Reduced Plasma Guanylin Levels Following Enterotoxigenic Escherichia coli-Induced Diarrhea
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Infection Study
2.2. Specimen Collection
2.3. Clinical Evaluation
2.4. Biochemical Analyses
2.5. Statistical Analyses
2.6. Ethical Statement
3. Results
3.1. Clinical Evaluations
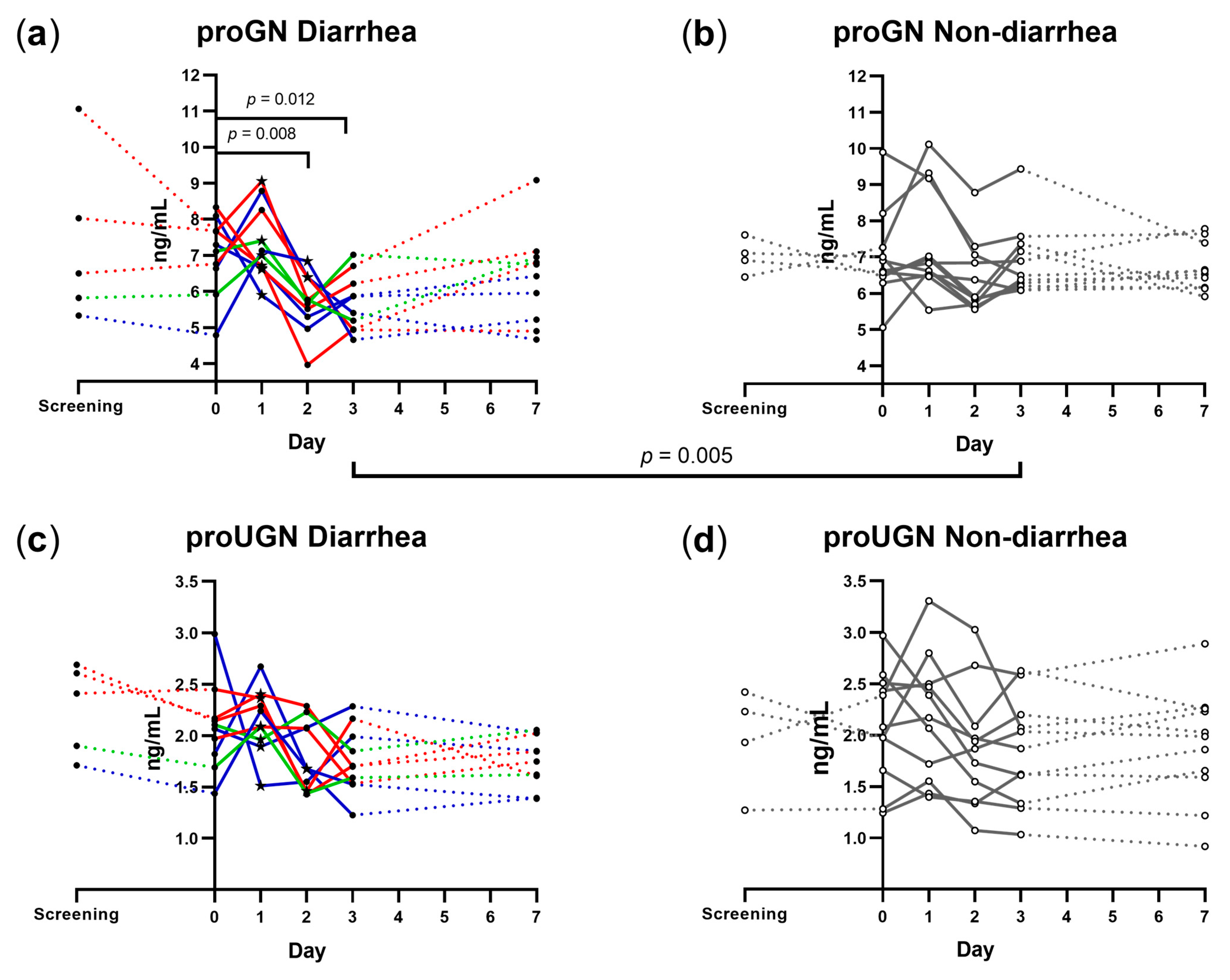
3.2. Plasma proGN and proUGN
3.3. Correlation between Plasma proGN and proUGN Concentrations
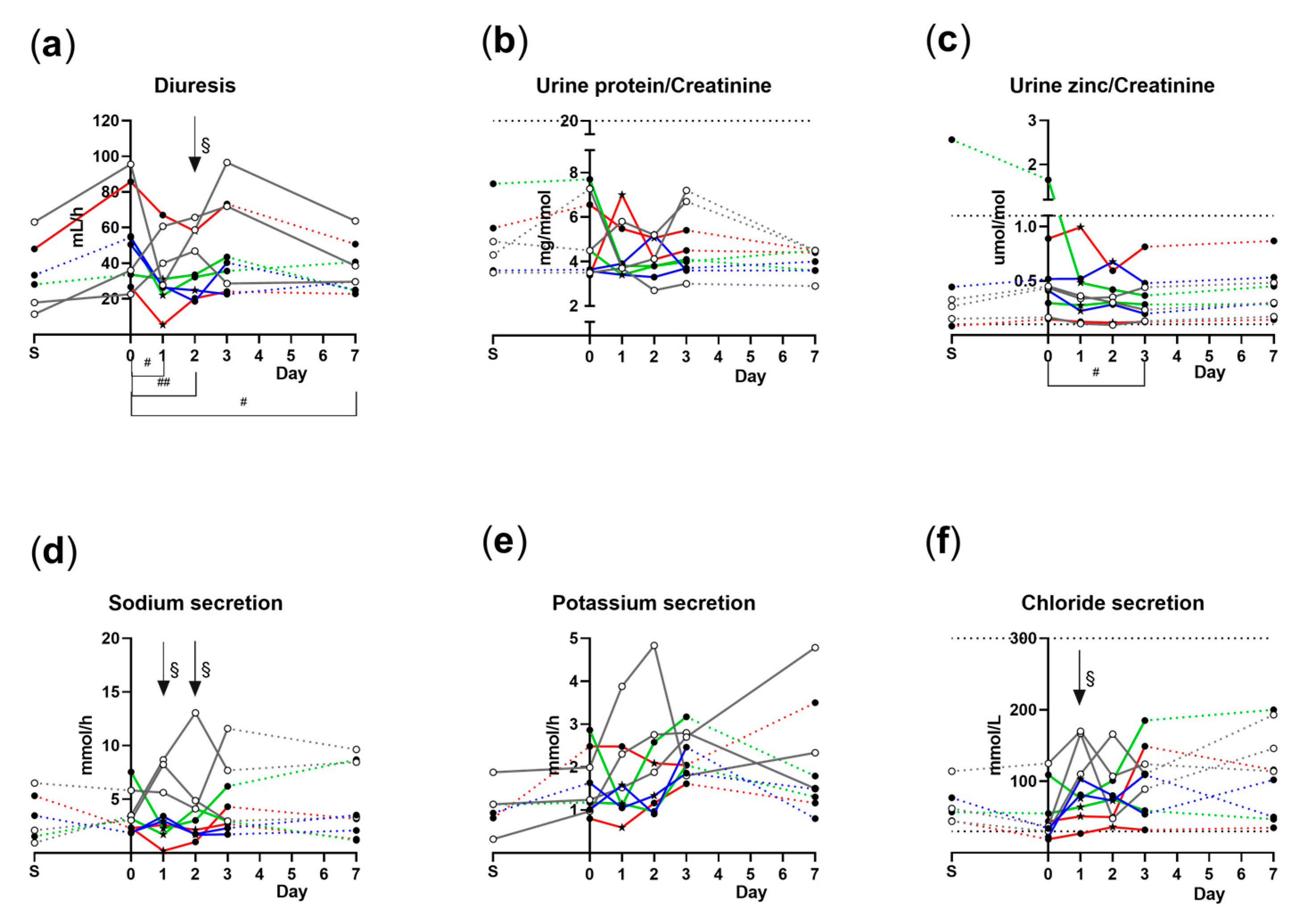
3.4. Overnight Diuresis and Urine Analyte Concentrations
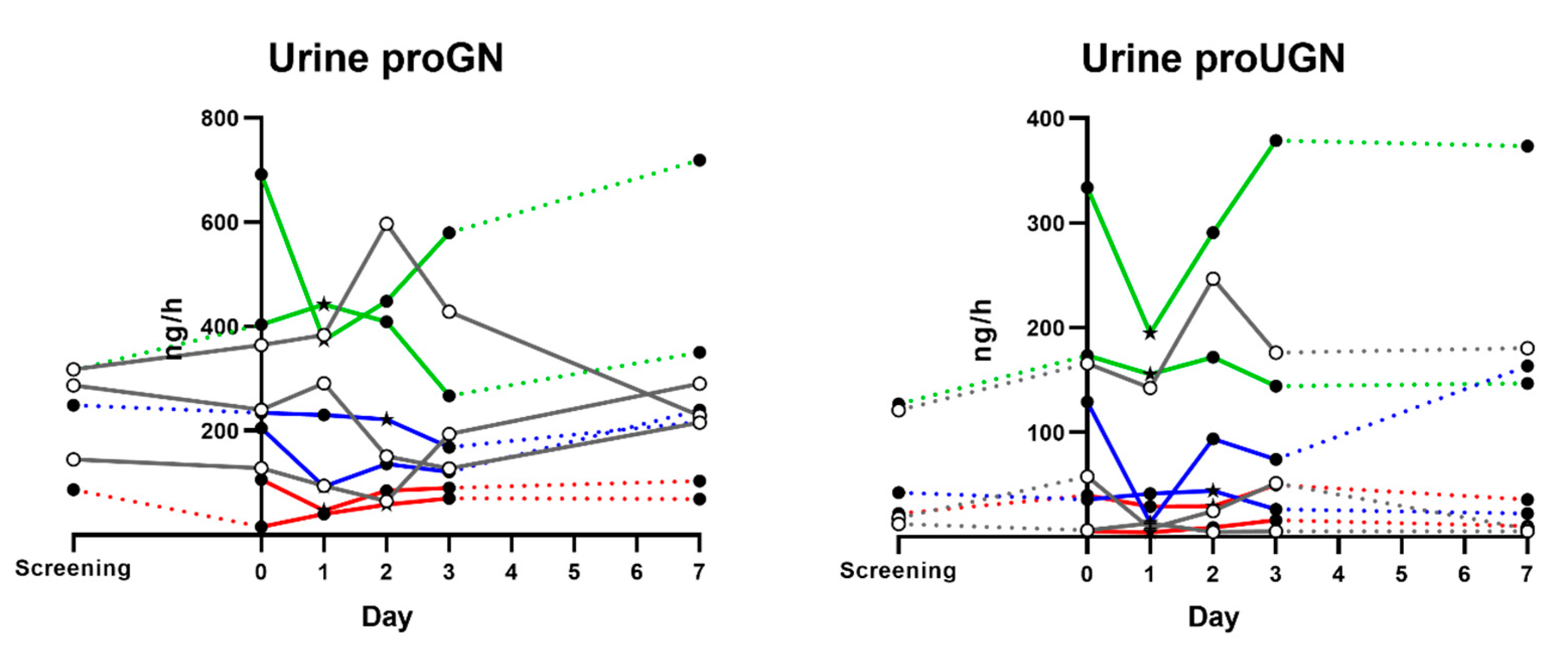
3.5. Urine proGN and proUGN Secretion Rates
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keely, S.J.; Barrett, K.E. Intestinal secretory mechanisms and diarrhea. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G405–G420. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M. Mechanisms of diarrhea. Curr. Gastroenterol. Rep. 2010, 12, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Currie, M.G.; Fok, K.F.; Kato, J.; Moore, R.J.; Hamra, F.K.; Duffin, K.L.; Smith, C.E. Guanylin: An endogenous activator of intestinal guanylate cyclase. Proc. Natl. Acad. Sci. USA 1992, 89, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Hamra, F.K.; Forte, L.R.; Eber, S.L.; Pidhorodeckyj, N.V.; Krause, W.J.; Freeman, R.H.; Chin, D.T.; Tompkins, J.A.; Fok, K.F.; Smith, C.E.; et al. Uroguanylin: Structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc. Natl. Acad. Sci. USA 1993, 90, 10464–10468. [Google Scholar] [CrossRef]
- de Sauvage, F.J.; Keshav, S.; Kuang, W.J.; Gillett, N.; Henzel, W.; Goeddel, D.V. Precursor structure, expression, and tissue distribution of human guanylin. Proc. Natl. Acad. Sci. USA 1992, 89, 9089–9093. [Google Scholar] [CrossRef]
- Miyazato, M.; Nakazato, M.; Matsukura, S.; Kangawa, K.; Matsuo, H. Uroguanylin gene expression in the alimentary tract and extra-gastrointestinal tissues. FEBS Lett. 1996, 398, 170–174. [Google Scholar] [CrossRef]
