The Immunogenicity and Properties of a Whole-Cell ETEC Vaccine Inactivated with Psoralen and UVA Light in Comparison to Formalin
Abstract
1. Introduction
2. Materials and Methods
3. Results
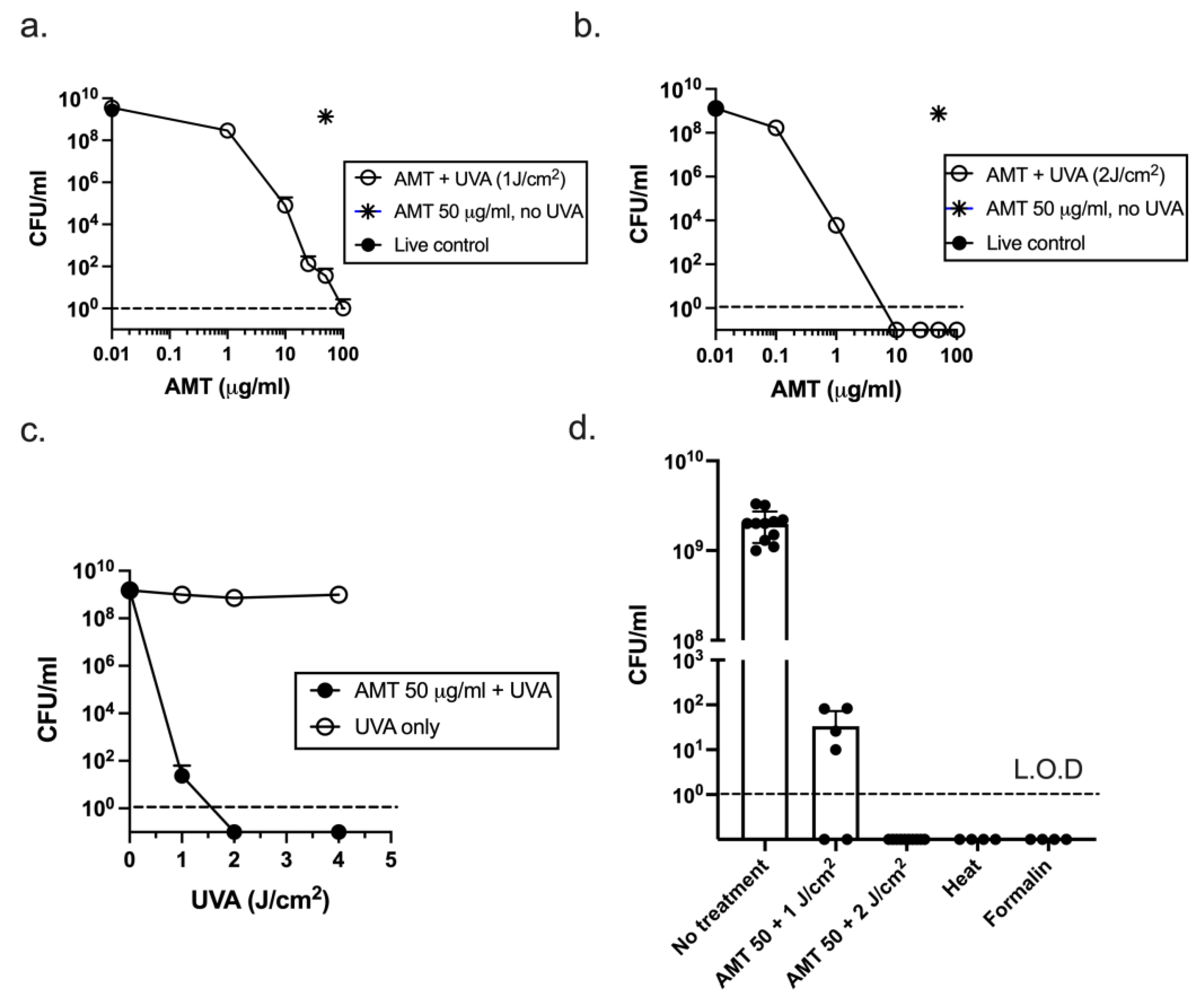
3.1. Psoralen + UVA (PUVA) Inactivation of ETEC
3.2. Metabolic Activity of PUVA–ETEC
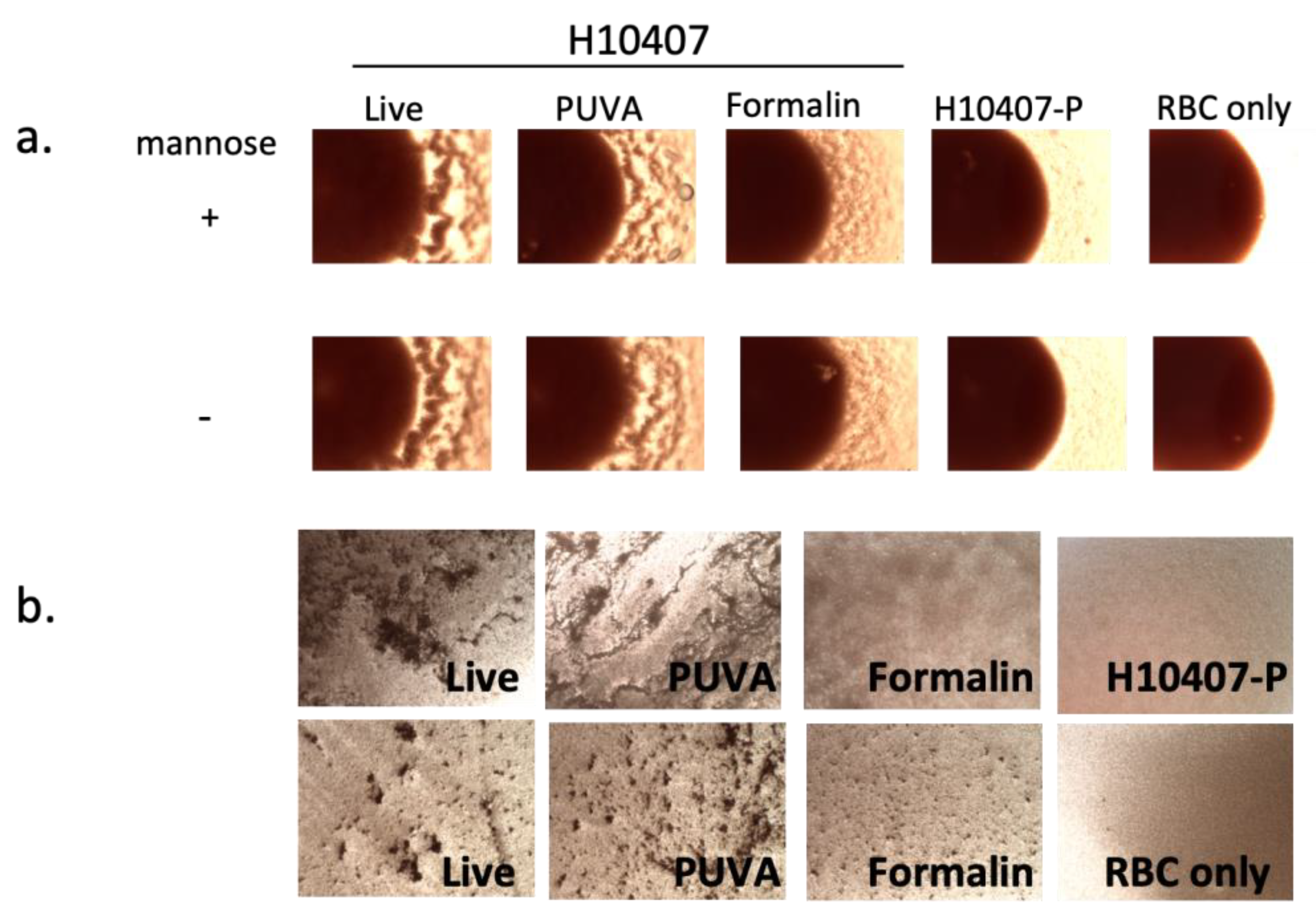
3.3. CFA/I-Mediated MRHA after PUVA and Formalin Inactivation of ETEC
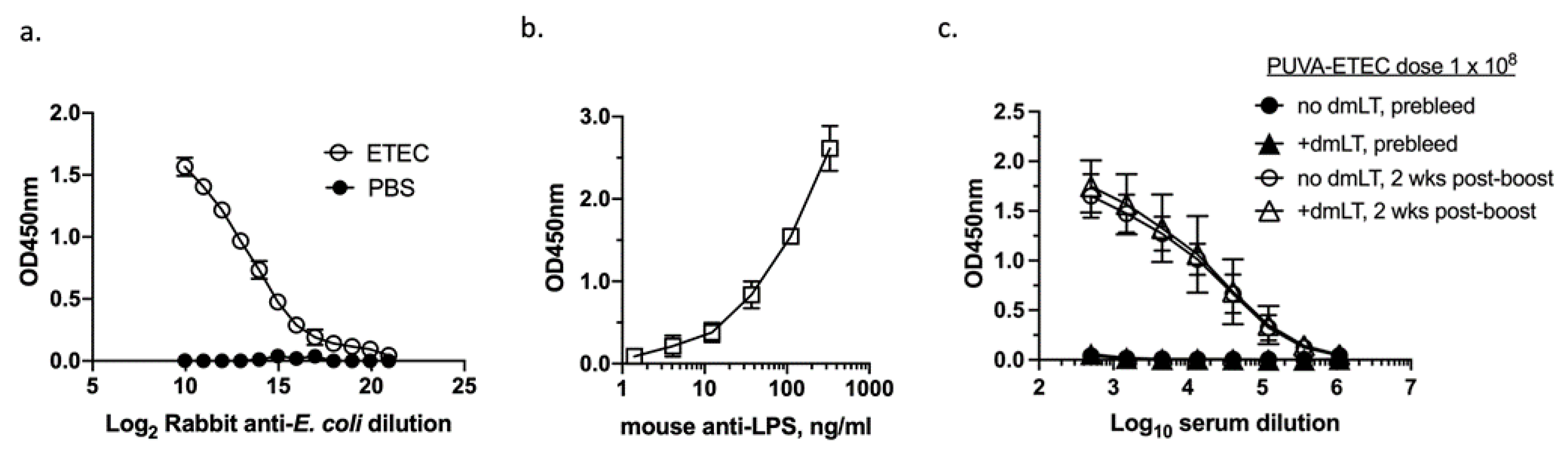
3.4. Immunogenicity of PUVA-Inactivated ETEC (PUVA–ETEC) Vaccine in Mice
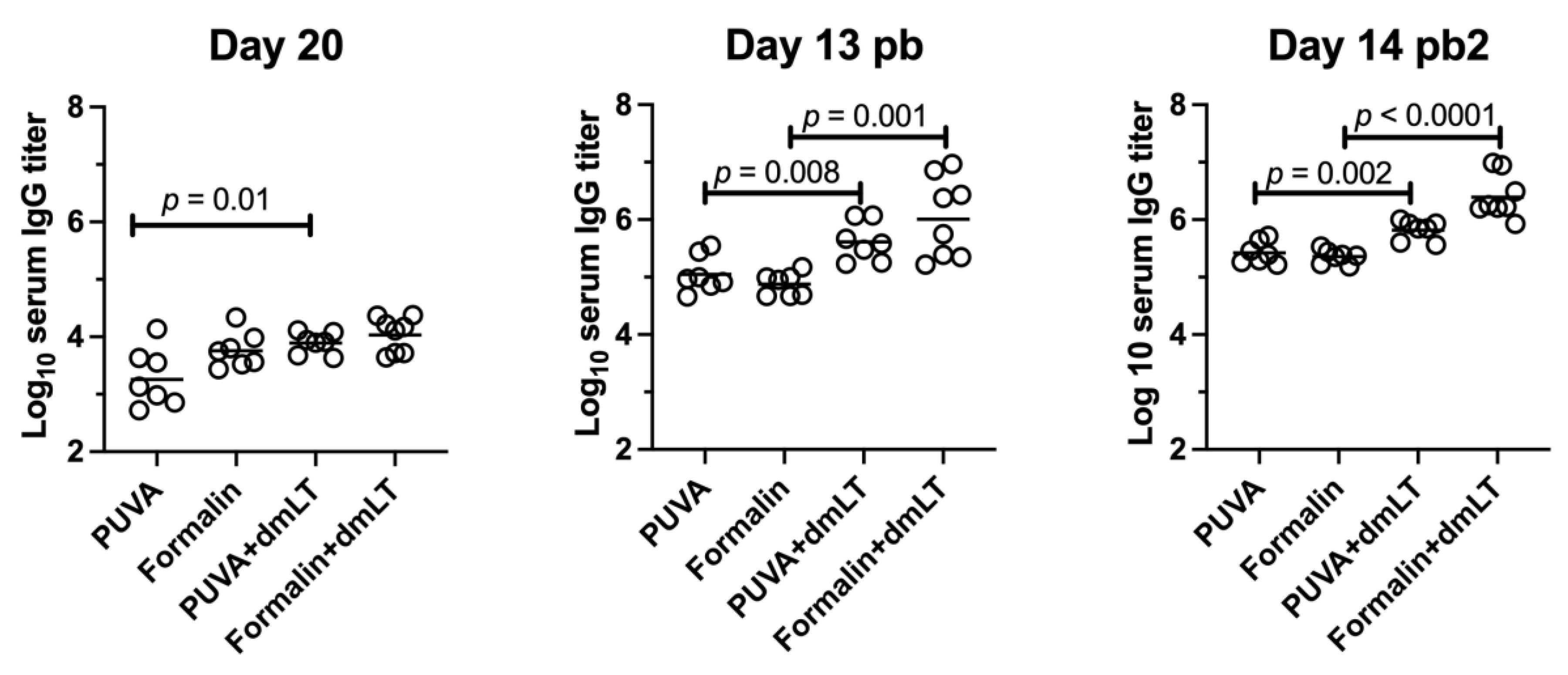
3.5. Immunogenicity of PUVA–ETEC as Compared to Formalin–ETEC Vaccines in Mice
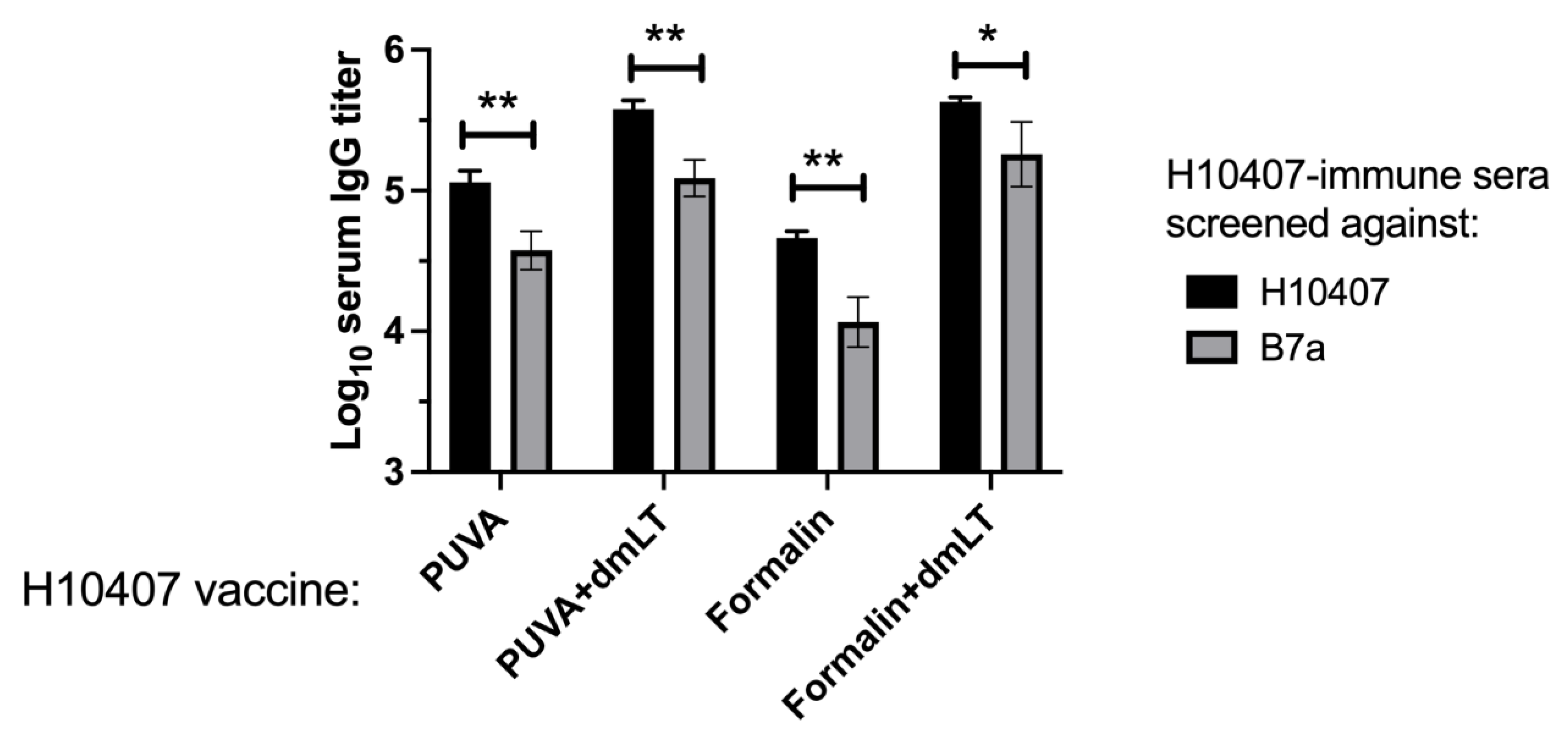
3.6. Reactivity of Immune Sera with Heterologous ETEC Strain
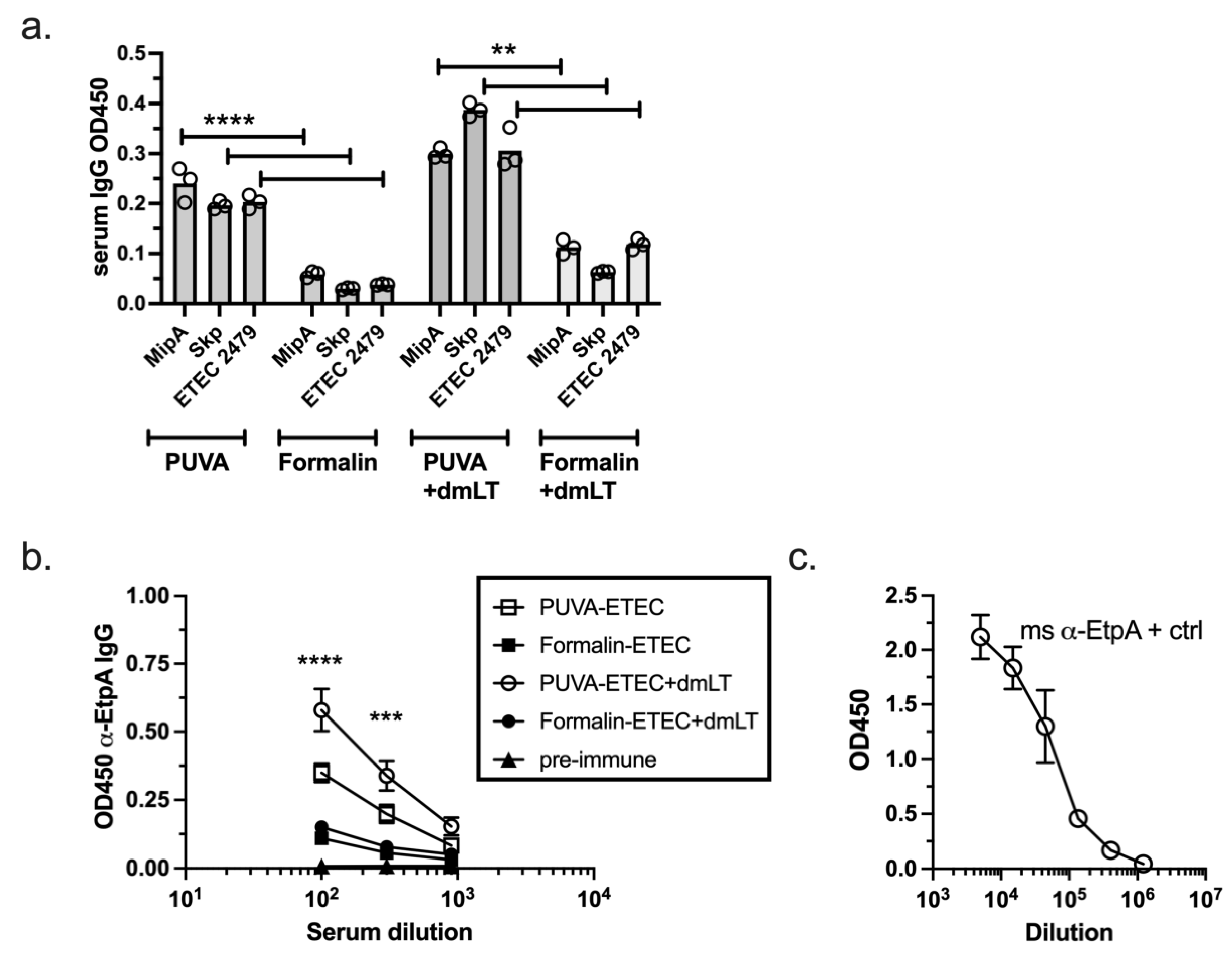
3.7. IgG Response Raised against Conserved ETEC Proteins
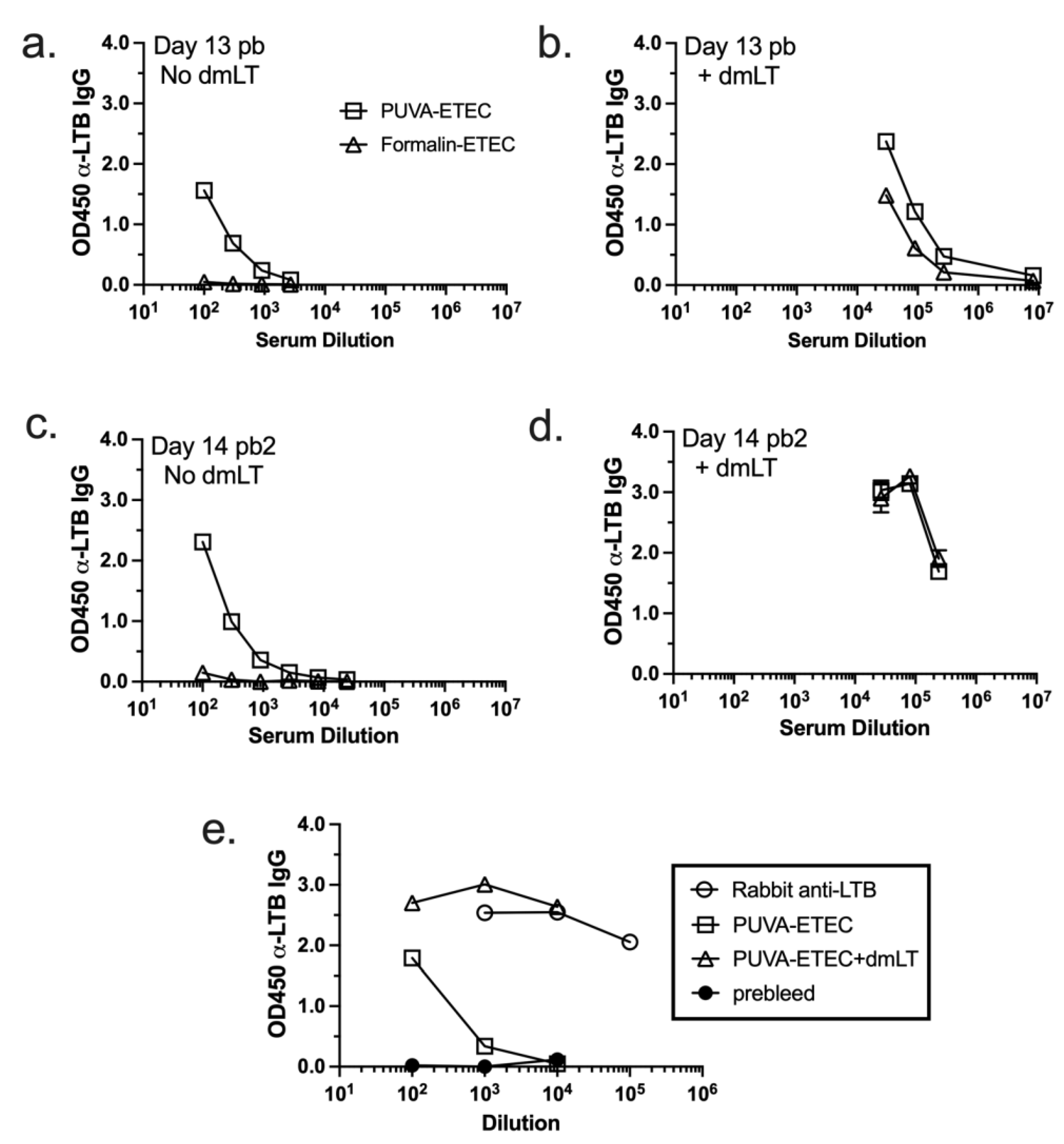
3.8. IgG Response Raised against Heat-Labile Toxin B Subunit (LTB)
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cederna, J.B.; Klinzman, D.; Stapleton, J.T. Hepatitis A virus-specific humoral and cellular immune responses following immunization with a formalin-inactivated hepatitis A vaccine. Vaccine 1999, 18, 892–898. [Google Scholar] [CrossRef]
- Kaminski, R.W.; Wu, M.; Turbyfill, K.R.; Clarkson, K.; Tai, B.; Bourgeois, A.L.; Van De Verg, L.L.; Walker, R.I.; Oaks, E.V. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin. Vaccine Immunol. 2014, 21, 366–382. [Google Scholar] [CrossRef]
- Fan, Y.; Mu, Y.; Lu, L.; Tian, Y.; Yuan, F.; Zhou, B.; Yu, C.; Wang, Z.; Li, X.; Lei, S.; et al. Hydrogen peroxide-inactivated bacteria induces potent humoral and cellular immune responses and releases nucleic acids. Int. Immunopharmacol. 2019, 69, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, P.H. The effect of formaldehyde, hydrogen peroxide and genetic detoxification of pertussis toxin on epitope recognition by murine monoclonal antibodies. Vaccine 1996, 14, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, L.; Dasgupta, U.; Clements, J.D. Influence of strain viability and antigen dose on the use of attenuated mutants of Salmonella as vaccine carriers. Vaccine 1994, 12, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Wood, D.J.; Minor, P.D. Antigenic structure of poliovirus in inactivated vaccines. J. Gen. Virol. 1993, 74 Pt 4, 685–690. [Google Scholar] [CrossRef]
