Virulence Factor Genes in Invasive Escherichia coli Are Associated with Clinical Outcomes and Disease Severity in Patients with Sepsis: A Prospective Observational Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Design and Patients of the FAPIC Study
2.3. Ethical Considerations
2.4. Microbiological Diagnostics and Bacterial Isolates
2.5. Patient Data Collection
2.6. Whole Genome Sequencing
2.7. Control Samples
2.8. Bioinformatic Analyses
2.9. Statistical Analyses
2.10. Role of the Funding Source
3. Results
3.1. Patient Demographics
3.2. Clinical Isolate Characteristics
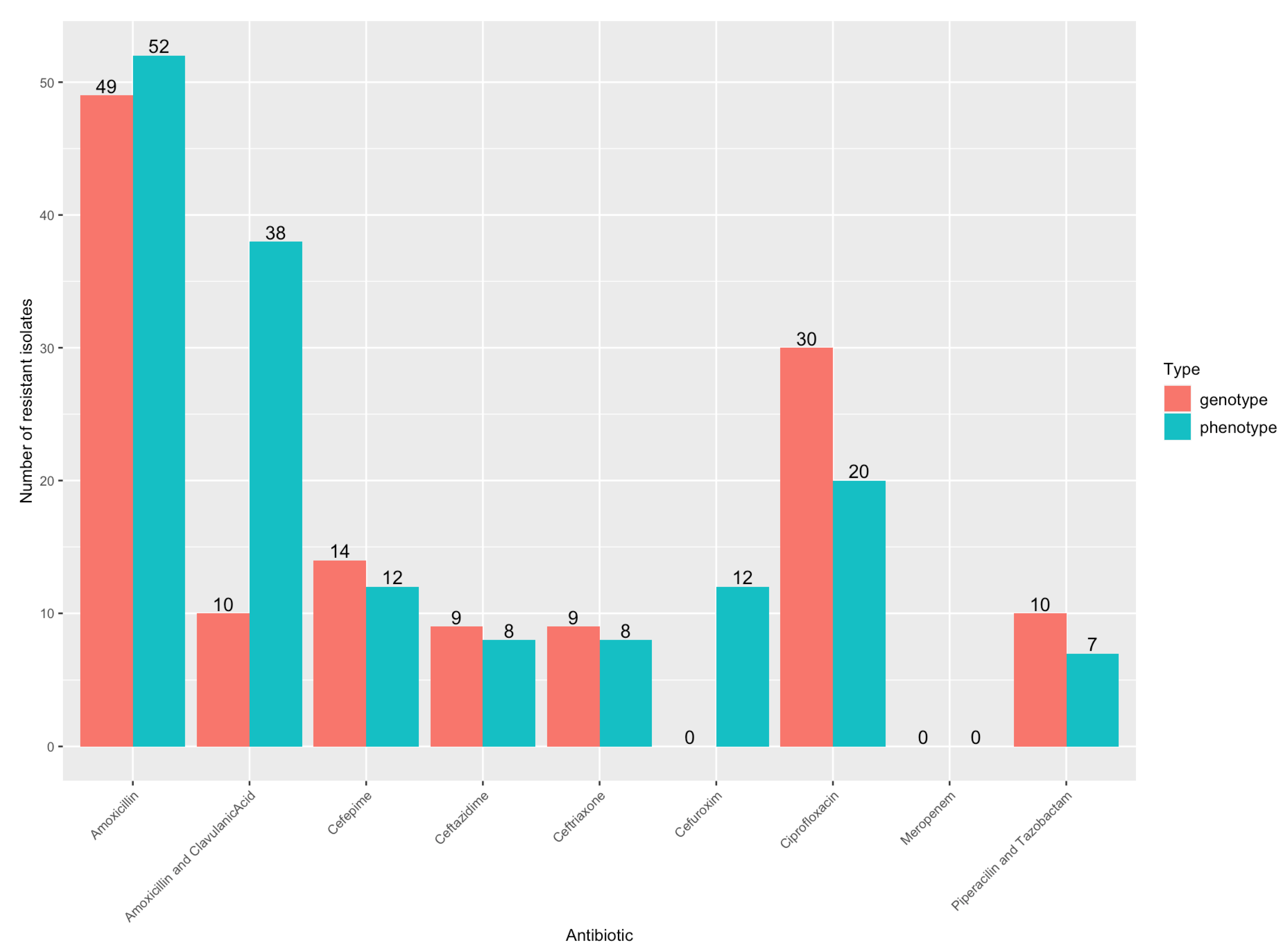
3.3. Antibiotic Resistance
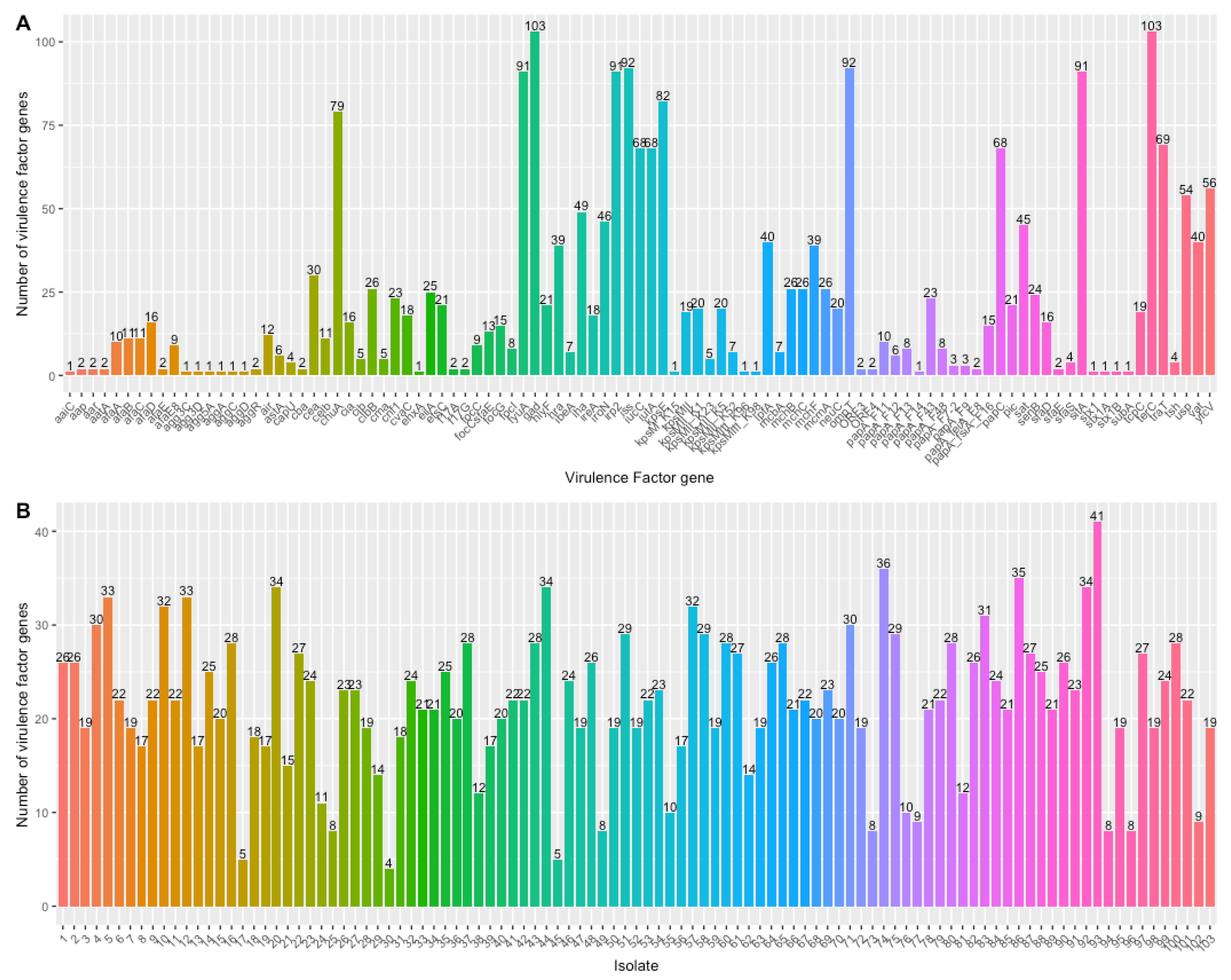
3.4. Virulence Genes
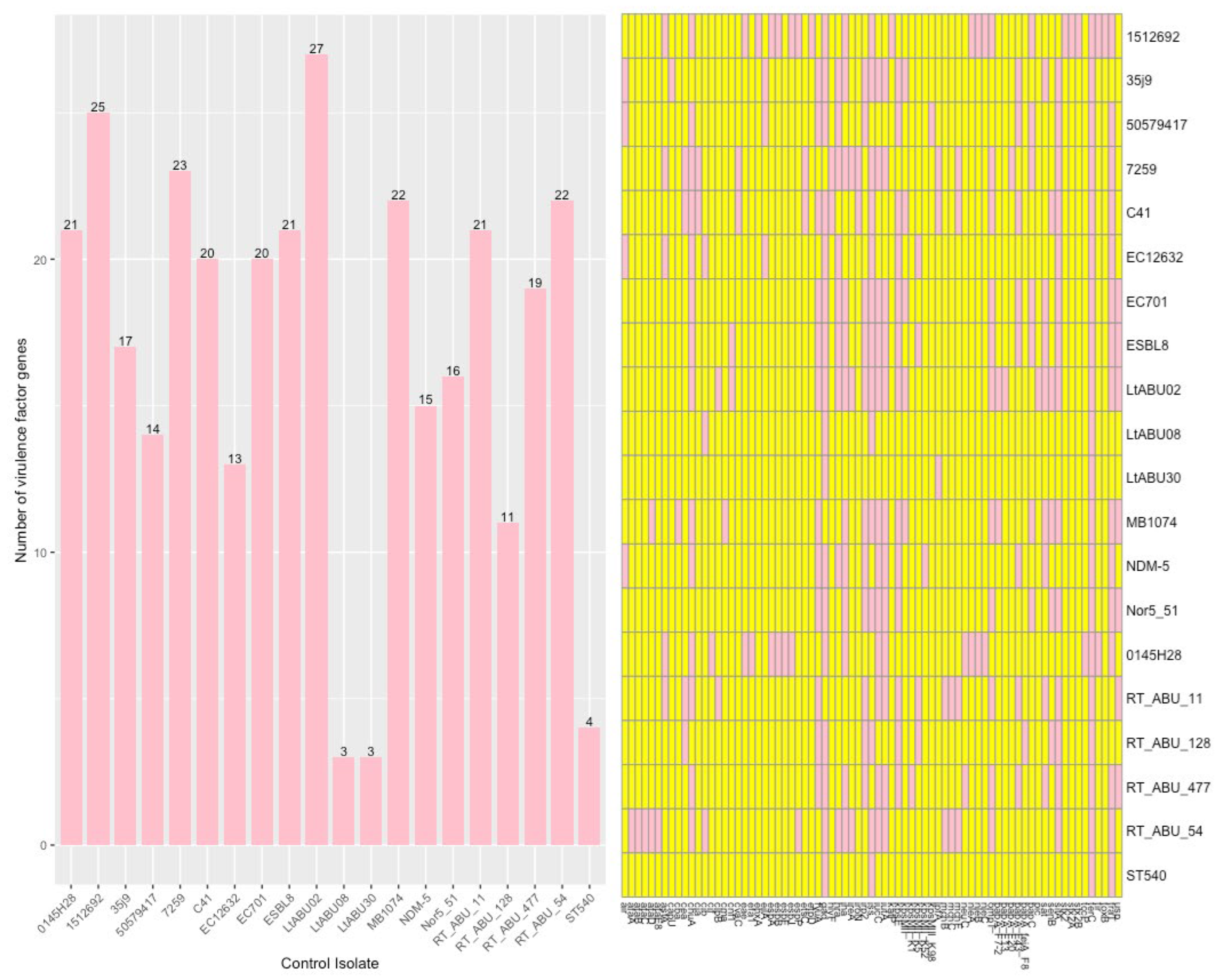
