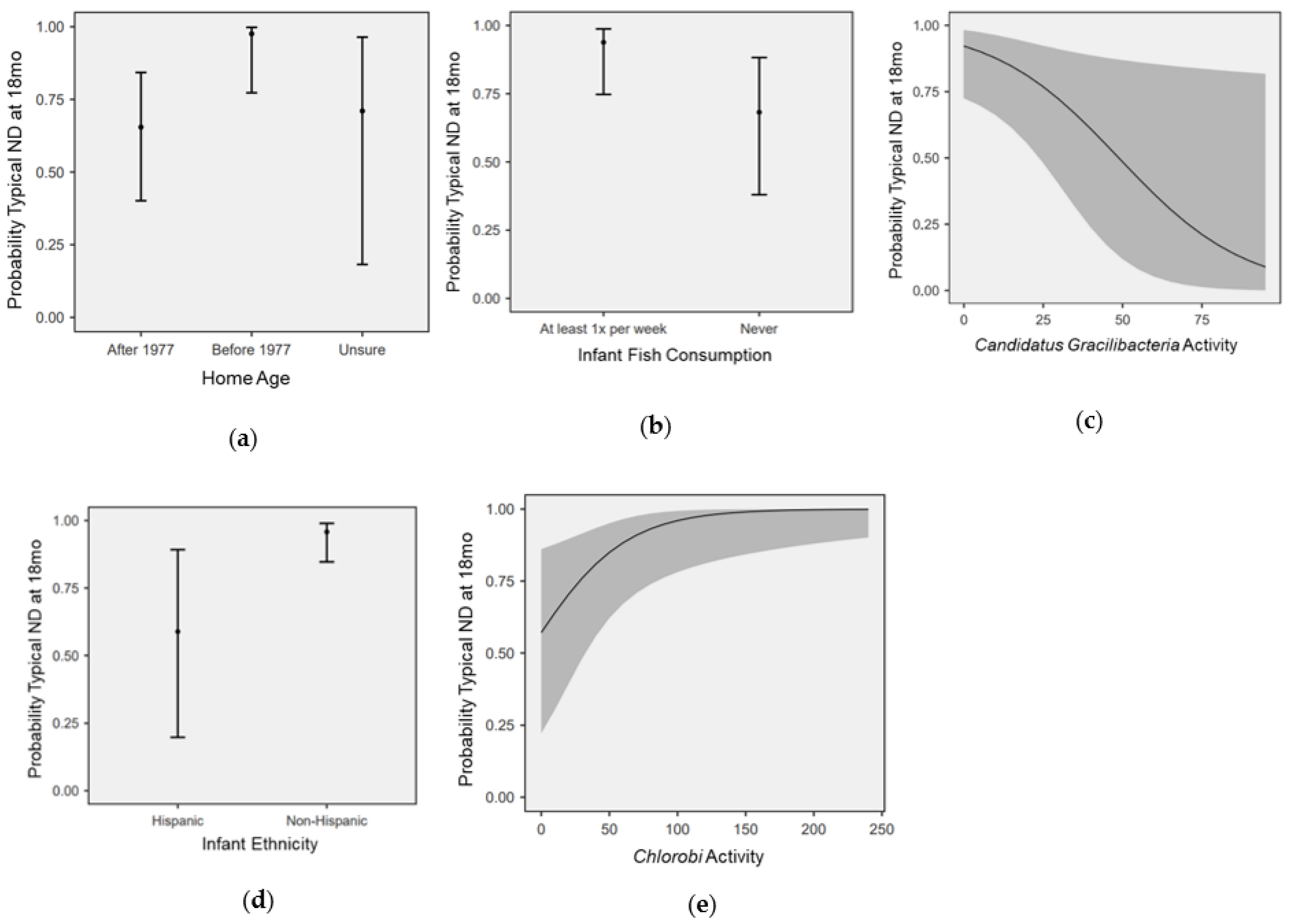
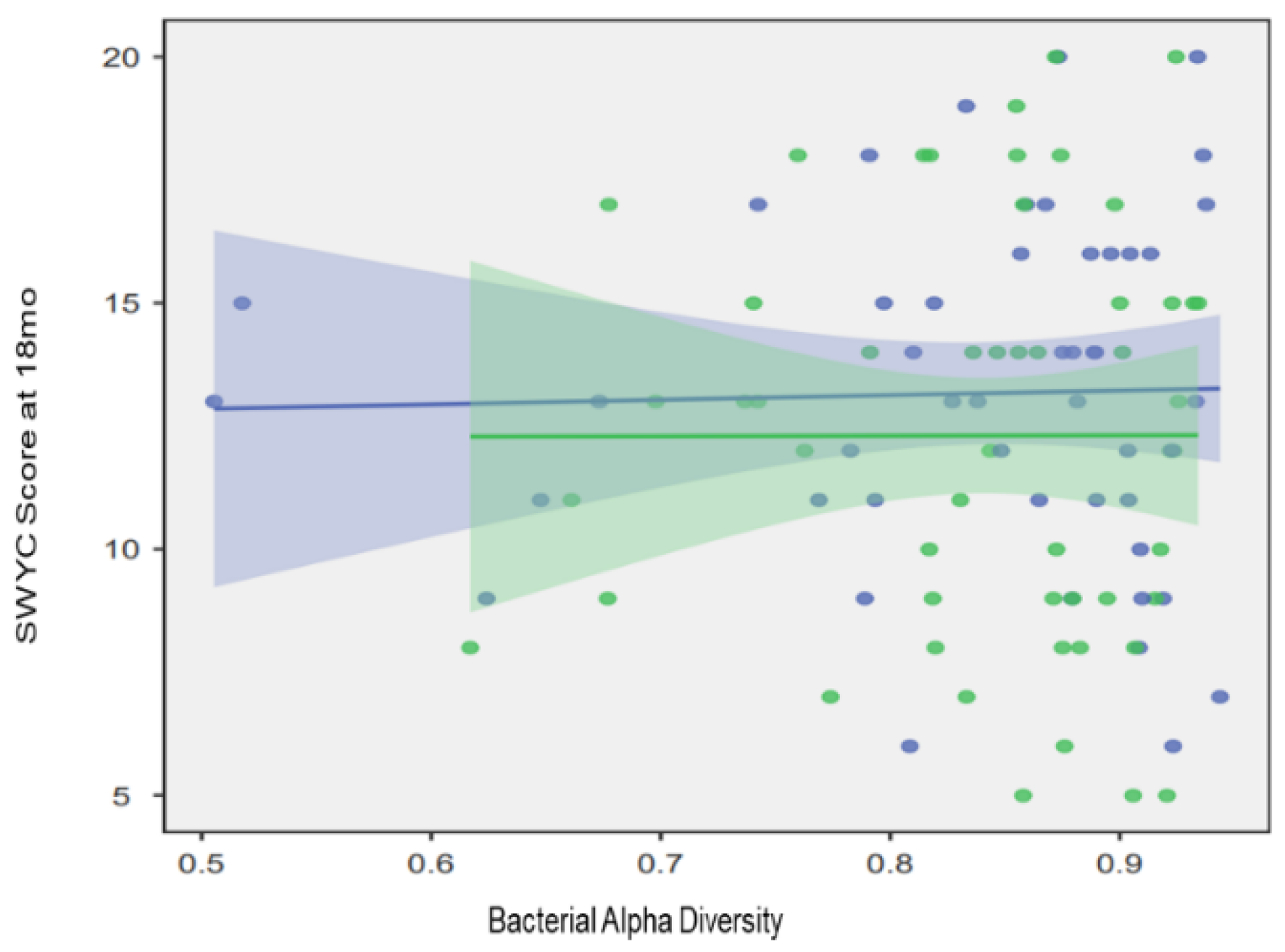
3.4. Modeling the Effects of Nutrition, SDOH, and Microbial Activity on ND
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korkmaz, B. Theory of Mind and Neurodevelopmental Disorders of Childhood. Pediatr. Res. 2011, 69, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.J. The genetics of neurodevelopmental disease. Curr. Opin. Neurobiol. 2011, 21, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, R.; Szatmari, P. Genetic and neurodevelopmental influences in autistic disorder. Can. J. Psychiatry 2003, 48, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.M.; Li, K. Poverty, toxic stress, and education in children born preterm. Nurs. Res. 2019, 68, 275–284. [Google Scholar] [CrossRef]
- Castagna, A.; Mascheroni, E.; Fustinoni, S.; Montirosso, R. Air pollution and neurodevelopmental skills in preschool-and school-aged children: A systematic review. Neurosci. Biobehav. Rev. 2022, 136, 104623. [Google Scholar] [CrossRef]
- Dórea, J.G. Environmental exposure to low-level lead (Pb) co-occurring with other neurotoxicants in early life and neurodevelopment of children. Environ. Res. 2019, 177, 108641. [Google Scholar] [CrossRef]
- Bellinger, D.C. A strategy for comparing the contributions of environmental chemicals and other risk factors to neurodevelopment of children. Environ. Health Perspect. 2012, 120, 501–507. [Google Scholar] [CrossRef]
- Kelly, J.R.; Minuto, C.; Cryan, J.F.; Clarke, G.; Dinan, T.G. Cross talk: The microbiota and neurodevelopmental disorders. Front. Neurosci. 2017, 11, 490. [Google Scholar] [CrossRef]
- Olsen, I.; Hicks, S.D. Oral microbiota and autism spectrum disorder (ASD). J. Oral. Microbiol. 2020, 12, 1702806. [Google Scholar] [CrossRef]
- Clark, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Cortes-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef]