- Tano, Y.; Shimizu, H.; Martin, J.; Nishimura, Y.; Simizu, B.; Miyamura, T. Antigenic characterization of a formalin-inactivated poliovirus vaccine derived from live-attenuated Sabin strains. Vaccine 2007, 25, 7041–7046. [Google Scholar] [CrossRef]
- Murphy, B.R.; Walsh, E.E. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J. Clin. Microbiol. 1988, 26, 1595–1597. [Google Scholar] [CrossRef]
- Delrue, I.; Verzele, D.; Madder, A.; Nauwynck, H.J. Inactivated virus vaccines from chemistry to prophylaxis: Merits, risks and challenges. Expert. Rev. Vaccines 2012, 11, 695–719. [Google Scholar] [CrossRef]
- Schneider, K.; Wronka-Edwards, L.; Leggett-Embrey, M.; Walker, E.; Sun, P.; Ondov, B.; Wyman, T.H.; Rosovitz, M.J.; Bohn, S.S.; Burans, J.; et al. Psoralen Inactivation of Viruses: A Process for the Safe Manipulation of Viral Antigen and Nucleic Acid. Viruses 2015, 7, 5875–5888. [Google Scholar] [CrossRef]
- Hanson, C.V.; Riggs, J.L.; Lennette, E.H. Photochemical inactivation of DNA and RNA viruses by psoralen derivatives. J. Gen. Virol. 1978, 40, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Dubensky, T.W., Jr.; Skoble, J.; Lauer, P.; Brockstedt, D.G. Killed but metabolically active vaccines. Curr. Opin. Biotechnol. 2012, 23, 917–923. [Google Scholar] [CrossRef]
- Cimino, G.D.; Gamper, H.B.; Isaacs, S.T.; Hearst, J.E. Psoralens as photoactive probes of nucleic acid structure and function: Organic chemistry, photochemistry, and biochemistry. Annu. Rev. Biochem. 1985, 54, 1151–1193. [Google Scholar] [CrossRef] [PubMed]
- Groene, W.S.; Shaw, R.D. Psoralen preparation of antigenically intact noninfectious rotavirus particles. J. Virol. Methods 1992, 38, 93–102. [Google Scholar] [CrossRef]
- Maves, R.C.; Castillo Ore, R.M.; Porter, K.R.; Kochel, T.J. Immunogenicity of a psoralen-inactivated dengue virus type 1 vaccine candidate in mice. Clin. Vaccine Immunol. 2010, 17, 304–306. [Google Scholar] [CrossRef][Green Version]
