Redistribution and Activation of CD16brightCD56dim NK Cell Subset to Fight against Omicron Subvariant BA.2 after COVID-19 Vaccination
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Lymphocyte Subset Detection
2.3. Cytokine Profile Analysis
2.4. Perforin and Granzyme B Content by Lymphocyte Cells
2.5. Lymphocyte Function
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Patients with Omicron BA.2 Infection
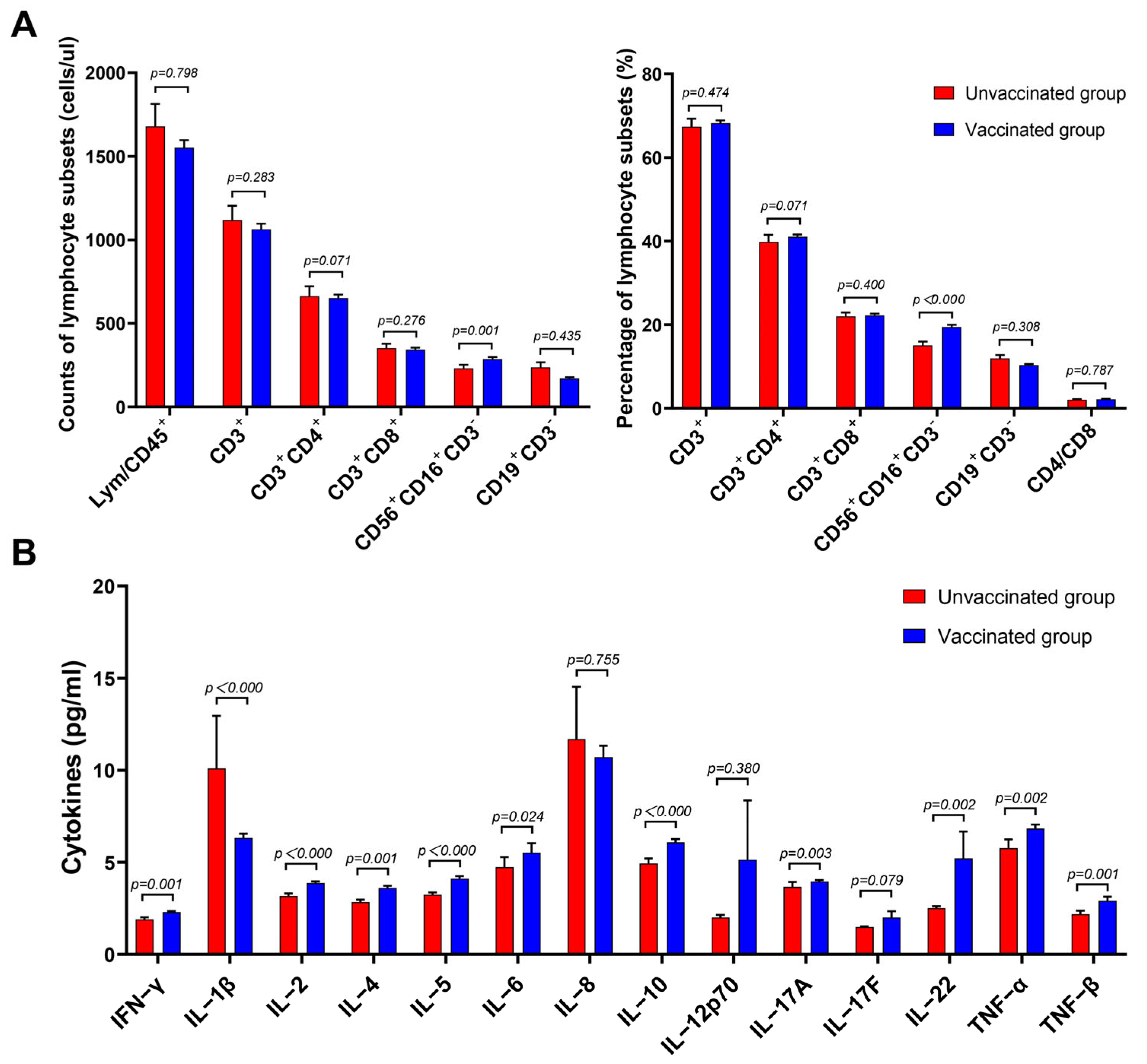
3.2. Lymphocyte Subsets and Cytokines of Patients with Omicron BA.2 on Hospital Admission
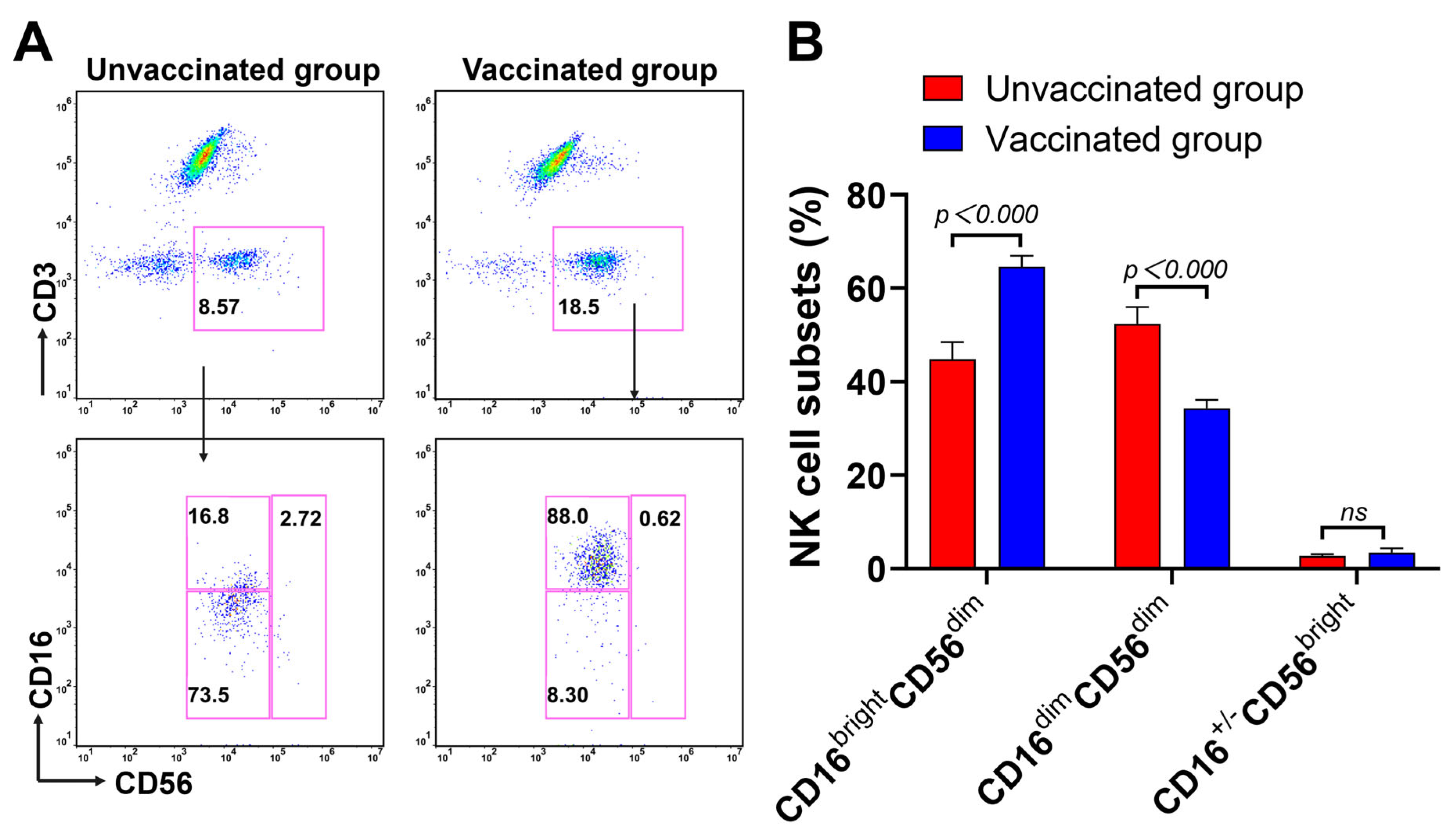
3.3. Expansion of CD16brightCD56dim NK Cells Subsets during Omicron BA.2 Infection after Vaccination
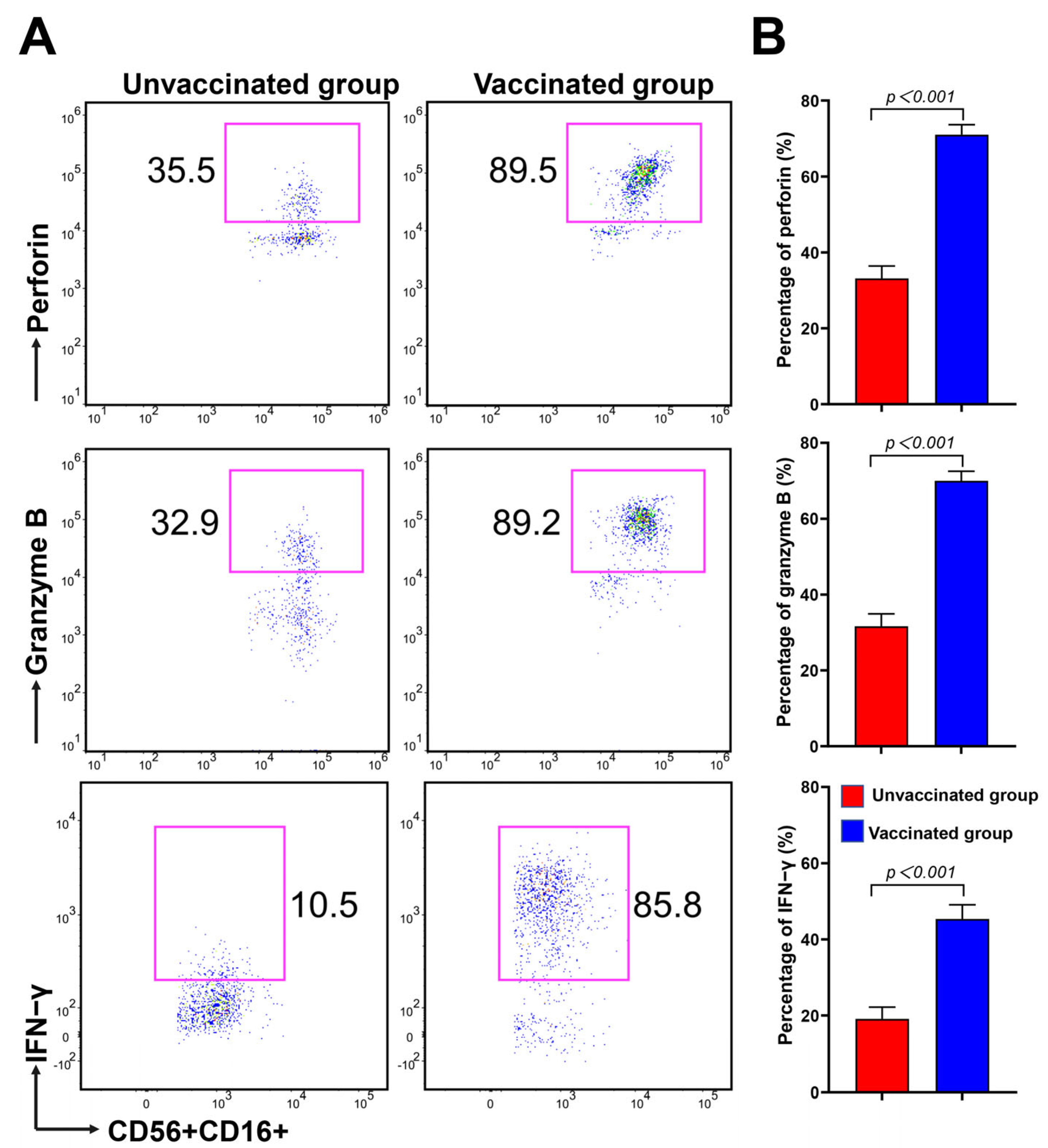
3.4. CD16brightCD56dim NK Cells Have Strong Potential in Cytokine Secretion and Cytotoxicity during Omicron BA.2 Infection after Vaccination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Wang, R.; Wang, M.; Wei, G.W. Mutations Strengthened SARS-CoV-2 Infectivity. J. Mol. Biol. 2020, 432, 5212–5226. [Google Scholar] [CrossRef]
- Barouch, D.H. COVID-19 Vaccines—Immunity, Variants, Boosters. N. Engl. J. Med. 2022, 387, 1011–1020. [Google Scholar] [CrossRef]
- Desingu, P.A.; Nagarajan, K.; Dhama, K. Emergence of Omicron third lineage BA.3 and its importance. J. Med. Virol. 2022, 94, 1808–1810. [Google Scholar] [CrossRef]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef]
- Kopsidas, I.; Karagiannidou, S.; Kostaki, E.G.; Kousi, D.; Douka, E.; Sfikakis, P.P.; Moustakidis, S.; Kokkotis, C.; Tsaopoulos, D.; Tseti, I.; et al. Global Distribution, Dispersal Patterns, and Trend of Several Omicron Subvariants of SARS-CoV-2 across the Globe. Trop. Med. Infect. Dis. 2022, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Lyngse, F.P.; Kirkeby, C.T.; Denwood, M.; Christiansen, L.E.; Mølbak, K.; Møller, C.H.; Skov, R.L.; Krause, T.G.; Rasmussen, M.; Sieber, R.N.; et al. Transmission of SARS-CoV-2 Omicron VOC subvariants BA.1 and BA.2: Evidence from Danish Households. Nature Commun. 