New Potential Biological Limiters of the Main Esca-Associated Fungi in Grapevine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Media, and Growth Conditions
2.2. Growth Rate
2.3. In Vitro Dual Culture Interactions
2.4. Interaction on Detached Grapevine Canes
2.5. Statistical Analysis
3. Results
3.1. Growth Rate
3.2. In Vitro Dual Culture Interactions
3.3. Interaction on Detached Grapevine Canes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Graniti, A.; Surico, G.; Mugnai, L. Esca of grapevine: A disease complex or a complex of diseases? Phytopathol. Mediterr. 2000, 39, 16–20. [Google Scholar] [CrossRef]
- Surico, G. Towards a redefinition of the diseases within the esca complex of grapevine. Phytopathol. Mediterr. 2009, 48, 5–10. [Google Scholar] [CrossRef]
- Bruno, G.L.; Ippolito, M.P.; Mannerucci, F.; Bragazzi, L.; Tommasi, F. Physiological responses of ‘Italia’ grapevines infected with Esca pathogens. Phytopathol. Mediterr. 2021, 60, 321–336. [Google Scholar] [CrossRef]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2012, 62, 243–265. [Google Scholar] [CrossRef]
- Surico, G.; Mugnai, L.; Marchi, G. The Esca Disease Complex. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Ciancio, A., Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 119–136. [Google Scholar]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef]
- Fischer, M. Biodiversity and geographic distribution of basidiomycetes causing esca-associated white rot in grapevine: A worldwide perspective. Phytopathol. Mediterr. 2006, 45, 30–42. [Google Scholar] [CrossRef]
- Mostert, L.; Groenewald, J.Z.; Summerbell, R.C.; Gams, W.; Crous, P.W. Taxonomy and pathology of Togninia (Diaporthales) and its Phaeoacremonium anamorphs. Stud. Mycol. 2006, 54, 1–113. [Google Scholar] [CrossRef]
- Mostert, L.; Hallen, F.; Fourie, P.; Crous, P.W. A review of Phaeoacremonium species involved in Petri disease and esca of grapevines. Phytopathol. Mediterr. 2006, 45, S12–S29. [Google Scholar] [CrossRef]
- Claverie, M.; Notaro, M.; Fontaine, F.; Wery, J. Current knowledge on Grapevine Trunk Diseases with complex etiology: A systemic approach. Phytopathol. Mediterr. 2020, 59, 29–53. [Google Scholar] [CrossRef]
- Gramaje, D.; Armengol, J.; Salazar, D.; López-Cortés, I.; García-Jiménez, J. Effect of hot-water treatments above 50 °C on grapevine viability and survival of Petri disease pathogens. Crop Prot. 2009, 28, 280–285. [Google Scholar] [CrossRef]
