Fluorescent Nanoparticle Uptake by Myzocytosis and Endocytosis in Colpodella sp. ATCC 50594
Abstract
1. Introduction
2. Materials and Methods
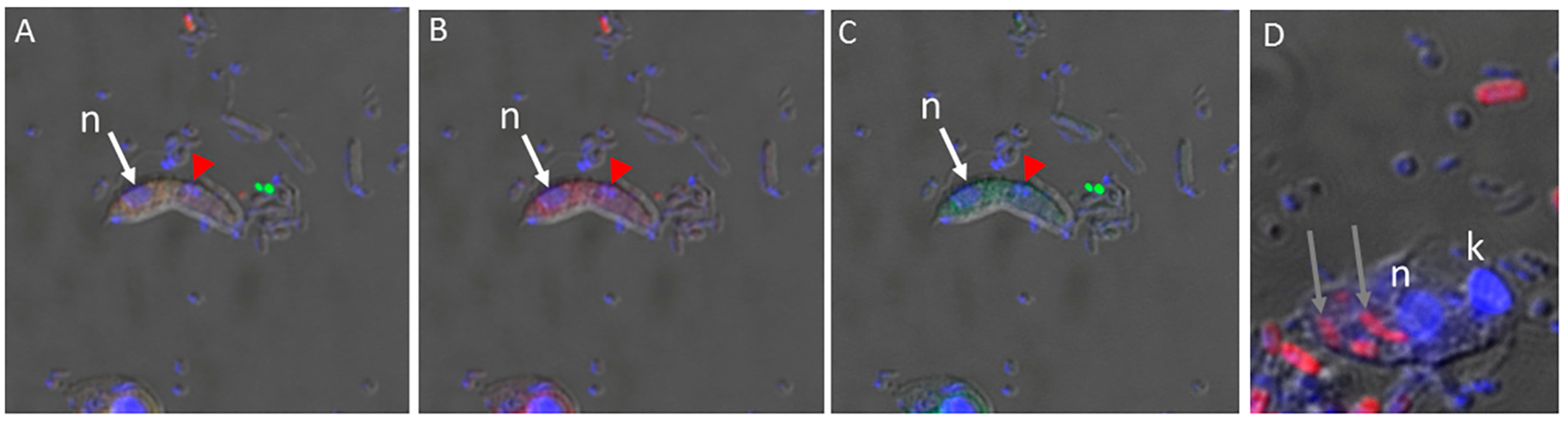
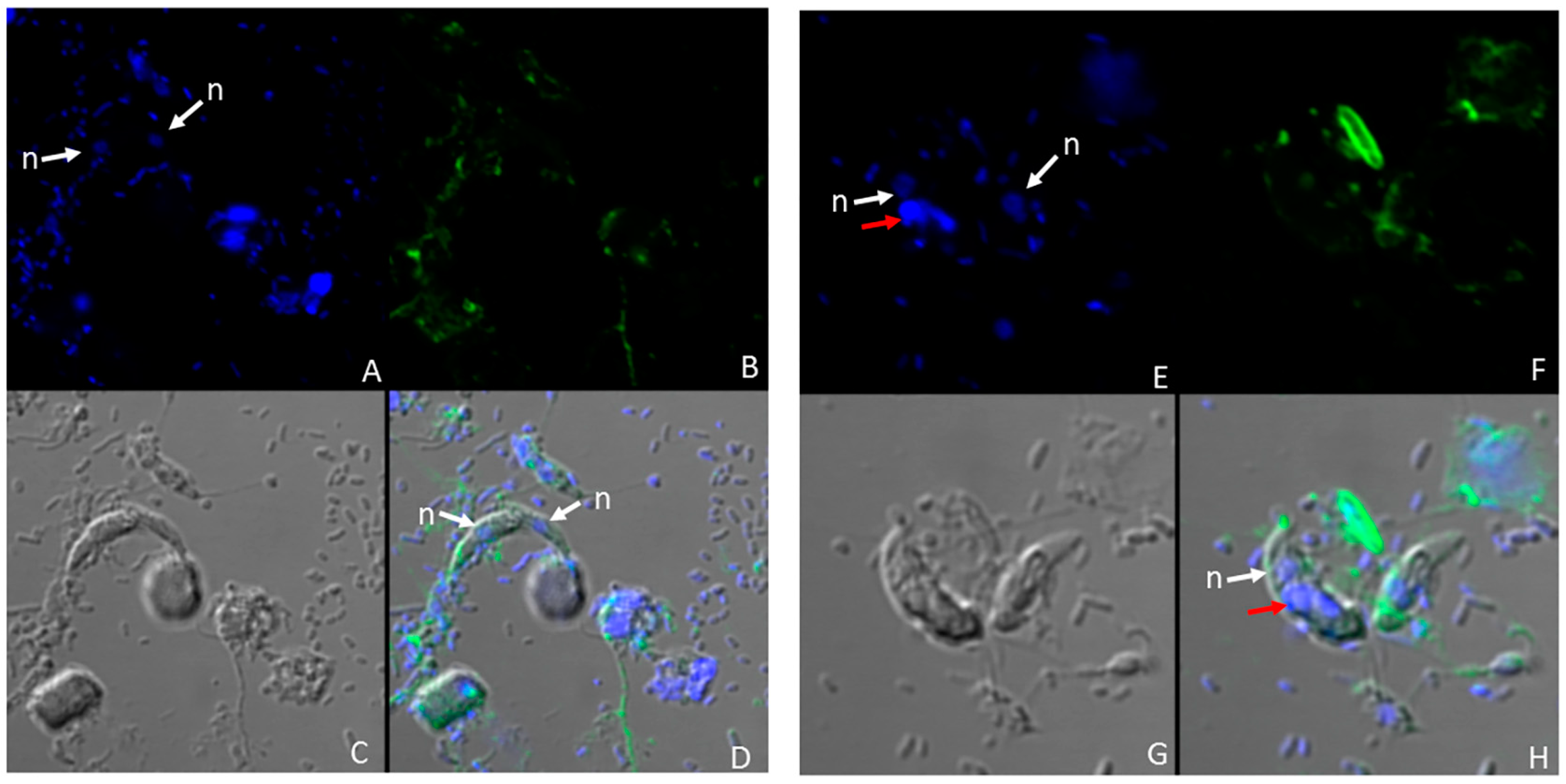
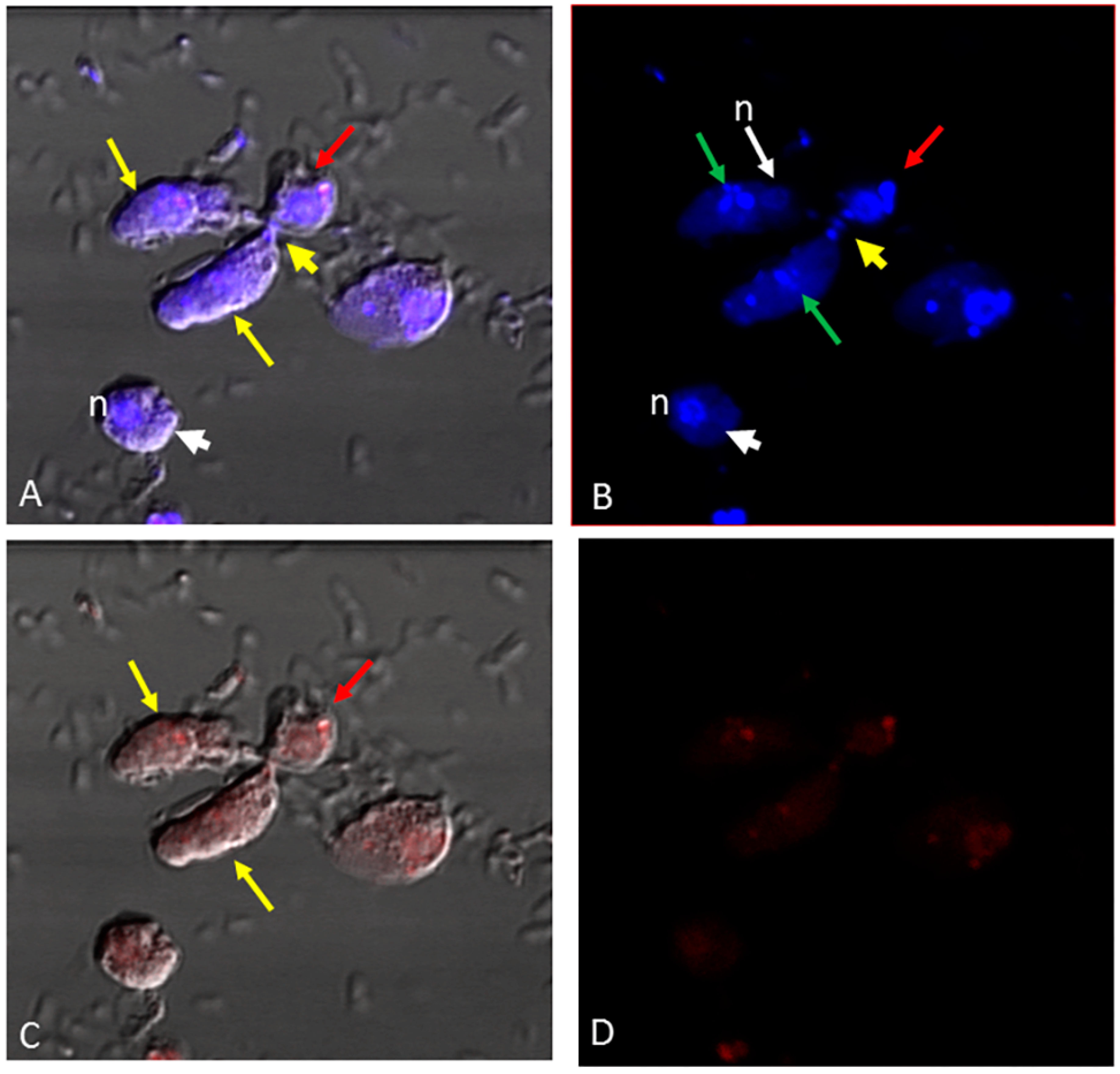
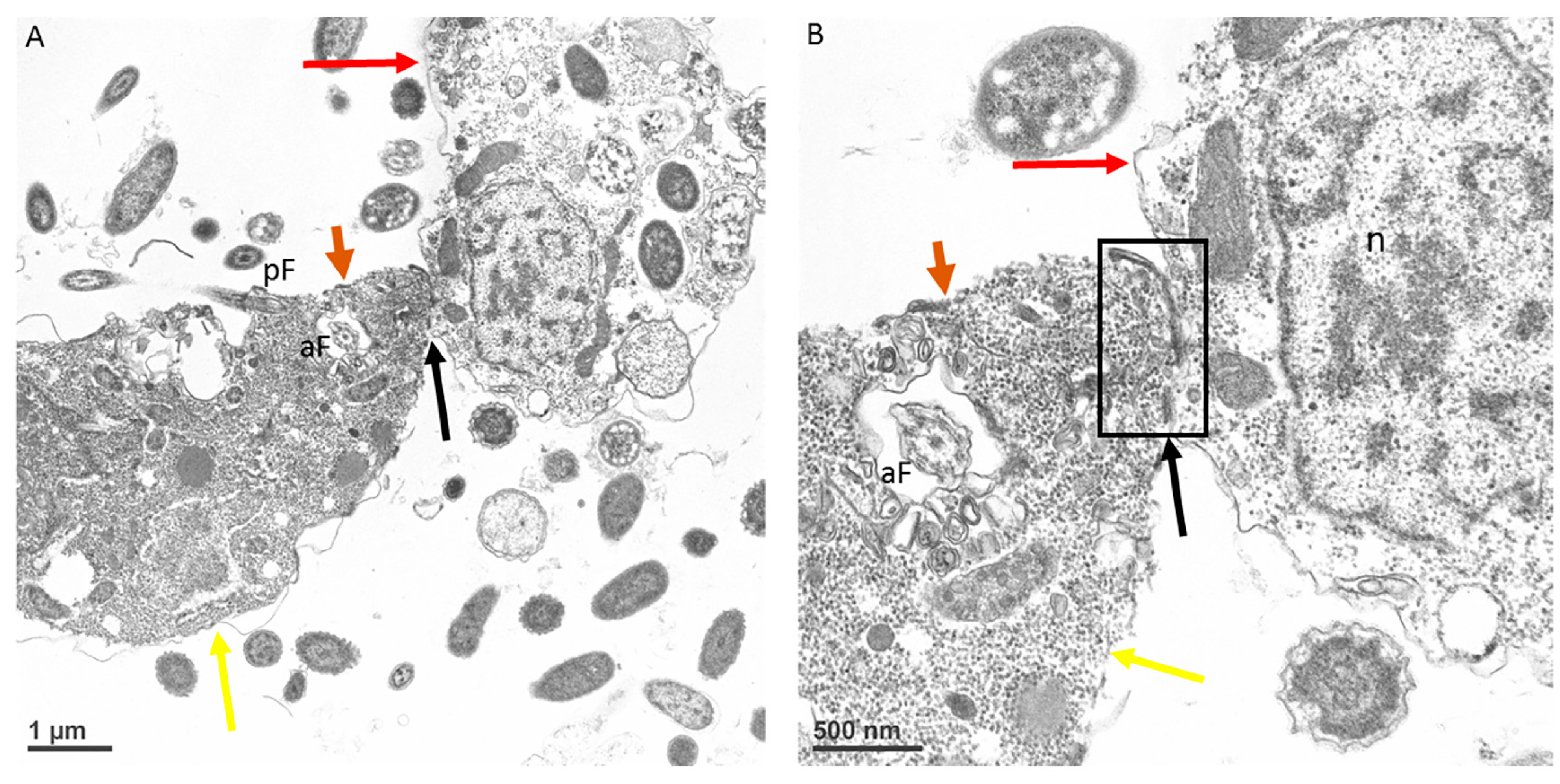
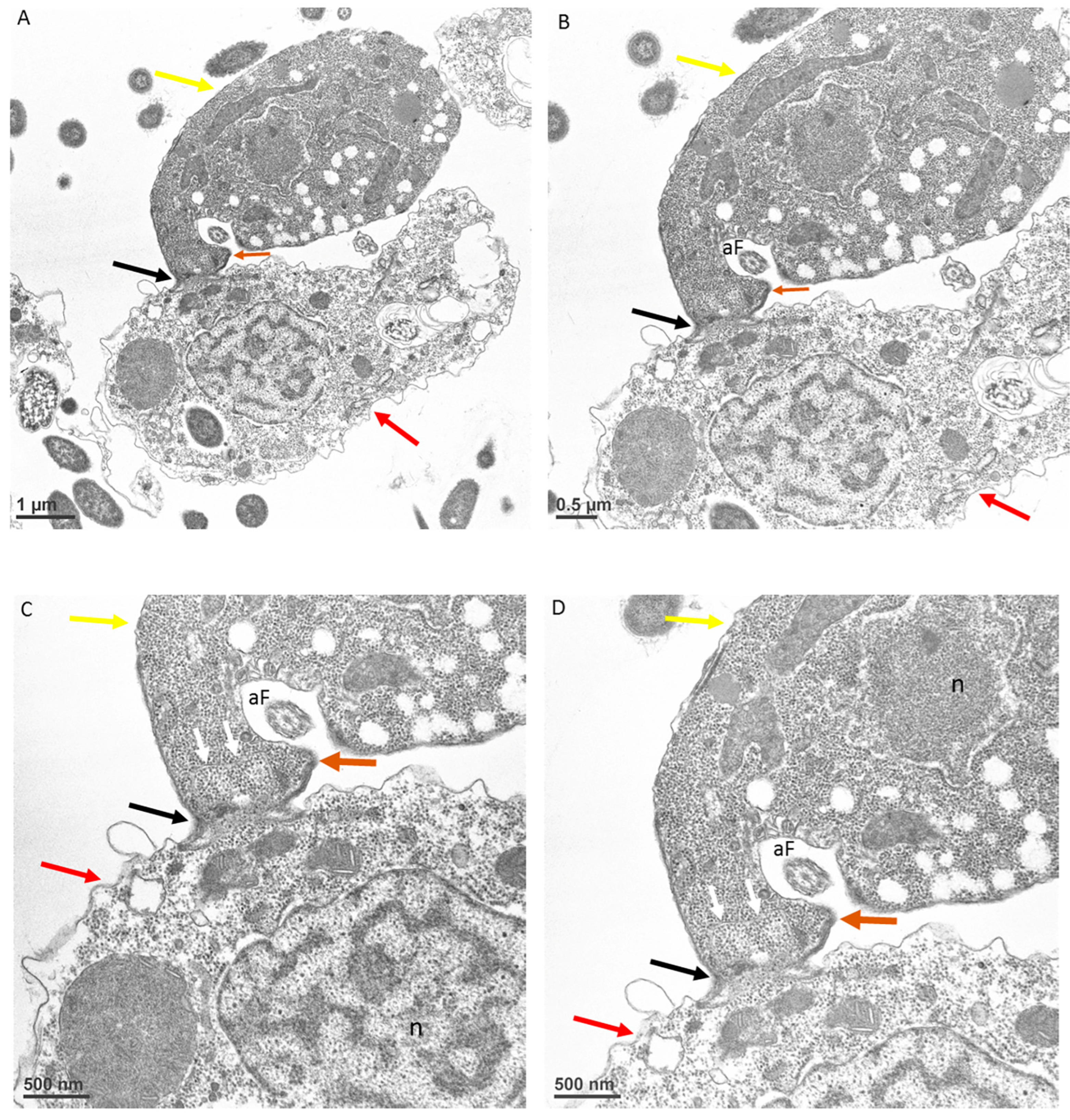
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kuvardina, O.N.; Leander, B.S.; Aleshin, V.V.; Mylnikov, A.P.; Keeling, P.J.; Simdyanov, T.G. The phylogeny of colpodellids (Alveolata) using small subunit rRNAgene sequences suggests they are the free-living sister group to apicomplexans. J. Euk. Microbiol. 2002, 49, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Janouškovec, J.; Paskerova, G.G.; Miroliubova, T.S.; Mikhailov, K.V.; Birley, T.; Aleoshin, V.V.; Simdyanov, T.G. Apicom-plexan-like parasites are polyphyletic and widely but selectively dependent on cryptic plastid organelles. Elife 2019, 16, e49662. [Google Scholar]
- Cavalier-Smith, T.; Chao, E.E. Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates (phylum Myzozoa nom. Nov.). Eur. J. Protistol. 2004, 40, 185–212. [Google Scholar]
