Kinetics of SARS-CoV-2 Spike Antibodies after the Second and Third Dose of the BNT162b2 COVID-19 Vaccine and Association with Epidemiological Characteristics and Breakthrough Infection in a Cohort Study of Healthcare Workers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Total Antibody Detection against SARS-CoV-2
2.3. Neutralization Assays against SARS-CoV-2 Wild Type and Omicron Variant
2.4. Statistical Methods
3. Results
3.1. Study Population
3.2. Breakthrough Infections
3.3. Antibody Kinetics after Second and Third BNT162b2 COVID-19 Vaccine
3.4. Association of Antibody Levels with Epidemiological Data
4. Discussion
5. Public Health Implications of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Excler, J.-L.; Saville, M.; Privor-Dumm, L.; Gilbert, S.; Hotez, P.J.; Thompson, D.; Abdool-Karim, S.; Kim, J.H. Factors, Enablers and Challenges for COVID-19 Vaccine Development. BMJ Glob. Health 2023, 8, e011879. [Google Scholar] [CrossRef]
- Science Brief: COVID-19 Vaccines and Vaccination. Available online: https://pubmed.ncbi.nlm.nih.gov/34009769/ (accessed on 5 July 2023).
- Heinz, F.X.; Stiasny, K. Distinguishing Features of Current COVID-19 Vaccines: Knowns and Unknowns of Antigen Presentation and Modes of Action. NPJ Vaccines 2021, 6, 104. [Google Scholar] [CrossRef]
- Cari, L.; Naghavi Alhosseini, M.; Mencacci, A.; Migliorati, G.; Nocentini, G. Differences in the Expression Levels of SARS-CoV-2 Spike Protein in Cells Treated with MRNA-Based COVID-19 Vaccines: A Study on Vaccines from the Real World. Vaccines 2023, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-Spike Protein Interaction in COVID-19: Get the Ball Rolling with a Novel Receptor and Therapeutic Target. Sci. Total Environ. 2022, 808, 152072. [Google Scholar] [CrossRef] [PubMed]
- Petrović, V.; Vuković, V.; Patić, A.; Marković, M.; Ristić, M. Immunogenicity of BNT162b2, BBIBP-CorV, Gam-COVID-Vac and ChAdOx1 NCoV-19 Vaccines Six Months after the Second Dose: A Longitudinal Prospective Study. Vaccines 2022, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Michos, A.; Tatsi, E.-B.; Filippatos, F.; Dellis, C.; Koukou, D.; Efthymiou, V.; Kastrinelli, E.; Mantzou, A.; Syriopoulou, V. Association of Total and Neutralizing SARS-CoV-2 Spike -Receptor Binding Domain Antibodies with Epidemiological and Clinical Characteristics after Immunization with the 1st and 2nd Doses of the BNT162b2 Vaccine. Vaccine 2021, 39, 5963–5967. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Sharma, N.; Anwer, M.K.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Bhatia, S.; et al. There Is Nothing Exempt from the Peril of Mutation—The Omicron Spike. Biomed. Pharmacother. 2022, 148, 112756. [Google Scholar] [CrossRef]