- Di Marco, S.; Mazzullo, A.; Calzarano, F.; Cesari, A. The control of esca: Status and perspectives. Phytopathol. Mediterr. 2000, 39, 232–240. [Google Scholar] [CrossRef]
- Roblin, G.; Luinia, E.; Fleurat-Lessarda, P.; Larignon, P.; Berjeauda, J.M. Towards a preventive and/or curative treatment of esca in grapevine trunk disease: General basis in the elaboration of treatments to control plant pathogen attacks. Crop Prot. 2019, 116, 156–169. [Google Scholar] [CrossRef]
- Mesguida, O.; Haidar, R.; Yacoub, A.; Dreux-Zigha, A.; Berthon, J.-Y.; Guyoneaud, R.; Attard, E.; Rey, P. Microbial Biological Control of Fungi Associated with Grapevine Trunk Diseases: A Review of Strain Diversity, Modes of Action, and Advantages and Limits of Current Strategies. J. Fungi 2023, 9, 638. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Urbez-Torres, S.; Rooney-Latham, S.; Eskalen, A.; Smith, R.J.; Gubler, W.D. Evaluation of pruning wound susceptibility and protection against fungi associated with grapevine trunk diseases. Am. J. Enol. Vitic. 2010, 61, 113–119. [Google Scholar] [CrossRef]
- Di Marco, S.; Osti, F.; Calzarano, F.; Roberti, R.; Veronesi, A.; Amalfitano, C. Effects of grapevine applications of fosetyl-aluminium formulations for downy mildew control on “esca” and associated fungi. Phytopathol. Mediterr. 2011, 50, S285–S299. [Google Scholar] [CrossRef]
- Gramaje, D.; Aroca, A.; Raposo, R.; García-Jiménez, J.; Armengol, J. Evaluation of fungicides to control Petri disease pathogens in the grapevine propagation process. Crop Prot. 2009, 28, 1091–1097. [Google Scholar] [CrossRef]
- Chervin, J.; Romeo-Oliván, A.; Fournier, S.; Puech-Pages, V.; Dumas, B.; Jacques, A.; Marti, G. Modification of Early Response of Vitis vinifera to Pathogens Relating to Esca Disease and Biocontrol Agent Vintec® Revealed By Untargeted Metabolomics on Woody Tissues. Front. Microbiol. 2022, 13, 835463. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Rashkov, I.; Naydenov, M. Eco-Friendly Hybrid PLLA/Chitosan/Trichoderma asperellum Nanomaterials as Biocontrol Dressings against Esca Disease in Grapevines. Polymers 2022, 14, 2356. [Google Scholar] [CrossRef]