3.5. Control Samples
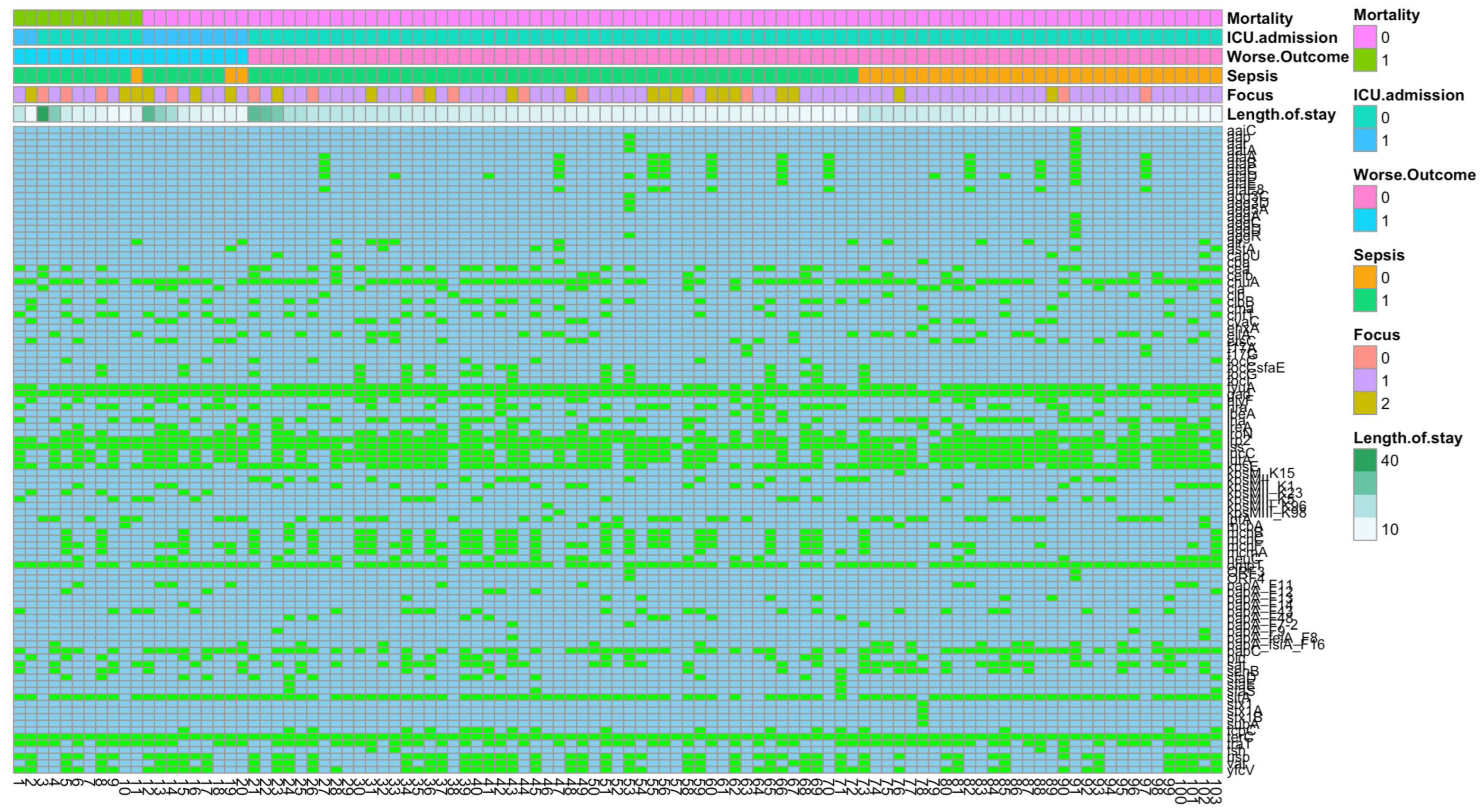
3.6. VF and Clinical Outcome
3.6.1. Mortality
3.6.2. ICU Admission
3.6.3. Sepsis
3.6.4. Infection Focus
3.7. BLAST Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desvaux, M.; Dalmasso, G.; Beyrouthy, R.; Barnich, N.; Delmas, J.; Bonnet, R. Pathogenicity Factors of Genomic Islands in Intestinal and Extraintestinal Escherichia coli. Front. Microbiol. 2020, 11, 2065. [Google Scholar] [CrossRef]
- Fröding, I.; Hasan, B.; Sylvin, I.; Coorens, M.; Nauclér, P.; Giske, C.G. Extended-Spectrum-β-Lactamase- and Plasmid AmpC-Producing Escherichia coli Causing Community-Onset Bloodstream Infection: Association of Bacterial Clones and Virulence Genes with Septic Shock, Source of Infection, and Recurrence. Antimicrob. Agents Chemother. 