- Kita, T.; Smith, C.E.; Fok, K.F.; Duffin, K.L.; Moore, W.M.; Karabatsos, P.J.; Kachur, J.F.; Hamra, F.K.; Pidhorodeckyj, N.V.; Forte, L.R.; et al. Characterization of human uroguanylin: A member of the guanylin peptide family. Am. J. Physiol. 1994, 266, F342–F348. [Google Scholar] [CrossRef]
- Brierley, S.M. Guanylate cyclase-C receptor activation: Unexpected biology. Curr. Opin. Pharmacol. 2012, 12, 632–640. [Google Scholar] [CrossRef]
- Forte, L.R. Guanylin regulatory peptides: Structures, biological activities mediated by cyclic GMP and pathobiology. Regul. Pept. 1999, 81, 25–39. [Google Scholar] [CrossRef]
- Kuhn, M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol. Rev. 2016, 96, 751–804. [Google Scholar] [CrossRef]
- Albano, F.; Brasitus, T.; Mann, E.A.; Guarino, A.; Giannella, R.A. Colonocyte basolateral membranes contain Escherichia coli heat-stable enterotoxin receptors. Biochem. Biophys. Res. Commun. 2001, 284, 331–334. [Google Scholar] [CrossRef]
- Moss, N.G.; Riguera, D.A.; Fellner, R.C.; Cazzolla, C.; Goy, M.F. Natriuretic and antikaliuretic effects of uroguanylin and prouroguanylin in the rat. Am. J. Physiol. Ren. Physiol. 2010, 299, F1433–F1442. [Google Scholar] [CrossRef]
- Nokihara, K.; Wray, V.; Ando, E.; Naruse, S.; Hayakawa, T. Synthesis, solution structure, binding activity, and cGMP activation of human guanylin and its disulfide isomer. Regul. Pept. 1997, 70, 111–120. [Google Scholar] [CrossRef]
- Hamra, F.K.; Eber, S.L.; Chin, D.T.; Currie, M.G.; Forte, L.R. Regulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidity. Proc. Natl. Acad. Sci. USA 1997, 94, 2705–2710. [Google Scholar] [CrossRef]
- Sindiće, A.; Başoglu, C.; Cerçi, A.; Hirsch, J.R.; Potthast, R.; Kuhn, M.; Ghanekar, Y.; Visweswariah, S.S.; Schlatter, E. Guanylin, uroguanylin, and heat-stable euterotoxin activate guanylate cyclase C and/or a pertussis toxin-sensitive G protein in human proximal tubule cells. J. Biol. Chem. 2002, 277, 17758–17764. [Google Scholar] [CrossRef]
