Herpes Simplex Virus Type 1 Infection Induces the Formation of Tunneling Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Virus
2.3. MTT Assay
2.4. Drug Treatment
2.5. Immunofluorescence Staining
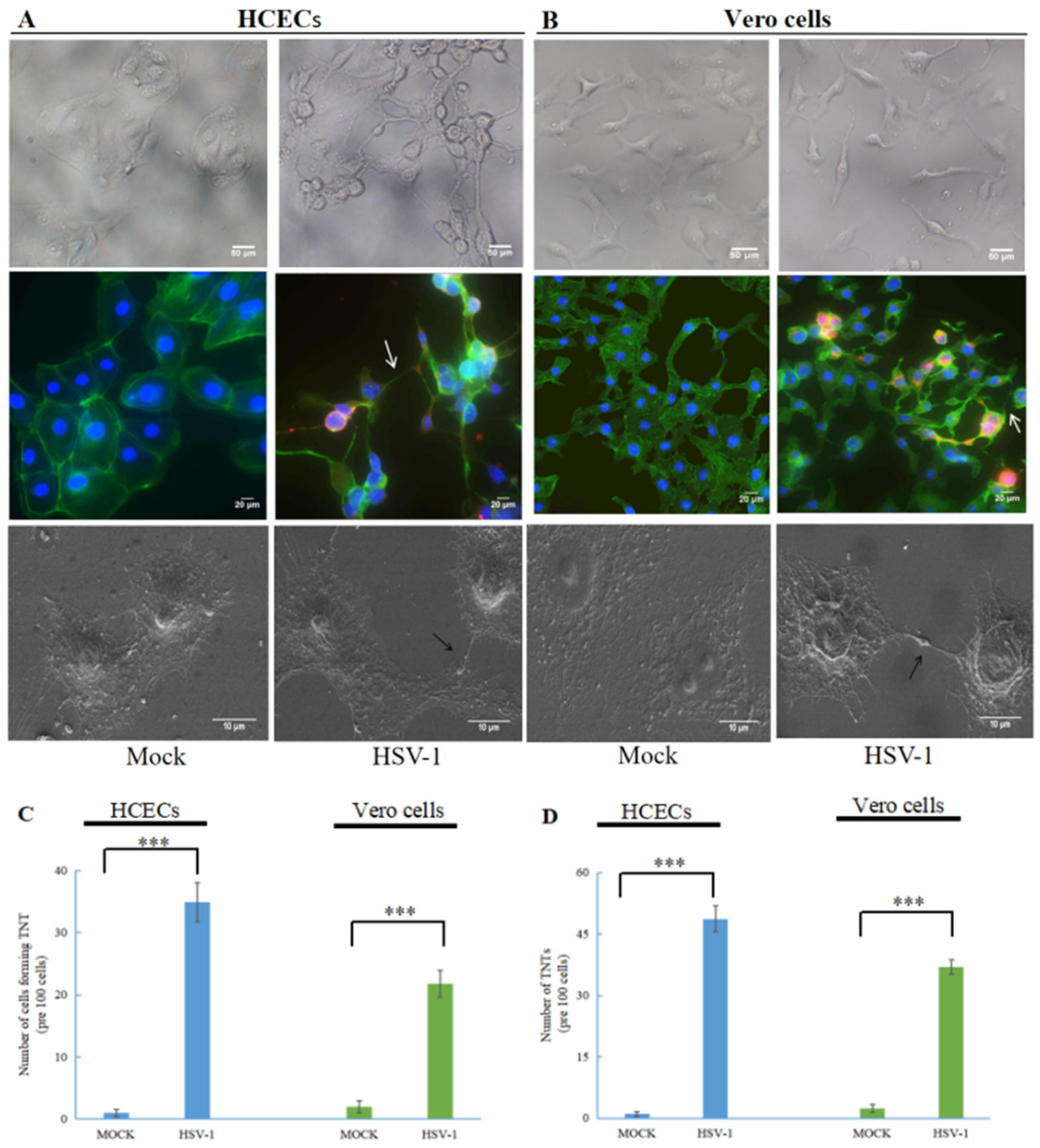
2.6. TNT Counting
2.7. Scanning Electron Microscopy (SEM)
2.8. Transmission Electron Microscopy (TEM)
2.9. Quantitative Polymerase Chain Reaction (qPCR)
2.10. Plaque Assays
2.11. Statistical Analysis
3. Results
3.1. Human Herpes Virus Type 1 (HSV-1) Induces the Formation of TNTs
3.2. TNTs Are Involved in HSV-1 Transmission
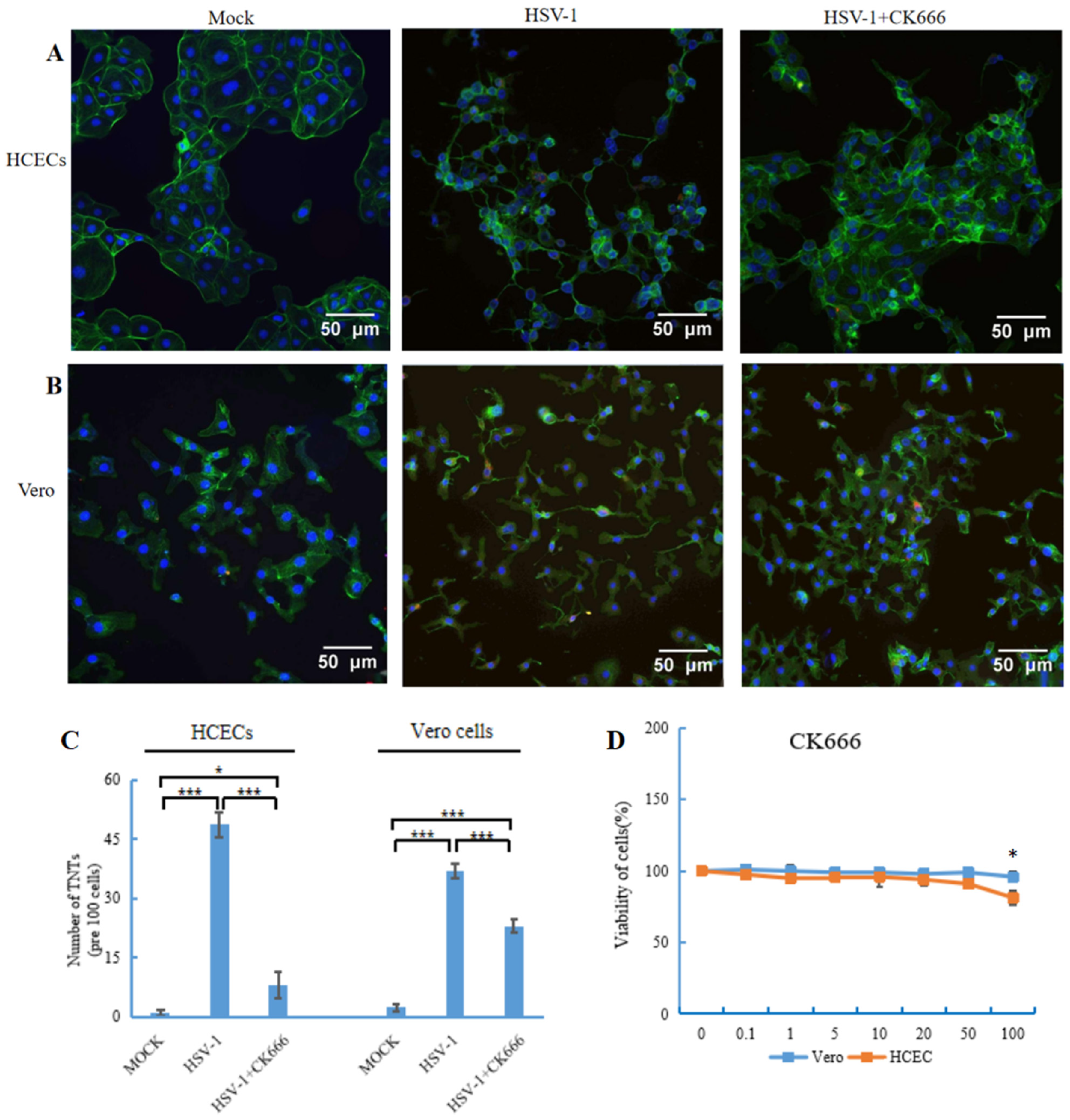
3.3. CK666 Inhibits TNT Formation Induced by HSV-1 Infection
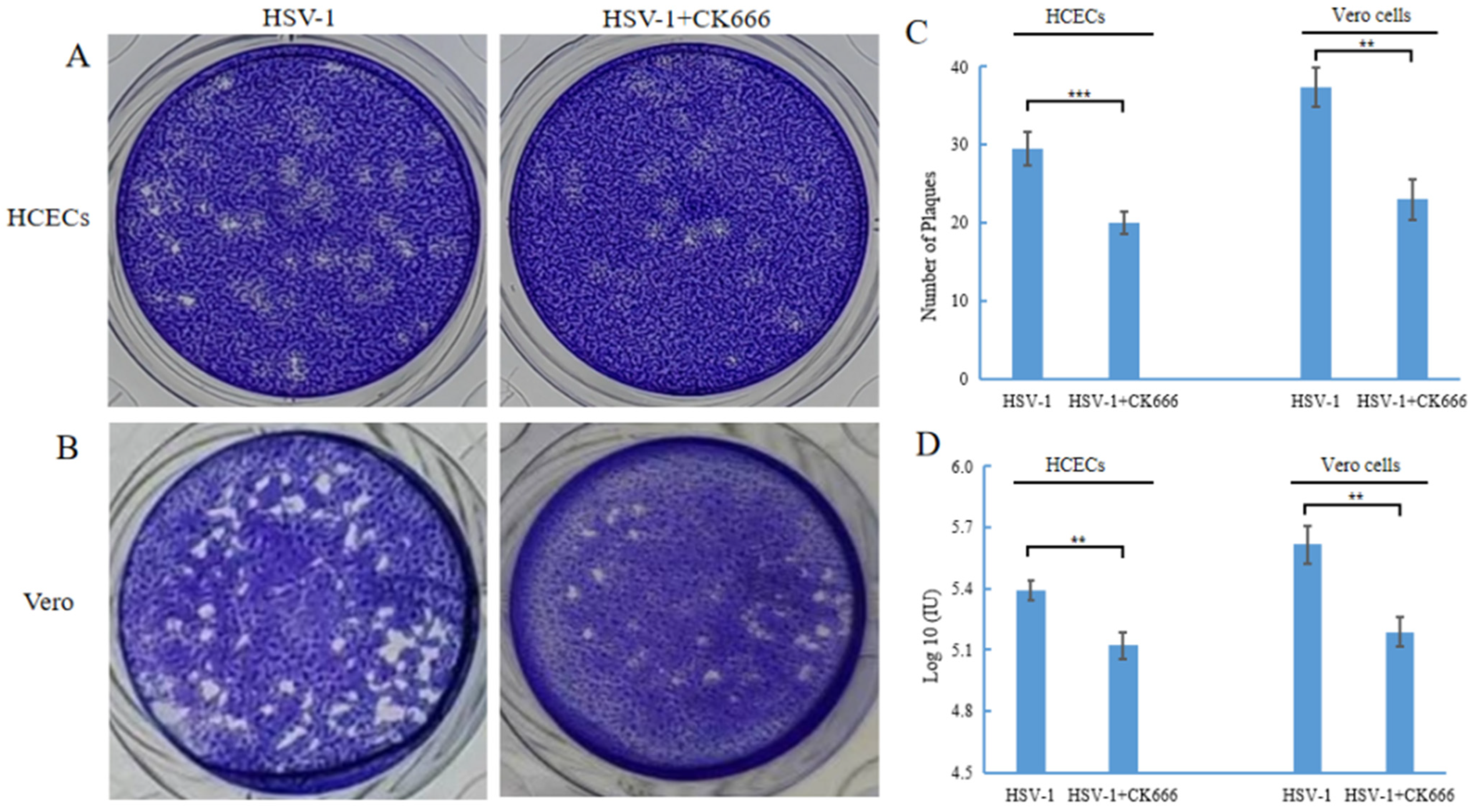
3.4. CK666 Can Weaken Intercellular HSV-1 Spread
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McQuillan, G.M.; Kruszon-Moran, D.; Flagg, E.W.; Paulose-Ram, R. Faculty Opinions recommendation of Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14–49: United States, 2015–2016; National Center for Health Statistics: Atlanta, GA, USA, 2018; pp. 1–8. [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.E.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.-H. Nanotubular Highways for Intercellular Organelle Transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Sowinski, S.; Jolly, C.; Berninghausen, O.; Purbhoo, M.A.; Chauveau, A.; Köhler, K.; Oddos, S.; Eissmann, P.; Brodsky, F.M.; Hopkins, C.; et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 2008, 10, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ganti, K.; Han, J.; Manicassamy, B.; Lowen, A.C. Rab11a mediates cell-cell spread and reassortment of influenza A virus genomes via tunneling nanotubes. PLoS Pathog. 2021, 17, e1009321. [Google Scholar] [CrossRef] [PubMed]
