1. Introduction
Haemogregarines are blood parasites of the phylum Apicomplexa with a life-cycle involving a vertebrate as an intermediate host and an invertebrate as a definitive host and vector. They are currently classified into four families: the Haemogregarinidae which comprise three genera, namely
Haemogregarina,
Cyrilia, and
Desseria; the Hepatozoidae with a single genus,
Hepatozoon; the Karyolysidae with two genera, namely
Hemolivia and
Karyolysus; and the Dactylosomatidae, also with two genera,
Babesiosoma and
Dactylosoma [
1]. Whereas the intermediate host encompasses all types of vertebrates, i.e., fishes, mammals, amphibians, reptiles, and birds, the vector host encompasses solely leeches and acarines. Since Siddall [
2] and his work based on a cladistic approach of morphological characters, the systematics of haemogregarines have been greatly improved. Siddal [
2] was the first to show
Haemogregarina as a polyphyletic taxon. He thus transferred all species infecting snakes, lizards, crocodilians, and birds, which were originally described as
Haemogregarina spp., into the genus
Hepatozoon and retained all other species infecting fishes, amphibians, and chelonians in the genus
Haemogregarina sensu lato. In addition, based on other informative characters, the distinction of
Haemogregarina sensu stricto was allowed to accommodate mostly
Haemogregarina spp. infecting chelonians. Smith [
3] completed the classification revised by Siddall [
2] in transferring certain haemogregarine species from one genus to another. Following the advent of molecular data in phylogenetics by the end of the 1990s, certain haemogregarines have been investigated for their phylogenetic position within the Apicomplexa—more particularly, species of
Hepatozoon [
4,
5,
6]. The first comprehensive study that included the taxa of the four recognized families was conducted by Barta et al. [
7], although
Hepatozoon species were still over-represented in their phylogeny in comparison to the other taxa, namely
Hemolivia,
Dactylosoma,
Bebesiosoma, and
Haemogregarina. Later on, the non-monophyly of
Hepatozoon species was evidenced [
8,
9,
10] following an investigation of the phylogenetic relationships of species of the four haemogregarine familes. In addition to the paraphyly of
Hepatozoon species, the paraphyly of the Karyolysidae was also illustrated [
11].
While the monophyly of particular genera and their relationships within the Adeleorina remain to be investigated using molecular markers, numerous questions have been raised regarding the genetic diversity of haemogregarines infecting vertebrate hosts, especially freshwater chelonians. Extensive phylogenetic investigations during the ten past years on
Haemogregarina stepanowi Danilewsky 1885 have demonstrated that this species is able to infest a large diversity of the turtle species of North Africa and Western Europe: among others, the European pond turtle
Emys orbicularis (Linnaeus); and the Sicilian pond turtle
Emys trinacris Fritz, Fattizzo, Guicking, Tripepi, Pennisi, Lenk, Joger, and Wink of the Emydidae; the Caspian turtle
Mauremys caspica (Gmelin); the Mediterranean pond turtle
Mauremys leprosa (Schweigger); and the Western Caspian turtle
Mauremys rivulata (Valenciennes) of the Geoemydidae [
12,
13,
14]. Although the intermediate host specificity for this parasite was believed to be very low, Attia El Hili et al. [
15] further evidenced from 18S-rRNA gene sequences the occurrence of distinct species of
Haemogregarina within the same chelonian host species, in both
M. leprosa and
E. orbicularis turtles, thus suggesting coinfection. Based on genetic data, one of these haemogregarine species could be considered with certainty to be
H. stepanowi. All of these results were soon confirmed from an analysis of target COI sequences obtained from the same isolates of blood samples [
16]. Nine distinct COI haplotypes were revealed corresponding to three molecular lineages, each corresponding to a distinct
Haemogregarina species, as previously advocated from an analysis of nuclear markers [
15]. A similarly high
Haemogregarina genetic diversity has since been evidenced from mitochondrial markers within the Savanna side-necked turtle
Podocnemis vogli Müller [
17], as well as from nuclear markers within the yellow-spotted Amazon river turtle
Podocnemis unifilis Troschel [
18] and
E. orbicularis [
19]. Due to the low host specificity of
H. stepanowi against its intermediate host on the one hand [
12,
13,
14] and the high diversity of
Haemogregarina spp. in certain turtle species on the other [
16,
17,
18,
19], the host specificity of
Haemogregarina could act against leeches—namely
Placobdella as the definitive host—as claimed earlier by Attia El Hili et al. [
16].