- Ben Chaabene, R.; Lentini, G.; Soldati-Favre, D. Biogenesis and discharge of the rhoptries: Key organelles for entry and hijack of host cells by the Apicomplexa. Mol. Microbiol. 2021, 115, 453–465. [Google Scholar] [CrossRef]
- Valigurová, A.; Paskerova, G.G.; Diakin, A.; Kováčiková, M.; Simdyanov, T.G. Protococcidian Eleutheroschizon duboscqi, an Unusual Apicomplexan Interconnecting Gregarines and Cryptosporidia. PLoS ONE 2015, 10, e0125063. [Google Scholar] [CrossRef]
- Cowman, A.F.; Tonkin, C.J.; Tham, W.H.; Duraisingh, M.T. The Molecular Basis of Erythrocyte Invasion by Malaria Parasites. Cell Host Microbe 2017, 22, 232–245. [Google Scholar]
- Valigurová, A.; Florent, I. Nutrient Acquisition and Attachment Strategies in Basal Lineages: A Tough Nut to Crack in the Evolutionary Puzzle of Apicomplexa. Microorganisms 2021, 9, 1430. [Google Scholar] [CrossRef]
- Valigurová, A.; Hofmannová, L.; Koudela, B.; Vávra, J. An ultrastructural comparison of the attachment sites between Gregarina steini and Cryptosporidium muris. J. Eukaryot. Microbiol. 2007, 54, 495–510. [Google Scholar] [CrossRef]
