Impact of Bacillus cereus on the Human Gut Microbiota in a 3D In Vitro Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Fecal Microbiota and Bacterial Strains
2.2. In Vitro Adhesion of B. cereus to Mucins
2.3. Preparation of Mucin-Coated Electrospun Gelatin Structures
2.4. Preparation of B. cereus Suspensions and Culture Supernatants
2.5. In Vitro Microbiota Culture on the EGM Scaffolds and Addition of B. cereus
2.6. Genomic DNA Extraction
2.7. Real-Time Quantitative PCR
2.8. 16S rRNA Gene Sequencing and Metagenomic Analysis
2.9. Statistical Analysis
3. Results
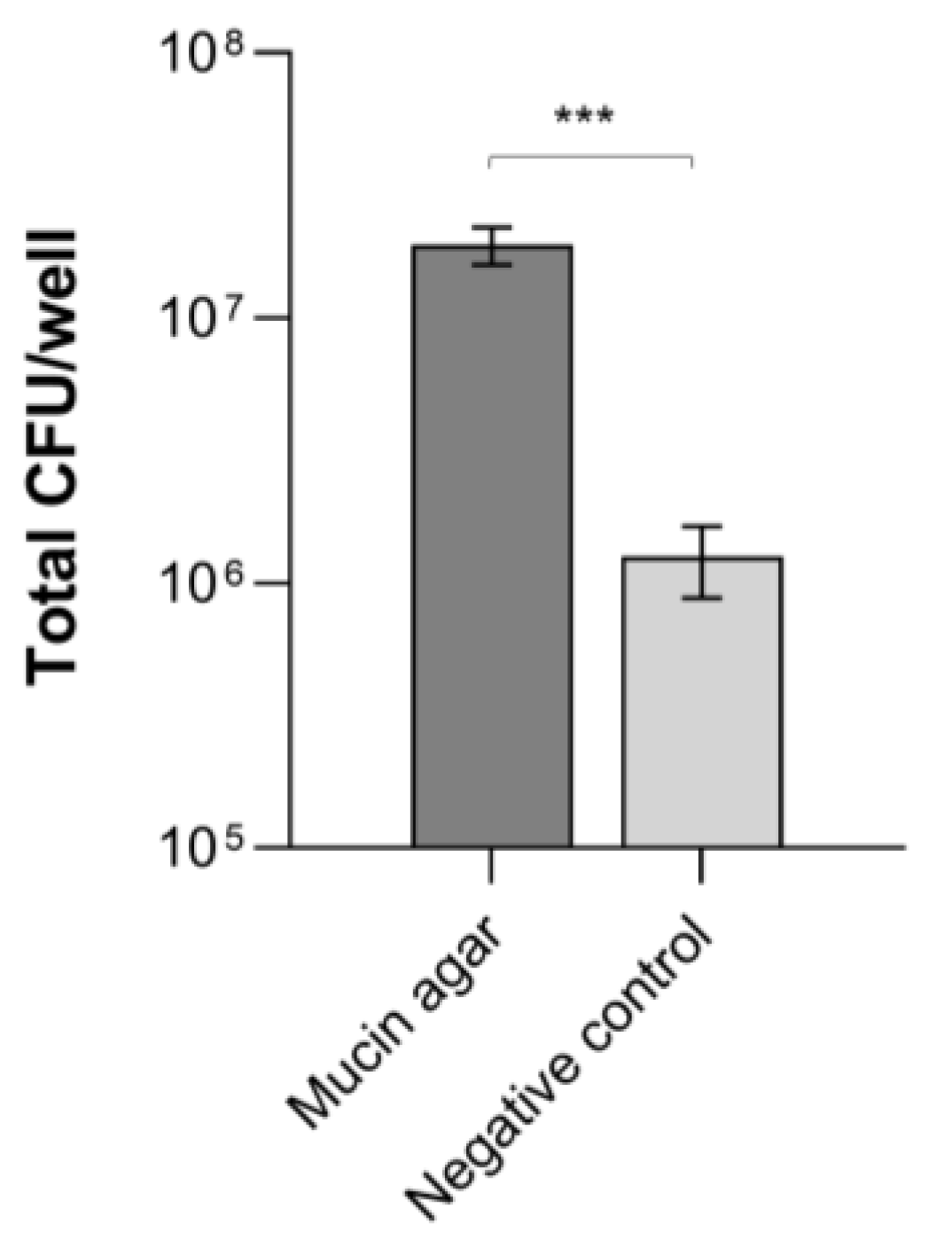
3.1. B. cereus Adhesion to Mucins
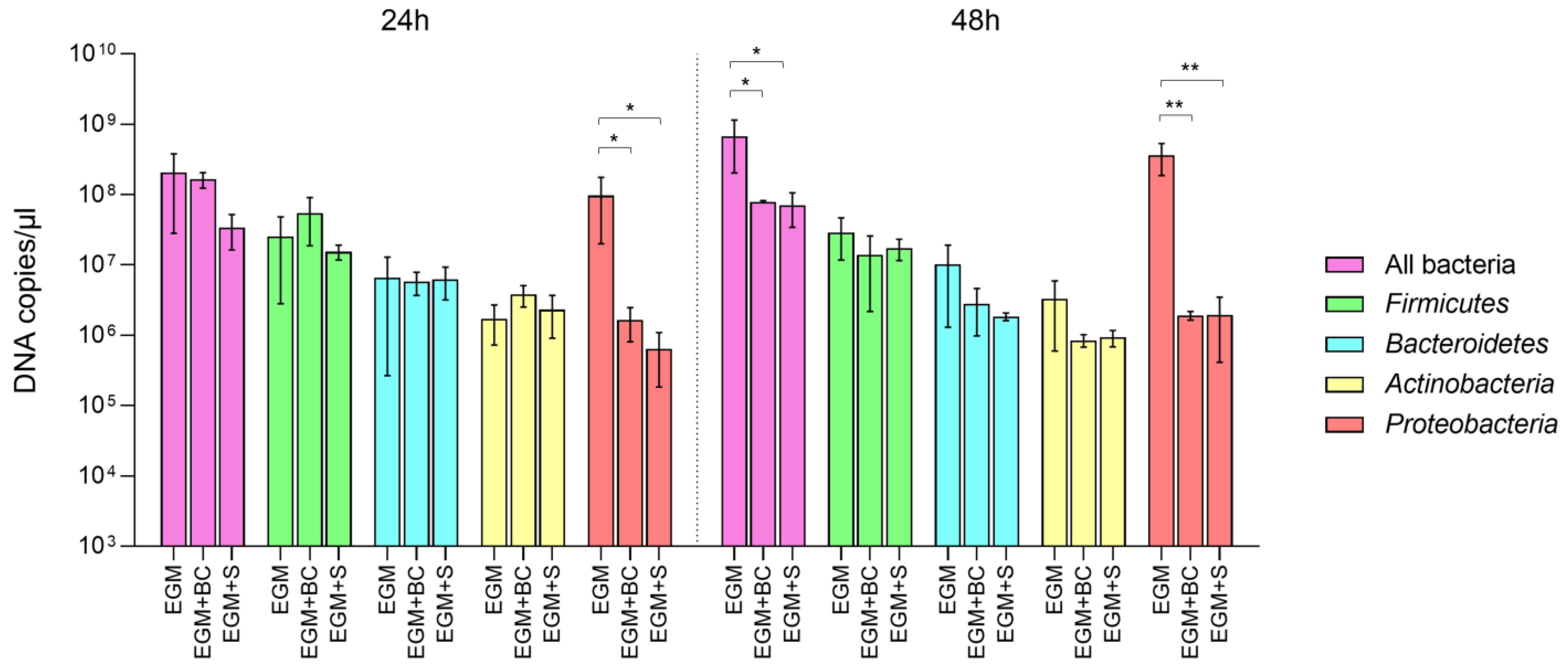
3.2. Absolute Quantification of Bacterial Populations by Real-Time qPCR
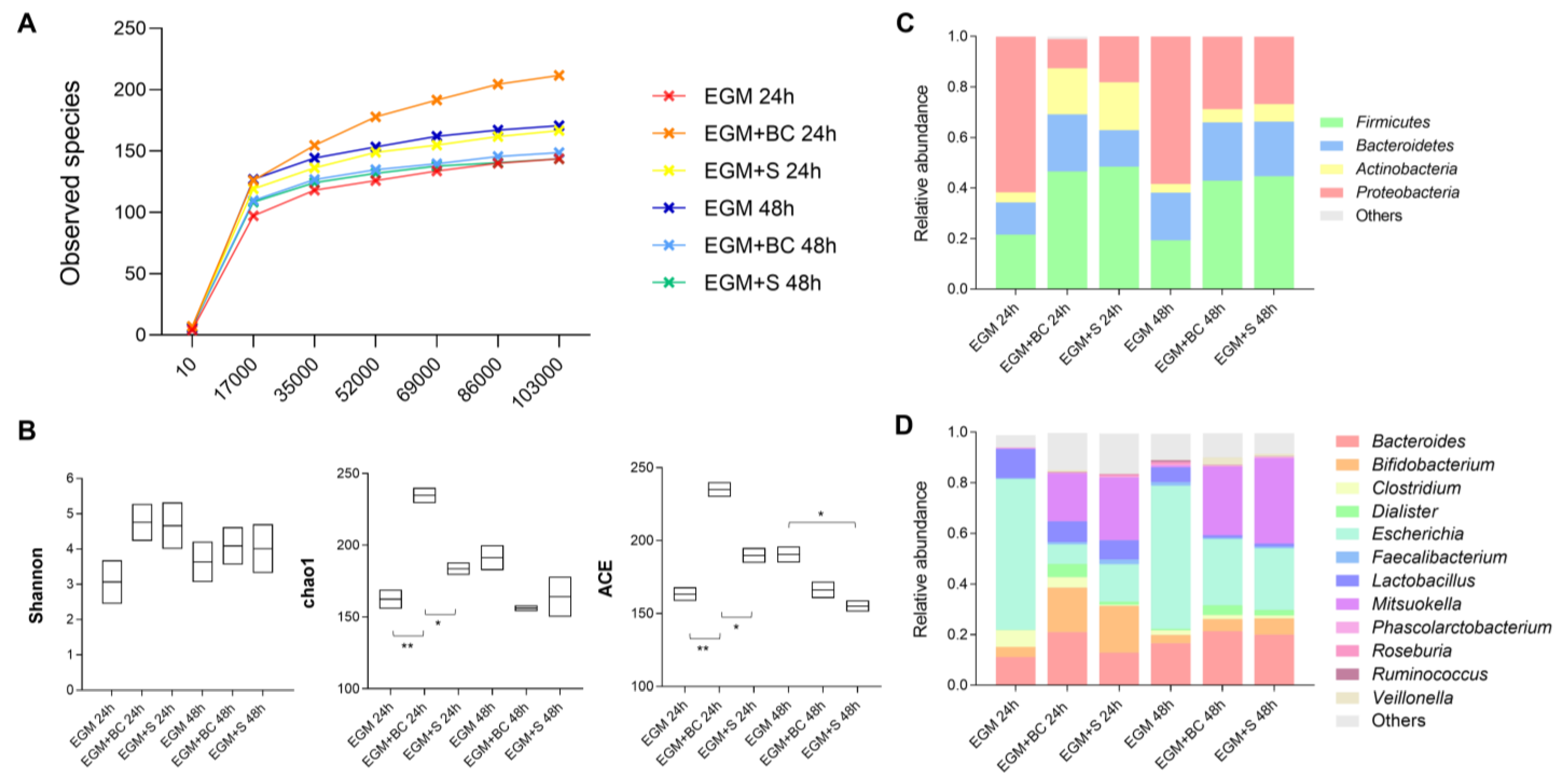
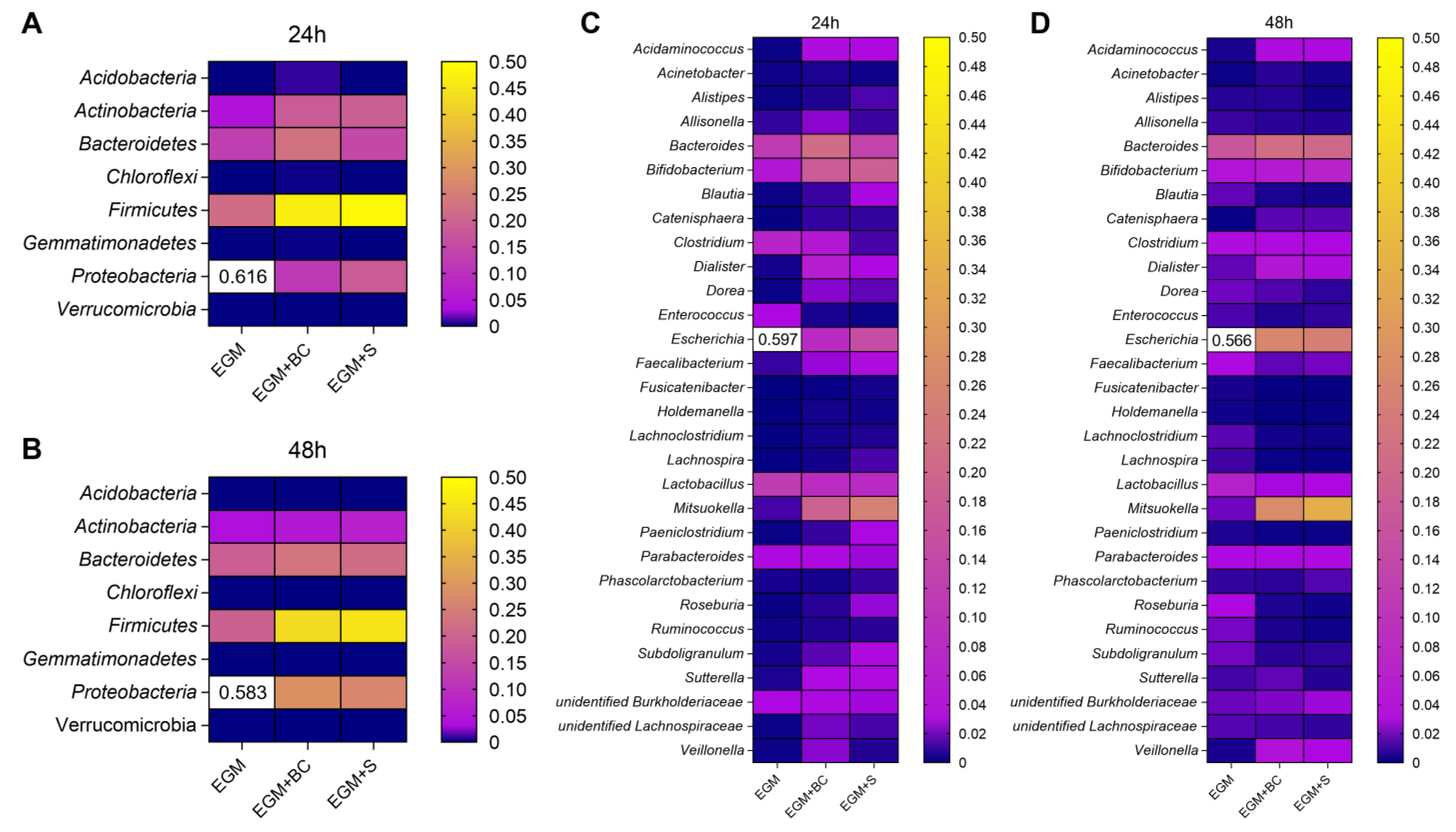
3.3. Qualitative Analysis of Bacterial Populations by 16S rRNA Gene Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Biagini, F.; Daddi, C.; Calvigioni, M.; De Maria, C.; Zhang, Y.S.; Ghelardi, E.; Vozzi, G. Designs and methodologies to recreate in vitro human gut microbiota models. Bio-Des. Manuf. 2022, 6, 298–318. [Google Scholar] [CrossRef]
- Qi, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhai, Q. In vitro models to study human gut-microbiota interactions: Applications, advances, and limitations. Microbiol. Res. 2023, 70, 127336. [Google Scholar] [CrossRef]