Placobdella is a widespread genus of blood-feeding leeches belonging to the family Glossiphoniidae (Clitellata, Hirudinea, Rhynchobdellida). It includes 25 recognized species [
20], most of which display a Nearctic distribution [
21].
Placobdella leeches are common ectoparasites of freshwater vertebrates. It has been shown that host specificity differs among
Placobdella spp. Certain leeches exhibit an opportunistic generalist behavior with a wide range of host species: for instance,
Placobdella montifera Moore 1906 [
22,
23] and
Placobdella phalera (Graf 1899) [
24] which infest a great many species;
Placobdella multilineata Moore 1953 which has been reported in 17 species and subspecies of reptiles, such as alligators, crocodiles, snakes, and turtles, as well as in amphiumas [
25]; and
Placobdella ornata (Verrill 1872) which infests turtles, as well as fish, amphibians, birds, and humans [
22]. In contrast, certain other leeches exhibit a very strict host specificity. This is the case for
Placobdella cryptobranchii (Johnson and Klemm 1977) [
26] which has been reported from a single amphibian host species, i.e., the Ozark hellbender
Cryptobranchus alleganiensis bishopi Grobman [
22,
24,
26], although Moser et al. [
27] claimed an additional host species, i.e., the Mudpuppy
Necturus maculosus (Rafinesque). This was also the case for
Placobdella appalachiensis Moser and Hopkins 2014 which was reported from a single host species, i.e., the Eastern hellbender
Cryptobranchus alleganiensis (Daudin) [
28].
Placobdella costata (Fr Müller 1846), the type species of the genus
Placobdella, was considered as the only European representative of the genus until Soors et al. [
29] reported
Placobdella ornata (Verril 1872) in Europe following the introduction of its host. While it was first thought to infect a single turtle species, i.e.,
E. orbicularis across its entire geographical distribution, from Western Europe to Russia [
30], as well as in Algeria [
31] and Tunisia [
15],
P. costata is now known to infect certain other turtles. This has indeed been reported from
M. caspica in Iran [
32,
33], from
E. trinacris in Sicilia [
34], and from
M. leprosa in Spain [
35,
36], Morocco [
37], and Tunisia [
15]. While it is now well received from the investigation of molecular markers that
Placobdella is a monophyletic genus, certain cryptic diversity was evidenced within several species, among which are
Placobdella mexicana Moore 1898 and
Placobdella ringueleti López-Jiménez and Oceguera-Figueroa 2009 [
38]. Based on COI and ITS1 genetic variations, Kvist et al. [
39] also illustrated seven independent lineages within
P. costata, suggesting at least five unique leech species across Central and Eastern Europe, as well as in Algeria. Independent phylogenetic relationships were shown among Italian populations of
P. costata and their two host species
E. orbicularis and
E. trinacris [
40]; thus, Kvist et al. [
39] concluded that species diversity and speciation within
P. costata were likely not the result of speciation, isolation, or dispersal of the host species.
Hence, considering the low specificity of H. stepanowi towards its intermediate chelonian hosts, the high diversity of Haemogregarina spp. within turtles, as well as the cryptic species diversity within P. costata, we aimed to investigate the genetic diversity within haemogregarines and leeches infecting North African freshwater turtles. Our objectives were to compare phylogenetic patterns between hosts and their parasites in order to identify processes of parasite speciation.