- Hosseinian, S.; de Assis, R.; Khalil, G.; Luu, M.K.; Jain, A.; Horvath, P.; Nakajima, R.; Palma, A.M.; Hoang, A.; Razzak, E.; et al. Analysis and Comparison of SARS-CoV-2 Variant Antibodies and Neutralizing Activity for 6 Months after a Booster MRNA Vaccine in a Healthcare Worker Population. Front. Immunol. 2023, 14, 1166261. [Google Scholar] [CrossRef]
- Lipsitch, M.; Krammer, F.; Regev-Yochay, G.; Lustig, Y.; Balicer, R.D. SARS-CoV-2 Breakthrough Infections in Vaccinated Individuals: Measurement, Causes and Impact. Nat. Rev. Immunol. 2022, 22, 57–65. [Google Scholar] [CrossRef]
- Tan, S.T.; Kwan, A.T.; Rodríguez-Barraquer, I.; Singer, B.J.; Park, H.J.; Lewnard, J.A.; Sears, D.; Lo, N.C. Infectiousness of SARS-CoV-2 Breakthrough Infections and Reinfections during the Omicron Wave. Nat. Med. 2023, 29, 358–365. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Raw, R.K.; Rees, J.; Chadwick, D.R. Increased Adverse Events Following Third Dose of BNT162b2/Pfizer Vaccine in Those with Previous COVID-19, but Not with Concurrent Influenza Vaccine. PLoS Glob. Public Health 2023, 3, e0001053. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, R.; Isomura, E.T.; Matsunaga, K.; Kubota, K. Young Age, Female Sex, and No Comorbidities Are Risk Factors for Adverse Reactions after the Third Dose of BNT162b2 COVID-19 Vaccine against SARS-CoV-2: A Prospective Cohort Study in Japan. Vaccines 2022, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Mofaz, M.; Yechezkel, M.; Guan, G.; Brandeau, M.L.; Patalon, T.; Gazit, S.; Yamin, D.; Shmueli, E. Self-Reported and Physiologic Reactions to Third BNT162b2 MRNA COVID-19 (Booster) Vaccine Dose. Emerg. Infect. Dis. 2022, 28, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Mat Ripen, A.; Leong, C.T.; Lee, J.V.; Yen, C.H.; Chand, A.K.; Koh, K.; Abdul Rahim, N.A.B.; Gokilavanan, V.; Mohamed, N.N.E.B.; et al. COVID-19 Breakthrough Infections and Humoral Immune Response among BNT162b2 Vaccinated Healthcare Workers in Malaysia. Emerg. Microbes Infect. 2022, 11, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Cortese, P.; Amato, F.; Davino, A.; De Franchis, R.; Esposito, S.; Zollo, I.; Di Domenico, M.; Solito, E.; Zarrilli, F.; Gentile, L.; et al. The Immune Response to SARS-CoV-2 Vaccine in a Cohort of Family Pediatricians from Southern Italy. Cells 2023, 12, 1447. [Google Scholar] [CrossRef]
