Antibacterial Properties of Methanolic Leaf Extracts of Melia azedarach L. against Gram-Positive and Gram-Negative Pathogenic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Origins
2.2. Preparation of the Methanolic Extracts
2.3. HPLC-DAD Analyses
2.4. Disc Diffusion Assay
2.5. Determination of the Minimal Inhibitory and Bactericidal Concentrations
2.6. Antibiofilm Activity
2.7. Membrane Integrity
2.8. Reactive Oxygen Species Production (ROS)
2.9. Evaluation of Lipid Peroxidation
2.10. Assessment of Antioxidant Enzyme Activity
2.11. Statistical Analyses
3. Results
3.1. HPLC-DAD Analysis for Chemical Compound Identification and Quantification
3.2. Antimicrobial Activity
3.3. Anti-Biofilm Activity
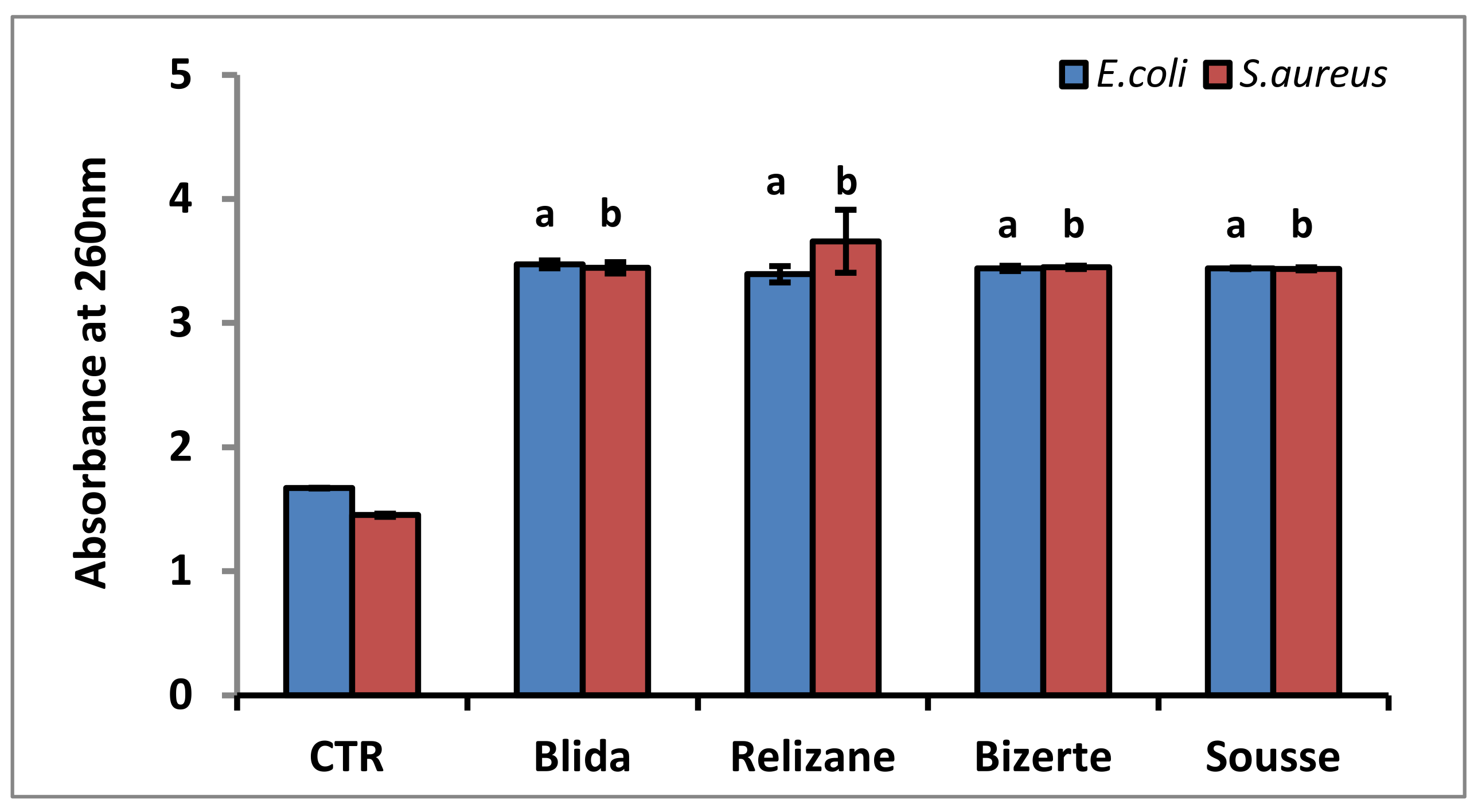
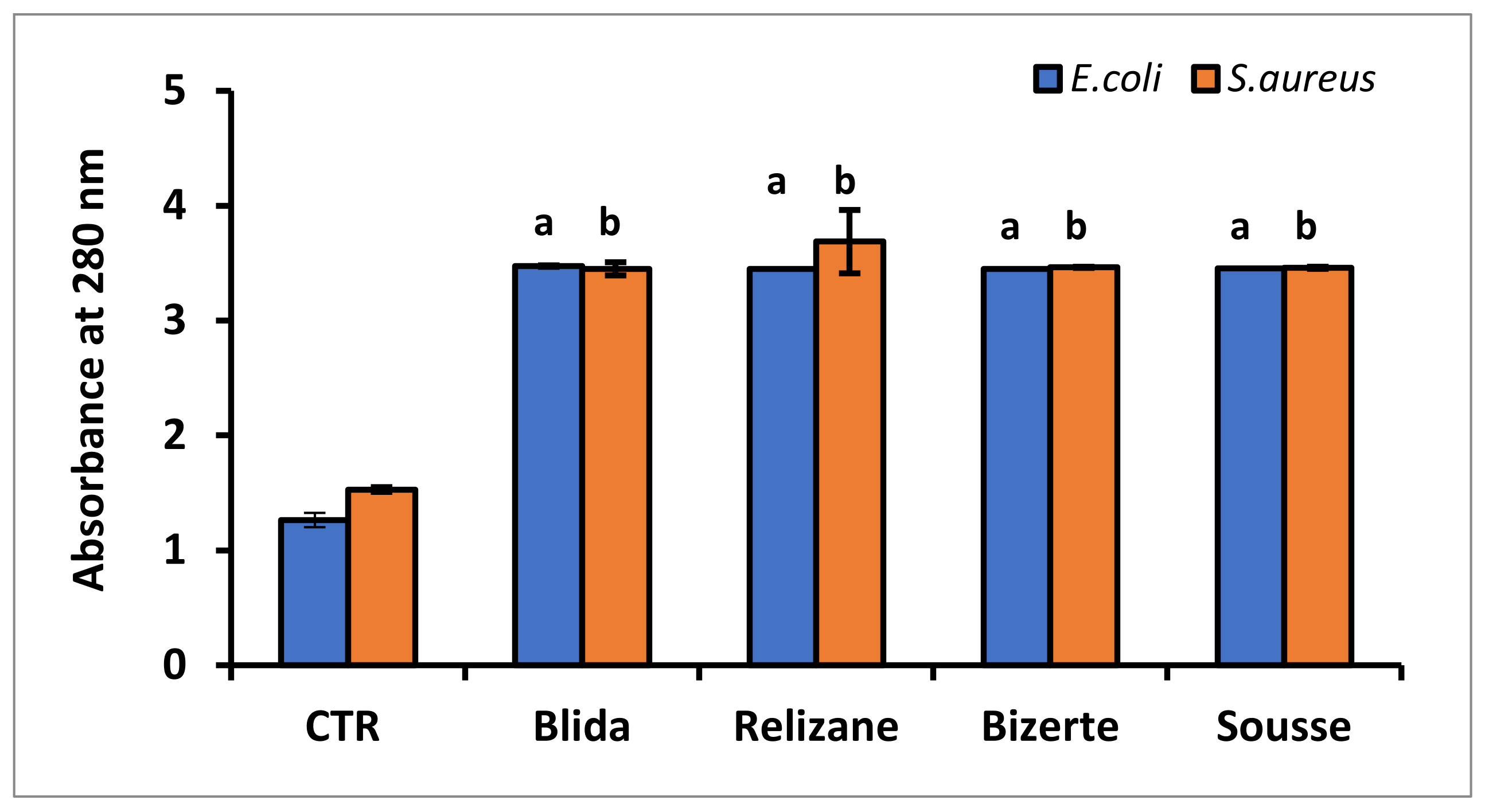
3.4. Membrane Integrity
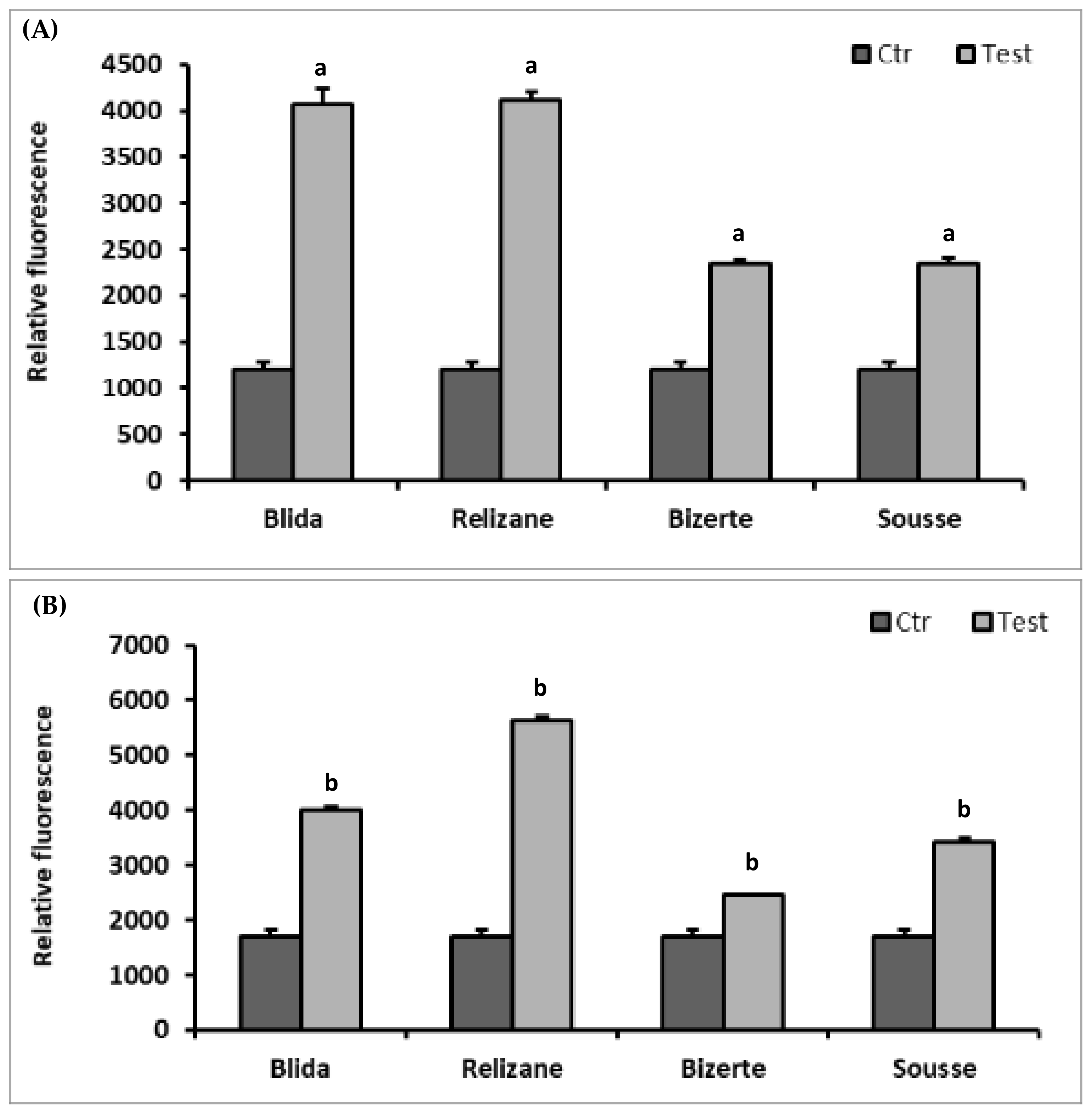
3.5. Reactive Oxygen Species (ROS)
3.6. Evaluation of MDA Lipid Peroxidation
3.7. Evaluation of Production of Superoxide Dismutase (SOD) and Catalase (CAT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nocedo-Mena, D.; Garza-González, E.; González-Ferrara, M.; Camacho-Corona, M.d.R. Antibacterial Activity of Cissus incisa Extracts against Multidrug-Resistant Bacteria. Curr. Top. Med. Chem. 2020, 20, 318–323. [Google Scholar] [CrossRef]
- Mohsen, S.; Dickinson, J.A.; Somayaji, R. Update on the adverse effects of antimicrobial therapies in community practice. Can. Fam. Physician 2020, 66, 651–659. [Google Scholar]
- Paunova-Krasteva, T.; Hemdan, B.A.; Dimitrova, P.D.; Damyanova, T.; El-Feky, A.M.; Elbatanony, M.M.; Stoitsova, S.; El-Liethy, M.A.; El-Taweel, G.E.; El Nahrawy, A.M.; et al. Hybrid Chitosan/CaO-Based Nanocomposites Doped with Plant Extracts from Azadirachta indica and Melia azedarach: Evaluation of Antibacterial and Antibiofilm Activities. Bionanoscience 2023, 13, 88–102. [Google Scholar] [CrossRef]
- Mohamed, A. Evaluation of antimicrobial activity of different solvent extracts of Saussurea lappa. World J. Pharm. Pharm. Sci. 2017, 6, 12–18. [Google Scholar] [CrossRef][Green Version]