2. Materials and Methods
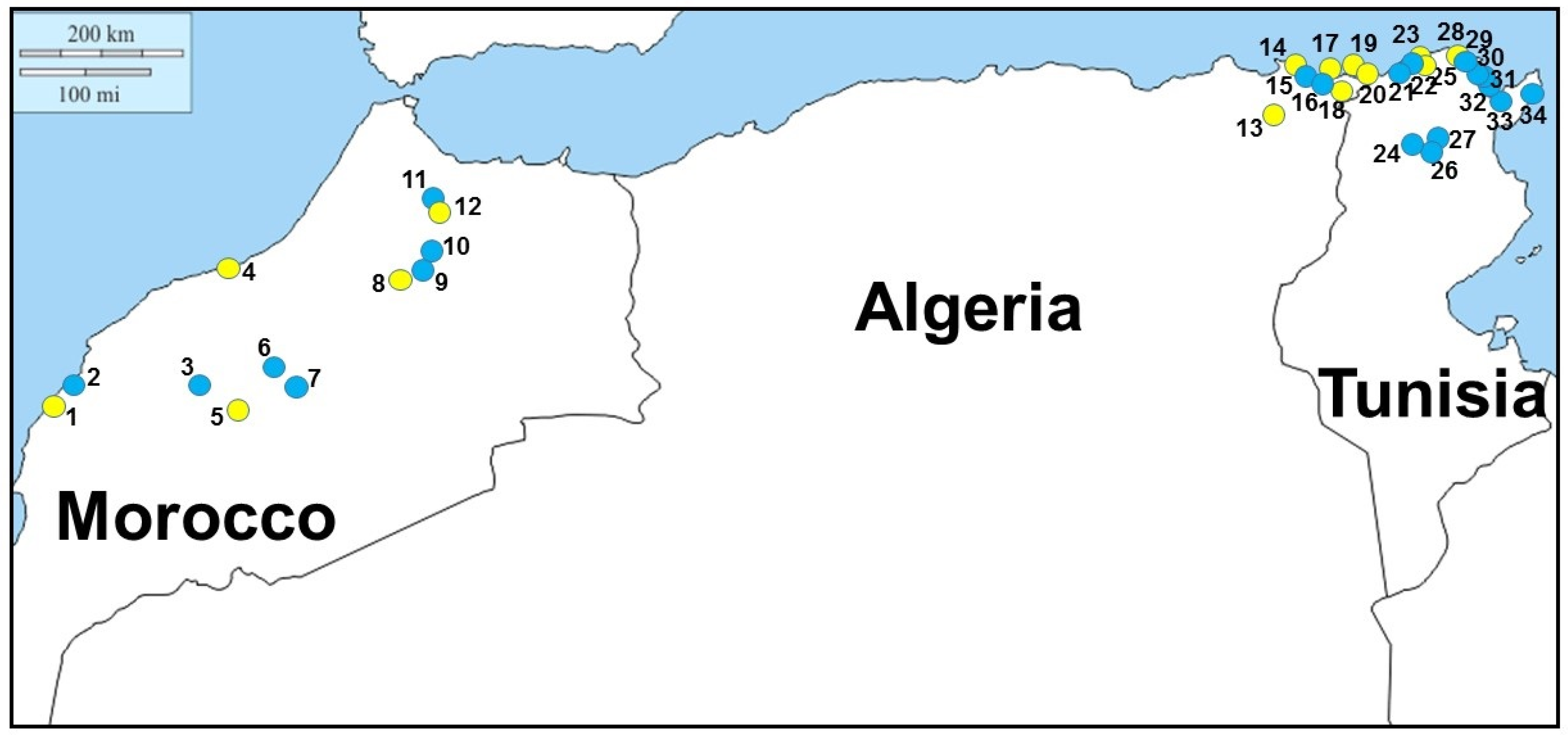
Field-work investigations were carried out on 2019 and 2020, mainly in freshwater aquatic environments of Morocco, Algeria, and Tunisia suitable for
E. orbicularis and
M. leprosa (see
Figure 1 and
Table 1 for GPS localities). Sampling sessions were conducted during the peak activity period of both turtles, from March to November. Traps were baited with fish and set in waters with plastic bottles to maintain them at the surface water and to prevent turtles’ drowning. They were firmly attached to the bank of the water bodies with a solid rope and checked every day, usually between one to three days, before being removed. Trapped turtles were marked individually on the marginal scutes for further capture–mark–recapture procedures. They were sexed, measured, weighted, and checked for ectoparasites. Leeches usually occur at the base of the anterior and/or posterior limbs. When present, they were collected and preserved in 70% alcohol for molecular systematic studies. Blood was also collected from each specimen from the dorsal coccygeal vein running in the midline of the turtle’s tail with the help of 1-mL insulin syringes. A drop of blood was immediately spread out on a glass slide, fixed with a few drops of methanol after air drying, and stored temporarily until staining in the lab. Of the remaining blood, one half was stored in 90% ethanol for molecular systematic studies of the host and their haemoparasites, and the second half was preserved into two distinct Microtainer
® tubes with anticoagulant that were immediately frozen in liquid nitrogen before being transported to the lab where they were finally stored at −80 °C for further biochemical analyses. Investigated turtles were released at the same place of capture in the field.