- Santoro, A.; Capri, A.; Petrone, D.; Colavita, F.; Meschi, S.; Matusali, G.; Mizzoni, K.; Notari, S.; Agrati, C.; Goletti, D.; et al. SARS-CoV-2 Breakthrough Infections According to the Immune Response Elicited after MRNA Third Dose Vaccination in COVID-19-Naïve Hospital Personnel. Biomedicines 2023, 11, 1247. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wu, M.; Harvey, R.; Schmitt, A.M.; Tippu, Z.; Shum, B.; Farag, S.; Rogiers, A.; et al. Omicron Neutralising Antibodies after Third COVID-19 Vaccine Dose in Patients with Cancer. Lancet 2022, 399, 905–907. [Google Scholar] [CrossRef]
- Belik, M.; Jalkanen, P.; Lundberg, R.; Reinholm, A.; Laine, L.; Väisänen, E.; Skön, M.; Tähtinen, P.A.; Ivaska, L.; Pakkanen, S.H.; et al. Comparative Analysis of COVID-19 Vaccine Responses and Third Booster Dose-Induced Neutralizing Antibodies against Delta and Omicron Variants. Nat. Commun. 2022, 13, 2476. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Meister, T.; Kolde, A.; Fischer, K.; Pisarev, H.; Kolde, R.; Kalda, R.; Suija, K.; Tisler, A.; Uusküla, A. A Retrospective Cohort Study of Incidence and Risk Factors for Severe SARS-CoV-2 Breakthrough Infection among Fully Vaccinated People. Sci. Rep. 2023, 13, 8531. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Cosma, C.; Bonfante, F.; Della Rocca, F.; Barbaro, F.; Santarossa, C.; Dall’olmo, L.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. Neutralizing Antibody Titers Six Months after Comirnaty Vaccination: Kinetics and Comparison with SARS-CoV-2 Immunoassays. Clin. Chem. Lab. Med. 2021, 60, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Lo Sasso, B.; Agnello, L.; Giglio, R.V.; Gambino, C.M.; Ciaccio, A.M.; Vidali, M.; Ciaccio, M. Longitudinal Analysis of Anti-SARS-CoV-2 S-RBD IgG Antibodies before and after the Third Dose of the BNT162b2 Vaccine. Sci. Rep. 2022, 12, 8679. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Balado, M.M.; Bulnes-Ramos, Á.; Garrido-Rodríguez, V.; Olivas-Martínez, I.; Lozano, C.; González-Escribano, M.F.; Leal, M.; Pacheco, Y.M. Longitudinal Age Differences in Humoral Responses to the COVID-19 Vaccine in the Elderly Are Lost after the Third Dose. J. Infect. 2023, 86, 154–225. [Google Scholar] [CrossRef]
- Igari, H.; Asano, H.; Murata, S.; Yoshida, T.; Kawasaki, K.; Kageyama, T.; Ikeda, K.; Koshikawa, H.; Okuda, Y.; Urushihara, M.; et al. Antibody Responses and SARS-CoV-2 Infection after BNT162b2 MRNA Booster Vaccination among Healthcare Workers in Japan. J. Infect. Chemother. 2022, 28, 1483–1488. [Google Scholar] [CrossRef]
- Nomura, Y.; Sawahata, M.; Nakamura, Y.; Kurihara, M.; Koike, R.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; et al. Age and Smoking Predict Antibody Titres at 3 Months after the Second Dose of the BNT162b2 COVID-19 Vaccine. Vaccines 2021, 9, 1042. [Google Scholar] [CrossRef]