2020, 64, e02351-19. [Google Scholar] [CrossRef] [PubMed]
- Biggel, M.; Xavier, B.B.; Johnson, J.R.; Nielsen, K.L.; Frimodt-Møller, N.; Matheeussen, V.; Goossens, H.; Moons, P.; Van Puyvelde, S. Horizontally acquired papGII-containing pathogenicity islands underlie the emergence of invasive uropathogenic Escherichia coli lineages. Nat. Commun. 2020, 11, 5968. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Leventogiannis, K.; Norrby-Teglund, A.; Dimopoulos, G.; Pantazi, A.; Orfanos, S.E.; Rovina, N.; Tsangaris, I.; Gkavogianni, T.; Botsa, E.; et al. Macrophage activation-like syndrome: An immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- CDC Collecting Cultures: A Clinician Guide. 2019. Available online: https://www.cdc.gov/antibiotic-use/core-elements/collecting-cultures.html (accessed on 13 August 2022).
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.A.; Korobeynikov, A.; Lapidus, A.; Prjibelski, A.D.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J. Comput. Biol. 2013, 20, 714–737. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206-14. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; Garcìa-Fernandez, A.; Larsen, M.; Lund, O.; Voldby Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- CC BY-NC-SA 3.0 IGO; Global Report on the Epidemiology and Burden of Sepsis: Current Evidence, Identifying Gaps and Future Directions. World Health Organization: Geneva, Switzerland, 2020.