- Starling, P.; Charlton, K.; McMahon, A.T.; Lucas, C. Fish intake during pregnancy and foetal neurodevelopment—A systematic review of the evidence. Nutrients 2015, 7, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Avella-Garcia, C.B.; Julvez, J. Seafood Intake and Neurodevelopment: A Systematic Review. Curr. Environ. Health Rep. 2014, 1, 46–77. [Google Scholar] [CrossRef]
- Sheldrick, R.C.; Perrin, E.C. Evidence-based milestones for surveillance of cognitive, language, and motor development. Acad. Pediatr. 2013, 13, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Elliott, L.; Arbes, S.J.; Harvey, E.S.; Lee, R.C.; Salo, P.M.; Cohn, R.D.; London, S.J.; Zeldin, D.C. Dust weight and asthma prevalence in the National Survey of Lead and Allergens in Housing (NSLAH). Environ. Health Perspect. 2007, 115, 215–220. [Google Scholar] [CrossRef]
- Thompson, F.E.; Midthune, D.; Kahle, L.; Dodd, K.W. Development and Evaluation of the National Cancer Institute’s Dietary Screener Questionnaire Scoring Algorithms. J. Nutr. 2017, 147, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Fein, S.B.; Labiner-Wolfe, J.; Shealy, K.R.; Li, R.; Chen, J.; Grummer-Strawn, L.M. Infant Feeding Practices Study II: Study methods. Pediatrics 2008, 122 (Suppl. S2), S28–S35. [Google Scholar] [CrossRef]
- Hicks, S.D.; Jacob, P.; Middleton, F.A.; Perez, O.; Gagnon, Z. Distance running alters peripheral microRNAs implicated in metabolism, fluid balance, and myosin regulation in a sex-specific manner. Physiol. Genom. 2018, 50, 658–667. [Google Scholar] [CrossRef]
- Sittiprapaporn, P.; Bumrungpert, A.; Suyajai, P.; Stough, C. Effectiveness of fish Oil-DHA supplementation for cognitive function in Thai children: A randomized, doubled-blind, two-dose, placebo-controlled clinical trial. Foods 2022, 11, 2595. [Google Scholar] [CrossRef]
- Al-Ghannami, S.S.; Al-Adawi, S.; Ghebremeskel, K.; Hussein, I.S.; Min, Y.; Jeyaseelan, L.; Al-Shammakhi, S.M.; Mabry, R.M.; Al-Oufi, H.S. Randomized open-label trial of docosahexaenoic acid–enriched fish oil and fish meal on cognitive and behavioral functioning in Omani children. Nutrition 2019, 57, 167–172. [Google Scholar] [CrossRef]