- García-Díaz, M.; Cendra, M.D.M.; Alonso-Roman, R.; Urdániz, M.; Torrents, E.; Martínez, E. Mimicking the intestinal host-pathogen interactions in a 3D in vitro model: The role of the mucus layer. Pharmaceutics 2022, 14, 1552. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Garrido, J.J.; Denis, S.; Jiménez-Marín, A.; Beaumont, M.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Pathogen challenge and dietary shift alter microbiota composition and activity in a mucin-associated in vitro model of the piglet colon (MPigut-IVM) simulating weaning transition. Front. Microbiol. 2021, 12, 703421. [Google Scholar] [CrossRef]
- Wiese, M.; Schuren, F.H.J.; Smits, W.K.; Kuijper, E.J.; Ouwens, A.; Heerikhuisen, M.; Vigsnaes, L.; van den Broek, T.J.; de Boer, P.; Montijn, R.C.; et al. 2′-Fucosyllactose inhibits proliferation of Clostridioides difficile ATCC 43599 in the CDi-screen, an in vitro model simulating Clostridioides difficile infection. Front. Cell. Infect. Microbiol. 2022, 12, 991150. [Google Scholar] [CrossRef]
- Biagini, F.; Calvigioni, M.; Lapomarda, A.; Vecchione, A.; Magliaro, C.; De Maria, C.; Montemurro, F.; Celandroni, F.; Mazzantini, D.; Mattioli-Belmonte, M.; et al. A novel 3D in vitro model of the human gut microbiota. Sci. Rep. 2020, 10, 21499. [Google Scholar] [CrossRef]
- Biagini, F.; Calvigioni, M.; De Maria, C.; Magliaro, C.; Montemurro, F.; Mazzantini, D.; Celandroni, F.; Mattioli-Belmonte, M.; Ghelardi, E.; Vozzi, G. Study of the adhesion of the human gut microbiota on electrospun structures. Bioengineering 2022, 9, 96. [Google Scholar] [CrossRef]