- Yamamoto, S.; Oshiro, Y.; Inamura, N.; Nemoto, T.; Horii, K.; Okudera, K.; Konishi, M.; Ozeki, M.; Mizoue, T.; Sugiyama, H.; et al. Durability and Determinants of Anti-SARS-CoV-2 Spike Antibodies Following the Second and Third Doses of MRNA COVID-19 Vaccine. Clin. Microbiol. Infect. 2023, in press. [Google Scholar] [CrossRef]
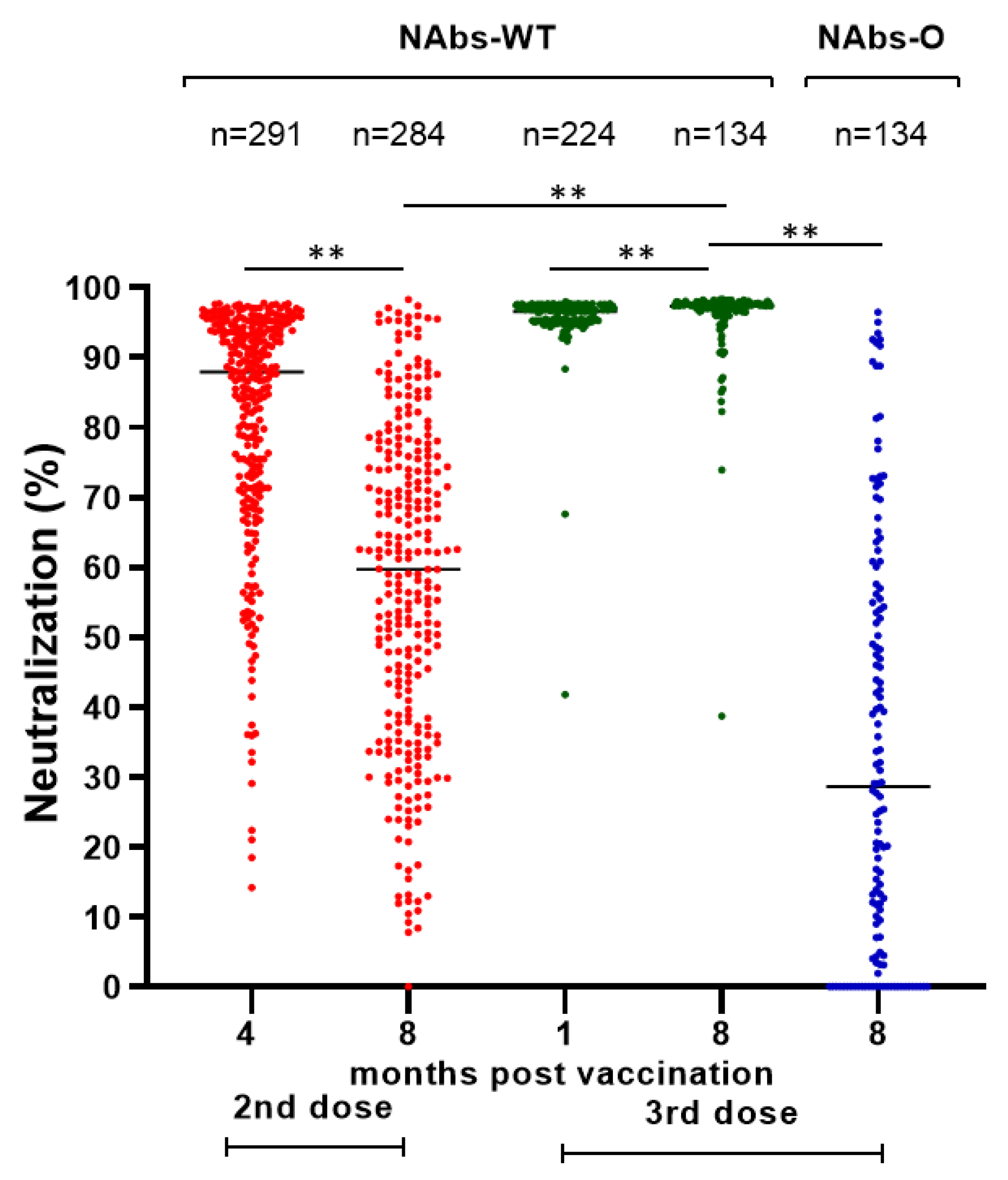
| Breakthrough Infection (BI) | After the Second Dose | After the Third Dose | Study Population n = 486 | ||
|---|---|---|---|---|---|
| No n = 463 | Yes n = 23 | No n = 294 | Yes n = 169 | ||
| Age (years) | 48.8 (39–56.3) | 41 (31–49) | 52 (42–58) | 43 (35–53) | 49 (38–56) |
| BMI (kg/m3) | 24.4 (22–27.4) | 23.1 (20.8–25.7) | 24.4 (22–27.4) | 24.3 (22–27.4) | 24.4 (22–27.3) |
| Sex n (%) | |||||
| Male | 105 (22.7) | 4 (17.4) | 73 (24.8) | 33 (19.5) | 109 (22.4) |
| Female | 358 (77.3) | 19 (82.6) | 221 (75.2) | 136 (80.5) | 377 (77.6) |
| Smoking n (%) | |||||
| Yes | 132 (28.5) | 5 (21.7) | 88 (29.9) | 45 (26.6) | 137 (28.2) |
| No | 331 (71.5) | 18 (78.3) | 206 (70.1) | 124 (73.4) | 349 (71.8) |
| Autoimmune disease n (%) | |||||
| Yes | 89 (19.2) | 8 (34.8) | 56 (19.0) | 34 (20.1) | 97 (20.0) |
| No | 374 (80.8) | 15 (65.2) | 238 (81.0) | 135 (79.9) | 389 (80.0) |
| Underlying disease n (%) | |||||
| Yes | 180 (38.8) | 6 (26.1) | 122 (41.5) | 57 (33.7) | 186 (38.3) |
| No | 283 (61.2) | 17 (73.9) | 172 (58.5) | 112 (66.3) | 300 (61.7) |
| Breakthrough Infection (BI) | TAbs-WT (U/mL) | |||
|---|---|---|---|---|
| After Second Dose | After Third Dose | |||
| 4 Months (n = 443) | 8 Months (n = 408) | 1 Month (n = 386) | 8 Months (n = 347) | |
| No | n = 437 | n = 397 | n = 370 | n = 216 |
| 703.6 (716.1) | 413.1 (479.6) | 17,241.5 (14,479.0) | 4441.5 (6,037.0) | |
| Yes | n = 6 | n = 11 | n = 16 | n = 131 |
| 5,017.5 (11,185.4) | 6,551.0 (14,909.0) | 24,863.5 (8671.5) | 25,000.0 (9,498.0) | |
| p-value 1 | 0.022 | <0.001 | 0.036 | <0.001 |
| p-value 2 | <0.001 | |||
| TAbs-WT (U/mL) | NAbs-WT (%) | NAbs-O (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| After Second Dose | After Third Dose | After Second Dose | After Third Dose | ||||||
| 4 Months | 8 Months | 1 Month | 8 Months | 4 Months | 8 Months | 1 Month | 8 Months | 8 Months | |
| Sex | |||||||||
| Male | n = 94 | n = 85 | n = 76 | n = 45 | n = 64 | n = 61 | n = 42 | n = 25 | n = 5 |
| 659.5 (704.7) | 363.4 (435.2) | 20,629.5 (15,174.0) | 6063.0 (6786.0) | 85.5 (26.6) | 56.3 (42.5) | 96.9 (2.3) | 97.5 (1.0) | 39.7 (56.6) | |
| Female | n = 393 | n = 312 | n = 294 | n = 171 | n = 231 | n = 227 | n = 184 | n = 109 | n = 109 |
