Remdesivir: Effectiveness and Safety in Hospitalized Patients with COVID-19 (ReEs-COVID-19)—Analysis of Data from Daily Practice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
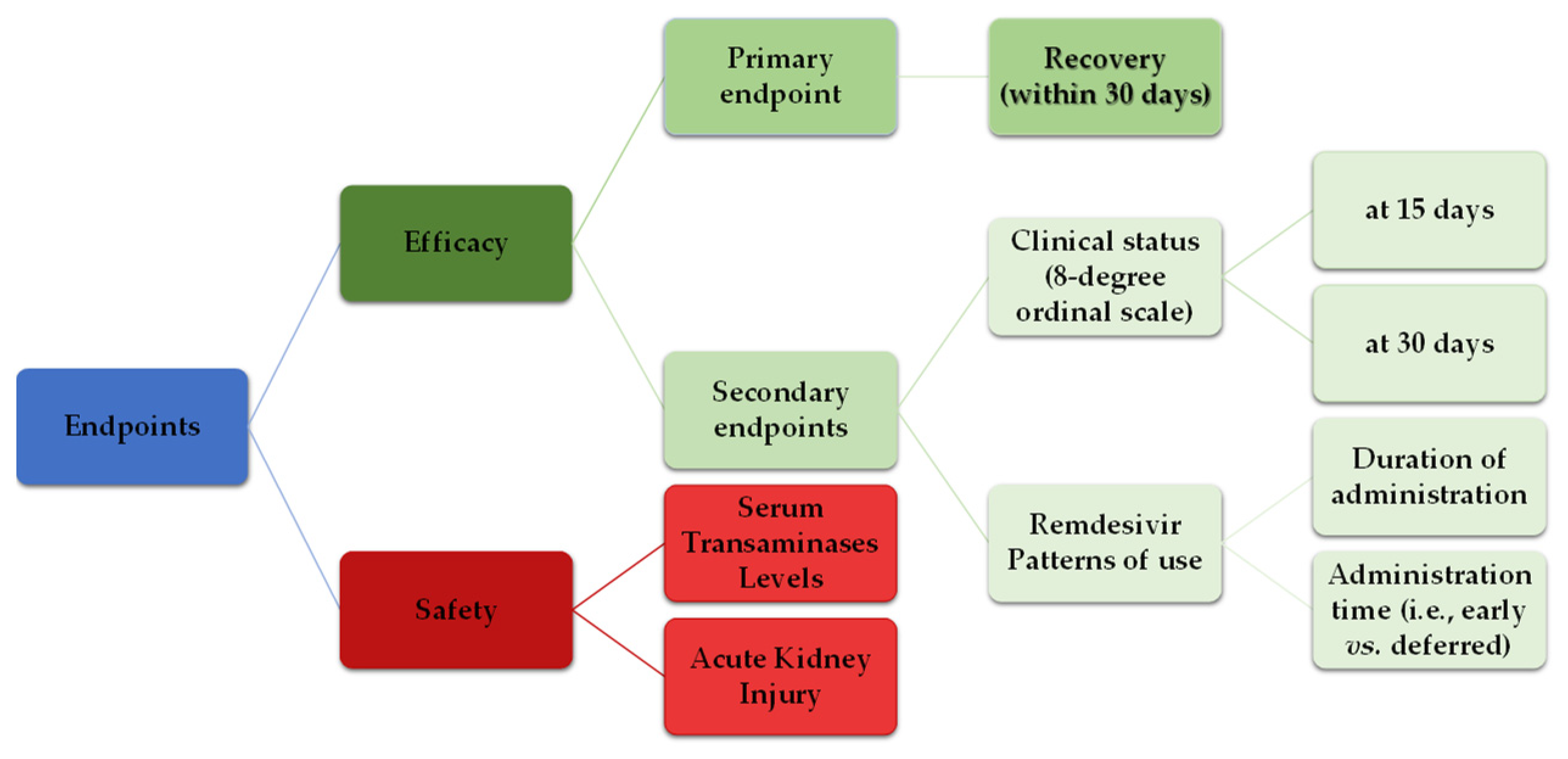
2.2. Endpoints
2.3. Ethics
2.4. Statistical Analysis
3. Results
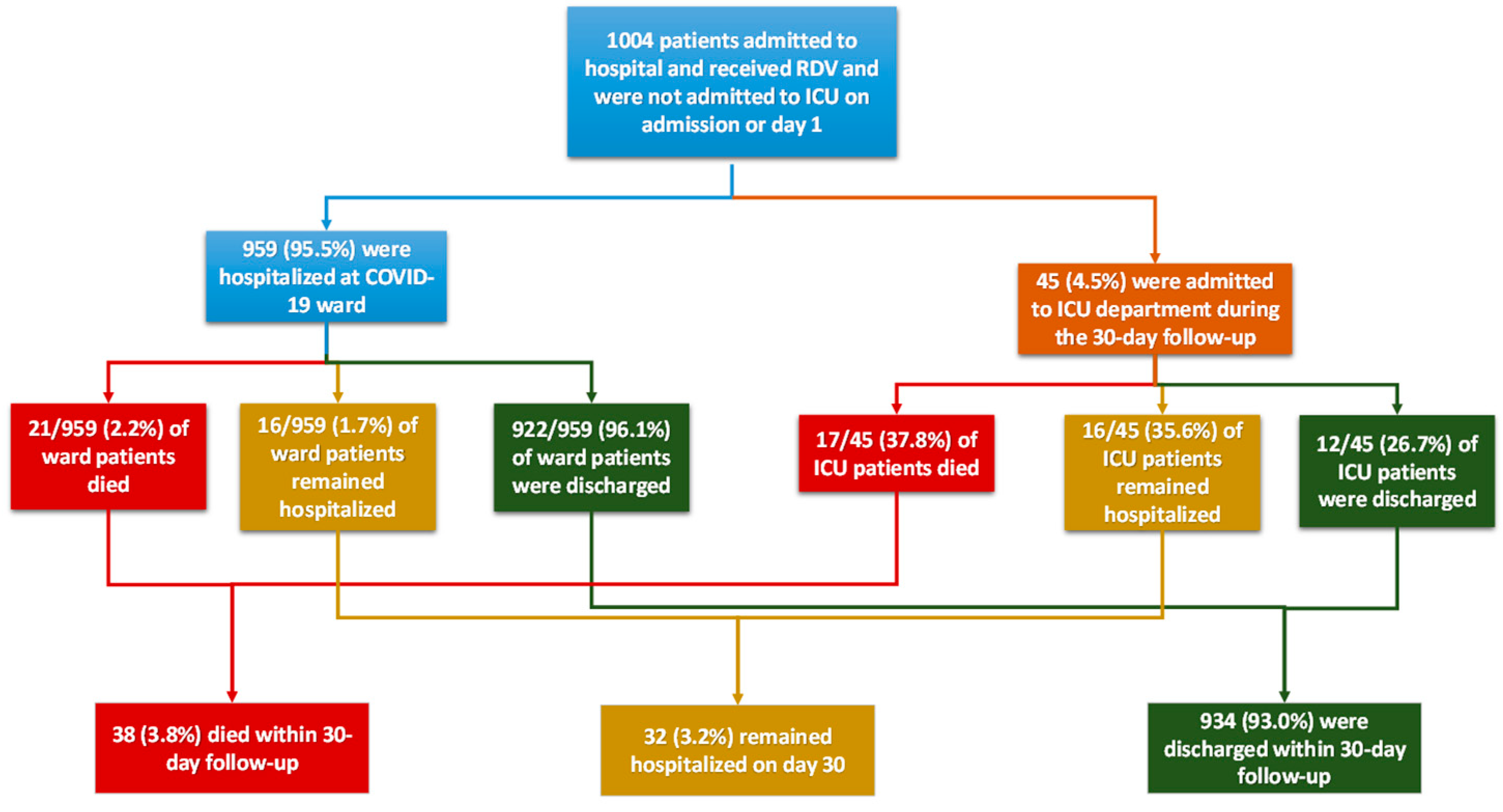
3.1. Patients’ Characteristics
3.2. Remdesivir Patterns