- Barišić, I.; Mitteregger, D.; Hirschl, A.M.; Noehammer, C.; Wiesinger-Mayr, H. High diversity of beta-lactamases in the General Hospital Vienna verified by whole genome sequencing and statistical analysis. Infect. Genet. Evol. 2014, 27, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Qasemi, A.; Rahimi, F.; Katouli, M. Genetic diversity and virulence characteristics of biofilm-producing uropathogenic Escherichia coli. Int. Microbiol. 2022, 25, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Kuskowski, M.A.; Gajewski, A.; Soto, S.; Horcajada, J.P.; De Anta, M.T.J.; Vila, J. Extended virulence genotypes and phylogenetic background of Escherichia coli isolates from patients with cystitis, pyelonephritis, or prostatitis. J. Infect. Dis. 2005, 191, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Logue, C.M.; Johnson, J.R.; Kuskowski, M.A.; Sherwood, J.S.; Barnes, H.J.; DebRoy, C.; Wannemuehler, Y.M.; Obata-Yasuoka, M.; Spanjaard, L.; et al. Associations between multidrug resistance, plasmid content, and virulence potential among extraintestinal pathogenic and commensal Escherichia coli from humans and poultry. Foodborne Pathog. Dis. 2012, 9, 37–46. [Google Scholar] [CrossRef]
- Micenková, L.; Beňová, A.; Frankovičová, L.; Bosák, J.; Vrba, M.; Ševčíková, A.; Kmeťová, M.; Šmajs, D. Human Escherichia coli isolates from hemocultures: Septicemia linked to urogenital tract infections is caused by isolates harboring more virulence genes than bacteraemia linked to other conditions. Int. J. Med. Microbiol. 2017, 307, 182–189. [Google Scholar] [CrossRef]
- Micenková, L.; Bosák, J.; Vrba, M.; Ševčíková, A.; Šmajs, D. Human extraintestinal pathogenic Escherichia coli strains differ in prevalence of virulence factors, phylogroups, and bacteriocin determinants. BMC Microbiol. 2016, 16, 218. [Google Scholar] [CrossRef]
- Huang, W.-C.; Liao, Y.-J.; Hashimoto, M.; Chen, K.-F.; Chu, C.; Hsu, P.-C.; Wang, S.; Teng, C.-H. cjrABC-senB hinders survival of extraintestinal pathogenic E. coli in the bloodstream through triggering complement-mediated killing. J. Biomed. Sci. 2020, 27, 86. [Google Scholar] [CrossRef]
- Diabate, M.; Munro, P.; Garcia, E.; Jacquel, A.; Michel, G.; Obba, S.; Goncalves, D.; Luci, C.; Marchetti, S.; Demon, D.; et al. Escherichia coli α-hemolysin counteracts the anti-virulence innate immune response triggered by the Rho GTPase activating toxin CNF1 during bacteremia. PLoS Pathog. 2015, 11, e1004732. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention Control Healthcare. Associated infections acquired in intensive care units. In Annual Epidemiological Report for 2017; ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- Skjøt-Rasmussen, L.; Ejrnæs, K.; Lundgren, B.; Hammerum, A.M.; Frimodt-Møller, N. Virulence factors and phylogenetic grouping of Escherichia coli isolates from patients with bacteraemia of urinary tract origin relate to sex and hospital- vs. community-acquired origin. Int. J. Med. Microbiol. 2012, 302, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Severinov, K.; Nair, S.K. Microcin C: Biosynthesis and mechanisms of bacterial resistance. Future Microbiol. 2012, 7, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.P.; Pandey, A.K.; Hesp, R.J.; Belogiannis, K.; Laver, J.R.; Shone, C.C.; Read, R.C. Genomes of Escherichia coli bacteraemia isolates originating from urinary tract foci contain more virulence-associated genes than those from non-urinary foci and neutropaenic hosts. J. Infect. 2018, 77, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Mora-Rillo, M.; Fernández-Romero, N.; Francisco, C.N.-S.; Díez-Sebastián, J.; Romero-Gómez, M.P.; Fernández, F.A.; López, J.R.A.; Mingorance, J. Impact of virulence genes on sepsis severity and survival in Escherichia coli bacteremia. Virulence 2015, 6, 93–100. [Google Scholar] [CrossRef]
- Freire, C.A.; Silva, R.M.; Ruiz, R.C.; Pimenta, D.C.; Bryant, J.A.; Henderson, I.R.; Barbosa, A.S.; Elias, W.P. Secreted Autotransporter Toxin (Sat) Mediates Innate Immune System Evasion. Front. Immunol. 2022, 13, 844878. [Google Scholar] [CrossRef]