- Meldrum, S.J.; D’Vaz, N.; Simmer, K.; Dunstan, J.A.; Hird, K.; Prescott, S.L. Effects of high-dose fish oil supplementation during early infancy on neurodevelopment and language: A randomised controlled trial. Br. J. Nutr. 2012, 108, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Uhlig, R.; Afshari, P.; Williams, J.; Chroneos, M.; Tierney-Aves, C.; Wagner, K.; Middleton, F.A. Oral microbiome activity in children with autism spectrum disorder. Autism Res. 2018, 11, 1286–1299. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, J.E.; Korrick, S.; Laranjo, N.; Carey, V.; Weinstock, G.M.; Gold, D.R.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Beigelman, A.; et al. Association of the infant gut microbiome with early childhood neurodevelopmental outcomes: An ancillary study to the VDAART randomized clinical trial. JAMA Netw. Open 2019, 2, e190905. [Google Scholar] [CrossRef] [PubMed]
- Camanocha, A.; Dewhirst, F.E. Host-associated bacterial taxa from Chlorobi, Chloroflexi, GN02, Synergistetes, SR1, TM7, and WPS-2 Phyla/candidate divisions. J. Oral. Microbiol. 2014, 6, 25468. [Google Scholar] [CrossRef]
- Naud, S.; Ibrahim, A.; Valles, C.; Maatouk, M.; Bittar, F.; Tidjani Alou, M.; Raoult, D. Candidate Phyla Radiation, an Underappreciated Division of the Human Microbiome, and Its Impact on Health and Disease. Clin. Microbiol. Rev. 2022, 35, e00140-21. [Google Scholar] [CrossRef]
- Sieber, C.M.; Paul, B.G.; Castelle, C.J.; Hu, P.; Tringe, S.G.; Valentine, D.L.; Andersen, G.L.; Banfield, J.F. Unusual metabolism and hypervariation in the genome of a gracilibacterium (Bd1-5) from an oil-degrading community. MBio 2019, 10, e02128-19. [Google Scholar] [CrossRef]
- Brumbaugh, J.E.; Vohr, B.R.; Bell, E.F.; Bann, C.M.; Travers, C.P.; McGowan, E.C.; Harmon, H.M.; Carlo, W.A.; Duncan, A.F.; Hintz, S.R.; et al. Early-Life Outcomes in Relation to Social Determinants of Health for Children Born Extremely Preterm. J. Pediatr. 2023, 25, 113443. [Google Scholar] [CrossRef]