- Mylnikov, A.P.; Mylnikova, Z.M. Feeding spectra and pseudoconoid structure in predatory alveolate flagellates. Inland Water Biol. 2008, 1, 210–216. [Google Scholar] [CrossRef]
- Mylnikov, A.P. Ultrastructure and phylogeny of colpodellids (Colpodellida, Alveolata). Biol. Bull. 2009, 36, 582–590. [Google Scholar] [CrossRef]
- Olmo, J.L.; Esteban, G.F.; Finlay, B.J. New records of the ectoparasitic flagellate Colpodella gonderi on non-Colpoda ciliates. J. Int. Microbiol. 2011, 14, 207–211. [Google Scholar]
- Koreny, L.; Mercado-Saavedra, B.N.; Klinger, C.M.; Barylyuk, K.; Butterworth, S.; Hirst, J.; Rivera-Cuevas, Y.; Zaccai, N.R.; Holzer, V.J.C.; Klingl, A.; et al. Stable endocytic structures navigate the complex pellicle of apicomplexan parasites. Nat. Commun. 2023, 14, 2167. [Google Scholar]
- Wan, W.; Dong, H.; Lai, D.H.; Yang, J.; He, K.; Tang, X.; Liu, Q.; Hide, G.; Zhu, X.Q.; Sibley, L.D.; et al. The Toxoplasma micropore mediates endocytosis for selective nutrient salvage from host cell compartments. Nat. Commun. 2023, 14, 977. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.L.; Keeling, P.J.; Krause, P.J.; Horak, A.; Bent, S.; Rollend, L.; Hua, X.G. Colpodella spp.–like Parasite Infection in Woman, China. Emerg. Infect. Dis. 2012, 18, 125–127. [Google Scholar] [CrossRef]