- Vaishampayan, A.; Grohmann, E. Antimicrobials Functioning through ROS-Mediated Mechanisms: Current Insights. Microorganisms 2021, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Della Bona, A.; Nedel, F. Evaluation of Melia azedarach Extracts against Streptococcus mutans. J. Med. Food 2015, 18, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant Natural Flavonoids against Multidrug Resistant Pathogens. Adv. Sci. 2021, 8, e2100749. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, R.; Meera, R.S.; Kalidhar, S.B. Chem components Insectisidal Prop Bakyain (Melia azedarach L.)—A Review. Agric. Rev. 2003, 24, 101–115. [Google Scholar]
- Zahoor, M.; Ahmed, M.; Naz, S.; Ayaz, M. Cytotoxic, antibacterial and antioxidant activities of extracts of the bark of Melia azedarach (China Berry). Nat. Prod. Res. 2015, 29, 1170–1172. [Google Scholar] [CrossRef]
- Hemdan, B.A.; Mostafa, A.; Elbatanony, M.M.; El-Feky, A.M.; Paunova-Krasteva, T.; Stoitsova, S.; El-Liethy, M.A.; El-Taweel, G.E.; Abu Mraheil, M. Bioactive Azadirachta indica and Melia azedarach leaves extracts with anti-SARS-CoV-2 and antibacterial activities. PLoS ONE 2023, 18, e0282729. [Google Scholar] [CrossRef]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, L.; Bahrin, L.; Babii, C.; Stefan, M.; Birsa, M. Synthetic flavonoids with antimicrobial activity: A review. J. Appl. Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef]
- Lewis, K. The Science of Antibiotic Discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, F.; De Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Ong, K.S.; Cheow, Y.L.; Lee, S.M. The role of reactive oxygen species in the antimicrobial activity of pyochelin. J. Adv. Res. 2017, 8, 393–398. [Google Scholar] [CrossRef]
- Trinh, P.-C.; Thao, L.-T.-T.; Ha, H.-T.-V.; Nguyen, T. DPPH-Scavenging and Antimicrobial Activities of Asteraceae Medicinal Plants on Uropathogenic Bacteria. Liu I-M, editor. Evidence-Based Complement. Altern. Med. 2020, 2020, 7807026. [Google Scholar]