- Daraignes, L.; Gerbore, J.; Yacoub, A.; Dubois, L.; Romand, C.; Zekri, O.; Roudet, J.; Chambon, P.; Fermaud, M. Efficacy of P. oligandrum affected by its association with bacterial BCAs and rootstock effect in controlling grapevine trunk diseases. Biol. Control 2018, 119, 59–67. [Google Scholar] [CrossRef]
- Kotze, C.; Van Niekerk, J.; Halleen, F.; Mostert, L.; Fourie, P. Evaluation of biocontrol agents for grapevine pruning wound protection against trunk pathogen infection. Phytopathol. Mediterr. 2011, 50, 247–263. [Google Scholar] [CrossRef]
- Alfonzo, A.; Conigliaro, G.; Torta, L.; Burruano, S.; Moschetti, G. Antagonism of Bacillus subtilis Strain AG1 against Vine Wood Fungal Pathogens. Phytopathol. Mediterr. 2009, 48, 155–158. [Google Scholar] [CrossRef]
- Haidar, R.; Roudet, J.; Bonnard, O.; Dufour, M.C.; Corio-Costet, M.F.; Fert, M.; Gautier, T.; Deschamps, A.; Fermaud, M. Screening and modes of action of antagonistic bacteria to control the fungal pathogen Phaeomoniella chlamydospora involved in grapevine trunk diseases. Microbiol. Res. 2016, 192, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Andreolli, M.; Zapparoli, G.; Angelini, E.; Lucchetta, G.; Lampis, S.; Vallini, G. Pseudomonas protegens MP12: A Plant Growth-Promoting endophytic bacterium with broad-spectrum antifungal activity against grapevine phytopathogens. Microbiol. Res. 2018, 219, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Haidar, R.; Yacoub, A.; Vallance, J.; Compant, S.; Antonielli, L.; Saad, A.; Habenstein, B.; Kauffmann, B.; Grélard, A.; Loquet, A.; et al. Bacteria associated with wood tissues of Esca-diseased grapevines: Functional diversity and synergy with Fomitiporia mediterranea to degrade wood components. Environ. Microbiol. 2021, 23, 6104–6121. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, J.M.; González-García, S.; Cobos, R.; Olego, M.Á.; Ibañez, A.; Díez-Galán, A.; Garzón-Jimeno, E.; Coque, J.J.R. Use of endophytic and rhizosphere actinobacteria from grapevine plants to reduce nursery fungal graft infections that lead to young grapevine decline. Appl. Environ. Microbiol. 2017, 83, e01564-17. [Google Scholar] [CrossRef]
- del Pilar Martínez-Diz, M.; Díaz-Losada, E.; Andrés-Sodupe, M.; Bujanda, R.; Maldonado-González, M.M.; Ojeda, S.; Yacoub, A.; Rey, P.; Gramaje, D. Field evaluation of biocontrol agents against black-foot and Petri diseases of grapevine. Pest Manag. Sci. 2021, 77, 697–708. [Google Scholar] [CrossRef]