- Whitaker, T.L.; Witte, D.P.; Scott, M.C.; Cohen, M.B. Uroguanylin and guanylin: Distinct but overlapping patterns of messenger RNA expression in mouse intestine. Gastroenterology 1997, 113, 1000–1006. [Google Scholar] [CrossRef]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef]
- Jiang, Z.D.; DuPont, H.L. Etiology of travellers’ diarrhea. J. Travel. Med. 2017, 24, S13–S16. [Google Scholar] [CrossRef]
- Field, M.; Graf, L.H., Jr.; Laird, W.J.; Smith, P.L. Heat-stable enterotoxin of Escherichia coli: In vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc. Natl. Acad. Sci. USA 1978, 75, 2800–2804. [Google Scholar] [CrossRef]
- Schulz, S.; Green, C.K.; Yuen, P.S.; Garbers, D.L. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell 1990, 63, 941–948. [Google Scholar] [CrossRef]
- Kaar, G.; Dieplinger, B.; Gabriel, C.; Haltmayer, M.; Mueller, T. Proguanylin and prouroguanylin--assay evaluation and clinical analyte characterization. Clin. Chim. Acta 2011, 412, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Dieplinger, B. The guanylin peptide family and the proposed gastrointestinal-renal natriuretic signaling axis. Kidney Int. 2012, 82, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- von Volkmann, H.L.; Bronstad, I.; Tronstad, R.R.; Dizdar, V.; Nylund, K.; Hanevik, K.; Hausken, T.; Gilja, O.H.; Fiskerstrand, T. Plasma levels of guanylins are reduced in patients with Crohn’s disease. Scand. J. Gastroenterol. 2020, 55, 449–453. [Google Scholar] [CrossRef] [PubMed]
- von Volkmann, H.L.; Brønstad, I.; Gilja, O.H.; Tronstad, R.R.; Sangnes, D.A.; Nortvedt, R.; Hausken, T.; Dimcevski, G.; Fiskerstrand, T.; Nylund, K. Prolonged intestinal transit and diarrhea in patients with an activating GUCY2C mutation. PLoS ONE 2017, 12, e0185496. [Google Scholar] [CrossRef]
- Fan, X.; Hamra, F.K.; London, R.M.; Eber, S.L.; Krause, W.J.; Freeman, R.H.; Smith, C.E.; Currie, M.G.; Forte, L.R. Structure and activity of uroguanylin and guanylin from the intestine and urine of rats. Am. J. Physiol. 1997, 273, E957–E964. [Google Scholar] [CrossRef]