- Freire, C.A.; Santos, A.C.M.; Pignatari, A.C.; Silva, R.M.; Elias, W.P. Serine protease autotransporters of Enterobacteriaceae (SPATEs) are largely distributed among Escherichia coli isolated from the bloodstream. Braz. J. Microbiol. 2020, 51, 447–454. [Google Scholar] [CrossRef]
- Guignot, J.; Chaplais, C.; Coconnier-Polter, M.H.; Servin, A.L. The secreted autotransporter toxin, Sat, functions as a virulence factor in Afa/Dr diffusely adhering Escherichia coli by promoting lesions in tight junction of polarized epithelial cells. Cell. Microbiol. 2007, 9, 204–221. [Google Scholar] [CrossRef]
- Vieira, P.C.G.; Espinoza-Culupú, A.O.; Nepomuceno, R.; Alves, M.R.; Lebrun, I.; Elias, W.P.; Ruiz, R.C. Secreted autotransporter toxin (Sat) induces cell damage during enteroaggregative Escherichia coli infection. PLoS ONE 2020, 15, e0228959. [Google Scholar] [CrossRef]
- Johnson, J.R.; Jelacic, S.; Schoening, L.M.; Clabots, C.; Shaikh, N.; Mobley, H.L.; Tarr, P.I. The IrgA homologue adhesin Iha is an Escherichia coli virulence factor in murine urinary tract infection. Infect. Immun. 2005, 73, 965–971. [Google Scholar] [CrossRef]
- Clark, J.R.; Maresso, A.M. Comparative Pathogenomics of Escherichia coli: Polyvalent Vaccine Target Identification through Virulome Analysis. Infect. Immun. 2021, 89, e0011521. [Google Scholar] [CrossRef]
- Johnson, J.R.; Russo, T.A.; Tarr, P.I.; Carlino, U.; Bilge, S.S.; Vary, J.C., Jr.; Stell, A.L. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroN (E. coli), among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 2000, 68, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Merino, I.; Porter, S.B.; Johnston, B.D.; Clabots, C.; Shaw, E.; Horcajada, J.P.; Cantón, R.; Ruiz-Garbajosa, P.; Johnson, J.R. Virulence genes and subclone status as markers of experimental virulence in a murine sepsis model among Escherichia coli sequence type 131 clinical isolates from Spain. PLoS ONE 2017, 12, e0188838. [Google Scholar] [CrossRef]
- Parkkinen, J.; Hacker, J.; Korhonen, T.K. Enhancement of tissue plasminogen activator-catalyzed plasminogen activation by Escherichia coli S fimbriae associated with neonatal septicaemia and meningitis. Thromb. Haemost. 1991, 65, 483–486. [Google Scholar]
- Sokolowska-Köhler, W.; Schönian, G.; Bollmann, R.; Schubert, A.; Parschau, J.; Seeberg, A.; Presber, W. Occurrence of S and F1C/S-related fimbrial determinants and their expression in Escherichia coli strains isolated from extraintestinal infections. FEMS Immunol. Med. Microbiol. 1997, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Roos, V.; Schembri, M.A.; Ulett, G.C.; Klemm, P. Asymptomatic bacteriuria Escherichia coli strain 83972 carries mutations in the foc locus and is unable to express F1C fimbriae. Microbiology 2006, 152 Pt 6, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-T.; Cheng, M.-F.; Tseng, F.-C.; Chen, Y.-S.; Lee, S.S.-J.; Chang, T.-H.; Lin, H.-H.; Hung, C.-H.; Wang, J.-L. Bloodstream infection with extended-spectrum beta-lactamase-producing Escherichia coli: The role of virulence genes. J. Microbiol. Immunol. Infect. 2019, 52, 947–955. [Google Scholar] [CrossRef]
- Daga, A.P.; Koga, V.L.; Soncini, J.G.M.; De Matos, C.M.; Perugini, M.R.E.; Pelisson, M.; Kobayashi, R.; Vespero, E.C. Escherichia coli Bloodstream Infections in Patients at a University Hospital: Virulence Factors and Clinical Characteristics. Front. Cell. Infect. Microbiol. 2019, 9, 191. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Mingorance, J.; Fernández-Romero, N.; Serrano, L.; López-Cerero, L.; Pascual, A. Outcome of bacteraemia due to extended-spectrum β-lactamase-producing Escherichia coli: Impact of microbiological determinants. J. Infect. 2013, 67, 27–34. [Google Scholar] [CrossRef]