- Appleton, A.A.; Holdsworth, E.A.; Kubzansky, L.D. A systematic review of the interplay between social determinants and environmental exposures for early-life outcomes. Curr. Environ. Health Rep. 2016, 3, 287–301. [Google Scholar] [CrossRef]
- Fraiman, Y.S.; Barrero-Castillero, A.; Litt, J.S. Implications of racial/ethnic perinatal health inequities on long-term neurodevelopmental outcomes and health services utilization. In Seminars in Perinatology; WB Saunders: Philadelphia, PA, USA, 2022; p. 151660. [Google Scholar]
- Shonkoff, J.P.; Slopen, N.; Williams, D.R. Early childhood adversity, toxic stress, and the impacts of racism on the foundations of health. Annu. Rev. Public Health 2021, 42, 115–134. [Google Scholar] [CrossRef]
- Leigh, J.P.; Du, J. Brief report: Forecasting the economic burden of autism in 2015 and 2025 in the United States. J. Autism Dev. Disord. 2015, 45, 4135–4139. [Google Scholar] [CrossRef]
- Hirai, A.H.; Kogan, M.D.; Kandasamy, V.; Reuland, C.; Bethell, C. Prevalence and Variation of Developmental Screening and Surveillance in Early Childhood. JAMA Pediatr. 2018, 172, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Carbone, P.S.; Campbell, K.; Wilkes, J.; Stoddard, G.J.; Huynh, K.; Young, P.C.; Gabrielsen, T.P. Primary Care Autism Screening and Later Autism Diagnosis. Pediatrics 2020, 146, e20192314. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.N.; Wieckowski, A.T.; Dieckhaus, M.F.S.; Dai, Y.G.; Zhang, F.; Dumont-Mathieu, T.; Barton, M.; Fein, D.; Robins, D.L. Primary Care Clinician and Child Characteristics Impacting Autism Surveillance. Brain Sci. 2022, 13, 18. [Google Scholar] [CrossRef] [PubMed]
| Characteristics and SDOH | All N = 142 | Typical ND N = 118 | NDD N = 24 |
|---|---|---|---|
| Female sex, n (%) | 77 (54) | 65 (55) | 12 (50) |
| White race, n (%) | 102 (71) | 86 (72) | 16 (66) |
| Hispanic ethnicity, n (%) | 23 (16) | 17 (14) | 6 (25) |
| Maternal college education, n (%) | 120 (84) | 99 (83) | 21 (87) |