- Bustamante, M.I.; Elfar, K.; Eskalen, A. Evaluation of the Antifungal Activity of Endophytic and Rhizospheric Bacteria against Grapevine Trunk Pathogens. Microorganisms 2022, 10, 2035. [Google Scholar] [CrossRef]
- Yacoub, A.; Gerbore, J.; Magnin, N.; Chambon, P.; Dufour, M.C.; Corio-Costet, M.F.; Guyoneaud, R.; Rey, P. Ability of Pythium oligandrum strains to protect Vitis vinifera L., by inducing plant resistance against Phaeomoniella chlamydospora, a pathogen involved in Esca, a grapevine trunk disease. Biol. Control 2016, 92, 7–16. [Google Scholar] [CrossRef]
- Yacoub, A.; Magnin, N.; Gerbore, J.; Haidar, R.; Bruez, E.; Compant, S.; Guyoneaud, R.; Rey, P. The biocontrol Root-Oomycete, Pythium oligandrum, triggers grapevine resistance and shifts in the transcriptome of the trunk pathogenic fungus, Phaeomoniella chlamydospora. Int. J. Mol. Sci. 2020, 21, 6876. [Google Scholar] [CrossRef]
- Del Frari, G.; Cabral, A.; Nascimento, T.; Boavida Ferreira, R.; Oliveira, H. Epicoccum layuense a potential biological control agent of esca-associated fungi in grapevine. PLoS ONE 2019, 14, e0213273. [Google Scholar] [CrossRef]
- Aloi, F.; Reggiori, G.; Bigot, A.; Montermini, P.; Bortolotti, R.; Nannini, F.; Osti, L.; Mugnai, L.; Di Marco, S. Remedier® (Trichoderma asperellum and Trichoderma gamsii): A new opportunity to control the esca disease complex. Five years of results of field trials in Italy. Phytopathol. Mediterr. 2015, 54, 420–436. [Google Scholar]
- Silva-Valderrama, I.; Toapanta, D.; Miccono, M.d.l.A.; Lolas, M.; Díaz, G.A.; Cantu, D.; Castro, A. Biocontrol Potential of Grapevine Endophytic and Rhizospheric Fungi Against Trunk Pathogens. Front. Microbiol. 2021, 11, 614620. [Google Scholar] [CrossRef]
- Wallis, C.M. Nutritional Niche Overlap Analysis as a method to identify potential biocontrol fungi against trunk pathogens. BioControl 2021, 66, 559–571. [Google Scholar] [CrossRef]
- Di Marco, S.; Osti, F. Applications of Trichoderma to prevent Phaeomoniella chlamydospora infections in organic nurseries. Phytopathol. Mediterr. 2007, 46, 11. [Google Scholar] [CrossRef]
- Di Marco, S.; Osti, F.; Cesari, A. Experiments on the control of Esca by Trichoderma. Phytopathol. Mediterr. 2004, 43, 108–115. [Google Scholar] [CrossRef]