Blood smears on slides were stained with 10% Giemsa for 20 min in the laboratory, air dried, and examined by optical microscopy using a ×40 objective for the first screening. A Leica digital camera using a ×100 objective was used to capture images for biometric measurements and developmental-stage identification according to Dvoráková et al. [
12,
41]. A total of 422 blood smears were prepared and examined, including 406 from
M. leprosa collected at 12 localities of Morocco, six of Algeria, and 14 of Tunisia, and 16 from
E. orbicularis collected at five localities of Algeria.
DNA extractions were performed with the E.Z.N.A Tissue DNA Kit (Omega bio-tek, Norcross, GA, USA) following recommendations of the supplier for blood and tissue samples preserved in ethanol. DNA extracts were resuspended in approximately 200 µL of Tris-EDTA 1X elution buffer before use for PCR. The cytochrome c oxidase I (COI) of leeches was amplified using the forward LCO Plac 5′-AYTCAACTAATCAYAAAGAYATTGG-3′ and reverse HCO Plac 5′-TADACTTCWGGRTGACCAAAAAATCA-3′ primers which were designed for
Placobdella spp. [
38]. The COI of haemogregarines was amplified using the forward HemoFor4 5′-TGGACATTATACCCACCTTTAAG-3′ and reverse HemoRev4 5′-ATACAACCCATAGCTAGTATCAT-3′ primers which were designed for
Haemogregarina spp. [
16]. PCR assays of COI were conducted in a final volume of 25 μL comprising 1X buffer, 1.5 mM MgCl
2, 0.2 mM dNTPs, 0.4 mM primers, 0.75 units GoTaq Polymerase (Promega, Charbonnières-les-Bains, France), and DNA (3 µL) under the following conditions: an initial step of 5 min at 95 °C for long denaturation; followed by 35 cycles of 30 s at 95 °C for denaturation, 30 s at 48 °C for annealing, and 1 min at 72 °C for elongation; with a final step of 10 min at 72 °C for terminal extension. The success of the PCR amplifications was controlled following electrophoresis in gels of 1% agarose stained with ethidium bromide. When amplifications were successful, they yielded PCR fragments of approximately 700 bp for the COI of leeches and 465 bp for the COI of haemogregarines which were subsequently sequenced by the Genoscreen Company (Lille, France).
COI chromatograms were edited with Chromas 2.2.6 (Technelysium Pty Ltd., Brisbane, Australia), and the resulting new sequences obtained for leeches and haemogregarines were respectively grouped in two distinct files with certain others that were extracted from GenBank. The final COI leech data set included 78 COI sequences corresponding to 23 distinct
Placobdella species on the one hand and two
Helobdella species used for outgroup comparisons on the other. At this stage,
P. costata was considered as a single species and referred as
P. costata sensu lato. Conversely, the final COI protozoan data set included 86 COI sequences corresponding to seven species of Piroplasmida, 17 species of Haemosporida, and 30 species of Eucoccidiorida, including nine putative
Haemogregarina species from two South American turtles, and
Scrippsiella sweeneyae Balech 1965 used for outgroup comparisons [
16]. At this stage,
H. stepanowi was considered as a single species and referred as
H. stepanowi sensu lato. The COI sequences included in both data sets were aligned independently using Clustal W [
42] implemented in MEGA7 [
43] under default parameters.
For the Bayesian analysis, a GTR + I + G model was selected independently for the two COI partitions following the hierarchical likelihood ratio tests (hLRTs) implemented in Modeltest 3.06 [
44]. Therefore, six types of substitutions and invariable-gamma rates with four gamma rate categories were applied for each partition, evolutionary parameters being estimated independently. The leech COI partition included 689 characters, while the protozoan COI partition included 448 characters. Bayesian analyses were run using MrBayes 3.04b [
45], with four chains running for five million generations and sampled every 100 cycles. Consensus trees for both data sets were drawn, after removing the first 5000 trees as the burn-in phase, and opened with TreeGraph 2 [
46]. These were converted into a phylogram, and only nodes supported by more than 0.75 posterior probabilities were shown.