- Díaz, J.M.; Dozois, C.M.; Avelar-González, F.J.; Hernández-Cuellar, E.; Pokharel, P.; De Santiago, A.S.; Guerrero-Barrera, A.L. The Vacuolating Autotransporter Toxin (Vat) of Escherichia coli Causes Cell Cytoskeleton Changes and Produces Non-lysosomal Vacuole Formation in Bladder Epithelial Cells. Front. Cell. Infect. Microbiol. 2020, 10, 299. [Google Scholar] [CrossRef]
- Nichols, K.B.; Totsika, M.; Moriel, D.G.; Lo, A.W.; Yang, J.; Wurpel, D.J.; Rossiter, A.E.; Strugnell, R.A.; Henderson, I.R.; Ulett, G.C.; et al. Molecular Characterization of the Vacuolating Autotransporter Toxin in Uropathogenic Escherichia coli. J. Bacteriol. 2016, 198, 1487–1498. [Google Scholar] [CrossRef]
- Kang, H.; Thomas, R.M. Bacteria and Sepsis: Microbiome to the Rescue? J. Clin. Med. 2021, 10, 3578. [Google Scholar] [CrossRef]
- Miller, W.D.; Keskey, R.; Alverdy, J.C. Sepsis and the Microbiome: A Vicious Cycle. J. Infect. Dis. 2021, 223 (Suppl. S2), S264–S269. [Google Scholar] [CrossRef]
- Neugent, M.L.; Hulyalkar, N.V.; Nguyen, V.H.; Zimmern, P.E.; De Nisco, N.J. Advances in Understanding the Human Urinary Microbiome and Its Potential Role in Urinary Tract Infection. mBio 2020, 11, e00218-20. [Google Scholar] [CrossRef]
- Horwitz, D.; McCue, T.; Mapes, A.C.; Ajami, N.J.; Petrosino, J.F.; Ramig, R.F.; Trautner, B.W. Decreased microbiota diversity associated with urinary tract infection in a trial of bacterial interference. J. Infect. 2015, 71, 358–367. [Google Scholar] [CrossRef]
- Lipworth, S.; Vihta, K.D.; Chau, K.; Barker, L.; George, S.; Kavanagh, J.; Davies, T.; Vaughan, A.; Andersson, M.; Jeffery, K.; et al. Ten-year longitudinal molecular epidemiology study of Escherichia coli Klebsiella species bloodstream infections in Oxfordshire, UK. Genome Med. 2021, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Jauneikaite, E.; Honeyford, K.; Blandy, O.; Mosavie, M.; Pearson, M.; Ramzan, F.A.; Ellington, M.J.; Parkhill, J.; Costelloe, C.E.; Woodford, N.; et al. Bacterial genotypic and patient risk factors for adverse outcomes in Escherichia coli bloodstream infections: A prospective molecular epidemiological study. J. Antimicrob. Chemother. 2022, 77, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Merino, I.; Hernández-García, M.; Turrientes, M.-C.; Pérez-Viso, B.; López-Fresneña, N.; Diaz-Agero, C.; Maechler, F.; Fankhauser-Rodriguez, C.; Kola, A.; Schrenzel, J.; et al. Emergence of ESBL-producing Escherichia coli ST131-C1-M27 clade colonizing patients in Europe. J. Antimicrob. Chemother. 2018, 73, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.W. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin. Microbiol. Infect. 2014, 20, 380–390. [Google Scholar] [CrossRef]
- Da Silva, G.J.; Mendonça, N. Association between antimicrobial resistance and virulence in Escherichia coli. Virulence 2012, 3, 18–28. [Google Scholar] [CrossRef]
| Total, n = 113 Patients | ||
|---|---|---|
| Age (Median, IQR) | 74 (66.5–84) | |
| Sex (female) | 54 (52.4%) | |
| Diagnosis of Infection | ||
| Secondary BSI | 98 (86.7%) | |
| Urosepsis | 75 (66.4%) | |
| Intra-abdominal infection | 23 (20.4%) | |
| 11 (47.8%) | |
| 8 (34.7%) | |
| 1 (4.3%) | |
| 1 (4.3%) | |
| 1 (4.3%) | |
| 1 (4.3%) | |
| Primary BSI | 15 (12.4%) | |
| Endocarditis | 1 (0.8%) | |
| SOFA score | ||
| Median (IQR) | 2 (1–4) | |
| SOFA = 0 | 10 (8.8%) | |
| SOFA = 1 | 22 (19.5%) | |
| SOFA = 2 | 29 (25.7%) | |
| SOFA = 3 | 22 (19.5%) | |
| SOFA = 4 | 16 (14.2%) | |
| SOFA = 5 | 7 (6.2%) | |
| SOFA = 6 | 4 (3.5%) | |
| SOFA = 7 | 1 (0.8%) | |
| SOFA = 8 | 2 (1.8%) | |
| ICU admission | 14 (12.4%) | |
| All-cause in-hospital mortality | 11 (9.7%) | |
| Length of stay (Median, IQR) | 6 (4–11) days | |
| Mortality | Survived | Died | p-Value | Relative Risk | p-Value | |
|---|---|---|---|---|---|---|
| kpsMII_K23 | Absent | 90 (91.8) | 8 (8.2) | 0.004 | 7.35 (2.77–19.51) | 0.009 |
| Present | 2 (40.0) | 3 (60.0) | ||||
| ICU Admission | Not Admitted | Admitted | p-Value | Relative Risk | p-Value | |
| kpsMII_K23 | Absent | 90 (91.8) | 8 (8.2) | 0.004 | 7.35 (2.77–19.51) | 0.009 |