- Martínez-Diz, M.d.P.; Díaz-Losada, E.; Díaz-Fernández, Á.; Bouzas-Cid, Y.; Gramaje, D. Protection of grapevine pruning wounds against Phaeomoniella chlamydospora and Diplodia seriata by commercial biological and chemical methods. Crop Prot. 2021, 143, 105465. [Google Scholar] [CrossRef]
- Geiger, A.; Karácsony, Z.; Geml, J.; Váczy, K.Z. Mycoparasitism capability and growth inhibition activity of Clonostachys rosea isolates against fungal pathogens of grapevine trunk diseases suggest potential for biocontrol. PLoS ONE 2022, 17, e0273985. [Google Scholar] [CrossRef]
- Gkikas, F.-I.; Tako, A.; Gkizi, D.; Lagogianni, C.; Markakis, E.A.; Tjamos, S.E. Paenibacillus alvei K165 and Fusarium oxysporum F2: Potential Biocontrol Agents against Phaeomoniella chlamydospora in Grapevines. Plants 2021, 10, 207. [Google Scholar] [CrossRef]
- Sparapano, L.; Bruno, G.L.; Campanella, A. Interactions between three fungi associated with esca of grapevine, and their secondary metabolites. Phytopathol. Mediterr. 2001, 40, S417–S422. [Google Scholar] [CrossRef]
- Sharfuddin, C.; Mohanka, R. In vitro antagonism of indigenous Trichoderma isolates against phytopathogen causing wilt of lentil. Int. J. Life Sci. Pharma Res. 2012, 2, 195–202. [Google Scholar]
- Badalyan, S.M.; Innocenti, G.; Garibyan, N.G. Antagonistic activity of xylotrophic mushrooms against pathogenic fungi of cereals in dual culture. Phytopathol. Mediterr. 2002, 41, 200–225. [Google Scholar] [CrossRef]
- Badalyan, S.M.; Innocenti, G.; Garibyan, N.G. Interactions between xylotrophic mushrooms and mycoparasitic fungi in dual culture. Phytopathol. Mediterr. 2004, 43, 44–48. [Google Scholar] [CrossRef]
- Koukol, O.; Mrnka, L.; Kulhankova, A.; Vosatka, M. Competition of Scleroconidioma sphagnicola with fungi decomposing spruce litter needles. Can. J. Bot. 2006, 84, 469–476. [Google Scholar] [CrossRef]
- Brglez, A.; Piškur, B.; Ogris, N. In vitro Interactions between Eutypella parasitica and some frequently isolated fungi from the wood of the dead branches of young sycamore maple (Acer pseudoplatanus). Forests 2020, 11, 1072. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Kothari, I.L.; Patel, M. Plant immunization. Indian J. Exp. Biol. 2004, 42, 244–252. [Google Scholar]