For the parsimony approach, a bootstrap test with 1000 replicates was applied for each data set following a heuristic search under PAUP* Version 4.0 b10 [
47] on the parsimony-informative characters, gaps being treated as missing data. The nearest neighbor interchange (NNI) branch-swapping option was used to find trees of minimal length. In order not to weight down the illustrated trees, only bootstrap proportions higher than 75% were indicated next to the branches within the clades
P. costata sensu lato and
H. stepanowi sensu lato.
For the distance analysis, a bootstrap test with 5000 replicates was applied for each data set. Minimum evolution (ME) trees were searched using the close-neighbor-interchange (CNI) algorithm [
48] at a search level of one for each replication based on Kimura two-parameter distances [
49] that were computed under the pairwise-deletion option. Evolutionary analyses were conducted in MEGA7 [
43]. Regarding parsimony, only bootstrap proportions higher than 75% were indicated next to the branches for the two clades of interest.
A total of 22 COI sequences from Placobdella rugosa (Verrill 1874) were retrieved from GenBank in order to investigate the molecular threshold within Placobdella. All of these sequences correspond to distinct leech specimens that were sampled at separate sites across Canada, which represents most of the geographic range of that species; thus, we expected that the intra-average distances measured within all these specimens may reflect the average genetic variations within each Placobdella species. Pairwise p-distances were calculated within P. rugosa and the average distance with its standard deviation (SD) was then estimated and given with the minimum and maximum distances between all investigated specimens. Finally, pairwise p-distances were calculated within and between haploclades of P. costata sensu lato to explore the species diversity.
The interspecific average pairwise COI p-distance was estimated to about 2.1% between two closely related valid distinct species, namely
Eimeria lancasterensis Joseph 1969 and
Eimeria sciurorum Galli-Valerio 1922, and further considered as the molecular threshold within
Haemogregarina [
16]. Therefore, pairwise p-distances were calculated within and between haploclades of
H. stepanowi sensu lato to explore the species diversity based on the molecular threshold.
3. Results
Following field-work investigations,
M. leprosa was sampled in 12 localities of Morocco, among which five showed the presence of leeches attached to turtles. Following blood observations, haemogregarines were observed within turtles in five localities: only three of them had leeches, the other two did not. The prevalence of haemogregarine infection varied from approximately 11% to 80% in localities where turtles were found to be infected (