- Raviprakash, K.; Sun, P.; Raviv, Y.; Luke, T.; Martin, N.; Kochel, T. Dengue virus photo-inactivated in presence of 1,5-iodonaphthylazide (INA) or AMT, a psoralen compound (4’-aminomethyl-trioxsalen) is highly immunogenic in mice. Hum. Vaccin. Immunother. 2013, 9, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Maves, R.C.; Ore, R.M.; Porter, K.R.; Kochel, T.J. Immunogenicity and protective efficacy of a psoralen-inactivated dengue-1 virus vaccine candidate in Aotus nancymaae monkeys. Vaccine 2011, 29, 2691–2696. [Google Scholar] [CrossRef]
- Sundaram, A.K.; Ewing, D.; Blevins, M.; Liang, Z.; Sink, S.; Lassan, J.; Raviprakash, K.; Defang, G.; Williams, M.; Porter, K.R.; et al. Comparison of purified psoralen-inactivated and formalin-inactivated dengue vaccines in mice and nonhuman primates. Vaccine 2020, 38, 3313–3320. [Google Scholar] [CrossRef]
- Brockstedt, D.G.; Bahjat, K.S.; Giedlin, M.A.; Liu, W.; Leong, M.; Luckett, W.; Gao, Y.; Schnupf, P.; Kapadia, D.; Castro, G.; et al. Killed but metabolically active microbes: A new vaccine paradigm for eliciting effector T-cell responses and protective immunity. Nat. Med. 2005, 11, 853–860. [Google Scholar] [CrossRef]
- Lankowski, A.J.; Hohmann, E.L. Killed but metabolically active Salmonella typhimurium: Application of a new technology to an old vector. J. Infect. Dis. 2007, 195, 1203–1211. [Google Scholar] [CrossRef]
- Le Gouellec, A.; Chauchet, X.; Laurin, D.; Aspord, C.; Verove, J.; Wang, Y.; Genestet, C.; Trocme, C.; Ahmadi, M.; Martin, S.; et al. A safe bacterial microsyringe for in vivo antigen delivery and immunotherapy. Mol. Ther. 2013, 21, 1076–1086. [Google Scholar] [CrossRef][Green Version]
- Meynet, E.; Laurin, D.; Lenormand, J.L.; Camara, B.; Toussaint, B.; Le Gouellec, A. Killed but metabolically active Pseudomonas aeruginosa-based vaccine induces protective humoral- and cell-mediated immunity against Pseudomonas aeruginosa pulmonary infections. Vaccine 2018, 36, 1893–1900. [Google Scholar] [CrossRef]