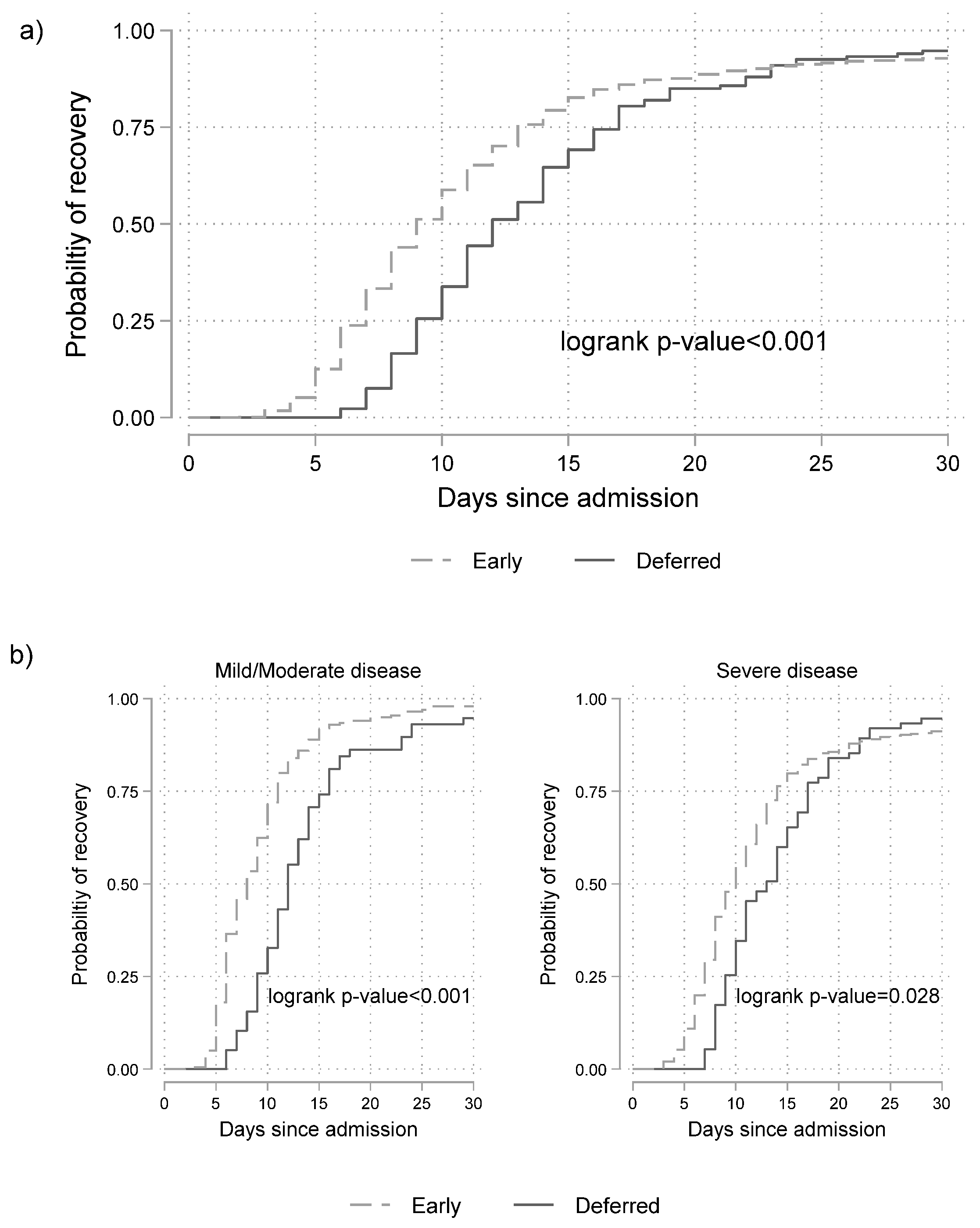
3.3. Effectiveness of Remdesivir
3.4. Safety Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid.who.int (accessed on 14 July 2023).
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.; Lim, M.A.; Pranata, R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. 2020, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Desai, S.S.; Kuy, S.; Henry, T.D.; Patel, A.N. Cardiovascular Disease, Drug Therapy, and Mortality in COVID-19. N. Engl. J. Med. 2020, 382, e102. [Google Scholar] [CrossRef]
- EMA Provides Recommendations on Compassionate Use of Remdesivir for COVID-19. Available online: https://www.ema.europa.eu/en/documents/press-release/ema-provides-recommendations-compassionate-use-remdesivir-covid-19_en.pdf (accessed on 26 October 2022).