Table 1). In Algeria,
M. leprosa was sampled in six localities, among which three showed the presence of leeches attached to turtles. Following blood observations, haemogregarines were observed within turtles in five localities: only three of them had leeches, the other two did not. The prevalence of haemogregarine infection varied from approximately 25% to 75% in localities where turtles were found to be infected.
Emys orbicularis was also sampled in five localities of Algeria—three times in syntopy with
M. leprosa—among which three localities showed the presence of leeches attached to turtles. Haemogregarines were observed within turtles in three localities, a single one had leeches, the other two did not. The prevalence of haemogregarine infection varied from approximately 50% to 75% in localities where turtles were found to be infected (
Table 1). In Tunisia,
M. leprosa was sampled in 14 localities, among which three showed the presence of leeches attached to turtles. Following blood observations, haemogregarines were observed within turtles in three localities: only two of them had leeches, the last one did not. The prevalence of haemogregarine infection varied from approximately 58% to 67% in localities where turtles were found to be infected (
Table 1).
In total, 31 leeches were processed for their COI, 11 collected from
M. leprosa sampled from four localities of Morocco, 15 collected from
M. leprosa and
E. orbicularis sampled from six localities of Algeria, and five collected from
M. leprosa sampled from three localities of Tunisia (
Table 2). They were characterized by six distinct haplotypes, C8a to C8c in Morocco, C5a to C5c in Algeria, and C5a to C5b in Tunisia. According to the phylogenetic reconstruction shown in
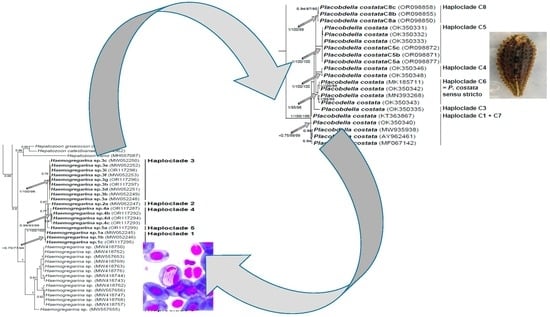
Figure 2,
P. costata sensu lato constitutes a robust clade that can be split into six distinct lineages, namely haploclades C1 + C7, C3, C4, C5, and C6 based on [
39], and Haploclade C8 based on the present results. Conversely, due to technical difficulties in amplifying the COI of haemogregarines from blood samples, only 13 sequences were obtained from
M. leprosa sampled from two localities of Morocco, from one locality of Algeria, and from two localities of Tunisia (
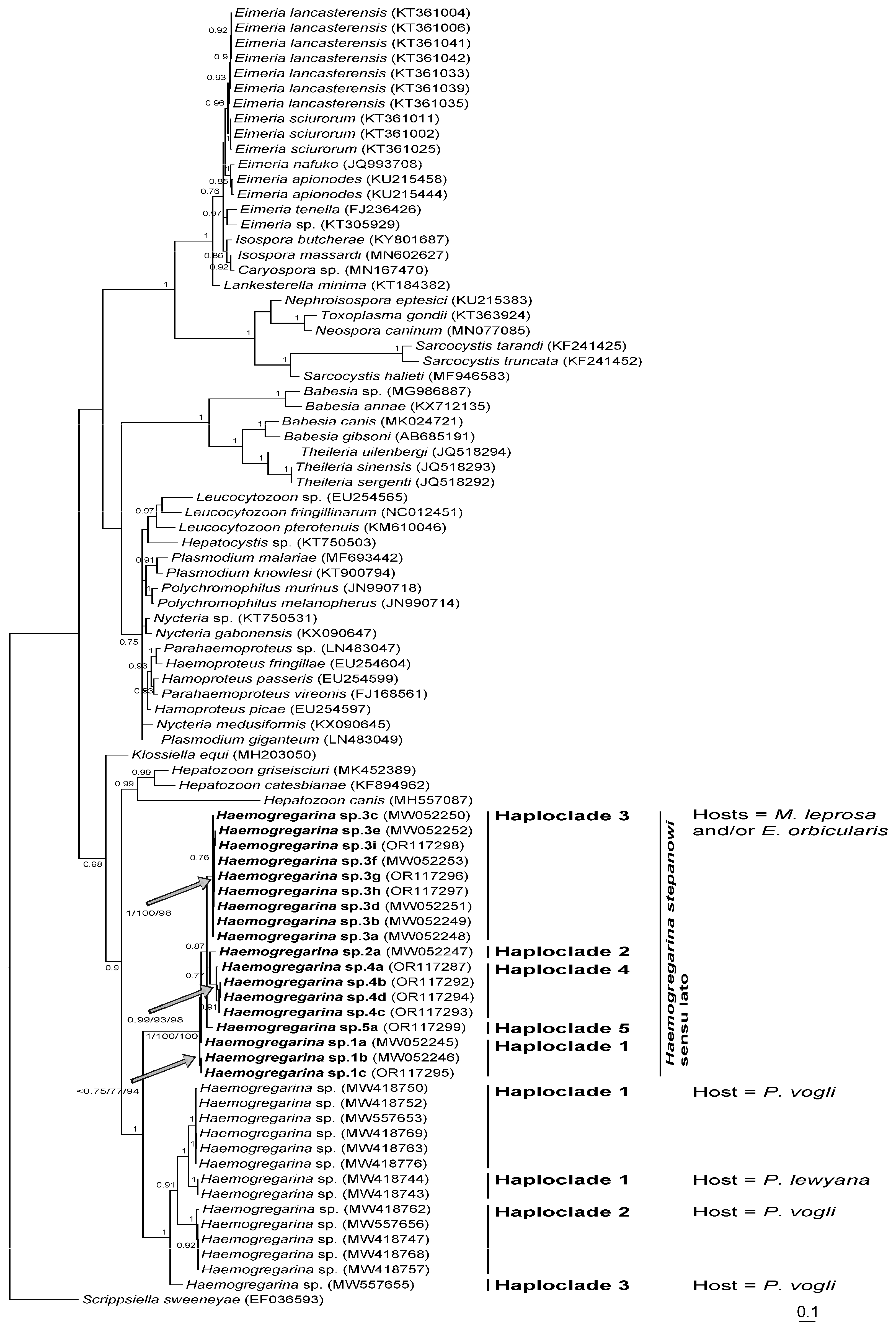
Table 3). These were characterized by nine distinct haplotypes: 4a to 4d in Morocco, 1c in Algeria, and 3g to 3i and 5a in Tunisia. According to the phylogenetic reconstruction shown in
Figure 3,
H. stepanowi sensu lato constitutes a robust clade that can be split into five distinct lineages, namely haploclades 1 to 3 based on Attia El Hili et al. [
16], and haploclades 4 and 5 based on the present results.