- Calvigioni, M.; Panattoni, A.; Biagini, F.; Donati, L.; Mazzantini, D.; Massimino, M.; Daddi, C.; Celandroni, F.; Vozzi, G.; Ghelardi, E. Development of an in vitro model of the gut microbiota enriched in mucus-adhering bacteria. Microbiol. Spectr. 2023, e0033623. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus group: Bacillus species with pathogenic potential. Microbiol. Spectr. 2019, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Calvigioni, M.; Cara, A.; Celandroni, F.; Mazzantini, D.; Panattoni, A.; Tirloni, E.; Bernardi, C.; Pinotti, L.; Stella, S.; Ghelardi, E. Characterization of a Bacillus cereus strain associated with a large feed-related outbreak of severe infection in pigs. J. Appl. Microbiol. 2022, 133, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Slaten, D.D.; Oropeza, R.I.; Werner, S.B. An outbreak of Bacillus cereus food poisoning: Are caterers supervised sufficiently? Public Health Rep. 1992, 107, 477–480. [Google Scholar] [PubMed]
- Gaulin, C.; Viger, Y.B.; Fillion, L. An outbreak of Bacillus cereus implicating a part-time banquet caterer. Can. J. Public Health 2002, 93, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Dierick, K.; Van Coillie, E.; Swiecicka, I.; Meyfroidt, G.; Devlieger, H.; Meulemans, A.; Hoedemaekers, G.; Fourie, L.; Heyndrickx, M.; Mahillon, J. Fatal family outbreak of Bacillus cereus-associated food poisoning. J. Clin. Microbiol. 2005, 43, 4277–4279. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.; Fortunato, F.; Tafuri, S.; Cozza, V.; Chironna, M.; Germinario, C.; Pedalino, B.; Prato, R. Lessons learnt from a birthday party: A Bacillus cereus outbreak, Bari, Italy, January 2012. Ann. dell’Istituto Super. Sanita 2013, 49, 391–394. [Google Scholar] [CrossRef]