- FDA Approves First Treatment for COVID-19. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19#:~:text=Today%2C%20the%20U.S.%20Food%20and,of%20COVID%2D19%20requiring%20hospitalization (accessed on 26 October 2022).
- Veklury (Remdesivir). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/veklury (accessed on 26 October 2022).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas Lopez, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Consortium, W.H.O.S.T. Remdesivir and three other drugs for hospitalised patients with COVID-19: Final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet 2022, 399, 1941–1953. [Google Scholar] [CrossRef]
- National Public Health Organization. Available online: https://eody.gov.gr/neos-koronaios-covid-19/ (accessed on 26 October 2022).
- KDIGO Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2 (Suppl. 1), 1–138. [Google Scholar]
- Mercado, M.G.; Smith, D.K.; Guard, E.L. Acute Kidney Injury: Diagnosis and Management. Am. Fam. Physician 2019, 100, 687–694. [Google Scholar]
- Cleveland, W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 1979, 74, 829–836. [Google Scholar] [CrossRef]
- Hellenic Society of Infectious Diseases. Therapeutic Algorithm of Adult Hospitalized Patients with COVID-19; Hellenic Society of Infectious Diseases: Athens, Greece, 2022. [Google Scholar]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Mozaffari, E.; Chandak, A.; Liang, S.; Gayle, J.; Thrun, M.; Hodgkins, P.; Haubrich, R.H. Treatment and outcomes of COVID-19 in the US: Are they different according to race? Top. Antivir. Med. 2021, 29, 141. [Google Scholar]
- Watanabe, J.H.; Kwon, J.; Nan, B.; Abeles, S.R.; Mehta, S.R. Examination of Medication Use Patterns by Age Group, Comorbidity, and Month in COVID-19 Positive Patients in a Large Statewide Health System During the Pandemic in 2020. J. Pharm. Technol. 2022, 38, 75–87. [Google Scholar] [CrossRef]
- Ader, F.; Bouscambert-Duchamp, M.; Hites, M.; Peiffer-Smadja, N.; Poissy, J.; Belhadi, D.; Diallo, A.; Le, M.P.; Peytavin, G.; Staub, T.; et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2022, 22, 209–221. [Google Scholar] [CrossRef]
- Olender, S.A.; Perez, K.K.; Go, A.S.; Balani, B.; Price-Haywood, E.G.; Shah, N.S.; Wang, S.; Walunas, T.L.; Swaminathan, S.; Slim, J.; et al. Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care. Clin. Infect. Dis. 2021, 73, e4166–e4174. [Google Scholar] [CrossRef]
- Polivka, L.; Gajdacsi, J.; Fazekas, L.; Sebok, S.; Barczi, E.; Hidvegi, E.; Sutto, Z.; Dinya, E.; Maurovich-Horvat, P.; Szabo, A.J.; et al. Long-term survival benefit of male and multimorbid COVID-19 patients with 5-day remdesivir treatment. J. Glob. Health 2022, 12, 05031. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, D.; Kulkarni, A.; Thyagaraj, V.; Balaji, V.; Kumar, T.A.; Rao, M.Y. A study to assess the outcome in COVID-19 confirmed cases receiving Remdesivir as compared to conventional therapy: Medical records-based audit. J. Family Med. Prim. Care 2022, 11, 3133–3137. [Google Scholar] [CrossRef]