- Jiang, J.-F.; Jiang, R.-R.; Chang, Q.-C.; Zheng, Y.-C.; Jiang, B.-G.; Sun, Y.; Jia, N.; Wei, R.; Bo, H.-B.; Huo, Q.-B.; et al. Potential novel tick-borne Colpodella species parasite infection in patient with neurological symptoms. PLoS Negl. Trop. Dis. 2018, 12, e0006546. [Google Scholar]
- Simdyanov, T.G.; Paskerova, G.G.; Valigurová, A.; Diakin, A.; Kováčiková, M.; Schrével, J.; Guillou, L.; Dobrovolskij, A.A.; Aleoshin, V.V. First Ultrastructural and Molecular Phylogenetic Evidence from the Blastogregarines, an Early Branching Lineage of Plesiomorphic Apicomplexa. Protist 2018, 169, 697–726. [Google Scholar] [PubMed]
- Schrével, J.; Valigurová, A.; Prensier, G.; Chambouvet, A.; Florent, I.; Guillou, L. Ultrastructure of Selenidium pendula, the Type Species of Archigregarines, and Phylogenetic Relations to Other Marine Apicomplexa. Protist 2016, 167, 339–368. [Google Scholar] [CrossRef]
- Nakada-Tsukui, K.; Nozaki, T. Trogocytosis in Unicellular Eukaryotes. Cells 2021, 10, 2975. [Google Scholar] [CrossRef]
- Wiser, M.F. Unique Endomembrane Systems and Virulence in Pathogenic Protozoa. Life 2021, 11, 822. [Google Scholar] [CrossRef]