2022, 13, 5760. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, G.W. Omicron BA.2 (B.1.1.529.2): High Potential for Becoming the Next Dominant Variant. J. Phys. Chem. Lett. 2022, 13, 3840–3849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Liang, Z.; Li, T.; Liu, S.; Cui, Q.; Nie, J.; Wu, Q.; Qu, X.; Huang, W.; et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg. Microbes Infect. 2022, 11, 1–5. [Google Scholar] [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Dai, L.; Gao, L.; Tao, L.; Hadinegoro, S.R.; Erkin, M.; Ying, Z.; He, P.; Girsang, R.T.; Vergara, H.; Akram, J.; et al. Efficacy and Safety of the RBD-Dimer-Based COVID-19 Vaccine ZF2001 in Adults. N. Engl. J. Med. 2022, 386, 2097–2111. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Mallapaty, S. China’s COVID vaccines have been crucial—Now immunity is waning. Nature 2021, 598, 398–399. [Google Scholar] [CrossRef]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, CD015477. [Google Scholar] [CrossRef]
- Pan, P.; Du, X.; Zhou, Q.; Cui, Y.; Deng, X.; Liu, C.; Hu, Z.; Chen, J.; Yu, X.; Shi, W. Characteristics of lymphocyte subsets and cytokine profiles of patients with COVID-19. Virol. J. 2022, 19, 57. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Belaid, B.; Lamara Mahammad, L.; Mihi, B.; Rahali, S.Y.; Djidjeli, A.; Larab, Z.; Berkani, L.; Berkane, I.; Sayah, W.; Merah, F.; et al. T cell counts and IL-6 concentration in blood of North African COVID-19 patients are two independent prognostic factors for severe disease and death. J. Leukoc. Biol. 2022, 111, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhou, Y.; Yu, J.; Mao, L.; Bosco, M.J.; Wang, J.; Lu, Y.; Mao, L.; Wu, X.; Wang, F.; et al. Establishment of the Reference Intervals of Lymphocyte Function in Healthy Adults Based on IFN-gamma Secretion Assay upon Phorbol-12-Myristate-13-Acetate/Ionomycin Stimulation. Front. Immunol. 