- Harzallah, H.J.; Kouidhi, B.; Flamini, G.; Bakhrouf, A.; Mahjoub, T. Chemical composition, antimicrobial potential against cariogenic bacteria and cytotoxic activity of Tunisian Nigella sativa essential oil and thymoquinone. Food Chem. 2011, 129, 1469–1474. [Google Scholar] [CrossRef]
- Prakash, O.; Nimonkar, Y.; Desai, D. A Recent Overview of Microbes and Microbiome Preservation. Indian J. Microbiol. 2020, 60, 297–309. [Google Scholar] [CrossRef]
- Magina, M.D.A.; Dalmarco, E.M.; Wisniewski, A., Jr.; Simionatto, E.L.; Dalmarco, J.B.; Pizzolatti, M.G.; Brighente, I.M.C. Chemical composition and antibacterial activity of essential oils of Eugenia species. J. Nat. Med. 2009, 63, 345–350. [Google Scholar] [CrossRef]
- Merghni, A.; Marzouki, H.; Hentati, H.; Aouni, M.; Mastouri, M. Antibacterial and antibiofilm activities of Laurus nobilis L. essential oil against Staphylococcus aureus strains associated with oral infections. Curr. Res. Transl. Med. 2016, 64, 29–34. [Google Scholar] [CrossRef]
- Abinaya, M.; Gayathri, M. Biodegradable collagen from Scomberomorus lineolatus skin for wound healing dressings and its application on antibiofilm properties. J. Cell. Biochem. 2019, 120, 15572–15584. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter Plate Assay for Assessment of Listeria monocytogenes Biofilm Formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- A Antropova, G.; Zheznyakovskaya, L.F.; Egorova, E.S.; I Okonenko, T.; Proshina, L.G. Possibilities of Using Biologically Active Substances of Iceland Moss. IOP Conf. Ser. Earth Environ. Sci. 2021, 852, 012008. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef]
- Shihad, A.; Sysa, A.; Khancheuski, M.; Gritskevitch, E.; Kvasyuk, E.; Lemiasheuski, V. Analysis of the antibacterial efficacy of modified purines derivatives. Int. J. Health Sci. 2022, 6, 11593–11607. [Google Scholar] [CrossRef]
- Martins, D.; McKay, G.; Sampathkumar, G.; Khakimova, M.; English, A.M.; Nguyen, D. Superoxide dismutase activity confers (p)ppGppmediated antibiotic tolerance to stationary-phase Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2018, 115, 9797–9802. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Acuña, L.G.; Calderón, I.L.; Elías, A.O.; Castro, M.E.; Vásquez, C.C. Expression of the yggE gene protects Escherichia coli from potassium tellurite-generated oxidative stress. Arch. Microbiol. 2009, 191, 473–476. [Google Scholar] [CrossRef]