- Terkes, V.; Lisica, K.; Marusic, M.; Verunica, N.; Tolic, A.; Morovic, M. Remdesivir Treatment in Moderately Ill COVID-19 Patients: A Retrospective Single Center Study. J. Clin. Med. 2022, 11, 5066. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Meira, F.; Cozar-Llisto, A.; Duenas, G.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Cardozo, C.; Hernandez-Meneses, M.; Alonso-Navarro, R.; et al. Real-life use of remdesivir in hospitalized patients with COVID-19. Rev. Esp. Quimioter. 2021, 34, 136–140. [Google Scholar] [CrossRef]
- Petrakis, V.; Rapti, V.; Akinosoglou, K.; Bonelis, C.; Athanasiou, K.; Dimakopoulou, V.; Syrigos, N.K.; Spernovasilis, N.; Trypsianis, G.; Marangos, M.; et al. Greek Remdesivir Cohort (GREC) Study: Effectiveness of Antiviral Drug Remdesivir in Hospitalized Patients with COVID-19 Pneumonia. Microorganisms 2022, 10, 1949. [Google Scholar] [CrossRef]
- Cholongitas, E.; Bali, T.; Georgakopoulou, V.E.; Giannakodimos, A.; Gyftopoulos, A.; Georgilaki, V.; Gerogiannis, D.; Basoulis, D.; Eliadi, I.; Karamanakos, G.; et al. Prevalence of abnormal liver biochemistry and its impact on COVID-19 patients’ outcomes: A single-center Greek study. Ann. Gastroenterol. 2022, 35, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Lytras, T.; Tsiodras, S. Total patient load, regional disparities and in-hospital mortality of intubated COVID-19 patients in Greece, from September 2020 to May 2021. Scand. J. Public Health 2022, 50, 671–675. [Google Scholar] [CrossRef]
- AbuRuz, S.; Al-Azayzih, A.; ZainAlAbdin, S.; Beiram, R.; Al Hajjar, M. Clinical characteristics and risk factors for mortality among COVID-19 hospitalized patients in UAE: Does ethnic origin have an impact. PLoS ONE 2022, 17, e0264547. [Google Scholar] [CrossRef]
- Bell, M.; Hergens, M.P.; Fors, S.; Tynelius, P.; de Leon, A.P.; Lager, A. Individual and neighborhood risk factors of hospital admission and death during the COVID-19 pandemic: A population-based cohort study. BMC Med. 2023, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.; Soderberg, M.; Lundberg, C.E.; Lindgren, M.; Santosa, A.; Edqvist, J.; Aberg, M.; Gisslen, M.; Robertson, J.; Cronie, O.; et al. COVID-19 in people aged 18-64 in Sweden in the first year of the pandemic: Key factors for severe disease and death. Glob. Epidemiol. 2022, 4, 100095. [Google Scholar] [CrossRef]
- Lim, H.; Palaiodimos, L.; Berto, C.G.; Tedunjaiye, O.; Malik, P.; Nagraj, S.; Choi, H.; Hti Lar Seng, N.S.; Kladas, M.; Kharawala, A.; et al. Remdesivir in the Treatment of COVID-19: A Propensity Score-Matched Analysis from a Public Hospital in New York City Assessing Renal and Hepatic Safety. J. Clin. Med. 2022, 11, 3132. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D.; Northwell, C.-R.C.; et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef]
- Wu, B.; Luo, M.; Wu, F.; He, Z.; Li, Y.; Xu, T. Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS. Front. Pharmacol. 2022, 13, 692828. [Google Scholar] [CrossRef] [PubMed]
- Gerard, A.O.; Laurain, A.; Fresse, A.; Parassol, N.; Muzzone, M.; Rocher, F.; Esnault, V.L.M.; Drici, M.D. Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database. Clin. Pharmacol. Ther. 2021, 109, 1021–1024. [Google Scholar] [CrossRef]
- Goldman, A.; Bomze, D.; Dankner, R.; Hod, H.; Meirson, T.; Boursi, B.; Maor, E. Cardiovascular adverse events associated with hydroxychloroquine and chloroquine: A comprehensive pharmacovigilance analysis of pre-COVID-19 reports. Br. J. Clin. Pharmacol. 2021, 87, 1432–1442. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Silva, N.A.O.; Zara, A.; Figueras, A.; Melo, D.O. Potential kidney damage associated with the use of remdesivir for COVID-19: Analysis of a pharmacovigilance database. Cad. Saude Publica 2021, 37, e00077721. [Google Scholar] [CrossRef]