2018, 9, 172. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Wang, X.; Wu, Y.; Ye, L.; Wei, H.; Sun, R.; Tian, Z.; Peng, H. CD49a(+)CD49b(+) NK cells induced by viral infection reflect an activated state of conventional NK cells. Sci. China Life Sci. 2020, 63, 1725–1733. [Google Scholar] [CrossRef]
- Scott, L.; Hsiao, N.Y.; Moyo, S.; Singh, L.; Tegally, H.; Dor, G.; Maes, P.; Pybus, O.G.; Kraemer, M.U.G.; Semenova, E.; et al. Track Omicron’s spread with molecular data. Science 2021, 374, 1454–1455. [Google Scholar] [CrossRef]
- Kawaoka, Y.; Uraki, R.; Kiso, M.; Iida, S.; Imai, M.; Takashita, E.; Kuroda, M.; Halfmann, P.; Loeber, S.; Maemura, T.; et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Xu, R.; Wang, W.; Zhang, W. As the SARS-CoV-2 virus evolves, should Omicron subvariant BA.2 be subjected to quarantine, or should we learn to live with it? Front. Public Health 2022, 10, 1039123. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, H.; Wilder-Smith, A. Does the World Still Need New COVID-19 Vaccines? N. Engl. J. Med. 2022, 386, 2140–2142. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Omicron thwarts some of the world’s most-used COVID vaccines. Nature 2022, 601, 311. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Qi, X.; Cao, Y.; Li, P.; Lu, L.; Wang, P.; Feng, Y.; Yang, J.; Wei, H.; Guo, L.; et al. Three doses of an inactivation-based COVID-19 vaccine induces cross-neutralizing immunity against the SARS-CoV-2 Omicron variant. Emerg. Microbes Infect. 2022, 11, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, R.K.; Chen, I.P.; Ma, T.; Syed, A.M.; Brazer, N.; Saldhi, P.; Simoneau, C.R.; Ciling, A.; Khalid, M.M.; Sreekumar, B.; et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature 2022, 607, 351–355. [Google Scholar] [CrossRef]
- Sheikh, A.; Kerr, S.; Woolhouse, M.; McMenamin, J.; Robertson, C.; Collaborators, E.I. Severity of omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): A national cohort study with nested test-negative design. Lancet Infect. Dis. 2022, 22, 959–966. [Google Scholar] [CrossRef]