- Rjiba-Touati, K.; Ayed-Boussema, I.; Guedri, Y.; Achour, A.; Bacha, H.; Abid-Essefi, S. Effect of recombinant human erythropoietin on mitomycin C-induced oxidative stress and genotoxicity in rat kidney and heart tissues. Hum. Exp. Toxicol. 2015, 35, 53–62. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Zimmermann, A.; Kainz, K.; Pietrocola, F.; Chen, G.; Maglioni, S.; Schiavi, A.; Nah, J.; Mertel, S.; Beuschel, C.B.; et al. The flavonoid 4,4’-dimethoxychalcone promotes autophagy-dependent longevity across species. Nat. Commun. 2019, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Vijayanands, S.; Wesely, E.G. Phytochemical studies of Melia azedrach and Murryaya koeingi. Int. J. Pharma Sci. Res. 2011, 2, 1298–1302. [Google Scholar]
- Jafari, S.; Saeidnia, S.; Hajimehdipoor, H.; Ardekani, M.R.S.; Faramarzi, M.A.; Hadjiakhoondi, A.; Khanavi, M. Cytotoxic evaluation of Melia azedarach in Comparison with, Azadirachta indica and its phytochemical investigation. Daru 2013, 21, 37. [Google Scholar] [CrossRef]
- Salib, J.Y.; Michael, H.N.; El-Nogoumy, S.I. New lactoyl glycoside quercetin from Melia azedarach leaves. Chem. Nat. Compd. 2008, 44, 13–15. [Google Scholar] [CrossRef]
- Aoudia, H.; Oomah, B.D.; Zaidi, F.; Zaidi-Yahiaoui, R.; Drover, J.C.G.; Harrison, J.E. Phenolics, antioxidant and anti-inflammatory activities of Melia azedarach extracts. Int. J. Appl. Res. Nat. Prod. 2013, 6, 19–29. [Google Scholar]
- Zhao, L.; Ji, Z.; Li, K.; Wang, B.; Zeng, Y.; Tian, S. HPLC-DAD analysis of Hyssopus Cuspidatus Boriss extract and mensuration of its antioxygenation property. BMC Complement. Med. Ther. 2020, 20, 228. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Zhang, L.; Wan, S.; Li, C.; Liu, S. Solvents effect on phenolics, iridoids, antioxidant activity, antibacterial activity, and pancreatic lipase inhibition activity of noni (Morinda citrifolia L.) fruit extract. Food Chem. 2022, 377, 131989. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Abraham, S.V.P.I.; Palani, A.; Ramaswamy, B.R.; Shunmugiah, K.P.; Arumugam, V.R. Antiquorum Sensing and Antibiofilm Potential of Capparis spinosa. Arch. Med Res. 2011, 42, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Saeloh, D.; Visutthi, M. Efficacy of Thai Plant Extracts for Antibacterial and Anti-Biofilm Activities against Pathogenic Bacteria. Antibiotics 2021, 10, 1470. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm Activity of Plant Polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Ren, X.; An, P.; Zhai, X.; Wang, S.; Kong, Q. The antibacterial mechanism of pterostilbene derived from xinjiang wine grape: A novel apoptosis inducer in Staphyloccocus aureus and Escherichia coli. LWT 2019, 101, 100–106. [Google Scholar] [CrossRef]
- Ouyang, J.; Feng, W.; Lai, X.; Chen, Y.; Zhang, X.; Rong, L.; Sun, F.; Chen, Y. Quercetin inhibits Pseudomonas aeruginosa biofilm formation via the vfr-mediated lasIR system. Microb. Pathog. 2020, 149, 104291. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Koorbanally, N.; Kudanga, T. Laccase-mediated modification of isorhamnetin improves antioxidant and antibacterial activities. Process. Biochem. 2022, 112, 53–61. [Google Scholar] [CrossRef]
- Esmail, A.; Chahboun, N.; Mennane, Z.; Amiyare, R.; Abed, H.; Barrahi, M.; Qebibo, A.; Ouhssine, M.; Berny, E.H. Study of antimicrobial activity of olive mille wastewater (OMWW) from Fez Boulman against some pathogenic strains. J. Mater. Environ. 2015, 6, 869–876. [Google Scholar]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur. Food Res. Technol. 2014, 238, 589–596. [Google Scholar] [CrossRef]
- Taleb, H.; Maddocks, S.E.; Morris, R.K.; Kanekanian, A.D. The Antibacterial Activity of Date Syrup Polyphenols against S. aureus and E. coli. Front. Microbiol. 2016, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Ghosh, D.; Bhattacharya, S.; Sarkar, S.; Karmakar, P.; Koley, H.; Gachhui, R. Antibacterial activity of polyphenolic fraction of Kombucha against Vibrio cholerae: Targeting cell membrane. Lett. Appl. Microbiol. 