- Armaly, Z.; Kinaneh, S.; Skorecki, K. Renal Manifestations of Covid-19: Physiology and Pathophysiology. J. Clin. Med. 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ng, J.H.; Bijol, V.; Jhaveri, K.D.; Wanchoo, R. Pathology of COVID-19-associated acute kidney injury. Clin. Kidney J. 2021, 14, i30–i39. [Google Scholar] [CrossRef] [PubMed]
- Giannitsioti, E.; Mavroudis, P.; Speggos, I.; Katsoulidou, A.; Pantazis, N.; Loupis, T.; Daniil, I.; Rekleiti, N.; Damianidou, S.; Louka, C.; et al. Real life treatment experience and outcome of consecutively hospitalised patients with SARS-CoV-2 pneumonia by Omicron-1 vs Delta variants. Infect. Dis. 2023, 10, 1–10. [Google Scholar] [CrossRef]
| Disease Severity at Baseline * | ||||
|---|---|---|---|---|
| Severe n = 746 (74.30%) | Mild/Moderate n = 258 (25.70%) | Overall n = 1004 (100%) | p-Value | |
| Age (years)—Mean (SD) | 62.6 (14.6) | 57.8 (15.6) | 61.3 (15.0) | <0.001 |
| Male | 454 (60.86%) | 154 (59.69%) | 608 (60.56%) | 0.768 |
| Greek origin | 685 (91.82%) | 233 (90.31%) | 918 (91.43%) | 0.437 |
| Residence | 0.152 | |||
| Rural | 60 (8.04%) | 14 (5.43%) | 74 (7.37%) | |
| Urban | 618 (82.84%) | 212 (82.17%) | 830 (82.67%) | |
| Semi-urban | 68 (9.12%) | 32 (12.40%) | 100 (9.96%) | |
| Smoking | 0.001 | |||
| Active Smoker | 49 (6.57%) | 19 (7.36%) | 68 (6.77%) | |
| Never Smoker | 303 (40.62%) | 152 (58.91%) | 455 (45.32%) | |
| Former Smoker | 140 (18.77%) | 32 (12.40%) | 172 (17.13%) | |
| Alcohol abuse | 7 (0.94%) | 2 (0.78%) | 9 (0.90%) | >0.999 |
| Region | 0.079 | |||
| Attica | 417 (55.90%) | 161 (62.40%) | 578 (57.57%) | |
| Rest of Greece | 329 (44.10%) | 97 (37.60%) | 426 (42.43%) | |
| Days from symptoms onset to admission—Median (IQR) Comorbidities | 6.0 (4.0, 8.0) | 6.0 (4.0, 8.0) | 6.0 (4.0, 8.0) | 0.282 |
| COPD | 55 (7.37%) | 7 (2.71%) | 62 (6.18%) | 0.006 |
| Diabetes mellitus | 188 (25.20%) | 48 (18.60%) | 236 (23.51%) | 0.033 |
| Obesity | 105 (14.08%) | 27 (10.47%) | 132 (13.15%) | 0.164 |
| CVD | 70 (9.38%) | 31 (12.02%) | 101 (10.06%) | 0.231 |
| Number of comorbidities | <0.001 | |||
| None | 192 (25.74%) | 100 (38.76%) | 292 (29.08%) | |
| One | 166 (22.25%) | 63 (24.42%) | 229 (22.81%) | |
| Two or more | 388 (52.01%) | 95 (36.82%) | 483 (48.11%) | |
| Baseline severity | <0.001 | |||
| Hospitalized, not requiring supplemental oxygen, but requiring ongoing medical care | 136 (18.23%) | 258 (100.00%) | 394 (39.24%) | |
| Hospitalized, requiring supplemental oxygen | 524 (70.24%) | 0 (0.00%) | 524 (52.19%) | |
| Hospitalized, on non-invasive ventilation or high flow oxygen devices | 86 (11.53%) | 0 (0.00%) | 86 (8.57%) | |
| Disease Severity at Baseline | ||||
|---|---|---|---|---|
| Severe n = 746 (74.30%) | Mild/Moderate n = 258 (25.70%) | Overall n = 1004 (100%) | ||
| Remdesivir administration | <0.001 | |||
| Deferred Administration | 75 (10.05%) * | 58 (22.48%) * | 133 (13.25%) | |
| Early Administration | 671 (89.95%) | 200 (77.52%) | 871 (86.75%) | |
| Duration of remdesivir administration (categories) | 0.674 | |||