- Skoble, J.; Beaber, J.W.; Gao, Y.; Lovchik, J.A.; Sower, L.E.; Liu, W.; Luckett, W.; Peterson, J.W.; Calendar, R.; Portnoy, D.A.; et al. Killed but metabolically active Bacillus anthracis vaccines induce broad and protective immunity against anthrax. Infect. Immun. 2009, 77, 1649–1663. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, J.A.; Babji, S.; Bodhidatta, L.; Gratz, J.; Haque, R.; Havt, A.; McCormick, B.J.; McGrath, M.; Olortegui, M.P.; Samie, A.; et al. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED). Lancet Glob. Health 2015, 3, e564–e575. [Google Scholar] [CrossRef]
- Qadri, F.; Saha, A.; Ahmed, T.; Al Tarique, A.; Begum, Y.A.; Svennerholm, A.M. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect. Immun. 2007, 75, 3961–3968. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Kaminski, R.W.; Porter, C.; Choy, R.K.M.; White, J.A.; Fleckenstein, J.M.; Cassels, F.; Bourgeois, L. Vaccines for Protecting Infants from Bacterial Causes of Diarrheal Disease. Microorganisms 2021, 9, 1382. [Google Scholar] [CrossRef]
- Gaastra, W.; Svennerholm, A.M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 1996, 4, 444–452. [Google Scholar] [CrossRef]
- Holmgren, J.; Bourgeois, L.; Carlin, N.; Clements, J.; Gustafsson, B.; Lundgren, A.; Nygren, E.; Tobias, J.; Walker, R.; Svennerholm, A.M. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine 2013, 31, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Norton, E.B.; Lawson, L.B.; Freytag, L.C.; Clements, J.D. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 2011, 18, 546–551. [Google Scholar] [CrossRef]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018, 3, e00215-18. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Akhtar, M.; Bhuiyan, T.R.; Chowdhury, M.I.; Ahmed, T.; Rafique, T.A.; Khan, A.; Rahman, S.I.A.; Khanam, F.; Lundgren, A.; et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: A double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 2020, 20, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Chowdhury, M.I.; Bhuiyan, T.R.; Kaim, J.; Ahmed, T.; Rafique, T.A.; Khan, A.; Rahman, S.I.A.; Khanam, F.; Begum, Y.A.; et al. Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled Phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Vaccine 2019, 37, 5645–5656. [Google Scholar] [CrossRef]