2018, 66, 145–152. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Vadivel, V. Catechin isolated from cashew nut shell exhibits antibacterial activity against clinical isolates of MRSA through ROS-mediated oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 8279–8297. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Godber, S.S. Noninvasive measures of oxidative stress status in humans. Free Radic. Biol. Med. 1991, 10, 177–184. [Google Scholar] [CrossRef]
- Van Acker, H.; Gielis, J.; Acke, M.; Cools, F.; Cos, P.; Coenye, T. The Role of Reactive Oxygen Species in Antibiotic-Induced Cell Death in Burkholderia cepacia Complex Bacteria. PLoS ONE 2016, 11, e0159837. [Google Scholar] [CrossRef]
- Gasser, V.; Baco, E.; Cunrath, O.; August, P.S.; Perraud, Q.; Zill, N.; Schleberger, C.; Schmidt, A.; Paulen, A.; Bumann, D.; et al. Catechol siderophores repress the pyochelin pathway and activate the enterobactin pathway in Pseudomonas aeruginosa: An opportunity for siderophore–antibiotic conjugates development. Environ. Microbiol. 2016, 18, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.-Y.; Li, J.; Hou, Y.-N.; Ma, K.; Yao, G.-D.; Liu, W.-W.; Hayashi, T.; Itoh, K.; Tashiro, S.-I.; Onodera, S.; et al. Concentration-dependent dual effects of silibinin on kanamycin-induced cells death in Staphylococcus aureus. Biomed. Pharmacother. 2018, 102, 782–791. [Google Scholar] [CrossRef] [PubMed]
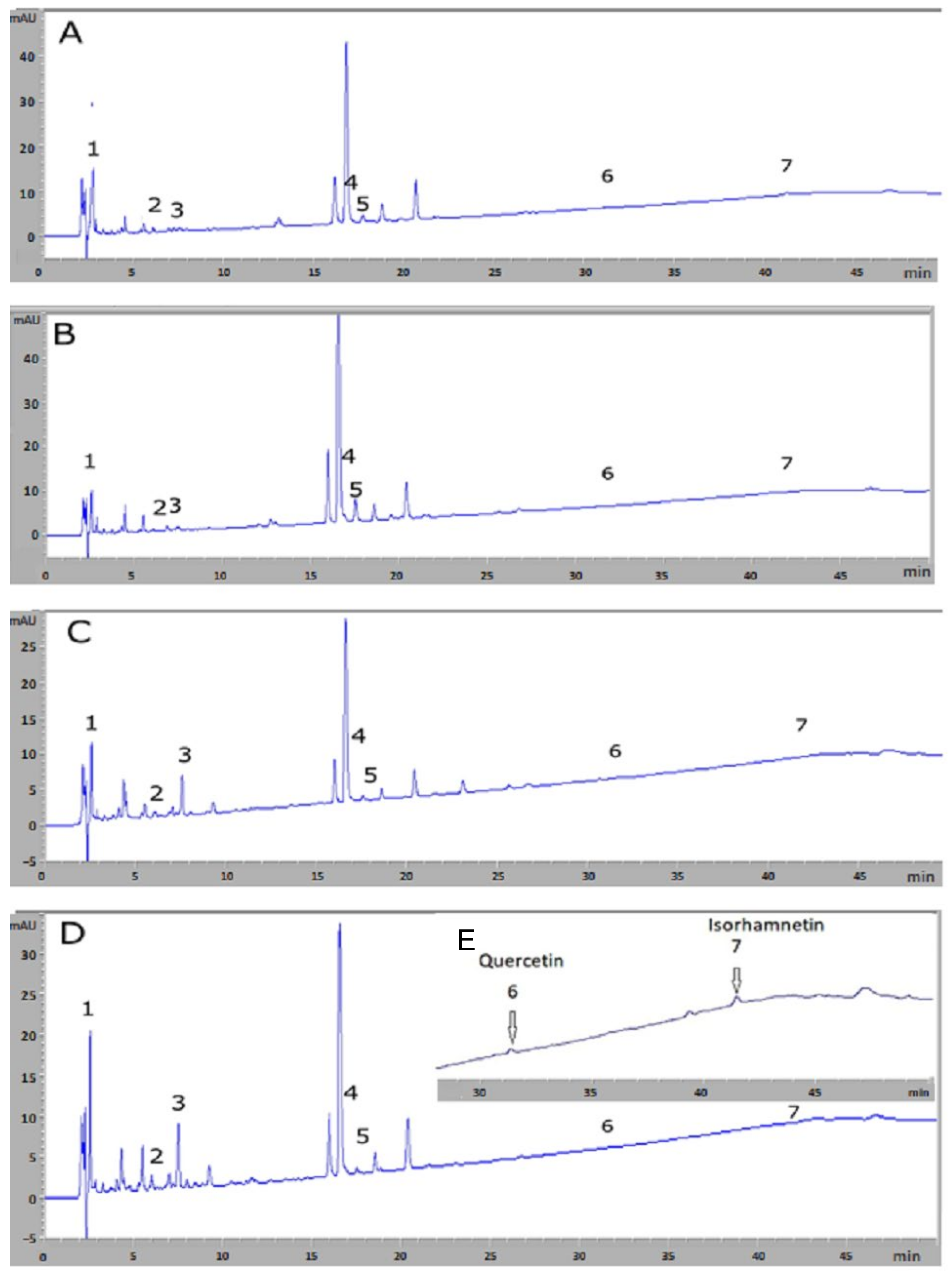
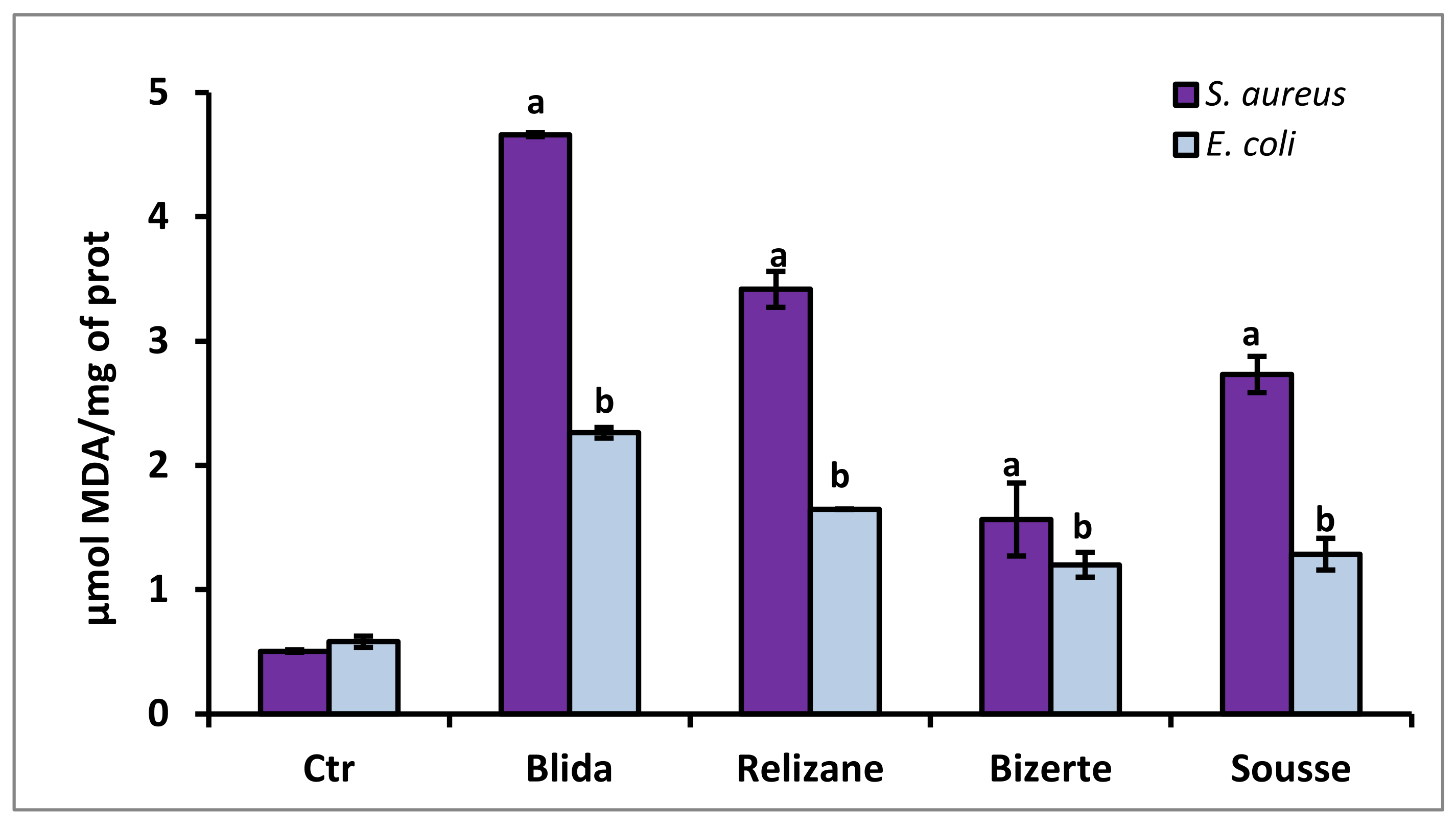

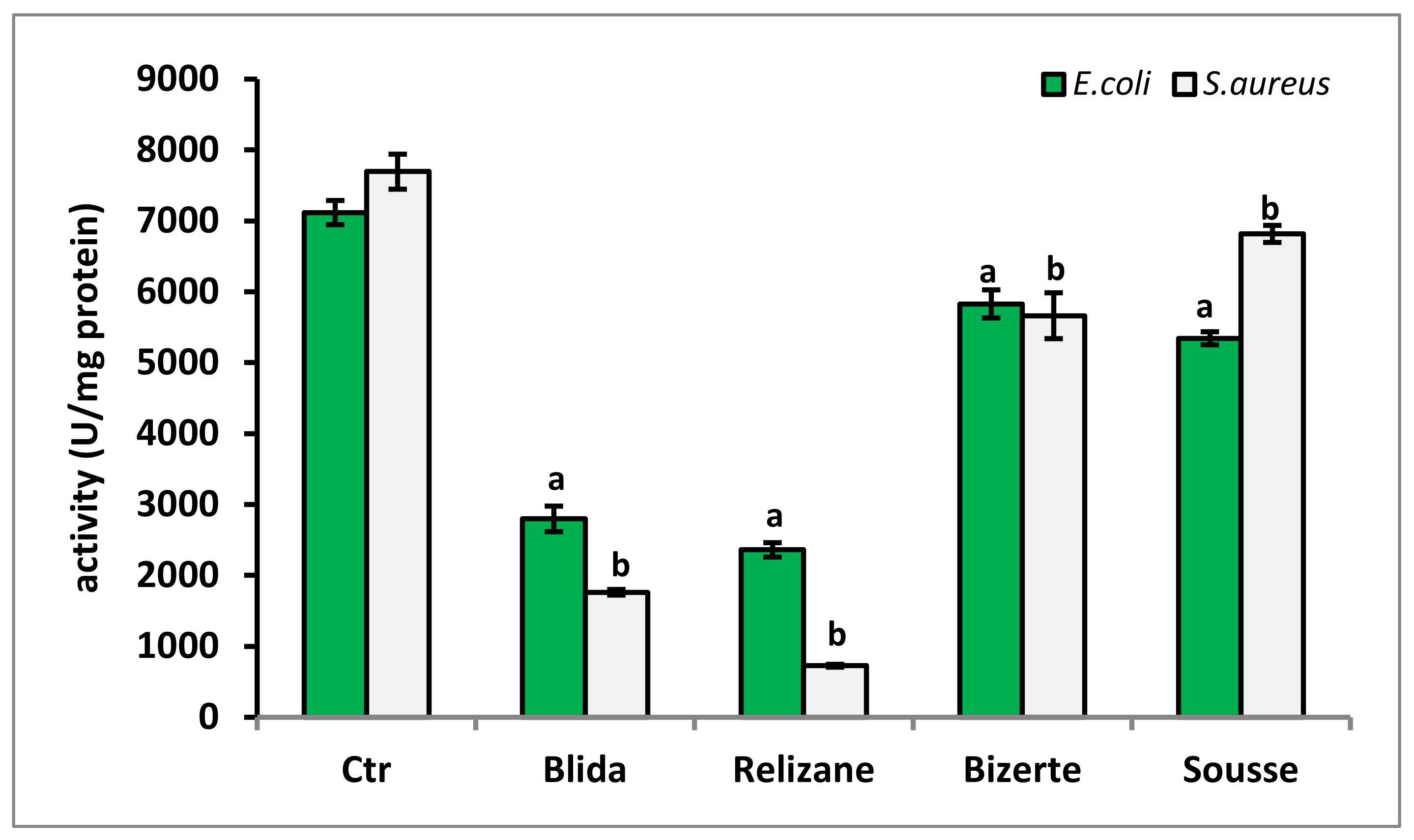
| Peak | Rt (min) | Chemical Compounds | Bizerte (µg/100 mL) | Sousse (µg/100 mL) | Relizane (µg/100 mL) | Blida (µg/100 mL) |
|---|---|---|---|---|---|---|
| 1 | 2.64 | Gallic acid | 6172.11 ± 34.48 a,b,c | 5121.29 ± 10.06 d,e | 6536.38 ± 20.98 f | 7601.04 ± 45.51 |
| 2 | 6.06 | Chlorogenic acid | 1257.62 ± 33.92 | 827.32 ± 16.99 d,e | 1527.56 ± 47.60 | 1529.57 ± 57.21 |
| 3 | 7.36 | Caffeic acid | 1298.99 ± 47.29 a,b,c | 1493.66 ± 50.78 d,e | 3761.98 ± 32.54 f | 3395.71 ± 64.66 |