- Lee, J.; Lozano-Ruiz, B.; Yang, F.M.; Fan, D.D.; Shen, L.; Gonzalez-Navajas, J.M. The Multifaceted Role of Th1, Th9, and Th17 Cells in Immune Checkpoint Inhibition Therapy. Front. Immunol. 2021, 12, 625667. [Google Scholar] [CrossRef]
- Gupta, G.; Shareef, I.; Tomar, S.; Kumar, M.S.N.; Pandey, S.; Sarda, R.; Singh, R.; Das, B.K.; Sinha, S. Th1/Th2/Th17 Cytokine Profile among Different Stages of COVID-19 Infection. Natl. Acad. Sci. Lett. 2022, 45, 363–369. [Google Scholar] [CrossRef]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; van den Akker, J.P.C.; Molenkamp, R.; Koopmans, M.P.G.; van Gorp, E.C.M.; et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5, eabd2071. [Google Scholar] [CrossRef]
- Richardson, J.R.; Gotz, R.; Mayr, V.; Lohse, M.J.; Holthoff, H.P.; Ungerer, M. SARS-CoV2 wild type and mutant specific humoral and T cell immunity is superior after vaccination than after natural infection. PLoS ONE 2022, 17, e0266701. [Google Scholar] [CrossRef]
- Lau, J.J.; Cheng, S.M.S.; Leung, K.; Lee, C.K.; Hachim, A.; Tsang, L.C.H.; Yam, K.W.H.; Chaothai, S.; Kwan, K.K.H.; Chai, Z.Y.H.; et al. Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population. Nat. Med. 2023, 29, 348–357. [Google Scholar] [CrossRef]
- Dhawan, M.; Priyanka; Choudhary, O.P. Emergence of Omicron sub-variant BA.2: Is it a matter of concern amid the COVID-19 pandemic? Int. J. Surg. 2022, 99, 106581. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, C.; Grifoni, A.; Muller, T.R.; Niessl, J.; Olofsson, A.; Humbert, M.; Hansson, L.; Osterborg, A.; Bergman, P.; et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat. Med. 2022, 28, 472–476. [Google Scholar] [CrossRef]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022, 603, 488–492. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef]