- Sakkestad, S.T.; Steinsland, H.; Skrede, S.; Lillebo, K.; Skutlaberg, D.H.; Guttormsen, A.B.; Zavialov, A.; Paavilainen, S.; Soyland, H.; Saevik, M.; et al. A new human challenge model for testing heat-stable toxin-based vaccine candidates for enterotoxigenic Escherichia coli diarrhea—Dose optimization, clinical outcomes, and CD4+ T cell responses. PLoS Negl. Trop. Dis. 2019, 13, e0007823. [Google Scholar] [CrossRef]
- Todnem Sakkestad, S.; Steinsland, H.; Skrede, S.; Kleppa, E.; Lillebo, K.; Saevik, M.; Soyland, H.; Rykkje Heien, A.; Gjerde Tellevik, M.; Barry, E.M.; et al. Experimental Infection of Human Volunteers with the Heat-Stable Enterotoxin-Producing Enterotoxigenic Escherichia coli Strain TW11681. Pathogens 2019, 8, 84. [Google Scholar] [CrossRef]
- Vedøy, O.B.; Hanevik, K.; Sakkestad, S.T.; Sommerfelt, H.; Steinsland, H. Proliferation of enterotoxigenic Escherichia coli strain TW11681 in stools of experimentally infected human volunteers. Gut Pathog. 2018, 10, 46. [Google Scholar] [CrossRef]
- Di Guglielmo, M.D.; Tonb, D.; He, Z.; Adeyemi, A.; van Golen, K.L. Pilot Study Measuring the Novel Satiety Hormone, Pro-Uroguanylin, in Adolescents With and Without Obesity. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 489–495. [Google Scholar] [CrossRef]
- Seeley, R.J.; Tschöp, M.H. Uroguanylin: How the gut got another satiety hormone. J. Clin. Investig. 2011, 121, 3384–3386. [Google Scholar] [CrossRef]
- Lan, D.; Niu, J.; Miao, J.; Dong, X.; Wang, H.; Yang, G.; Wang, K.; Miao, Y. Expression of guanylate cyclase-C, guanylin, and uroguanylin is downregulated proportionally to the ulcerative colitis disease activity index. Sci. Rep. 2016, 6, 25034. [Google Scholar] [CrossRef]
- Brenna, Ø.; Bruland, T.; Furnes, M.W.; Granlund, A.; Drozdov, I.; Emgård, J.; Brønstad, G.; Kidd, M.; Sandvik, A.K.; Gustafsson, B.I. The guanylate cyclase-C signaling pathway is down-regulated in inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 1241–1252. [Google Scholar] [CrossRef]