| 4 | 16.64 | Hyperoside | 182.08 ± 1.67 a,b | 371.45 ± 1.06 d,e | 72.83 ± 1.05 f | 179.85 ± 1.50 |
| 5 | 17.70 | Isoquercetin | 429.44 ± 0.48 a,b,c | 1054.13 ± 0.60 d,e | 140.43 ± 1.55 f | 148.71 ± 1.02 |
| 6 | 31.53 | Quercetin | Trace | 22.22 ± 0.71 d,e | 11.85 ± 0.90 f | 5.38 ± 0.96 |
| 7 | 41.79 | Isorhamnetin | 7.41 ± 0.59 a,b,c | 10.55 ± 0.57 d,e | 19.61 ± 1.17 f | 16.22 ± 0.78 |
| Origin | Concentration | S. aureus | S. epidermidis | E. coli | P. aeruginosa |
|---|---|---|---|---|---|
| Blida | 150 (mg/mL) | 13.5 ± 0.70 ** | 13 ± 0.04 *** | 11.5 ± 0.7 ** | 12.5 ± 0.35 |
| 300 (mg/mL) | 16 ± 0.08 | 16.5 ± 0.70 | 13.75 ± 0.35 | 13.5 ± 0.70 | |
| Relizane | 150 (mg/mL) | 9.75 ± 1.06 * | 14.25 ± 1.06 *** | 13 ± 0.06 *** | 9 ± 0.02 * |
| 300 (mg/mL) | 12.5 ± 0.70 | 19 ± 0.03 | 16.7 ± 0.70 | 10.5 ± 0.70 | |
| Bizerte | 150 (mg/mL) | 12.25 ± 0.35 ** | 9.5 ± 0.70 ** | 12.75 ± 0.35 *** | 8.5 ± 0.70 ** |
| 300 (mg/mL) | 15.5 ± 0.70 | 12.8 ± 0.28 | 14.5 ± 0.70 | 11 ± 0.02 | |
| Sousse | 150 (mg/mL) | 9.5 ± 0.70 * | 10.5 ± 0.70 ** | 10 ± 0.08 ** | 8 ± 0.05 ** |
| 300 (mg/mL) | 11.75 ± 0.35 | 13 ± 0.07 | 12 ± 0.09 | 9.5 ± 0.70 | |
| GEN | (10 µg) | 24 | 24 | 25 | 22 |
| Origin | MIC/MBC (mg/mL) | Bacterial Strains | |||
|---|---|---|---|---|---|
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | ||
| Blida | MIC | 31.25 | 31.25 | 31.25 | 125 |
| MBC | >250 | >250 | >250 | >250 | |
| Relizane | MIC | 62.5 | 31.25 | 31.25 | 125 |
| MBC | >250 | >250 | >250 | >250 | |
| Bizerte | MIC | 62.5 | 62.5 | 31.25 | 125 |
| MBC | >250 | >250 | >250 | >250 | |
| Sousse | MIC | 125 | 62.5 | 62.5 | 125 |
| MBC | >250 | >250 | >250 | >250 | |
| Origin | Concentration | Biofilm Eradication (%) | |||
|---|---|---|---|---|---|
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | ||
| Blida | MIC | 21.46 ± 2 a,b | 19.01 ± 2.3 a,b | 17.73 ± 0.7 a,b | 16.22 ± 1.4 a,b |
| MIC × 2 | 46.23± 1.4 c | 42.05 ± 1.5 c | 28.47 ± 0.5 c | 34.15 ± 2.7 c | |
| MIC × 4 | 65.52 ± 1.9 | 53.49 ± 3 | 47.55 ± 1.5 | 44.65 ± 2 | |
| Relizane | MIC | 15.47 ± 2.7 a,b | 21.12 ± 3.2 a,b | 32.66 ± 1.2 a,b | 11.79 ± 4.9 a,b |