- Graniti, A.; Bruno, G.; Sparapano, L. Three-Year Observation of Grapevines Cross-Inoculated with Esca-Associated Fungi. Phytopathol. Mediterr. 2001, 40, 376–386. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S. Induce systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Morón-Ríos, A.; Gómez-Cornelio, S.; Ortega-Morales, B.O.; De la Rosa-García, S.; Partida-Martínez, L.P.; Quintana, P.; Alayón-Gamboa, A.; Cappello-García, S.; Gonzáles-Gómez, S. Interactions between abundant fungal species influence the fungal community assemblage on limestone. PLoS ONE 2017, 12, e0188443. [Google Scholar] [CrossRef]
- Bell, D.K.; Wells, H.D.; Markham, C.R. In vitro antagonism of Trichoderma spp. against six fungal plant pathogens. Phytopathology 1982, 72, 379–382. [Google Scholar] [CrossRef]
- Sosnowski, M.; Emmett, B.; Clarke, K.; Wicks, T. Susceptibility of table grapes to black spot (anthracnose) disease. Aust. N. Z. Grapegrow. Winemak. 2007, 521a, 8–11. [Google Scholar]
- Mundy, D.C.; Robertson, S.M. Evaluation of single-node plantlets as a model system for grapevine trunk diseases. N. Z. Plant Protect. 2010, 63, 167–173. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Tomaselli, E.; Pollard-Flamand, J.; Boulé, J.; Gerin, D.; Pollastro, S. Characterization of Trichoderma isolates from southern Italy, and their potential biocontrol activity against grapevine trunk disease fungi. Phytopathol. Mediterr. 2020, 59, 425–439. [Google Scholar] [CrossRef]
- Sparapano, L.; Bruno, G.; Ciccarone, C.; Graniti, A. Infection of grapevines by some fungi associated with esca. II. Interaction among Phaeoacremonium chlamydosporum, P. aleophilum and Fomitiporia punctata. Phytopathol. Mediterr. 2000, 39, 53–58. [Google Scholar] [CrossRef]
- Kunz, C.; Sellam, O.; Bertheau, Y. Purification and characterization of a chitinase from the hyperparasitic fungus Aphanocladium album. Physiol. Mol. Plant Pathol. 1992, 40, 117–131. [Google Scholar] [CrossRef]
- Biali, M.; Dinoor, A.; Eshed, N.; Kenneth, R. Aphanocladium album, a fungus inducing teliospore production in rusts. Ann. Appl. Biol. 1972, 72, 37–42. [Google Scholar] [CrossRef]
- Sasanelli, N.; Ciccarese, F.; Papajová, I. Aphanocladium album by via sub-irrigation in the control of Pyrenochaeta lycopersici and Meloidogyne incognita on tomato in a plastic-house. Helminthologia 2008, 45, 137–142. [Google Scholar] [CrossRef]