- Ledford, H. ‘Killer’ immune cells still recognize Omicron variant. Nature 2022, 601, 307. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Lugli, E.; Marcenaro, E.; Mavilio, D. NK Cell Subset Redistribution during the Course of Viral Infections. Front. Immunol. 2014, 5, 390. [Google Scholar] [CrossRef]
- Casado, J.L.; Moraga, E.; Vizcarra, P.; Velasco, H.; Martin-Hondarza, A.; Haemmerle, J.; Gomez, S.; Quereda, C.; Vallejo, A. Expansion of CD56(dim)CD16(neg) NK Cell Subset and Increased Inhibitory KIRs in Hospitalized COVID-19 Patients. Viruses 2021, 14, 46. [Google Scholar] [CrossRef]
- Vietzen, H.; Danklmaier, V.; Zoufaly, A.; Puchhammer-Stockl, E. High-affinity FcgammaRIIIa genetic variants and potent NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC) responses contributing to severe COVID-19. Genet Med. 2022, 24, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.; Knoll, R.; Bonaguro, L.; ToVinh, M.; Raabe, J.; Astaburuaga-Garcia, R.; Schulte-Schrepping, J.; Kaiser, K.M.; Rieke, G.J.; Bischoff, J.; et al. Early IFN-alpha signatures and persistent dysfunction are distinguishing features of NK cells in severe COVID-19. Immunity 2021, 54, 2650–2669.e14. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, D.; Rodahl, I.; Varnaite, R.; Asgeirsson, H.; Glans, H.; Falck-Jones, S.; Vangeti, S.; Buggert, M.; Ljunggren, H.G.; Michaelsson, J.; et al. Comparison of Lung-Homing Receptor Expression and Activation Profiles on NK Cell and T Cell Subsets in COVID-19 and Influenza. Front. Immunol. 2022, 13, 834862. [Google Scholar] [CrossRef] [PubMed]
- Rieke, G.J.; van Bremen, K.; Bischoff, J.; To Vinh, M.; Monin, M.B.; Schlabe, S.; Raabe, J.; Kaiser, K.M.; Finnemann, C.; Odainic, A.; et al. Induction of NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC) against SARS-CoV-2 after natural infection is more potent than after vaccination. J. Infect. Dis. 2022, 225, 1688–1693. [Google Scholar] [CrossRef]