- Crothers, J.W.; Ross Colgate, E.; Cowan, K.J.; Dickson, D.M.; Walsh, M.; Carmolli, M.; Wright, P.F.; Norton, E.B.; Kirkpatrick, B.D. Intradermal fractional-dose inactivated polio vaccine (fIPV) adjuvanted with double mutant Enterotoxigenic Escherichia coli heat labile toxin (dmLT) is well-tolerated and augments a systemic immune response to all three poliovirus serotypes in a randomized placebo-controlled trial. Vaccine 2022, 40, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, J.; Sheikh, A.; Qadri, F. Novel antigens for enterotoxigenic Escherichia coli vaccines. Expert. Rev. Vaccines 2014, 13, 631–639. [Google Scholar] [CrossRef]
- Chakraborty, S.; Randall, A.; Vickers, T.J.; Molina, D.; Harro, C.D.; DeNearing, B.; Brubaker, J.; Sack, D.A.; Bourgeois, A.L.; Felgner, P.L.; et al. Human Experimental Challenge With Enterotoxigenic Escherichia coli Elicits Immune Responses to Canonical and Novel Antigens Relevant to Vaccine Development. J. Infect. Dis. 2018, 218, 1436–1446. [Google Scholar] [CrossRef]
- Kuhlmann, F.M.; Martin, J.; Hazen, T.H.; Vickers, T.J.; Pashos, M.; Okhuysen, P.C.; Gomez-Duarte, O.G.; Cebelinski, E.; Boxrud, D.; Del Canto, F.; et al. Conservation and global distribution of non-canonical antigens in Enterotoxigenic Escherichia coli. PLoS Negl. Trop. Dis. 2019, 13, e0007825. [Google Scholar] [CrossRef]
- Hays, M.P.; Kumar, A.; Martinez-Becerra, F.J.; Hardwidge, P.R. Immunization with the MipA, Skp, or ETEC_2479 Antigens Confers Protection against Enterotoxigenic E. coli Strains Expressing Different Colonization Factors in a Mouse Pulmonary Challenge Model. Front. Cell. Infect. Microbiol. 2016, 6, 181. [Google Scholar] [CrossRef]
- Evans, D.J., Jr.; Evans, D.G. Three characteristics associated with enterotoxigenic Escherichia coli isolated from man. Infect. Immun. 1973, 8, 322–328. [Google Scholar] [CrossRef]
- DuPont, H.L.; Formal, S.B.; Hornick, R.B.; Snyder, M.J.; Libonati, J.P.; Sheahan, D.G.; LaBrec, E.H.; Kalas, J.P. Pathogenesis of Escherichia coli diarrhea. N. Engl. J. Med. 1971, 285, 1–9. [Google Scholar] [CrossRef]
