Environmental Sampling Methods for Detection of Salmonella Infections in Laying Hens: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Questions
2.2. Search Strategy
2.3. Inclusion Criteria and Records Screening
- The study is performed in laying hens;
- Salmonella is the subject being studied;
- Type of sampling in the environment is described;
- Detection (or not) of Salmonella in the environment is described;
- The prevalence of Salmonella in the flock is described;
- The publication is written in English;
- The study is peer-reviewed;
- The study described is primary research;
- The full text of the study is available;
- Data are available on individual farm level (used to discriminate publications for the qualitative or quantitative data extraction).
2.4. Data Extraction
2.5. Data Analysis
2.5.1. Qualitative Analysis
2.5.2. Quantitative Analysis
3. Results
3.1. Literature Search and Screening
3.2. Qualitative Analysis
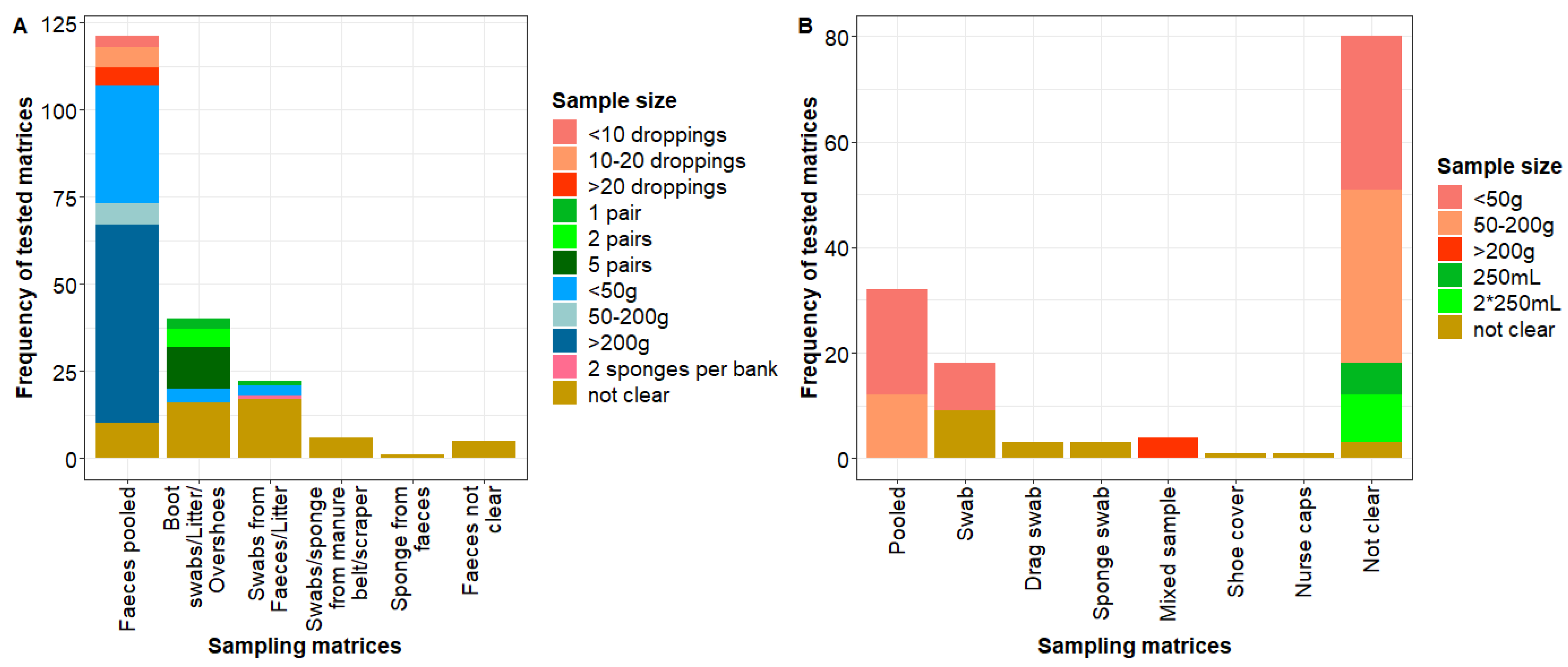
3.2.1. Overview of Environmental Sampling Matrices (Research Question One)
3.2.2. Overview of Matrices for Sampling Individual Hens (Research Question Two)
3.3. Quantitative Analysis
3.3.1. Detection of Positive Flocks Based on Sampling Individual Birds versus Environmental Matrices
3.3.2. Comparison of Proportion of Positive Samples between Environmental Matrices (Meta-Analysis—Research Question Three)
3.4. Use of ISO 6579-2002/ISO 6579-1:2017 for Detection of Salmonella (Research Question Four)
4. Discussion
- When the purpose is to detect infected flocks, environmental samples, particularly boot swabs would be the recommended samples, since they are more sensitive than those from individual hens and fewer numbers are required.