| <5 days | 70 (9.38%) | 19 (7.36%) | 89 (8.86%) | |
| 5 days | 575 (77.08%) | 205 (79.46%) | 780 (77.69%) | |
| 6 to 10 days | 98 (13.14%) | 34 (13.18%) | 132 (13.15%) | |
| >10 days | 3 (0.40%) | 0 (0.00%) | 3 (0.30%) | |
| Duration of remdesevir administration (days)—Median (IQR) (min-max) | 5.0 (5.0, 5.0) (1.0, 12.0) | 5.0 (5.0, 5.0) (1.0, 10.0) | 5.0 (5.0, 5.0) (1.0, 12.0) | 0.713 |
| Time between symptoms onset and remdesivir administration (days)—Median (IQR) (min,max) | 7 (4, 9) (0, 18) | 7 (5, 9) (0, 21) | 7 (4, 9) (0, 21) | 0.201 |
| Days from admission to Remdesivir administration—Median (IQR) (min,max) | 1 (1, 2) (1, 13) | 1 (1, 2) (1, 12) | 1 (1, 2) (1, 13) | <0.001 |
| Dexamethasone ** | 646 (86.60%) | 132 (51.16%) | 778 (77.49%) | <0.001 |
| Anticoagulants | 723 (96.92%) | 248 (96.12%) | 971 (96.71%) | 0.545 |
| Hazard Ratio (HR) | 95% C.I. | p-Value | |
|---|---|---|---|
| Remdesivir administration within first 2 days/after 2nd day since admission (mild/moderate disease at baseline) | 2.02 | (1.49, 2.74) | <0.001 |
| Remdesivir administration within 2 days/after 2nd day since admission (severe disease at baseline) | 1.27 | (1.00, 1.63) | 0.054 |
| Interaction: Disease severity X timing of Remdesivir | 0.022 | ||
| Number of co-existing conditions | 0.95 | (0.91, 1.00) | 0.038 |
| Age above or equal to 65/below 65 years | 0.62 | (0.53, 0.71) | <0.001 |
| Residence | |||
| Rural/Urban | 0.74 | (0.57, 0.96) | 0.023 |
| Semi-urban/Urban | 0.98 | (0.79, 1.22) | 0.850 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantazis, N.; Pechlivanidou, E.; Antoniadou, A.; Akinosoglou, K.; Kalomenidis, I.; Poulakou, G.; Milionis, H.; Panagopoulos, P.; Marangos, M.; Katsarolis, I.; et al. Remdesivir: Effectiveness and Safety in Hospitalized Patients with COVID-19 (ReEs-COVID-19)—Analysis of Data from Daily Practice. Microorganisms 2023, 11, 1998. https://doi.org/10.3390/microorganisms11081998
Pantazis N, Pechlivanidou E, Antoniadou A, Akinosoglou K, Kalomenidis I, Poulakou G, Milionis H, Panagopoulos P, Marangos M, Katsarolis I, et al. Remdesivir: Effectiveness and Safety in Hospitalized Patients with COVID-19 (ReEs-COVID-19)—Analysis of Data from Daily Practice. Microorganisms. 2023; 11(8):1998. https://doi.org/10.3390/microorganisms11081998
Chicago/Turabian StylePantazis, Nikos, Evmorfia Pechlivanidou, Anastasia Antoniadou, Karolina Akinosoglou, Ioannis Kalomenidis, Garyfallia Poulakou, Haralampos Milionis, Periklis Panagopoulos, Markos Marangos, Ioannis Katsarolis, and et al. 2023. "Remdesivir: Effectiveness and Safety in Hospitalized Patients with COVID-19 (ReEs-COVID-19)—Analysis of Data from Daily Practice" Microorganisms 11, no. 8: 1998. https://doi.org/10.3390/microorganisms11081998
APA StylePantazis, N., Pechlivanidou, E., Antoniadou, A., Akinosoglou, K., Kalomenidis, I., Poulakou, G., Milionis, H., Panagopoulos, P., Marangos, M., Katsarolis, I., Kazakou, P., Dimakopoulou, V., Chaliasou, A.-L., Rapti, V., Christaki, E., Liontos, A., Petrakis, V., Schinas, G., Biros, D., ... Touloumi, G. (2023). Remdesivir: Effectiveness and Safety in Hospitalized Patients with COVID-19 (ReEs-COVID-19)—Analysis of Data from Daily Practice. Microorganisms, 11(8), 1998. https://doi.org/10.3390/microorganisms11081998