- Jansens, R.J.J.; Broeck, W.V.D.; De Pelsmaeker, S.; Lamote, J.A.S.; Van Waesberghe, C.; Couck, L.; Favoreel, H.W. Pseudorabies Virus US3-Induced Tunneling Nanotubes Contain Stabilized Microtubules, Interact with Neighboring Cells via Cadherins, and Allow Intercellular Molecular Communication. J. Virol. 2017, 91, e00749-17. [Google Scholar] [CrossRef]
- Dagar, S.; Pathak, D.; Oza, H.V.; Mylavarapu, S.V.S. Tunneling nanotubes and related structures: Molecular mechanisms of formation and function. Biochem. J. 2021, 478, 3977–3998. [Google Scholar] [CrossRef] [PubMed]
- Drab, M.; Stopar, D.; Kralj-Iglič, V.; Iglič, A. Inception Mechanisms of Tunneling Nanotubes. Cells 2019, 8, 626. [Google Scholar] [CrossRef]
- Scheiblich, H.; Dansokho, C.; Mercan, D.; Schmidt, S.V.; Bousset, L.; Wischhof, L.; Eikens, F.; Odainic, A.; Spitzer, J.; Griep, A.; et al. Faculty Opinions recommendation of Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell 2021, 184, 5089–5106. [Google Scholar] [CrossRef]
- Hekmatshoar, Y.; Nakhle, J.; Galloni, M.; Vignais, M.L. The role of metabolism and tunneling nanotube-mediated intercellular mitochondria exchange in cancer drug resistance. Biochem. J. 2018, 475, 2305–2328. [Google Scholar] [CrossRef]
- Rotty, J.D.; Wu, C.; Bear, J.E. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat. Rev. Mol. Cell Biol. 2013, 14, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Rouiller, I.; Xu, X.P.; Amann, K.J.; Egile, C.; Nickell, S.; Nicastro, D.; Li, R.; Pollard, T.D.; Volkmann, N.; Hanein, D. The structural basis of actin filament branching by the Arp2/3 complex. J. Cell Biol. 2008, 180, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Chánez-Paredes, S.; Montoya-García, A.; Schnoor, M. Cellular and pathophysiological consequences of Arp2/3 complex inhibition: Role of inhibitory proteins and pharmacological compounds. Cell. Mol. Life Sci. 2019, 76, 3349–3361. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hou, F.; Yao, Y.