- Based on relative comparison in non-cage housing systems, pooled faeces seems to be superior to dust samples. For caged systems, dust gave better results. However, since the EU plans to phase out caged animal farming, this may be less relevant in the future. The use of pooled faeces is recommended for non-caged systems if samples other than, or in addition to, boot swabs are to be collected.
- The limited data available from the literature, unfortunately, does not allow for making recommendations on the best sample size or sample locations within the laying hen house. It is recommended to gain new experimental data to address this issue.
- The findings from this review suggest that there is room for improvement regarding reporting of the methods used. It is recommended to use uniform terminology in naming matrices used for sampling laying hens.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ReferencesEFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 6971. [Google Scholar]
- De Buck, J.; Van Immerseel, F.; Haesebrouck, F.; Ducatelle, R. Colonization of the chicken reproductive tract and egg contamination by Salmonella. J. Appl. Microbiol. 2004, 97, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Messens, W.; Grijspeerdt, K.; Herman, L. Eggshell penetration by Salmonella: A review. World′s Poult. Sci. J. 2005, 61, 71–86. [Google Scholar] [CrossRef]
- Arnold, M.E.; Carrique-Mas, J.J.; Davies, R.H. Sensitivity of environmental sampling methods for detecting Salmonella Enteritidis in commercial laying flocks relative to the within-flock prevalence. Epidemiol. Infect. 2010, 138, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.E.; Carrique-Mas, J.J.; McLaren, I.; Davies, R.H. A comparison of pooled and individual bird sampling for detection of Salmonella in commercial egg laying flocks. Prev. Vet. Med. 2011, 99, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.E.; Martelli, F.; McLaren, I.; Davies, R.H. Estimation of the rate of egg contamination from Salmonella-infected chickens. Zoonoses Public Health 2014, 61, 18–27. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific Opinion on the Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, 5596. [Google Scholar]
- Chanamé Pinedo, L.; Van Goethem, N.; Mallioris, P.; Pacholewicz, E.; Pijnacker, R.; Franz, E.; Mughini-Gras, L. Assessing potential determinants of the stagnating trend in Salmonella Enteritidis human infections in Europe and options for intervention: A multi-criteria decision analysis. One Health 2023, 16, 100535. [Google Scholar] [CrossRef]
- ISO 6579:2002; Microbiology of Food and Animal Feeding Stuffs: Horizontal Method for the Detection of Salmonella spp. International Standardization Organization: Geneva, Switzerland, 2002.
- EN ISO 6579-1; Microbiology of the Food Chain–Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2017.
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Kohl, C.; McIntosh, E.J.; Unger, S.; Haddaway, N.R.; Kecke, S.; Schiemann, J.; Wilhelm, R. Online tools supporting the conduct and reporting of systematic reviews and systematic maps: A case study on CADIMA and review of existing tools. Environ. Evid. 2018, 7, 8, Erratum in Environ. Evid. 2018, 7, 12. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Van Hoorebeke, S.; Van Immerseel, F.; Schulz, J.; Hartung, J.; Harisberger, M.; Barco, L.; Ricci, A.; Theodoropoulos, G.; Xylouri, E.; De Vylder, J.; et al. Determination of the within and between flock prevalence and identification of risk factors for Salmonella infections in laying hen flocks housed in conventional and alternative systems. Prev. Vet. Med. 2010, 94, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.; Van Hoorebeke, S.; Hald, B.; Hartung, J.; Van Immerseel, F.; Radtke, I.; Kabell, S.; Dewulf, J. The dynamics of Salmonella occurrence in commercial laying hen flocks throughout a laying period. Avian Pathol. 2011, 40, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Andoh, L.A.; Dalsgaard, A.; Obiri-Danso, K.; Newman, M.J.; Barco, L.; Olsen, J.E. Prevalence and antimicrobial resistance of Salmonella serovars isolated from poultry in Ghana. Epidemiol. Infect. 2016, 144, 3288–3299. [Google Scholar] [CrossRef] [PubMed]