| Present | 2 (40.0) | 3 (60.0) | ||||
| cvaC | Absent | 79 (92.9) | 6 (7.1) | 0.030 | 3.94 (1.35–11.50) | 0.026 |
| Present | 13 (72.2) | 5 (27.8) | ||||
| Sepsis | No | Yes | p-Value | Relative Risk | p-Value | |
| mchB | Absent | 32 (41.6) | 45 (58.4) | 0.003 | 1.58 (1.27–1.97) | 0.001 |
| Present | 2 (7.7) | 24 (92.3) | ||||
| mchC | Absent | 32 (41.6) | 45 (58.4) | 0.003 | 1.58 (1.27–1.97) | 0.001 |
| Present | 2 (7.7) | 24 (92.3) | ||||
| papA_fsiA_F16 | Absent | 24 (27.3) | 64 (72.7) | 0.007 | 0.46 (0.22–0.95) | 0.005 |
| Present | 10 (66.7) | 5 (33.3) | ||||
| sat | Absent | 13 (22.4) | 45 (77.6) | 0.017 | 0.69 (0.51–0.93) | 0.011 |
| Present | 21 (46.7) | 24 (53.3) | ||||
| senB | Absent | 21 (26.6) | 58 (73.4) | 0.023 | 0.62 (0.40–0.98) | 0.016 |
| Present | 13 (54.2) | 11 (45.8) | ||||
| iucC | Absent | 6 (17.1) | 29 (82.9) | 0.025 | 0.71 (0.55–0.91) | 0.014 |
| Present | 28 (41.2) | 40 (58.8) | ||||
| iutA | Absent | 6 (17.1) | 29 (82.9) | 0.025 | 0.71 (0.55–0.91) | 0.014 |
| Present | 28 (41.2) | 40 (58.8) | ||||
| iha | Absent | 12 (22.2) | 42 (77.8) | 0.025 | 0.71 (0.53–0.95) | 0.016 |
| Present | 22 (44.9) | 27 (55.1) | ||||
| sfaD | Absent | 33 (38.0) | 54 (62.0) | 0.029 | 1.51 (1.23–1.86) | 0.010 |
| Present | 1 (6.3) | 15 (94.7) | ||||
| cnf1 | Absent | 31 (38.8) | 49 (61.2) | 0.040 | 1.42 (1.12–1.80) | 0.019 |
| Present | 3 (13.0) | 20 (87.0) | ||||
| focG | Absent | 33 (37.5) | 55 (62.5) | 0.040 | 1.49 (1.21–1.84) | 0.016 |
| Present | 1 (6.7) | 14 (93.3) | ||||
| vat | Absent | 26 (41.3) | 37 (58.7) | 0.043 | 1.36 (1.05–1.76) | 0.026 |
| Present | 8 (20.0) | 32 (80.0) | ||||
| clbB | Absent | 30 (39.0) | 47 (61.0) | 0.048 | 1.39 (1.09–1.77) | 0.026 |
| Present | 4 (15.4) | 22 (84.6) | ||||
| mcmA | Absent | 30 (39.0) | 47 (61.0) | 0.049 | 1.39 (1.09–1.77) | 0.026 |
| Present | 4 (15.4) | 22 (84.6) |
| Infection Focus | Bloodstream Infection | Urosepsis | Abdominal Sepsis | p-Value | |
|---|---|---|---|---|---|
| papC | Absent | 9 (25.7) | 15 (42.9) | 11 (31.4) | 0.001354 |
| Present | 5 (7.4) | 53 (77.9) | 10 (14.7) | ||
| papA_fsiA_F16 | Absent | 14 (15.9) | 53 (60.2) | 21 (23.9) | 0.01091 |
| Present | 0 (0.0) | 15 (100.0) | 0 (0.0) | ||
| celb | Absent | 10 (10.9) | 61 (66.3) | 21 (22.8) | 0.02706 |
| Present | 4 (36.4) | 7 (63.6) | 0 (0.0) | ||
| f17A | Absent | 12 (11.9) | 68 (67.3) | 21 (20.8) | 0.001529 |
| Present | 2 (100.0) | 0 (0.0) | 0 (0.0) | ||
| f17G | Absent | 12 (11.9) | 68 (67.3) | 21 (20.8) | 0.001529 |
| Present | 2 (100.0) | 0 (0.0) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Onofrio, V.; Cartuyvels, R.; Messiaen, P.E.A.; Barišić, I.; Gyssens, I.C. Virulence Factor Genes in Invasive Escherichia coli Are Associated with Clinical Outcomes and Disease Severity in Patients with Sepsis: A Prospective Observational Cohort Study. Microorganisms 2023, 11, 1827. https://doi.org/10.3390/microorganisms11071827
D’Onofrio V, Cartuyvels R, Messiaen PEA, Barišić I, Gyssens IC. Virulence Factor Genes in Invasive Escherichia coli Are Associated with Clinical Outcomes and Disease Severity in Patients with Sepsis: A Prospective Observational Cohort Study. Microorganisms. 2023; 11(7):1827. https://doi.org/10.3390/microorganisms11071827
Chicago/Turabian StyleD’Onofrio, Valentino, Reinoud Cartuyvels, Peter E. A. Messiaen, Ivan Barišić, and Inge C. Gyssens. 2023. "Virulence Factor Genes in Invasive Escherichia coli Are Associated with Clinical Outcomes and Disease Severity in Patients with Sepsis: A Prospective Observational Cohort Study" Microorganisms 11, no. 7: 1827. https://doi.org/10.3390/microorganisms11071827
APA StyleD’Onofrio, V., Cartuyvels, R., Messiaen, P. E. A., Barišić, I., & Gyssens, I. C. (2023). Virulence Factor Genes in Invasive Escherichia coli Are Associated with Clinical Outcomes and Disease Severity in Patients with Sepsis: A Prospective Observational Cohort Study. Microorganisms, 11(7), 1827. https://doi.org/10.3390/microorganisms11071827