Regarding the average p-distance that was estimated within
P. rugosa, the COI molecular threshold can be considered as approximately 0.90% pairwise substitutions ± 0.0042 within
Placobdella (
Table 4). Therefore, according to the average p-distances that were estimated within haploclades of
P. costata sensu lato on the one hand, which varied from 0.30% for the minimal average distance to 1.44% for the maximal average distance (
Table 4), and between haploclades of
P. costata sensu lato on the other, which varied from 4.19% for the minimal average distance to 8.15% for the maximal distance (
Table 5), each haploclade can be considered as a distinct species, which is in agreement with Kvist et al. [
39]. If Haploclade C6 can be considered as
P. costata sensu stricto according to Kvist et al. [
39] (see
Figure 2), leeches that were collected from
M. leprosa and
E. orbicularis of Algeria and Tunisia can be regarded as the undescribed species corresponding to Haploclade C5 that was previously identified [
39]. Similarly, leeches that were collected from
M. leprosa of Morocco can be regarded as another undescribed species corresponding to Haploclade C8 identified here for the first time.
Considering the importance of molecular COI of 2.1% pairwise substitutions [
16], the average p-distances that were estimated within haploclades of
H. stepanowi sensu lato on the one hand, which varied from 0.43% for the minimal average distance to 1.61% for the maximal average distance (
Table 6), and between haploclades of
H. stepanowi sensu lato on the other, which varied from 6.71% for the minimal average distance to 8.21% for the maximal distance (
Table 7), suggest that each haploclade can be considered as a distinct species. While it is still difficult to assign one haplotype to
H. stepanowi sensu stricto, haploclades 1 and 3 characterizing haemogregarines sampled from
M. leprosa of Algeria and Tunisia, respectively, were previously identified in Attia El Hili et al. [
16]. Conversely, haploclades 4 and 5 characterizing haemogregarines sampled from
M. leprosa of Morocco and Tunisia, respectively, were identified here for the first time.
4. Discussion
Regarding the genetic diversity of
P. costata leeches sampled in Morocco, Algeria, and Tunisia, we can consider that two species are well differentiated in the Maghreb. According to Kvist et al. [
39],
P. costata is phylogenetically split into seven independent lineages in the Northern Mediterranean basin, Tunisia, and Algeria—at least five of which correspond to distinct species based on COI genetic variations. Among the mitochondrial haploclades that were illustrated in [
39], the C5 haploclade, which groups three different haplotypes characterizing leeches collected in Algeria and Tunisia, was also recovered in our study from leeches sampled in the same area (Haplotypes C5a–c), C5a–b being found in Algeria and Tunisia, and C5c being restricted to Algeria. In addition, we identified a new distinct haploclade that for convenience we named Haploclade C8. The latter also contained three distinct haplotypes (C8a–c) that characterized leeches that were collected in four distant localities of Morocco: C8a being found in three distinct localities, and C8b–c being restricted in the fourth locality. While the levels of COI variation clearly indicated that the C8 haploclade could also be considered as a distinct species, the phylogenetic relationships between all haploclades did not allow the resolution of the deepest nodes in the tree as already illustrated in [
39], thus suggesting a rapid diversification of leeches. Based on phylogeographic studies of several amphibian and reptile species across their geographical distribution area in the Maghreb (Morocco, Algeria, and Tunisia), an Eastern–Western speciation pattern was evidenced for most of the investigated species [
50]. This speciation pattern was very similar to the phylogeography of
M. leprosa that supported its northwestern origin before the species colonized Europe [
51]. It was impossible to consider a sister group relationship between the C5 and C8 haploclades, and at least two leech species infect
M. leprosa in its home range of Africa; thus, it is very unlikely that leeches in the Maghreb co-diverged along with their chelonian hosts. Furthermore, C5b was found in both turtle species, i.e.,