- Al-Abri, S.S.; Al-Jardani, A.K.; Al-Hosni, M.S.; Kurup, P.J.; Al-Busaidi, S.; Beeching, N.J. A hospital acquired outbreak of Bacillus cereus gastroenteritis, Oman. J. Infect. Public Health 2011, 4, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Enosi Tuipulotu, D.; Mathur, A.; Ngo, C.; Man, S.M. Bacillus cereus: Epidemiology, virulence factors, and host-pathogen interactions. Trends Microbiol. 2021, 29, 458–471. [Google Scholar] [CrossRef]
- Dietrich, R.; Jessberger, N.; Ehling-Schulz, M.; Märtlbauer, E.; Granum, P.E. The food poisoning toxins of Bacillus cereus. Toxins 2021, 13, 98. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Mazzantini, D.; Celandroni, F.; Salvetti, S.; Gueye, S.A.; Lupetti, A.; Senesi, S.; Ghelardi, E. FlhF is required for swarming motility and full pathogenicity of Bacillus cereus. Front. Microbiol. 2016, 7, 1644. [Google Scholar] [CrossRef] [PubMed]
- Huijboom, L.; Tempelaars, M.; Fan, M.; Zhu, Y.; Boeren, S.; van der Linden, E.; Abee, T. L-tyrosine modulates biofilm formation of Bacillus cereus ATCC 14579. Res. Microbiol. 2023, 18, 104072. [Google Scholar] [CrossRef] [PubMed]
- Mazzantini, D.; Calvigioni, M.; Celandroni, F.; Lupetti, A.; Ghelardi, E. In vitro assessment of probiotic attributes for strains contained in commercial formulations. Sci. Rep. 2022, 12, 21640. [Google Scholar] [CrossRef]