- Patterson, M.; Ward, H.; Halvai, D.; Holm Nilsen, H.A.; Reeves, S. Postprandial regulation of prouroguanylin in humans of a healthy weight and those who are overweight or with obesity. Peptides 2020, 123, 170179. [Google Scholar] [CrossRef]
- Fleckenstein, J.M.; Hardwidge, P.R.; Munson, G.P.; Rasko, D.A.; Sommerfelt, H.; Steinsland, H. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 2010, 12, 89–98. [Google Scholar] [CrossRef]
- Wang, D.; He, Y.; Liu, K.; Deng, S.; Fan, Y.; Liu, Y. Sodium Humate Alleviates Enterotoxigenic Escherichia coli-Induced Intestinal Dysfunction via Alteration of Intestinal Microbiota and Metabolites in Mice. Front. Microbiol. 2022, 13, 809086. [Google Scholar] [CrossRef]
- Barton, J.R.; Londregran, A.K.; Alexander, T.D.; Entezari, A.A.; Bar-Ad, S.; Cheng, L.; Lepore, A.C.; Snook, A.E.; Covarrubias, M.; Waldman, S.A. Intestinal neuropod cell GUCY2C regulates visceral pain. J. Clin. Investig. 2023, 133, e165578. [Google Scholar] [CrossRef]
- Vedøy, O.B.; Steinsland, H.; Sakkestad, S.T.; Sommerfelt, H.; Hanevik, K. Strong Association between Diarrhea and Concentration of Enterotoxigenic Escherichia coli Strain TW10722 in Stools of Experimentally Infected Volunteers. Pathogens 2023, 12, 283. [Google Scholar] [CrossRef]
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A multidisciplinary consensus on dehydration: Definitions, diagnostic methods and clinical implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef]
- Roy, S.K.; Tomkins, A.M.; Ara, G.; Jolly, S.P.; Khatun, W.; Chowdhury, R.; Chakrabarty, B. Impact of zinc deficiency on vibrio cholerae enterotoxin-stimulated water and electrolyte transport in animal model. J. Health Popul. Nutr. 2006, 24, 42–47. [Google Scholar]
- Di Leo, V.; D’Incà, R.; Barollo, M.; Tropea, A.; Fries, W.; Mazzon, E.; Irato, P.; Cecchetto, A.; Sturniolo, G.C. Effect of zinc supplementation on trace elements and intestinal metallothionein concentrations in experimental colitis in the rat. Dig. Liver Dis. 2001, 33, 135–139. [Google Scholar] [CrossRef]