M. leprosa and
E. orbicularis, thus strengthening the conclusions of Vecchioni et al. [
40] who clearly showed that the speciation of
P. costata leeches infecting
E. orbicularis and
E. trinacris in Italy was not the result of co-divergence processes.
Regarding the COI genetic diversity of
H. stepanowi revealed during this study on the blood of freshwater turtles from Morocco, Algeria, and Tunisia, and that already evidenced in [
16], we can assume that at least five haemogregarine species are well differentiated in the Maghreb. Based on our phylogenetic tree,
H. stepanowi sensu lato was phylogenetically split into five independent lineages in the Southern Mediterranean basin: Haploclades 2, 3, and 5 being restricted to Tunisia, Haploclade 1 to Tunisia and Algeria, and Haploclade 4 to Morocco. From the same mitochondrial marker, three independent lineages within the host
P. vogli of South America were also reported [
17]. Surprisingly, a fourth molecular lineage infecting the Rio Magdalena River turtle
Podocnemis lewyana Duméril was found nested within that complex of haploclades [
17], thus suggesting the occurrence of at least four undescribed species infecting two distinct host species (see
Figure 3). While levels of COI genetic variations clearly indicate the occurrence of several haemogregarine species within the same host species in sympatry, even in syntopy, which is illustrated by numerous cases of coinfections, it is still extremely difficult to link a particular molecular lineage to a specific morphological species, as also reported in [
17]. While the first molecular investigations on
Haemogregarina have shown from nuclear markers that
H. stepanowi possesses a wide distribution though Europe, Turkey, and the Middle East to Iran with a very low host-specificity [
12], the most recent advents in molecular systematics of
H. stepanowi have shown that this species could be actually split into a great number of distinct cryptic haemogregarine species [
15,
19,
52,
53], infecting
E. orbicularis and
M. leprosa on the one hand and additional hosts such as
M. rivulata and the invasive turtle species
Trachemys scripta (Thunberg) [
19] on the other. Therefore, one may wonder whether the haemogregarine distribution is correlated to that of its definitive host, namely the
Placobdella leech.
Investigating the links between host and parasite diversities to assess evolutionary processes requires well-resolved host and parasite phylogenies. It is also of utmost importance to set up exhaustive sampling procedures as far as possible in order to reduce the biases due to missing data in the field. Regarding leech and haemogregarine phylogenies, while a single leech species was found in Morocco (Haploclade C8), a single haemogregarine species was also evidenced in the same geographical area (Haploclade 4). Neither of these two haploclades were found in Algeria and Tunisia, suggesting a close correspondence between the parasite and its definitive host in Morocco. However, given the technical difficulties encountered in amplifying the COI of the haemoparasites, we did not obtain any information about parasite genotypes infecting turtles in Oued Zat and Oued Sebou localities—while we did for leeches. Additional data would therefore be needed to make conclusions. Concerning the eastern part of the investigated area, Algeria and Tunisia, while a single leech species was found across the sampled turtles (Haploclade C5), three distinct haemogregarine species were evidenced in the same geographical area, i.e., Haploclade 1 in Lac Noir (Algeria), Haploclade 3 in Ghayada (Tunisia), and Haploclade 5 in El Garia (Tunisia). Although none of these parasite haploclades were ever evidenced in Morocco, such as the host haploclade that was restricted to Algeria and Tunisia, it is still difficult to make conclusions regarding a close correspondence between the parasite and its definitive host in the eastern part of Maghreb because of the occurrence of the three distinct parasite lineages. However, it should be noted that no leeches were recovered from El Garia (Tunisia), which prevented linking Parasite Lineage 5 to a particular leech haploclade. Similarly, and as explained above, the difficulties encountered in amplifying the COI of haemogregarines did not allow for the genotyping of parasites in the Algerian Madjen Belhriti, Brabtia, and Lac Tonga, or Tunisian El Hania localities, while the genotypes of leeches were recovered. Additional data should help to provide conclusions regarding strict host (leech) and parasite (haemogregarine) specificity.
Although an Eastern–Western speciation pattern is apparent for both leeches and haemogregarines, with a certain level of geographical host fidelity for parasites, we cannot make definitive conclusions regarding co-speciation patterns between haemoparasites and leeches. The diversity of haemogregarines is the highest in the eastern part of the Maghreb; thus, we may also expect a higher leech diversity in that area, which is currently not the case. For this reason, deeper sampling should be conducted to collect more leeches on the one hand, and in as many localities as possible on the other. Lastly, the genotyping of haemogregarines was performed directly from blood samples of turtles; thus, we recommend that future studies genotype haemogregarines directly from leech blood extracts. Indeed, while host–parasite specificity seems to be very low within turtles, we still cannot reject the hypothesis of a very strict host–parasite specificity within leeches.