- Sánchez, B.; Arias, S.; Chaignepain, S.; Denayrolles, M.; Schmitter, J.M.; Bressollier, P.; Urdaci, M.C. Identification of surface proteins involved in the adhesion of a probiotic Bacillus cereus strain to mucin and fibronectin. Microbiology 2009, 155 Pt 5, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Tsilia, V.; Van den Abbeele, P.; Van de Wiele, T. Improved in vitro assay for determining the mucin adherence of bacteria sensitive to Triton X-100 treatment. Folia Microbiol. 2015, 60, 435–442. [Google Scholar] [CrossRef]
- Tsilia, V.; Kerckhof, F.M.; Rajkovic, A.; Heyndrickx, M.; Van de Wiele, T. Bacillus cereus NVH 0500/00 can adhere to mucin but cannot produce enterotoxins during gastrointestinal simulation. Appl. Environ. Microbiol. 2015, 82, 289–296. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Mohr, A.K.; Da Riol, C.; Märtlbauer, E. Porcine gastric mucin triggers toxin production of enteropathogenic Bacillus cereus. Infect. Immun. 2019, 87, e00765-18. [Google Scholar] [CrossRef]
- Hatanaka, M.; Nakamura, Y.; Maathuis, A.J.; Venema, K.; Murota, I.; Yamamoto, N. Influence of Bacillus subtilis C-3102 on microbiota in a dynamic in vitro model of the gastrointestinal tract simulating human conditions. Benef. Microbes 2012, 3, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Morita, H.; Aoyagi, Y.; Sasaki, K.; Sasaki, D.; Kondo, A.; Nakamura, T. Effective bifidogenic growth factors cyclo-Val-Leu and cyclo-Val-Ile produced by Bacillus subtilis C-3102 in the human colonic microbiota model. Sci. Rep. 2020, 10, 7591. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, F.; Zhou, L.; Fan, Y.; Zhao, B.; Xi, W.; Wang, C.; Zhu, F.; Ma, X.; Wang, W.; et al. Characterizing serum amino acids in schizophrenic patients: Correlations with gut microbes. J. Psychiatr. Res. 2022, 153, 125–133. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, G.; Byun, M.S.; Lee, J.H.; Yi, D.; Park, H.; Lee, D.Y.; KBASE Research Group. Gut microbiome alterations in preclinical Alzheimer’s disease. PLoS ONE 2022, 17, e0278276. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Fu, Y.; Sun, T.Y.; Jiang, Z.; Miao, Z.; Shuai, M.; Gou, W.; Ling, C.W.; Yang, J.; Wang, J.; et al. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome 2020, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Bamola, V.D.; Kapardar, R.; Lal, B.; Sharma, A.; Chaudhry, R. A metagenomic assessment of gut microbiota in Indian colon cancer patients. J. Cancer Res. Ther. 2022, 18, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Levine, U.Y.; Bearson, S.M.; Stanton, T.B. Mitsuokella jalaludinii inhibits growth of Salmonella enterica serovar Typhimurium. Vet. Microbiol. 2012, 159, 115–122. [Google Scholar] [CrossRef]
- Johansson, M.E.; Jakobsson, H.E.; Holmén-Larsson, J.; Schütte, A.; Ermund, A.; Rodríguez-Piñeiro, A.M.; Arike, L.; Wising, C.; Svensson, F.; Bäckhed, F.; et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 2015, 18, 582–592. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. Biomed. Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Bowers, A.A.; Acker, M.G.; Young, T.S.; Walsh, C.T. Generation of thiocillin ring size variants by prepeptide gene replacement and in vivo processing by Bacillus cereus. J. Am. Chem. Soc. 2012, 134, 10313–10316. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvigioni, M.; Panattoni, A.; Biagini, F.; Donati, L.; Mazzantini, D.; Massimino, M.; Daddi, C.; Celandroni, F.; Vozzi, G.; Ghelardi, E. Impact of Bacillus cereus on the Human Gut Microbiota in a 3D In Vitro Model. Microorganisms 2023, 11, 1826. https://doi.org/10.3390/microorganisms11071826
Calvigioni M, Panattoni A, Biagini F, Donati L, Mazzantini D, Massimino M, Daddi C, Celandroni F, Vozzi G, Ghelardi E. Impact of Bacillus cereus on the Human Gut Microbiota in a 3D In Vitro Model. Microorganisms. 2023; 11(7):1826. https://doi.org/10.3390/microorganisms11071826
Chicago/Turabian StyleCalvigioni, Marco, Adelaide Panattoni, Francesco Biagini, Leonardo Donati, Diletta Mazzantini, Mariacristina Massimino, Costanza Daddi, Francesco Celandroni, Giovanni Vozzi, and Emilia Ghelardi. 2023. "Impact of Bacillus cereus on the Human Gut Microbiota in a 3D In Vitro Model" Microorganisms 11, no. 7: 1826. https://doi.org/10.3390/microorganisms11071826
APA StyleCalvigioni, M., Panattoni, A., Biagini, F., Donati, L., Mazzantini, D., Massimino, M., Daddi, C., Celandroni, F., Vozzi, G., & Ghelardi, E. (2023). Impact of Bacillus cereus on the Human Gut Microbiota in a 3D In Vitro Model. Microorganisms, 11(7), 1826. https://doi.org/10.3390/microorganisms11071826