- Tobias, J.; Svennerholm, A.M.; Holmgren, J.; Lebens, M. Construction and expression of immunogenic hybrid enterotoxigenic Escherichia coli CFA/I and CS2 colonization fimbriae for use in vaccines. Appl. Microbiol. Biotechnol. 2010, 87, 1355–1365. [Google Scholar] [CrossRef]
- Evans, D.G.; Evans, D.J., Jr.; Tjoa, W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: Correlation with colonization factor. Infect. Immun. 1977, 18, 330–337. [Google Scholar] [CrossRef]
- Evans, D.G.; Silver, R.P.; Evans, D.J., Jr.; Chase, D.G.; Gorbach, S.L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect. Immun. 1975, 12, 656–667. [Google Scholar] [CrossRef]
- Petermann, K.; Vordenbaumen, S.; Pyun, J.C.; Braukmann, A.; Bleck, E.; Schneider, M.; Jose, J. Autodisplay of 60-kDa Ro/SS-A antigen and development of a surface display enzyme-linked immunosorbent assay for systemic lupus erythematosus patient sera screening. Anal. Biochem. 2010, 407, 72–78. [Google Scholar] [CrossRef]
- O’Dowd, A.; Maciel, M., Jr.; Poole, S.T.; Jobling, M.G.; Rollenhagen, J.E.; Woods, C.M.; Sincock, S.A.; McVeigh, A.L.; Gregory, M.J.; Maves, R.C.; et al. Evaluation of the Immunogenicity and Protective Efficacy of an Enterotoxigenic Escherichia coli CFA/I Adhesin-Heat-Labile Toxin Chimera. Infect. Immun. 2020, 88, e00252. [Google Scholar] [CrossRef]
- Satterwhite, T.K.; Evans, D.G.; DuPont, H.L.; Evans, D.J., Jr. Role of Escherichia coli colonisation factor antigen in acute diarrhoea. Lancet 1978, 2, 181–184. [Google Scholar] [CrossRef]
- Baqar, S.; Applebee, L.A.; Bourgeois, A.L. Immunogenicity and protective efficacy of a prototype Campylobacter killed whole-cell vaccine in mice. Infect. Immun. 1995, 63, 3731–3735. [Google Scholar] [CrossRef]
- Kumar, A.; Hays, M.; Lim, F.; Foster, L.J.; Zhou, M.; Zhu, G.; Miesner, T.; Hardwidge, P.R. Protective Enterotoxigenic Escherichia coli Antigens in a Murine Intranasal Challenge Model. PLoS Negl. Trop. Dis. 2015, 9, e0003924. [Google Scholar] [CrossRef]
- Luo, Q.; Vickers, T.J.; Fleckenstein, J.M. Immunogenicity and Protective Efficacy against Enterotoxigenic Escherichia coli Colonization following Intradermal, Sublingual, or Oral Vaccination with EtpA Adhesin. Clin. Vaccine Immunol. 2016, 23, 628–637. [Google Scholar] [CrossRef]