- Leoni, C.; Piancone, E.; Sasanelli, N.; Bruno, G.L.; Manzari, C.; Pesole, G.; Ceci, L.R.; Volpicella, M. Plant Health and Rhizosphere Microbiome: Effects of the Bionematicide Aphanocladium album in Tomato Plants Infested by Meloidogyne javanica. Microorganisms 2020, 8, 1922. [Google Scholar] [CrossRef]
- D’Ambrosio, G.; Cariddi, C.; Mannerucci, F.; Bruno, G.L. In vitro screening of new biological limiters against some of the main soil-borne phytopathogens. Sustainability 2022, 14, 2693. [Google Scholar] [CrossRef]
- Palizi, P.; Goltapeh, E.M.; Pourjam, E.; Safaie, N. Potential of Oyster mushrooms for the biocontrol of sugar beet nematode (Heterodera schachtii). J. Plant Prot. Res. 2009, 49, 27–33. [Google Scholar] [CrossRef]
- TariqJaveed, M.; Farooq, T.; Al-Hazmi, A.S.; Hussain, M.D.; Rehman, A.U. Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. J. Invertebr. Pathol. 2021, 183, 107626. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, L.E.; Marra, R.; Woo, L.S.; Lorito, M. Trichoderma-plant-pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. For agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Sati, O.P.; Walia, S. Comparative evaluation of two Trichoderma harzianum strains for major secondary metabolite production and antifungal activity. Nat. Prod. Res. 2015, 29, 914–920. [Google Scholar] [CrossRef]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biot. 2010, 87, 787–799. [Google Scholar] [CrossRef]
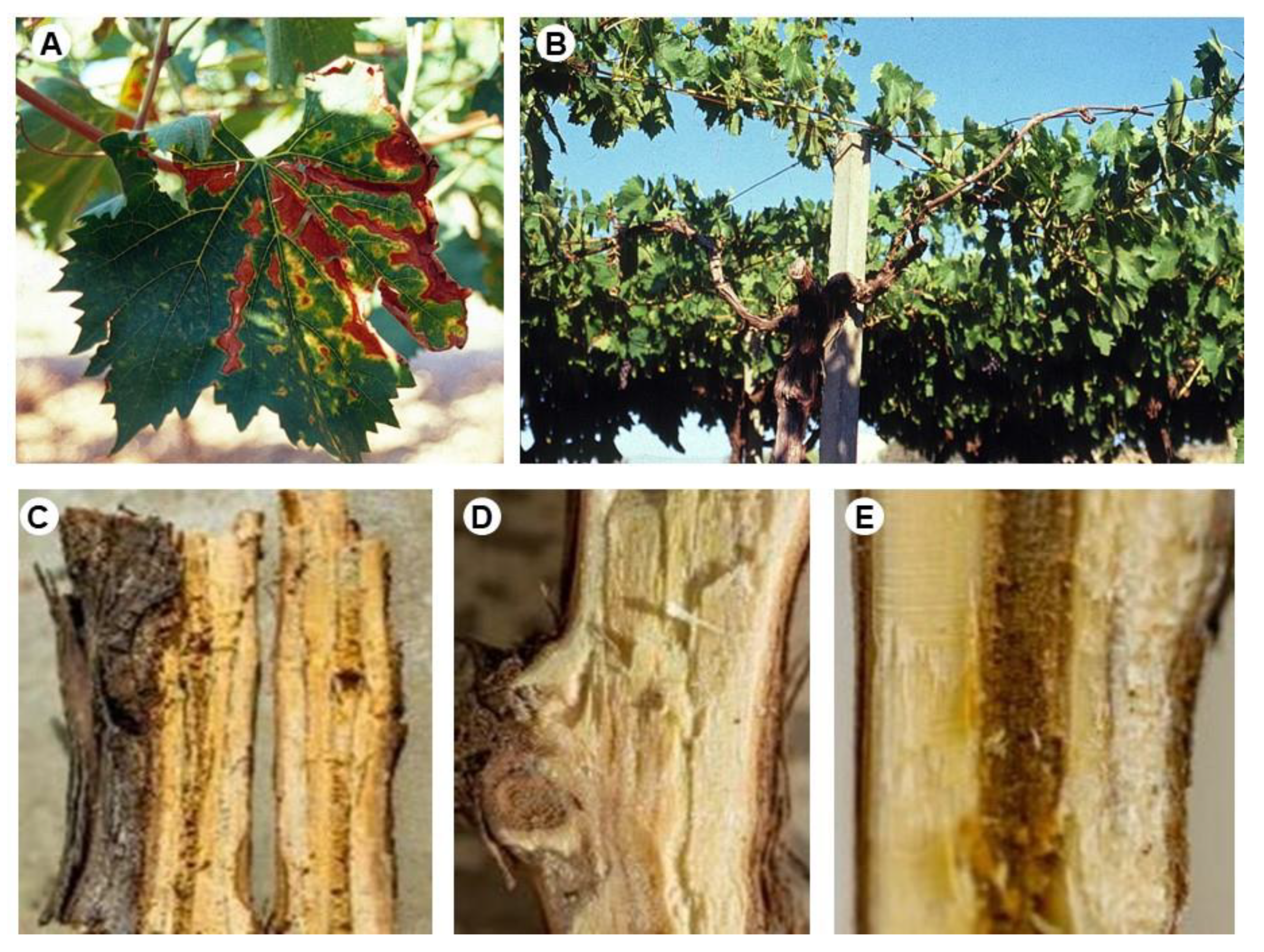
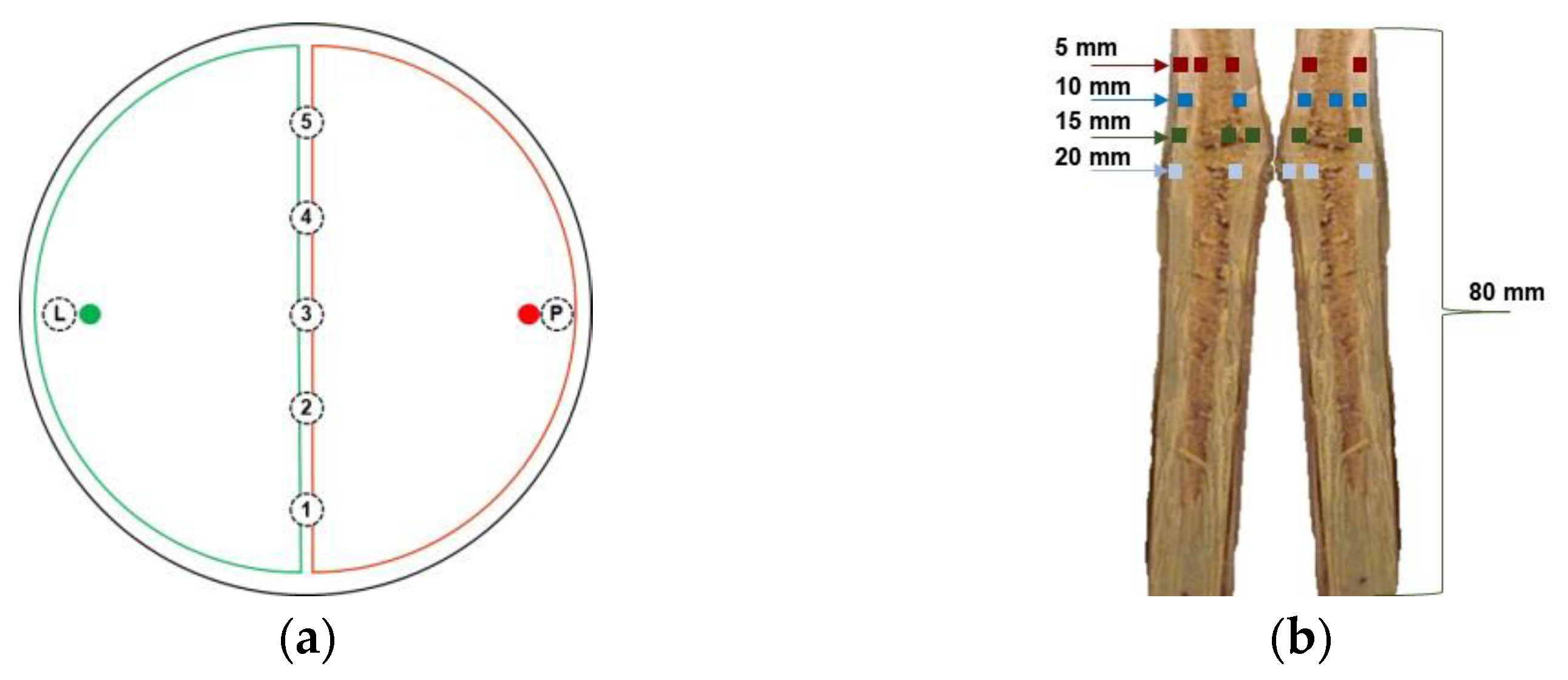
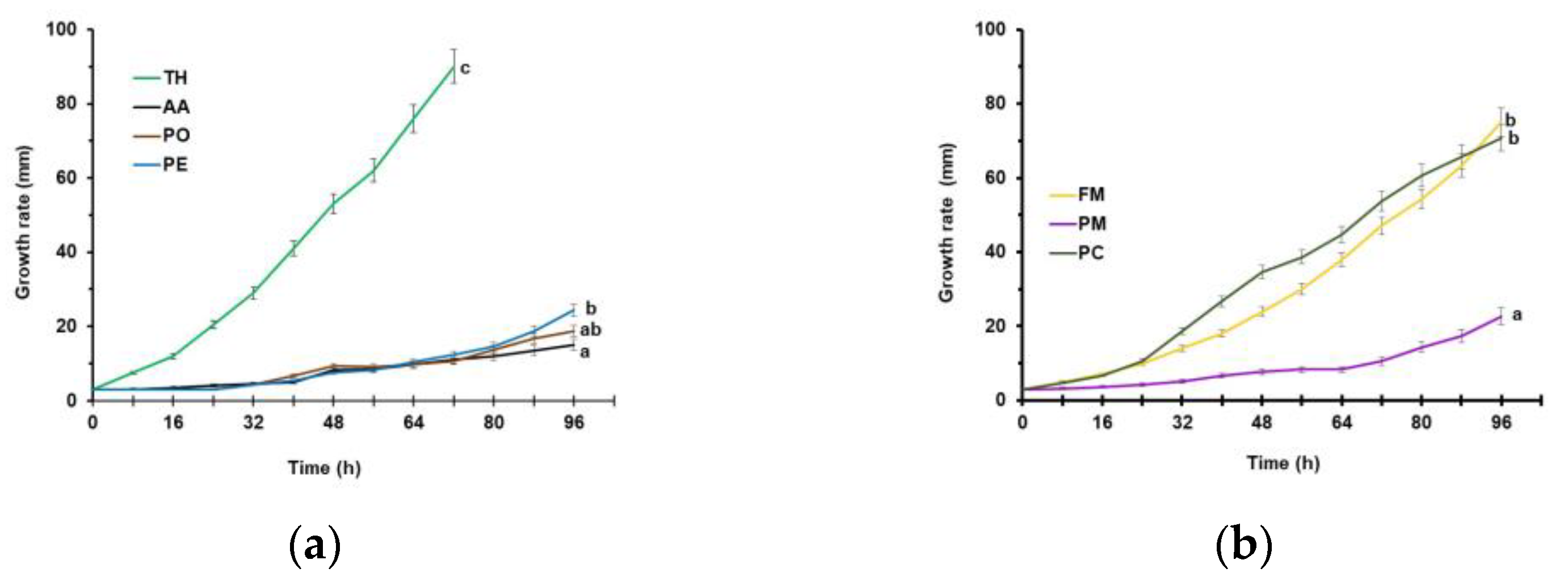



| Organism | Code | Acronym |
|---|---|---|
| Potential biological limiters | ||
| Aphanocladium album | DiSSPA 1 MX95 | AA |
| Pleurotus ostreatus | DiSSPA ALPO | PO |
| Pleurotus eryngii | DiSSPA AL142 | PE |
| Trichoderma harzianum | DiSSPA TH07.1-NC | TH |
| Target pathogens | ||
| Fomitiporia mediterranea | DiSSPA Fme22.12 | FM |
| Phaeoacremonium minimum | DiSSPA Pm22.53 | PM |
| Phaeomoniella chlamydospora | DiSSPA Pc22.65 | PC |
| Phytopathogenic | Biological Limiters 2 | |||
|---|---|---|---|---|
| Organisms | AA | PO | PE | TH |
| FM | 72 ±5.06 a | 88 ± 5.06 a | 88 ± 5.06 a | 56 ± 5.06 a |
| PM | 96 ± 5.06 b | 88 ± 5.06 a | 88 ± 5.06 a | 64 ± 5.06 b |
| PC 3 | 96 ± 5.06 b | 88 ± 7.16 a | 88 ± 7.16 a | 64 ± 7.16 b |
| PC8 3 | NT 4 | NT | NT | 64 ± 5.06 b |
| Biological Limiters | Phytopathogens 3 | |||
|---|---|---|---|---|