- Yadavalli, R.; Sam-Yellowe, T.Y. Developmental stages identified in the trophozoite of the free-living Alveolate flagellate Colpodella sp. (Apicomplexa). J. Int. Microbiol. 2017, 20, 178–183. [Google Scholar]
- Sam-Yellowe, T.Y.; Yadavalli, R. Giemsa staining and antibody characterization of Colpodella sp. (Apicomplexa). J. Microbiol. Modern Tech. 2018, 3, 103. [Google Scholar]
- Sam-Yellowe, T.Y.; Getty, T.A.; Addepalli, K.; Walsh, A.M.; Williams-Medina, A.R.; Fujioka, H.; Peterson, J.W. Novel life cycle stages of Colpodella sp. (Apicomplexa) identified using Sam-Yellowe’s trichrome stains and confocal and electron microscopy. Int. Microbiol. 2022, 25, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Getty, T.A.; Peterson, J.W.; Fujioka, H.; Walsh, A.M.; Sam-Yellowe, T.Y. Colpodella sp. (ATCC 50594) Life Cycle: Myzocytosis and Possible Links to the Origin of Intracellular Parasitism. Trop. Med. Infect. Dis. 2021, 6, 127. [Google Scholar] [PubMed]
- Sam-Yellowe, T.Y.; Fujioka, H.; Peterson, J.W. Ultrastructure of Myzocytosis and Cyst Formation, and the Role of Actin in Tubular Tether Formation in Colpodella sp. (ATCC 50594). Pathogens 2022, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Sam-Yellowe, T.Y.; Addepalli, K.; Yadavalli, R.; Peterson, J.W. New trichrome stains identify cysts of Colpodella sp. (Apicomplexa) and Bodo caudatus. J. Int. Microbiol. 2019, 23, 303–311. [Google Scholar] [CrossRef]
- McGregor, A.L.; Hsia, C.R.; Lammerding, J. Squish and squeeze-the nucleus as a physical barrier during migration in confined environments. Curr. Opin. Cell Biol. 2016, 40, 32–40. [Google Scholar] [CrossRef]
- Gubbels, M.-J.; Duraisingh, M.T. Evolution of apicomplexan secretory organelles. Int. J. Parasitol. 2012, 42, 1071–1081. [Google Scholar] [CrossRef]
- Simdyanov, T.G.; Kuvardina, O.N. Fine structure and putative feeding mechanism of the archigregarine Selenidium orientale (Apicomplexa: Gregarinomorpha). Eur. J. Protistol. 2007, 43, 17–25. [Google Scholar] [CrossRef]
- Schnepf, E.; Deichgräber, G. “Myzocytosis”, a kind of endocytosis with implications to compartmentation in endosymbiosis. Naturwissenschaften 1984, 71, 218–219. [Google Scholar] [CrossRef]