- Richardson, S.I.; Madzorera, V.S.; Spencer, H.; Manamela, N.P.; van der Mescht, M.A.; Lambson, B.E.; Oosthuysen, B.; Ayres, F.; Makhado, Z.; Moyo-Gwete, T.; et al. SARS-CoV-2 Omicron triggers cross-reactive neutralization and Fc effector functions in previously vaccinated, but not unvaccinated, individuals. Cell Host Microbe 2022, 30, 880–886. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 508) | Unvaccinated Group (n = 102) | Vaccinated Group (n = 406) | p-Value |
|---|---|---|---|---|
| Age, median (IQR *), years | 45.00 (17.75–67.00) | 33.00 (3.50–70.50) | 47.00 (19.00–66.00) | 0.066 |
| Male/female, n (%) | 239 (47.04)/269 (52.95) | 49 (48.04)/53 (51.96) | 190 (46.80)/216 (53.20) | 0.822 |
| Fever | <0.001 | |||
| Low (37.3–38 °C), n (%) | 35 (6.89) | 12 (11.76) | 23 (5.67) | |
| Moderate (38.1–39 °C), n (%) | 27 (5.31) | 16 (15.68) | 11 (2.71) | |
| High (39–41 °C), n (%) | 7 (1.38) | 4 (3.92) | 3 (0.74) | |
| Cough, n (%) | 261 (51.37) | 65 (63.73) | 196 (48.28) | 0.110 |
| Expectoration, n (%) | 46 (9.06) | 11 (10.78) | 35 (8.62) | 0.696 |
| Nasal congestion, n (%) | 41 (8.07) | 11 (10.78) | 30 (7.39) | 0.202 |
| Chills, n (%) | 3 (0.59) | 1 (0.98) | 2 (0.49) | 1.000 |
| Sore throat, n (%) | 49 (9.64) | 14 (13.72) | 35 (8.62) | 0.139 |
| Swollen tonsils, n (%) | 4 (0.78) | 1 (0.98) | 3 (0.74) | 1.000 |
| Myalgia, n (%) | 13 (2.56) | 3 (2.94) | 10 (2.46) | 1.000 |
| Fatigue, n (%) | 9 (1.77) | 2 (1.96) | 7 (1.72) | 1.000 |
| Anorexia, n (%) | 3 (0.60) | 1 (0.98) | 2 (0.50) | 1.000 |
| Short of breath, n (%) | 9 (1.77) | 5 (4.90) | 4 (0.99) | 0.012 |
| Chest tightness, n (%) | 23 (4.53) | 9 (8.82) | 14 (3.45) | 0.016 |
| Chest pain, n (%) | 3 (0.60) | 1 (0.98) | 2 (0.49) | 1.000 |
| Nausea or vomiting, n (%) | 7 (1.38) | 4 (3.92) | 3 (0.74) | 0.031 |
| Abdominal pain, n (%) | 4 (0.79) | 3 (2.94) | 1 (0.25) | 0.014 |
| Diarrhoea, n (%) | 2 (0.40) | 1 (0.98) | 1 (0.25) | 0.806 |
| Headache, n (%) | 13 (2.56) | 7 (6.86) | 6 (1.48) | 0.003 |
| Dizziness, n (%) | 28 (5.51) | 4 (3.92) | 24 (5.91) | 0.586 |