- Roy, K.; Hamilton, D.J.; Fleckenstein, J.M. Cooperative role of antibodies against heat-labile toxin and the EtpA Adhesin in preventing toxin delivery and intestinal colonization by enterotoxigenic Escherichia coli. Clin. Vaccine Immunol. 2012, 19, 1603–1608. [Google Scholar] [CrossRef]
- Horstman, A.L.; Kuehn, M.J. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. J. Biol. Chem. 2002, 277, 32538–32545. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, A.L.; Wierzba, T.F.; Walker, R.I. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 2016, 34, 2880–2886. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The Diversity of Escherichia coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef]
- Jalili, Z.; Saleh, M.; Bouzari, S.; Pooya, M. Characterization of killed but metabolically active uropathogenic Escherichia coli strain as possible vaccine candidate for urinary tract infection. Microb. Pathog. 2018, 122, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Hanawalt, P.C.; Crowley, D.J.; Ford, J.M.; Ganesan, A.K.; Lloyd, D.R.; Nouspikel, T.; Smith, C.A.; Spivak, G.; Tornaletti, S. Regulation of nucleotide excision repair in bacteria and mammalian cells. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.M.; Acott, J.D.; Courcelle, C.T.; Courcelle, J. Limited Capacity or Involvement of Excision Repair, Double-Strand Breaks, or Translesion Synthesis for Psoralen Cross-Link Repair in Escherichia coli. Genetics 2018, 210, 99–112. [Google Scholar] [CrossRef]
- Osorio, M.; Bray, M.D.; Walker, R.I. Vaccine potential for inactivated shigellae. Vaccine 2007, 25, 1581–1592. [Google Scholar] [CrossRef]
- Roy, K.; Hamilton, D.; Allen, K.P.; Randolph, M.P.; Fleckenstein, J.M. The EtpA exoprotein of enterotoxigenic Escherichia coli promotes intestinal colonization and is a protective antigen in an experimental model of murine infection. Infect. Immun. 2008, 76, 2106–2112. [Google Scholar] [CrossRef]
- Porter, C.K.; Riddle, M.S.; Tribble, D.R.; Louis Bougeois, A.; McKenzie, R.; Isidean, S.D.; Sebeny, P.; Savarino, S.J. A systematic review of experimental infections with enterotoxigenic Escherichia coli (ETEC). Vaccine 2011, 29, 5869–5885. [Google Scholar] [CrossRef]