- Hoppe, H.C.; van Schalkwyk, D.A.; Wiehart, U.I.; Meredith, S.A.; Egan, J.; Weber, B.W. Antimalarial quinolines and artemisinin inhibit endocytosis in Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 2370–2378. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sam-Yellowe, T.Y.; Asraf, M.M.; Peterson, J.W.; Fujioka, H. Fluorescent Nanoparticle Uptake by Myzocytosis and Endocytosis in Colpodella sp. ATCC 50594. Microorganisms 2023, 11, 1945. https://doi.org/10.3390/microorganisms11081945
Sam-Yellowe TY, Asraf MM, Peterson JW, Fujioka H. Fluorescent Nanoparticle Uptake by Myzocytosis and Endocytosis in Colpodella sp. ATCC 50594. Microorganisms. 2023; 11(8):1945. https://doi.org/10.3390/microorganisms11081945
Chicago/Turabian StyleSam-Yellowe, Tobili Y., Mary M. Asraf, John W. Peterson, and Hisashi Fujioka. 2023. "Fluorescent Nanoparticle Uptake by Myzocytosis and Endocytosis in Colpodella sp. ATCC 50594" Microorganisms 11, no. 8: 1945. https://doi.org/10.3390/microorganisms11081945
APA StyleSam-Yellowe, T. Y., Asraf, M. M., Peterson, J. W., & Fujioka, H. (2023). Fluorescent Nanoparticle Uptake by Myzocytosis and Endocytosis in Colpodella sp. ATCC 50594. Microorganisms, 11(8), 1945. https://doi.org/10.3390/microorganisms11081945