| Pulmonary infection, n (%) | 43 (8.46) | 17 (16.67) | 26 (6.40) | <0.001 |
| Hypertension, n (%) | 80 (15.75) | 20 (19.61) | 60 (14.78) | 0.252 |
| Coronary heart disease, n (%) | 14 (2.76) | 5 (4.90) | 9 (2.21) | 0.139 |
| Cerebral peduncle, n (%) | 14 (2.76) | 4 (3.92) | 10 (2.46) | 0.554 |
| Diabetes mellitus, n (%) | 20 (3.94) | 7 (6.86) | 13 (3.20) | 0.069 |
| Allergy, n (%) | 14 (2.76) | 4 (3.92) | 10 (2.46) | 0.598 |
| Underlying pulmonary diseases, n (%) | 15 (2.95) | 5 (4.90) | 10 (2.46) | 0.147 |
| Chronic liver or kidney disease, n (%) | 22 (4.33) | 7 (6.86) | 15 (3.69) | 0.136 |
| Malignancy, n (%) | 10 (1.97) | 4 (3.92) | 6 (1.48) | 0.182 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Xiang, T.; Xu, F.; Jiang, Y.; Zhong, L.; Peng, Y.; Le, A.; Zhang, W.; Liu, Y. Redistribution and Activation of CD16brightCD56dim NK Cell Subset to Fight against Omicron Subvariant BA.2 after COVID-19 Vaccination. Microorganisms 2023, 11, 940. https://doi.org/10.3390/microorganisms11040940
Peng H, Xiang T, Xu F, Jiang Y, Zhong L, Peng Y, Le A, Zhang W, Liu Y. Redistribution and Activation of CD16brightCD56dim NK Cell Subset to Fight against Omicron Subvariant BA.2 after COVID-19 Vaccination. Microorganisms. 2023; 11(4):940. https://doi.org/10.3390/microorganisms11040940
Chicago/Turabian StylePeng, Huiyun, Tianxin Xiang, Fei Xu, Yuhuan Jiang, Lipeng Zhong, Yanqi Peng, Aiping Le, Wei Zhang, and Yang Liu. 2023. "Redistribution and Activation of CD16brightCD56dim NK Cell Subset to Fight against Omicron Subvariant BA.2 after COVID-19 Vaccination" Microorganisms 11, no. 4: 940. https://doi.org/10.3390/microorganisms11040940
APA StylePeng, H., Xiang, T., Xu, F., Jiang, Y., Zhong, L., Peng, Y., Le, A., Zhang, W., & Liu, Y. (2023). Redistribution and Activation of CD16brightCD56dim NK Cell Subset to Fight against Omicron Subvariant BA.2 after COVID-19 Vaccination. Microorganisms, 11(4), 940. https://doi.org/10.3390/microorganisms11040940