- Kumar, P.; Luo, Q.; Vickers, T.J.; Sheikh, A.; Lewis, W.G.; Fleckenstein, J.M. EatA, an immunogenic protective antigen of enterotoxigenic Escherichia coli, degrades intestinal mucin. Infect. Immun. 2014, 82, 500–508. [Google Scholar] [CrossRef]
- Baqar, S.; Bourgeois, A.L.; Schultheiss, P.J.; Walker, R.I.; Rollins, D.M.; Haberberger, R.L.; Pavlovskis, O.R. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine 1995, 13, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Helander, A.; Grewal, H.M.; Gaastra, W.; Svennerholm, A.M. Detection and characterization of the coli surface antigen 6 of enterotoxigenic Escherichia coli strains by using monoclonal antibodies. J. Clin. Microbiol. 1997, 35, 867–872. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westcott, M.M.; Blevins, M.; Wierzba, T.F.; Morse, A.E.; White, K.R.; Sanders, L.A.; Sanders, J.W. The Immunogenicity and Properties of a Whole-Cell ETEC Vaccine Inactivated with Psoralen and UVA Light in Comparison to Formalin. Microorganisms 2023, 11, 2040. https://doi.org/10.3390/microorganisms11082040
Westcott MM, Blevins M, Wierzba TF, Morse AE, White KR, Sanders LA, Sanders JW. The Immunogenicity and Properties of a Whole-Cell ETEC Vaccine Inactivated with Psoralen and UVA Light in Comparison to Formalin. Microorganisms. 2023; 11(8):2040. https://doi.org/10.3390/microorganisms11082040
Chicago/Turabian StyleWestcott, Marlena M., Maria Blevins, Thomas F. Wierzba, Alexis E. Morse, Kinnede R. White, Leigh Ann Sanders, and John W. Sanders. 2023. "The Immunogenicity and Properties of a Whole-Cell ETEC Vaccine Inactivated with Psoralen and UVA Light in Comparison to Formalin" Microorganisms 11, no. 8: 2040. https://doi.org/10.3390/microorganisms11082040
APA StyleWestcott, M. M., Blevins, M., Wierzba, T. F., Morse, A. E., White, K. R., Sanders, L. A., & Sanders, J. W. (2023). The Immunogenicity and Properties of a Whole-Cell ETEC Vaccine Inactivated with Psoralen and UVA Light in Comparison to Formalin. Microorganisms, 11(8), 2040. https://doi.org/10.3390/microorganisms11082040