| MIC × 2 | 50.47 ± 2.3 c | 41.54 ± 3.2 c | 40.73 ± 1.3 c | 51.4 ± 4.3 c | |
| MIC × 4 | 72.17 ± 2.2 | 53.72 ± 0.4 | 60.26 ± 4.7 | 55.56 ± 0.2 | |
| Bizerte | MIC | 17.15 ± 0.8 a,b | 22.02 ± 2.4 a,b | 8.37 ± 1.3 a,b | 6.29 ± 2.7 a,b |
| MIC × 2 | 39.05 ± 0.6 c | 28.11 ± 2.0 c | 22.97 ± 1.8 c | 23.28 ± 2.4 c | |
| MIC × 4 | 47.22 ± 0.6 | 36.61 ± 0.5 | 33.37 ± 1.7 | 50.51 ± 2.1 | |
| Sousse | MIC | 16.13 ± 1.1 a,b | 17.4 ± 7.6 a,b | 23.25 ± 2.1 a,b | 26.65 ± 2.5 a,b |
| MIC × 2 | 34.65 ± 1.1 c | 26.29 ± 7.7 c | 35.08 ± 0.2 c | 46.68 ± 2.7 c | |
| MIC × 4 | 49.38 ± 1 | 57.4 ± 1.9 | 47.56 ± 1.5 | 59.95 ± 0.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touzout, S.N.; Merghni, A.; Laouani, A.; Boukhibar, H.; Alenazy, R.; Alobaid, A.; Alenazy, M.; Ben-Attia, M.; Saguem, K.; El-Bok, S. Antibacterial Properties of Methanolic Leaf Extracts of Melia azedarach L. against Gram-Positive and Gram-Negative Pathogenic Bacteria. Microorganisms 2023, 11, 2062. https://doi.org/10.3390/microorganisms11082062
Touzout SN, Merghni A, Laouani A, Boukhibar H, Alenazy R, Alobaid A, Alenazy M, Ben-Attia M, Saguem K, El-Bok S. Antibacterial Properties of Methanolic Leaf Extracts of Melia azedarach L. against Gram-Positive and Gram-Negative Pathogenic Bacteria. Microorganisms. 2023; 11(8):2062. https://doi.org/10.3390/microorganisms11082062
Chicago/Turabian StyleTouzout, Soraya Naila, Abderrahmen Merghni, Aicha Laouani, Halima Boukhibar, Rawaf Alenazy, Abdulmohsen Alobaid, Mustafa Alenazy, Mossadok Ben-Attia, Khaled Saguem, and Safia El-Bok. 2023. "Antibacterial Properties of Methanolic Leaf Extracts of Melia azedarach L. against Gram-Positive and Gram-Negative Pathogenic Bacteria" Microorganisms 11, no. 8: 2062. https://doi.org/10.3390/microorganisms11082062
APA StyleTouzout, S. N., Merghni, A., Laouani, A., Boukhibar, H., Alenazy, R., Alobaid, A., Alenazy, M., Ben-Attia, M., Saguem, K., & El-Bok, S. (2023). Antibacterial Properties of Methanolic Leaf Extracts of Melia azedarach L. against Gram-Positive and Gram-Negative Pathogenic Bacteria. Microorganisms, 11(8), 2062. https://doi.org/10.3390/microorganisms11082062