- Kiefer, M.C.; Motyka, N.I.; Clements, J.D.; Bitoun, J.P. Enterotoxigenic Escherichia coli Heat-Stable Toxin Increases the Rate of Zinc Release from Metallothionein and Is a Zinc- and Iron-Binding Peptide. mSphere 2020, 5, e00146-20. [Google Scholar] [CrossRef] [PubMed]

| No. of Volunteers (Females) | Mean Age, (SD) (Years) | Strain Used, No. of Volunteers | Mean Diarrheal Stool Output/24 h (Grams) | Mean Time Until Diarrhea (h) | ||
|---|---|---|---|---|---|---|
| TW 11681 | TW 10722 | |||||
| Non-diarrhea (ND) | 11 (10) | 24.5 (3.2) | 7 | 4 | Not applicable | Not applicable |
| Diarrhea (D) (all) | 10 (9) | 23.5 (1.4) | 2 | 8 | 472 | 33 |
| Mild | 4 (4) | 24.2 (2.1) | 2 | 2 | 375 | 42 |
| Moderate | 2 (1) | 23.5 (0.0) | 0 | 2 | 465 | 25 |
| Severe | 4 (4) | 22.8 (0.5) | 0 | 4 | 572 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brønstad, I.; von Volkmann, H.L.; Sakkestad, S.T.; Steinsland, H.; Hanevik, K. Reduced Plasma Guanylin Levels Following Enterotoxigenic Escherichia coli-Induced Diarrhea. Microorganisms 2023, 11, 1997. https://doi.org/10.3390/microorganisms11081997
Brønstad I, von Volkmann HL, Sakkestad ST, Steinsland H, Hanevik K. Reduced Plasma Guanylin Levels Following Enterotoxigenic Escherichia coli-Induced Diarrhea. Microorganisms. 2023; 11(8):1997. https://doi.org/10.3390/microorganisms11081997
Chicago/Turabian StyleBrønstad, Ingeborg, Hilde Løland von Volkmann, Sunniva Todnem Sakkestad, Hans Steinsland, and Kurt Hanevik. 2023. "Reduced Plasma Guanylin Levels Following Enterotoxigenic Escherichia coli-Induced Diarrhea" Microorganisms 11, no. 8: 1997. https://doi.org/10.3390/microorganisms11081997
APA StyleBrønstad, I., von Volkmann, H. L., Sakkestad, S. T., Steinsland, H., & Hanevik, K. (2023). Reduced Plasma Guanylin Levels Following Enterotoxigenic Escherichia coli-Induced Diarrhea. Microorganisms, 11(8), 1997. https://doi.org/10.3390/microorganisms11081997