-F.; Pan, D. Efficient establishment of reactivatable latency by an acyclovir-resistant herpes simplex virus 1 thymidine kinase substitution mutant with reduced neuronal replication. Virology 2021, 556, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Wilson, D.W. HSV-1 Cytoplasmic Envelopment and Egress. Int. J. Mol. Sci. 2020, 21, 5969. [Google Scholar] [CrossRef]
- Hogue, I.B.; Bosse, J.B.; Hu, J.R.; Thiberge, S.Y.; Enquist, L.W. Cellular mechanisms of alpha herpesvirus egress: Live cell fluorescence microscopy of pseudorabies virus exocytosis. PLoS Pathog. 2014, 10, e1004535. [Google Scholar] [CrossRef]
- Amin, I.; Vajeeha, A.; Younas, S.; Afzal, S.; Shahid, M.; Nawaz, R.; Khan, M.U.; Idrees, M. HSV-1 Infection: Role of Viral Proteins and Cellular Receptors. Crit. Rev. Eukaryot. Gene Expr. 2019, 29, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kim, J.H.; Ranjan, P.; Metcalfe, M.G.; Cao, W.; Mishina, M.; Gangappa, S.; Guo, Z.; Boyden, E.S.; Zaki, S.; et al. Influenza virus exploits tunneling nanotubes for cell-to-cell spread. Sci. Rep. 2017, 7, 40360. [Google Scholar] [CrossRef]
- Omsland, M.; Pise-Masison, C.; Fujikawa, D.; Galli, V.; Fenizia, C.; Parks, R.W.; Gjertsen, B.T.; Franchini, G.; Andresen, V. Inhibition of Tunneling Nanotube (TNT) Formation and Human T-cell Leukemia Virus Type 1 (HTLV-1) Transmission by Cytarabine. Sci. Rep. 2018, 8, 11118. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Koganti, R.; Russell, G.; Sharma, A.; Shukla, D. Role of Tunneling Nanotubes in Viral Infection, Neurodegenerative Disease, and Cancer. Front. Immunol. 2021, 12, 680891. [Google Scholar] [CrossRef]
- Okura, T.; Taneno, A.; Oishi, E. Cell-to-Cell Transmission of Turkey Herpesvirus in Chicken Embryo Cells via Tunneling Nanotubes. Avian Dis. 2021, 65, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Mothes, W.; Sherer, N.M.; Jin, J.; Zhong, P. Virus Cell-to-Cell Transmission. J. Virol. 