| Married, n (%) | 109 (76) | 90 (76) | 19 (79) |
| Private health insurance, n (%) | 114 (80) | 92 (77) | 22 (91) |
| Home built before 1977, n (%) | 28 (19) | 27 (22) | 1 (4) |
| Household income < $25,000, n (%) | 12 (8) | 9 (7) | 3 (12) |
| Persons in household, average (range) | 3.9 (2–9) | 3.9 (2–9) | 3.8 (3–5) |
| Atmospheric pollution, n (%) | 38 (26) | 33 (27) | 5 (20) |
| Infant Nutrition | All N = 142 | Typical ND N = 118 | NDD N = 24 |
|---|---|---|---|
| Breastfeeding at 6 months, n (%) | 106 (74) | 90 (76) | 16 (66) |
| Dairy ≥ once daily, n (%) | 28 (26) | 24 (27) | 4 (21) |
| Soy ≥ once weekly, n (%) | 12 (11) | 11 (12) | 1 (5) |
| Juice ≥ once weekly, n (%) | 34 (31) | 28 (31) | 6 (31) |
| Fruit ≥ once daily, n (%) | 85 (79) | 70 (79) | 14 (73) |
| Vegetables ≥ once daily, n (%) | 86 (80) | 73 (82) | 13 (68) |
| Meat/chicken ≥ once daily, n (%) | 42 (39) | 35 (39) | 7 (36) |
| Fish ≥ once weekly, n (%) | 51 (47) | 46 (52) | 5 (26) * |
| Eggs ≥ once weekly, average (range) | 89 (83) | 76 (88) | 13 (68) |
| Sweets ≥ once weekly, n (%) | 50 (46) | 41 (46) | 9 (47) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keck-Kester, T.; Hicks, S.D. Infant Saliva Microbiome Activity Modulates Nutritional Impacts on Neurodevelopment. Microorganisms 2023, 11, 2111. https://doi.org/10.3390/microorganisms11082111
Keck-Kester T, Hicks SD. Infant Saliva Microbiome Activity Modulates Nutritional Impacts on Neurodevelopment. Microorganisms. 2023; 11(8):2111. https://doi.org/10.3390/microorganisms11082111
Chicago/Turabian StyleKeck-Kester, Terrah, and Steven D. Hicks. 2023. "Infant Saliva Microbiome Activity Modulates Nutritional Impacts on Neurodevelopment" Microorganisms 11, no. 8: 2111. https://doi.org/10.3390/microorganisms11082111
APA StyleKeck-Kester, T., & Hicks, S. D. (2023). Infant Saliva Microbiome Activity Modulates Nutritional Impacts on Neurodevelopment. Microorganisms, 11(8), 2111. https://doi.org/10.3390/microorganisms11082111