- Soria, M.C.; Soria, M.A.; Bueno, D.J.; Godano, E.I.; Gomez, S.C.; ViaButron, I.A.; Padin, V.M.; Roge, A.D. Salmonella spp. contamination in commercial layer hen farms using different types of samples and detection methods. Poult. Sci. 2017, 96, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Aimey, V.; Hunte, K.; Whitehall, P.; Sanford, B.; Trotman, M.; Delgado, A.; Lefrancois, T.; Shaw, J.; Hernandez, J. Prevalence of and examination of exposure factors for Salmonella on commercial egg-laying farms in Barbados. Prev. Vet. Med. 2013, 110, 489–496. [Google Scholar] [CrossRef]
- Davies, R.; Breslin, M. Effects of vaccination and other preventive methods for Salmonella Enteritidis on commercial laying chicken farms. Vet. Rec. 2003, 153, 673–677. [Google Scholar] [CrossRef]
- Sharma, S.; Fowler, P.D.; Pant, D.K.; Singh, S.; Wilkins, M.J. Prevalence of non-typhoidal Salmonella and risk factors on poultry farms in Chitwan, Nepal. Vet. World 2021, 14, 426–436. [Google Scholar] [CrossRef]
- Haque, A.K.M.Z.; Akter, M.R.; Islam, S.K.S.; Jahangir, A.; Neogi, S.B.; Yamasaki, S.; Kabir, S.M.L. Salmonella gallinarum in small-scale commercial layer flocks: Occurrence, molecular diversity and antibiogram. Vet. Sci. 2021, 8, 71. [Google Scholar] [CrossRef]
- Saravanan, S.; Purushothaman, V.; Murthy, T.R.; Sukumar, K.; Srinivasan, P.; Gowthaman, V.; Balusamy, M.; Atterbury, R.; Kuchipudi, S.V. Molecular Epidemiology of Nontyphoidal Salmonella in Poultry and Poultry Products in India: Implications for Human Health. Indian J. Microbiol. 2015, 55, 319–326. [Google Scholar] [CrossRef]
- Davies, R.H.; Breslin, M.F. Observations on the distribution and persistence of Salmonella enterica serovar Enteritidis phage type 29 on a cage layer farm before and after the use of competitive exclusion treatment. Br. Poult. Sci. 2003, 44, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Gole, V.C.; Caraguel, C.G.B.; Sexton, M.; Fowler, C.; Chousalkar, K.K. Shedding of Salmonella in single age caged commercial layer flock at an early stage of lay. Int. J. Food Microbiol. 2014, 189, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Gole, V.C.; Torok, V.; Sexton, M.; Caraguel, C.G.B.; Chousalkar, K.K. Association between indoor environmental contamination by Salmonella enterica and contamination of eggs on layer farms. J. Clin. Microbiol. 2014, 52, 3250–3258. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Li, Q.; Xu, G.; Zheng, J. Salmonella contamination in layer farms in China: Detection and genetic analysis. J. Poult. Sci. 2018, 55, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gole, V.C.; Woodhouse, R.; Caraguel, C.; Moyle, T.; Rault, J.L.; Sexton, M.; Chousalkar, K. Dynamics of Salmonella shedding and welfare of hens in free-range egg production systems. Appl. Environ. Microbiol. 2017, 83, e03313. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.E.; Martelli, F.; McLaren, I.; Davies, R.H. Estimation of the sensitivity of environmental sampling for detection of Salmonella in commercial layer flocks post-introduction of national control programmes. Epidemiol. Infect. 2014, 142, 1061–1069. [Google Scholar] [CrossRef]
- Matsumoto, A.; Miyama, M.; Murakami, S. Comparison of Salmonella isolation rates in different types of egg-layer hen houses in Chiba, Japan. Avian Dis. 2001, 45, 195–200. [Google Scholar] [CrossRef]
- Van Hoorebeke, S.; Van Immerseel, F.; De Vylder, J.; Ducatelle, R.; Haesebrouck, F.; Pasmans, F.; De Kruif, A.; Dewulf, J. Faecal sampling underestimates the actual prevalence of Salmonella in laying hen flocks. Zoonoses Public Health 2009, 56, 471–476. [Google Scholar]
- Huneau-Salaun, A.; Chemaly, M.; Le Bouquin, S.; Lalande, F.; Petetin, I.; Rouxel, S.; Virginie, M.; Philippe, F.; Rose, N. Risk factors for Salmonella enterica subsp. enterica contamination in 519 French laying hen flocks at the end of the laying period. Prev. Vet. Med. 2009, 89, 51–58. [Google Scholar] [CrossRef]




| Search Nr. | Keywords | No. Publications |
|---|---|---|
| CAB Abstracts on Ovid Platform | ||