2010, 84, 8360–8368. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Huber, M.T. Directed Egress of Animal Viruses Promotes Cell-to-Cell Spread. J. Virol. 2002, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.A. Release of HSV-1 Cell-Free Virions: Mechanisms, Regulation, and Likely Role in Human-Human Transmission. Viruses 2021, 13, 2395. [Google Scholar] [CrossRef] [PubMed]
- Herrada-Manchón, H.; Celada, L.; Rodríguez-González, D.; Fernández, M.A.; Aguilar, E.; Chiara, M.-D. Three-dimensional bioprinted cancer models: A powerful platform for investigating tunneling nanotube-like cell structures in complex microenvironments. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112357. [Google Scholar] [CrossRef]
- Kolba, M.D.; Dudka, W.; Zaręba-Kozioł, M.; Kominek, A.; Ronchi, P.; Turos, L.; Chroscicki, P.; Wlodarczyk, J.; Schwab, Y.; Klejman, A.; et al. Tunneling nanotube-mediated intercellular vesicle and protein transfer in the stroma-provided imatinib resistance in chronic myeloid leukemia cells. Cell Death Dis. 2019, 10, 817. [Google Scholar] [CrossRef]
- Dash, C.; Saha, T.; Sengupta, S.; Jang, H.L. Inhibition of Tunneling Nanotubes between Cancer Cell and the Endothelium Alters the Metastatic Phenotype. Int. J. Mol. Sci. 2021, 22, 6161. [Google Scholar] [CrossRef]
- Kimura, S.; Hase, K.; Ohno, H. The molecular basis of induction and formation of tunneling nanotubes. Cell Tissue Res. 2012, 352, 67–76. [Google Scholar] [CrossRef][Green Version]
- Delage, E.; Cervantes, D.C.; Pénard, E.; Schmitt, C.; Syan, S.; Disanza, A.; Scita, G.; Zurzolo, C. Differential identity of Filopodia and Tunneling Nanotubes revealed by the opposite functions of actin regulatory complexes. Sci. Rep. 2016, 6, 39632. [Google Scholar] [CrossRef]
- Ljubojevic, N.; Henderson, J.M.; Zurzolo, C. The Ways of Actin: Why Tunneling Nanotubes Are Unique Cell Protrusions. Trends Cell Biol. 2020, 31, 130–142. [Google Scholar] [CrossRef]