| 720.7 (713.0) | 429.8 (494.1) | 16,800.5 (14,019.0) | 4371.0 (6104.0) | 88.2 (21.0) | 61.2 (35.8) | 96.5 (1.8) | 97.2 (1.4) | 27.2 (49.5) | |
| p-value 1 | 0.213 | 0.529 | 0.670 | 0.092 | 0.654 | 0.643 | 0.016 | 0.140 | 0.212 |
| p-value 2 | 0.505 | 0.207 | |||||||
| Age groups (years) | |||||||||
| 20–29 | n = 35 | n = 32 | n = 25 | n = 9 | n = 24 | n = 24 | n = 15 | n = 5 | n = 5 |
| 1094.0 (881.1) | 716.5 (507.3) | 15,287.0 (12,391.0) | 6298.0 (8716.0) | 89.9 (7.8) | 66.5 (27.0) | 96.7 (1.6) | 97.6 (5.8) | 11.9 (53.5) | |
| 30–39 | n = 84 | n = 73 | n = 64 | n = 22 | n = 70 | n = 66 | n = 51 | n = 19 | n = 19 |
| 1002.0 (803.9) | 515.7 (447.7) | 18,134.0 (14,387.5) | 10,137.0 (16,353.0) | 92.4 (10.9) | 67.8 (23.3) | 95.5 (1.8) | 97.5 (1.4) | 52.7 (63.6) | |
| 40–49 | n = 105 | n = 95 | n = 85 | n = 44 | n = 74 | n = 71 | n = 56 | n = 33 | n = 33 |
| 691.8 (669.5) | 429.3 (362.8) | 18,065.0 (11,172.0) | 4683.0 (6817.0) | 86.3 (23.1) | 55.6 (37.4) | 96.8 (1.8) | 97.3 (0.8) | 31.8 (52.5) | |
| 50–59 | n = 136 | n = 130 | n = 133 | n = 91 | n = 85 | n = 87 | n = 72 | n = 51 | n = 51 |
| 638.6 (685.3) | 363.7 (393.9) | 19,623.0 (13,393.0) | 4371.0 (5316.0) | 86.7 (23.2) | 55.3 (39.9) | 96.7 (1.8) | 97.0 (1.4) | 22.2 (44.1) | |
| >60 | n = 77 | n = 67 | n = 63 | n = 50 | n = 42 | n = 40 | n = 32 | n = 26 | n = 26 |
| 483.1 (594.8) | 269.0 (326.2) | 12,797.0 (11,789.0) | 3372.0 (5283.0) | 76.1 (34.8) | 45.2 (35.0) | 96.8 (2.5) | 97.3 (1.7) | 27.2 (50.6) | |
| p-value 1 | <0.001 a,b,c,d,e,f,g | <0.001 a,b,c,d,e,g | 0.004 a,b,c | 0.054 | <0.001 b,c,d,f | <0.001 b,c,d,e | 0.391 | 0.379 | 0.490 |
| p-value 2 | <0.001 | 0.009 | |||||||
| Smoking | |||||||||
| No | n = 315 | n = 292 | n = 272 | n = 152 | n = 221 | n = 214 | n = 167 | n = 98 | n = 98 |
| 741.4 (782.2) | 437.0 (494.6) | 16,952.5 (14,029.5) | 4441.5 (5995.5) | 88.0 (20.5) | 62.3 (34.3) | 96.6 (1.8) | 97.4 (1.0) | 32.9 (50.0) | |
| Yes | n = 122 | n = 105 | n = 98 | n = 64 | n = 74 | n = 74 | n = 59 | n = 36 | n = 36 |
| 623.3 (674.7) | 327.3 (420.7) | 18,003.5 (14,715.0) | 4375.0 (6028.5) | 85.2 (23.3) | 50.8 (35.4) | 96.8 (1.7) | 97.0 (1.7) | 17.6 (50.0) | |
| p-value 1 | 0.012 | 0.009 | 0.919 | 0.813 | 0.049 | 0.003 | 0.477 | 0.620 | 0.180 |
| p-value 2 | 0.999 | 0.073 | |||||||
| Autoimmune diseases | |||||||||
| No | n = 353 | n = 318 | n = 296 | n = 174 | n = 242 | n = 236 | n = 181 | n = 108 | n = 108 |
| 716.2 (690.8) | 419.1 (457.9) | 17,396.5 (14,534.0) | 4550.5 (7490.0) | 87.8 (20.9) | 61.3 (36.2) | 96.7 (1.8) | 97.4 (1.1) | 34.9 (52.9) | |
| Yes | n = 84 | n = 79 | n = 74 | n = 42 | n = 53 | n = 52 | n = 45 | n = 26 | n = 26 |