| 1 | Salmonella.mp. | 68,799 |
| 2 | (layer* or laying or egg-laying).mp. | 263,394 |
| 3 | (environment* or prevalen* or monitor* or surveillance or boot? or swab*).mp. | 1,855,687 |
| 4 | 1 and 2 and 3 | 734 |
| Scopus | ||
| 1 | (TITLE-ABS-KEY (salmonella)) AND (TITLE-ABS-KEY (layer? OR laying OR egg-laying)) AND (TITLE-ABS-KEY (environment* OR prevalen* OR surveillance OR monitor* OR boot? OR swab?)) | 444 |
| Source of Samples | Number of Publications |
|---|---|
| Faecal material | 69 |
| Dust | 43 |
| Nest boxes | 15 |
| Feed | 26 |
| Water | 22 |
| Poultry house equipment | 37 |
| Pest | 21 |
| Poultry house areas | 21 |
| Environmental unspecified | 15 |
| Other | 6 |
| Type of Matrix | Number of Publications | ||
|---|---|---|---|
| Eggs | 34 | ||
| Part(s) of an egg used for diagnosis | Shells | 18 | |
| Contents | 19 | ||
| Whole | 2 | ||
| Non-specified | 8 | ||
| Cloacal swabs | 15 | ||
| Intestinal tract | 15 | ||
| Caeca | 13 | ||
| Intestines | 3 | ||
| Organs | 12 | ||
| Liver | 8 | ||
| Spleen | 10 | ||
| Heart | 3 | ||
| Gallbladder | 1 | ||
| Reproductive tract | 13 | ||
| Ovary | 13 | ||
| Oviduct | 8 | ||
| Upper reproductive tract | 1 | ||
| Uterus | 1 | ||
| Individual faecal droppings | 2 | ||
| Housing System | Environmental Sampling Matrices | Number of Publications | Flocks/Farms Positive | Flock/Farms Tested | Percentage Positive Flocks/Farms |
|---|---|---|---|---|---|
| Cage | Pooled faeces | 9 | 117 | 175 | 67 |
| Cage | Boot swabs | 2 | 5 | 5 | 100 |
| Cage | Dust | 7 | 91 | 123 | 74 |
| Cage | Other | 8 | 93 | 111 | 84 |
| Non-cage | Pooled faeces | 5 | 39 | 63 | 62 |
| Non-cage | Boot swabs | 5 | 35 | 92 | 38 |
| Non-cage | Dust | 4 | 39 | 83 | 47 |
| Non-cage | Nest box | 2 | 13 | 35 | 37 |
| Non-cage | Other | 3 | 24 | 62 | 39 |
| Non-specified | Pooled faeces | 4 | 64 | 119 | 54 |
| Non-specified | Pooled faeces or Boot swabs | 1 | 17 | 20 | 85 |
| Non-specified | Boot swabs | 1 | 1 | 1 | 100 |
| Non-specified | Dust | 3 | 61 | 99 | 62 |
| Non-specified | Other | 2 | 5 | 41 | 12 |
| Environmental Sampling Matrices | Number of Publications | Number of Flocks/Farms Tested | Number of Positive Flocks/Farms Based on Environmental Sampling/(%) | Number of Environmental Samples per Farm/Flock Median (Quartile 1–3) | Number of Positive Flocks/Farms Based on Sampling Hens/(%) | Number of Individual Samples per Farm/Flock Median (Quartile 1–3) |
|---|---|---|---|---|---|---|
| Pooled faeces | 10 | 257 | 168/65% | 10 (5–30) | 137/53% | 60 (40–100) |
| Pooled faeces or Boot swabs | 1 | 20 | 17/85% | 10 (5–10) | 16/80% | 296 (225–300) |
| Boot swabs | 7 | 66 | 32/48% | 2 (2–6) | 34/52% | 30 (30–100) |
| Dust | 8 | 271 | 165/61% | 10 (5–27) | 165/61% | 100 (30–100) |
| Nest box | 1 | 28 | 8/29% | 10 (10–10) | 4/14% | 30 (30–30) |
| Other | 7 | 86 | 53/62% | 20 (9–21) | 23/27% | 30 (30–60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacholewicz, E.; Wisselink, H.J.; Koene, M.G.J.; van der Most, M.; Gonzales, J.L. Environmental Sampling Methods for Detection of Salmonella Infections in Laying Hens: A Systematic Review and Meta-Analysis. Microorganisms 2023, 11, 2100. https://doi.org/10.3390/microorganisms11082100
Pacholewicz E, Wisselink HJ, Koene MGJ, van der Most M, Gonzales JL. Environmental Sampling Methods for Detection of Salmonella Infections in Laying Hens: A Systematic Review and Meta-Analysis. Microorganisms. 2023; 11(8):2100. https://doi.org/10.3390/microorganisms11082100
Chicago/Turabian StylePacholewicz, Ewa, Henk J. Wisselink, Miriam G. J. Koene, Marleen van der Most, and Jose L. Gonzales. 2023. "Environmental Sampling Methods for Detection of Salmonella Infections in Laying Hens: A Systematic Review and Meta-Analysis" Microorganisms 11, no. 8: 2100. https://doi.org/10.3390/microorganisms11082100
APA StylePacholewicz, E., Wisselink, H. J., Koene, M. G. J., van der Most, M., & Gonzales, J. L. (2023). Environmental Sampling Methods for Detection of Salmonella Infections in Laying Hens: A Systematic Review and Meta-Analysis. Microorganisms, 11(8), 2100. https://doi.org/10.3390/microorganisms11082100