- Dilna, A.; Deepak, K.; Damodaran, N.; Kielkopf, C.S.; Kagedal, K.; Ollinger, K.; Nath, S. Amyloid-β induced membrane damage instigates tunneling nanotube-like conduits by p21-activated kinase dependent actin remodulation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166246. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.; Gondaliya, P.; Patel, T. Tunneling Nanotube-Mediated Communication: A Mechanism of Intercellular Nucleic Acid Transfer. Int. J. Mol. Sci. 2022, 23, 5487. [Google Scholar] [CrossRef] [PubMed]
- Baggett, A.W.; Cournia, Z.; Han, M.S.; Patargias, G.; Glass, A.C.; Liu, S.Y.; Nolen, B.J. Structural characterization and computer-aided optimization of a small-molecule inhibitor of the Arp2/3 complex, a key regulator of the actin cytoskeleton. ChemMedChem 2012, 7, 1286–1294. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Shang, K.-T.; Ma, Q.-H.; Dong, Z.-Y.; Chen, Y.-H.; Yao, Y.-F. Herpes Simplex Virus Type 1 Infection Induces the Formation of Tunneling Nanotubes. Microorganisms 2023, 11, 1916. https://doi.org/10.3390/microorganisms11081916
Wang J, Shang K-T, Ma Q-H, Dong Z-Y, Chen Y-H, Yao Y-F. Herpes Simplex Virus Type 1 Infection Induces the Formation of Tunneling Nanotubes. Microorganisms. 2023; 11(8):1916. https://doi.org/10.3390/microorganisms11081916
Chicago/Turabian StyleWang, Jie, Kun-Te Shang, Qiong-Hong Ma, Zhao-Ying Dong, Yi-Hong Chen, and Yu-Feng Yao. 2023. "Herpes Simplex Virus Type 1 Infection Induces the Formation of Tunneling Nanotubes" Microorganisms 11, no. 8: 1916. https://doi.org/10.3390/microorganisms11081916
APA StyleWang, J., Shang, K.-T., Ma, Q.-H., Dong, Z.-Y., Chen, Y.-H., & Yao, Y.-F. (2023). Herpes Simplex Virus Type 1 Infection Induces the Formation of Tunneling Nanotubes. Microorganisms, 11(8), 1916. https://doi.org/10.3390/microorganisms11081916