| FM | PM | PC 4 | PC8 4 | |
| AA | 1.09 ± 0 a | 1.79 ± 0 b | 1.79 ± 0 b | NT 5 |
| PO | * | * | * | NT |
| PE | 1.79 ± 0 b | 1.09 ± 0 a | 1.09 ± 0 a | NT |
| TH | * | * | * | * |
| Treatments | Strains | Position 4 | ||||
|---|---|---|---|---|---|---|
| Cutting Surface 3 | Longitudinal Section (mm below the surface) | |||||
| 5 | 10 | 15 | 20 | |||
| CN 5 | NFI 7 | NFI | NFI | NFI | NFI | |
| WA 6 | NFI | NFI | NFI | NFI | NFI | |
| AA | AA | 100 | 100 | NFI | NFI | NFI |
| TH | TH | 100 | 100 | NFI | NFI | NFI |
| FM | FM | 100 | 100 | NFI | NFI | NFI |
| PM | PM | 100 | 100 | NFI | NFI | NFI |
| PC | PC | 100 | 100 | NFI | NFI | NFI |
| FM5-AA | AA FM | 100 0 | 100 0 | NFI | NFI | NFI |
| PM5-AA | AA PM | 100 0 | 100 0 | NFI | NFI | NFI |
| PC5-AA | AA PC | 100 0 | 100 0 | NFI | NFI | NFI |
| FM5-TH | TH FM | 100 0 | 100 0 | NFI | NFI | NFI |
| PM5-TH | TH PM | 100 0 | 100 0 | NFI | NFI | NFI |
| PC5-TH | TH PC | 100 0 | 100 0 | NFI | NFI | NFI |
| AA5-FM | AA FM | 100 0 | 100 0 | NFI | NFI | NFI |
| AA5-PM | AA PM | 100 0 | 100 0 | NFI | NFI | NFI |
| AA5-PC | AA PC | 100 0 | 100 0 | NFI | NFI | NFI |
| TH5-FM | TH FM | 100 0 | 100 0 | NFI | NFI | NFI |
| TH5-PM | TH PM | 100 0 | 100 0 | NFI | NFI | NFI |
| TH5-PC | TH PC | 100 0 | 100 0 | NFI | NFI | NFI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannerucci, F.; D’Ambrosio, G.; Regina, N.; Schiavone, D.; Bruno, G.L. New Potential Biological Limiters of the Main Esca-Associated Fungi in Grapevine. Microorganisms 2023, 11, 2099. https://doi.org/10.3390/microorganisms11082099
Mannerucci F, D’Ambrosio G, Regina N, Schiavone D, Bruno GL. New Potential Biological Limiters of the Main Esca-Associated Fungi in Grapevine. Microorganisms. 2023; 11(8):2099. https://doi.org/10.3390/microorganisms11082099
Chicago/Turabian StyleMannerucci, Francesco, Giovanni D’Ambrosio, Nicola Regina, Domenico Schiavone, and Giovanni Luigi Bruno. 2023. "New Potential Biological Limiters of the Main Esca-Associated Fungi in Grapevine" Microorganisms 11, no. 8: 2099. https://doi.org/10.3390/microorganisms11082099
APA StyleMannerucci, F., D’Ambrosio, G., Regina, N., Schiavone, D., & Bruno, G. L. (2023). New Potential Biological Limiters of the Main Esca-Associated Fungi in Grapevine. Microorganisms, 11(8), 2099. https://doi.org/10.3390/microorganisms11082099