| 643.3 (811.0) | 385.3 (547.9) | 16,692.0 (13,465.0) | 3518.0 (4475.0) | 87.0 (30.6) | 58.2 (36.3) | 96.5 (1.9) | 97.1 (2.2) | 15.9 (31.8) | |
| p-value 1 | 0.130 | 0.272 | 0.937 | 0.043 | 0.289 | 0.264 | 0.932 | 0.232 | 0.049 |
| p-value 2 | 0.029 | 0.154 | |||||||
| Underlying diseases | |||||||||
| No | n = 268 | n = 244 | n = 219 | n = 117 | n = 178 | n = 173 | n = 122 | n = 73 | n = 73 |
| 747.4 (767.5) | 438.5 (497.8) | 16,469.0 (14,666.0) | 4448.0 (8411.0) | 87.9 (18.4) | 62.2 (30.0) | 96.5 (1.9) | 97.2 (1.4) | 24.7 (52.4) | |
| Yes | n = 169 | n = 153 | n = 151 | n = 99 | n = 117 | n = 115 | n = 104 | n = 61 | n = 61 |
| 679.5 (689.6) | 398.6 (464.0) | 18,065.0 (13,643.0) | 4435.0 (5608.0) | 86.7 (25.9) | 55.3 (39.9) | 96.7 (1.8) | 97.4(1.2) | 32.1(45.5) | |
| p-value 1 | 0.283 | 0.071 | 0.307 | 0.755 | 0.125 | 0.054 | 0.090 | 0.488 | 0.610 |
| p-value 2 | 0.151 | 0.145 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatsi, E.-B.; Filippatos, F.; Dellis, C.; Dourdouna, M.-M.; Syriopoulou, V.; Michos, A. Kinetics of SARS-CoV-2 Spike Antibodies after the Second and Third Dose of the BNT162b2 COVID-19 Vaccine and Association with Epidemiological Characteristics and Breakthrough Infection in a Cohort Study of Healthcare Workers. Microorganisms 2023, 11, 2010. https://doi.org/10.3390/microorganisms11082010
Tatsi E-B, Filippatos F, Dellis C, Dourdouna M-M, Syriopoulou V, Michos A. Kinetics of SARS-CoV-2 Spike Antibodies after the Second and Third Dose of the BNT162b2 COVID-19 Vaccine and Association with Epidemiological Characteristics and Breakthrough Infection in a Cohort Study of Healthcare Workers. Microorganisms. 2023; 11(8):2010. https://doi.org/10.3390/microorganisms11082010
Chicago/Turabian StyleTatsi, Elizabeth-Barbara, Filippos Filippatos, Charilaos Dellis, Maria-Myrto Dourdouna, Vasiliki Syriopoulou, and Athanasios Michos. 2023. "Kinetics of SARS-CoV-2 Spike Antibodies after the Second and Third Dose of the BNT162b2 COVID-19 Vaccine and Association with Epidemiological Characteristics and Breakthrough Infection in a Cohort Study of Healthcare Workers" Microorganisms 11, no. 8: 2010. https://doi.org/10.3390/microorganisms11082010
APA StyleTatsi, E.-B., Filippatos, F., Dellis, C., Dourdouna, M.-M., Syriopoulou, V., & Michos, A. (2023). Kinetics of SARS-CoV-2 Spike Antibodies after the Second and Third Dose of the BNT162b2 COVID-19 Vaccine and Association with Epidemiological Characteristics and Breakthrough Infection in a Cohort Study of Healthcare Workers. Microorganisms, 11(8), 2010. https://doi.org/10.3390/microorganisms11082010








